Abstract
Background
Although both obesity and the metabolic syndrome (MetS) are known risk factors for decline in physical function, the joint association of obesity and metabolic alterations with risk of incident mobility limitation is unknown.
Methods
Data are from 2,984 women and men aged 70–79 years participating in the Health, Aging, and Body Composition Study without mobility limitation at baseline. Obesity was defined as body mass index greater than or equal to 30 kg/m2 and the MetS as meeting greater than or equal to 3 of the ATP III criteria. Mobility limitation was defined as any difficulty walking one-quarter mile or climbing 10 steps during two consecutive semiannual assessments for more than 6.5 years.
Results
Incidence of mobility limitation was 55% in women and 44% in men. In women, adjusted risk of developing mobility limitation was progressively greater in nonobese participants with the MetS (hazard ratio [HR] = 1.49, 95% confidence interval [CI] = 1.24–1.80), obese participants without the MetS (HR = 1.95, 95% CI = 1.51–2.53), and obese participants with the MetS (HR = 2.16, 95% CI = 1.78–2.63) relative to the nonobese without the MetS. In men, the corresponding adjusted HRs (95% CI) were 1.07 (0.87–1.32), 1.64 (1.19–2.25), and 1.41 (1.12–1.78). Elevated inflammatory markers partly explained the association between obesity, the MetS, and mobility limitation, particularly in nonobese and obese participants with the MetS.
Conclusions
Obesity itself, independent of its metabolic consequences, is a risk factor for mobility limitation among obese older adults. In addition, having the MetS increases the risk of functional decline in older nonobese women but not in men.
Keywords: Obesity, Metabolic syndrome, Mobility limitation, Inflammation, Older people
THE prevalence of overweight and obesity has increased considerably as consequences of excess energy intake and sedentary lifestyle in a modern society. The negative impact of obesity on the health and functioning of older adults is widely acknowledged (1). Obesity is a known risk factor for several chronic conditions, including type 2 diabetes, heart disease, and osteoarthritis (2,3). In addition, obesity predicts functional decline and future disability in older persons (4–7). Obesity can hamper mobility directly as the excess body weight carried in weight-bearing activities, such as walking and stair climbing, increases the burden to lower extremity muscles and joints and demands on the cardiorespiratory system.
Obesity, especially abdominal obesity, is also strongly associated with insulin resistance, dyslipidemia, and the metabolic syndrome (MetS) (8). The MetS is a cluster of cardiovascular risk factors, which is also associated with poorer physical functioning and predicts mobility limitation (9–13). The prevalence of the MetS is higher in obese persons even when the role of high waist circumference is factored out (14). However, it is also true that many obese persons show little or no metabolic alterations (15,16) and many nonobese people (body mass index [BMI] <30 kg/m2) display characteristic features of the MetS (16–18). Because few studies have examined the independent and joint associations of obesity and the MetS with physical function, whether obesity “per se” or its metabolic consequences constitutes the primary threat to mobility is unknown.
Given the known biomechanical consequences of excess weight, we hypothesize that among initially well-functioning older adults, obesity is a strong independent risk factor for mobility limitation, and the presence of the MetS confers additional risk because it is associated with excess cardiovascular morbidity. Because both the obesity and the MetS are associated with a proinflammatory state (19,20), which also poses a threat to physical function and mobility (21), we hypothesize that elevated inflammatory markers is one of the mechanisms that account for the association between obesity, the MetS, and mobility limitation. Thus, this prospective study aims to examine the independent and joint associations of obesity and the MetS with risk of developing mobility limitations and the role of inflammatory markers in mediating these associations in older adults.
METHODS
Study Population
The Health, Aging, and Body Composition (Health ABC) Study is a longitudinal cohort study consisting of 3,075 well-functioning, 70- to 79-year-old black and white men and women (22,23). Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible community-dwelling black residents in designated ZIP code areas surrounding Memphis, Tennessee, and Pittsburgh, Pennsylvania. Participants were eligible if they reported no difficulty walking one quarter of a mile, going up 10 steps without resting, or performing basic activities of daily living. Participants were excluded if they reported a history of active treatment for cancer in the prior 3 years, planned to move out of the study area within 3 years, or were enrolled in a lifestyle intervention.
Baseline data, collected between April 1997 and June 1998, included an in-person interview and a clinic-based examination, with evaluation of body composition, clinical and subclinical diseases, and physical functioning. Six and a half years of follow-up was used for this study. Of the 3,075 participants, we excluded those missing data on the MetS (n = 39) or mobility limitation (n = 2) and those who died within the first 6 months (n = 11) or those who were underweight (BMI <18.5 kg/m2) at baseline (n = 39), yielding a sample of 2,984 persons. All participants signed informed written consent forms approved by the institutional review boards of both clinical sites.
Adiposity
Body weight was measured using a standard balance beam scale to the nearest 0.1 kg. Height was measured barefoot to the nearest 0.1 cm using a stadiometer (Holtain Ltd., Crymych, UK). BMI was calculated as weight divided by height squared (kilogram per square meter). Obesity was defined as BMI greater than or equal to 30 kg/m2 (3). Waist circumference was measured with a flexible plastic tape measure to the nearest 0.1 cm at the level of the largest circumference at the end of expiration.
Metabolic Alterations and the MetS
MetS was defined following the updated National Cholesterol Education Program Adult Treatment Panel III definition in 2005 (24) as the presence of three or more of the following: (a) waist circumference greater than or equal to 102 cm for men and greater than or equal to 88 cm for women, (b) triglyceride level greater than or equal to 150 mg/dL or currently on drug treatment for high triglyceride, (c) high-density lipoprotein (HDL) cholesterol less than 40 mg/dL for men and less than 50 mg/dL for women or currently on drug treatment for low HDL cholesterol, (d) systolic blood pressure greater than or equal to 130 and/or diastolic blood pressure 85 mmHg or using antihypertensive medication, and (e) fasting glucose greater than or equal to 100 mg/dL or using antidiabetic medication. Systolic and diastolic blood pressures were determined using a conventional mercury sphygmomanometer with the participant in a seated position. Blood samples were drawn after an overnight fast and analyzed for triglyceride and HDL cholesterol using a chemical analyzer (Vitros 950; Johnson & Johnson, Raritan, NJ). Plasma glucose was determined using the automated glucose oxidase reaction (YSI 2300 Glucose Analyzer; Yellow Springs Instruments, Yellow Springs, OH).
Incident Mobility Limitation
All the participants were free of mobility limitation at baseline. Incident mobility limitation was defined as a self-report of any difficulty walking one quarter of a mile or climbing 10 steps at two consecutive semiannual follow-up assessments conducted for more than 6.5 years. Follow-ups occurred every 6 months, alternating between clinic visits (12, 24, 36, 48, 60, and 72 months after baseline) and telephone interviews (6, 18, 30, 42, 54, 66, and 78 months after baseline). It was required that mobility limitation needed to be present at two consecutive assessments because this selects participants with chronic functional limitation rather than those with temporary mobility limitation due to acute events or traumas.
Inflammatory Markers
A proinflammatory state may represent one pathway through which obesity and the MetS influence on muscle strength and physical function decline (19,21). Measures for the proinflammatory markers interleukin (IL)-6 and tumor necrosis factor (TNF)-α and for C-reactive protein (CRP) were obtained from frozen stored plasma or serum. Fasting blood samples were obtained in the morning, and after processing, the specimens were aliquoted into cryovials, frozen at −70°C, and shipped to the Health ABC Core Laboratory at the University of Vermont. Circulating levels of IL-6, TNF-α, and CRP were all measured in duplicate. Serum IL-6 and plasma TNF-α were measured by Quantikine HS ELISA Kits (R&D Systems, Minneapolis, MN). The coefficients of variation were 10.3% for IL-6 and 15.8% for TNF-α. Plasma levels of CRP were measured using enzyme-linked immunosorbent assay with anti-CRP antibodies (Calbiochem, San Diego, CA) with a coefficient of variation of 8.0%. There were some missing values in inflammatory markers, CRP (n = 8), IL-6 (n = 133), and TNF-α (n = 171), which were taken into account in the analysis.
Covariates
Age, race (white or black), sex, study site (Memphis or Pittsburgh), educational level (<12, 12, or >12 years), smoking status (current, former smoker, or never-smoker), alcohol consumption (none, <1 drink/d, or ≥1 drink/d), physical activity, and chronic conditions were all considered as possible confounders of the association of obesity, MetS, and incident mobility limitation. Physical activity of the past 7 days was assessed by questionnaire during an interview (25). Time spent climbing stairs, walking for exercise, walking for other purposes, aerobics, weight or circuit training, high-intensity exercise activities, and moderate-intensity exercise activities were obtained as well as information on the intensity level at which each activity was performed. A metabolic equivalent value was assigned to each activity and intensity combination and was used to calculate the number of kilocalories per week spent on those activities (26).
Presence of lung disease (asthma, chronic bronchitis, emphysema, or chronic obstructive pulmonary disease), heart disease (coronary heart disease or congestive heart failure), cerebrovascular disease, peripheral arterial disease, and osteoarthritis (hip or knee osteoarthritis) was determined using standardized algorithms considering self-reported physician-diagnosed disease and use of medications. Depressed mood was assessed with the Center for Epidemiological Studies-Depression scale. A cutoff score of 16 was used as a criterion for major depressive symptoms (27).
Statistical Analyses
Baseline characteristics of the study population are reported by sex according to obesity (yes or no) and the MetS (yes or no) groups as mean and median values and standard deviations for continuous variables and proportions for categorical variables. Comparisons within nonobese and obese groups were examined with chi-square test for categorical variables, Kruskal–Wallis test for skewed continuous variables, and t test for normally distributed continuous variables. The outcome of this study was incident mobility limitation. Person-time for each participant was calculated from the date of the baseline examination to the date of the first of the two consecutive self-reported mobility limitations, date of death, or date of the last study contact, whichever came first. After assessing the proportionality assumption with the interactions with time (log transformed), Kaplan–Meier survival function plots and Cox proportional hazard regression models were used to examine the individual and combined associations of obesity and the MetS on time to incident mobility limitation. Analyses were adjusted for covariates statistically associated with incident mobility limitation or with obesity and MetS, including age, race, study site, education, physical activity, smoking, alcohol use as well as lung, heart, peripheral arterial disease, cerebrovascular disease, osteoarthritis, and depression. The mediating role of inflammatory markers on the association between obesity and MetS groups and mobility limitation was examined by adding inflammatory markers in the fully adjusted Cox proportional hazard regression models (Model 2, Table 4). The proportional reductions in hazard ratios (HRs) were calculated based on the HRs in Model 2 and Model 3 (Table 4). A significant two-way interaction of sex and obesity and MetS groups on mobility limitation was found (p < .001); thus, analyses were stratified by sex. The interaction of race and obesity and MetS groups on mobility limitation was not significant. Analyses were performed using SAS 9.1 Statistical Package (SAS Institute, Inc., Cary, NC).
Table 4.
Risk of Developing Mobility Limitation in Women and Men Based on the Obesity and the MetS Status
| Incidence Rate/100 Persons | Model 1 |
Model 2 |
Model 3 |
||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Women | |||||||
| No obesity/no MetS | 9 | 1 | 1 | 1 | |||
| No obesity/MetS | 14 | 1.54 | 1.29–1.84 | 1.49 | 1.24–1.80 | 1.27 | 1.04–1.56 |
| Obesity/no MetS | 25 | 2.10 | 1.66–2.66 | 1.95 | 1.51–2.53 | 1.84 | 1.39–2.43 |
| Obesity/MetS | 26 | 2.46 | 2.05–2.95 | 2.16 | 1.78–2.63 | 1.79 | 1.44–2.22 |
| Men | |||||||
| No obesity/no MetS | 8 | 1 | 1 | 1 | |||
| No obesity/MetS | 10 | 1.25 | 1.03–1.52 | 1.07 | 0.87–1.32 | 0.96 | 0.77–1.20 |
| Obesity/no MetS | 13 | 1.52 | 1.13–2.04 | 1.64 | 1.19–2.25 | 1.54 | 1.10–2.15 |
| Obesity/MetS | 14 | 1.74 | 1.40–2.15 | 1.41 | 1.12–1.78 | 1.21 | 0.95–1.56 |
Notes: CI = confidence interval; HR = hazard ratio; MetS = metabolic syndrome.
Model 1: Adjusted for age, race, study site, and education. Model 2: Model 1 + adjusted for physical activity, smoking, alcohol use, lung disease, heart disease, peripheral arterial disease, cerebrovascular disease, osteoarthritis, and depression. Model 3: Model 2 + adjusted for C-reactive protein, interleukin-6, and tumor necrosis factor-α.
RESULTS
The mean age of the study population was 73.6 years (SD = 2.9), 51% were women and 59% were white race. Baseline characteristics according to obesity and the MetS status are shown in Table 1 for women and in Table 2 for men. Overall, 42% of women and 53% of men were nonobese and did not have the MetS, and 28% of nonobese women and 25% of nonobese men were classified as having the MetS. A small number of obese participants did not have the MetS, 9% of women and 7% of men. Last, 21% of women and 15% of men had both the obesity and the MetS.
Table 1.
The Characteristics of Women by the Obesity and MetS Status
| Overall (n = 1,527) | No Obesity/No MetS (n = 640) | No Obesity/MetS (n = 431) | p Value* | Obesity/No MetS (n = 140) | Obesity/MetS (n = 316) | p Value* | |
| Age, M (SD), y | 73.5 (2.9) | 73.6 (2.9) | 73.6 (2.9) | .99 | 73.4 (2.9) | 73.1 (2.8) | .78 |
| Race, % black | 46.23 | 38.75 | 33.41 | .08 | 75.71 | 65.82 | .04 |
| Site, % Memphis | 49.97 | 50.16 | 54.06 | .21 | 43.57 | 46.84 | .52 |
| Education, % >high school | 37.11 | 42.79 | 41.4 | .90 | 20.29 | 27.07 | .29 |
| Incident mobility limitation, % | 55.14 | 41.09 | 55.45 | <.001 | 73.57 | 75.00 | .75 |
| Body mass index, M (SD), kg/m2 | 27.9 (5.3) | 24.4 (2.9) | 26.2 (2.6) | <.001 | 34.2 (3.8) | 34.6 (3.6) | .67 |
| Number of MetS criteria met, M (SD) | 2.5 (1.2) | 1.4 (0.7) | 3.6 (0.7) | <.001 | 1.9 (0.3) | 3.7 (0.7) | <.001 |
| Abdominal obesity, % | 78.59 | 56.41 | 89.79 | <.001 | 99.29 | 99.05 | .80 |
| High triglyceride, % | 33.53 | 9.84 | 68.21 | <.001 | 0.71 | 48.73 | <.001 |
| Low HDL, % | 29.8 | 4.38 | 58.93 | <.001 | 6.43 | 51.90 | <.001 |
| Hyperglycemia, % | 31.59 | 5.63 | 50.00 | <.001 | 5.71 | 70.57 | <.001 |
| High blood pressure, % | 80.75 | 67.97 | 90.02 | <.001 | 78.57 | 94.94 | <.001 |
| Current smoker, % | 9.18 | 12.66 | 6.98 | .01 | 3.57 | 7.62 | .04 |
| Alcohol use | .31 | .85 | |||||
| % None | 57.93 | 52.81 | 56.74 | 67.86 | 65.51 | ||
| % <1 drink/d | 38.66 | 43.44 | 38.84 | 30.00 | 32.59 | ||
| % ≥1 drink/d | 3.41 | 3.75 | 4.42 | 2.14 | 1.90 | ||
| High- and moderate-intensity physical activity, median (SD), kcal/wk | 304.0 (1,305) | 442.2 (1,331) | 261.5 (1,567) | .004 | 199.0 (905) | 182.1 (932) | .97 |
| Lung disease, % | 10.41 | 9.05 | 9.67 | .73 | 9.70 | 14.52 | .17 |
| Heart disease, % | 15.66 | 12.4 | 18.85 | .004 | 13.43 | 18.95 | .16 |
| Cerebrovascular disease, % | 8.14 | 8.20 | 10.30 | .24 | 6.47 | 5.79 | .78 |
| Peripheral arterial disease, % | 3.70 | 2.38 | 5.04 | .02 | 4.55 | 4.22 | .88 |
| Osteoarthritis, % | 13.73 | 11.32 | 13.78 | .23 | 14.39 | 18.27 | .31 |
| Depression, % | 5.61 | 5.84 | 5.58 | .86 | 4.35 | 5.75 | .54 |
| C-reactive protein, median (SD) | 2.00 (4.59) | 1.46 (2.91) | 2.03 (5.70) | <.001 | 2.51 (5.27) | 3.17 (4.91) | .01 |
| Interleukin-6, median (SD) | 1.75 (1.95) | 1.40 (1.80) | 1.78 (1.94) | <.001 | 1.99 (1.99) | 2.26 (2.08) | <.001 |
| Tumor necrosis factor-α, median (SD) | 3.10 (1.61) | 2.80 (1.24) | 3.38 (1.92) | <.001 | 2.91 (1.19) | 3.37 (1.76) | <.001 |
Notes: HDL = high-density lipoprotein; MetS = metabolic syndrome.
Comparisons within nonobese and obese groups were performed with chi-square test for categorical variables, Kruskal–Wallis test for skewed continuous variables, and t test for normally distributed continuous variables.
Table 2.
The Characteristics of Men by the Obesity and MetS Status
| Overall (n = 1,457) | No Obesity/No MetS (n = 772) | No Obesity/MetS (n = 369) | p Value* | Obesity/No MetS (n = 96) | Obesity/MetS (n = 220) | p Value* | |
| Age, M (SD), y | 73.7 (2.9) | 73.9 (2.9) | 73.9 (2.8) | .99 | 73.3 (2.8) | 73.3 (2.7) | .99 |
| Race, % black | 36.44 | 39.77 | 23.85 | <.001 | 47.92 | 40.91 | .25 |
| Site, % Memphis | 49.97 | 51.81 | 46.61 | .10 | 55.21 | 46.82 | .17 |
| Education, % >high school | 47.59 | 49.35 | 47.43 | .11 | 38.54 | 45.66 | .07 |
| Incident mobility limitation, % | 43.72 | 38.86 | 44.17 | .09 | 55.21 | 55.00 | .97 |
| Body mass index, M (SD), kg/m2 | 27.1 (3.9) | 24.9 (2.5) | 27.0 (2.1) | <.001 | 32.3 (2.8) | 33.0 (2.5) | .14 |
| Number of MetS criteria met, M (SD) | 2.3 (1.3) | 1.3 (0.7) | 3.5 (0.6) | <.001 | 1.8 (0.4) | 3.7 (0.8) | <.001 |
| Abdominal obesity, % | 43.99 | 15.41 | 59.35 | <.001 | 92.71 | 97.27 | .06 |
| High triglyceride, % | 29.81 | 9.46 | 65.22 | <.001 | 3.13 | 53.64 | <.001 |
| Low HDL, % | 31.75 | 12.82 | 64.03 | <.001 | 3.13 | 56.82 | <.001 |
| Hyperglycemia, % | 42.96 | 23.58 | 69.11 | <.001 | 22.92 | 75.91 | <.001 |
| High blood pressure, % | 77.42 | 70.47 | 90.51 | <.001 | 53.13 | 90.45 | <.001 |
| Current smoker, % | 10.45 | 12.60 | 9.21 | .21 | 7.29 | 6.36 | .45 |
| Alcohol use | .02 | .97 | |||||
| % None | 42.27 | 39.79 | 48.37 | 41.67 | 40.91 | ||
| % <1 drink/d | 46.13 | 47.38 | 40.22 | 50.00 | 50.00 | ||
| % ≥1 drink/d | 11.6 | 12.83 | 11.41 | 8.33 | 9.09 | ||
| High- and moderate-intensity physical activity, median (SD), kcal/wk | 701.1 (2,336) | 751.4 (2,505) | 688.8 (2,381) | .87 | 708.8 (1,821) | 581.9 (2,379) | .45 |
| Lung disease, % | 10.14 | 9.66 | 10.44 | .68 | 9.38 | 11.68 | .55 |
| Heart disease, % | 28.15 | 23.23 | 36.74 | <.001 | 18.28 | 35.32 | .00 |
| Cerebrovascular disease, % | 8.04 | 7.97 | 9.29 | .46 | 4.35 | 7.73 | .28 |
| Peripheral arterial disease, % | 6.9 | 6.49 | 8.89 | .15 | 1.09 | 7.51 | .02 |
| Osteoarthritis, % | 7.38 | 5.28 | 9.29 | .01 | 5.32 | 12.33 | .06 |
| Depression, % | 3.67 | 3.40 | 3.80 | .73 | 2.17 | 5.00 | .25 |
| C-reactive protein, median (SD) | 1.48 (4.78) | 1.31 (5.54) | 1.47 (3.86) | .07 | 2.03 (2.63) | 2.06 (3.99) | .81 |
| Interleukin-6, median (SD) | 1.89 (1.89) | 1.75 (1.85) | 2.06 (2.07) | <.001 | 2.04 (1.40) | 2.27 (1.89) | .18 |
| Tumor necrosis factor-α, median (SD) | 3.27 (1.82) | 3.00 (1.70) | 3.80 (1.92) | <.001 | 2.76 (1.49) | 3.57 (1.95) | <.001 |
Notes: HDL = high-density lipoprotein; MetS = metabolic syndrome.
Comparisons within nonobese and obese groups were performed with chi-square test for categorical variables, Kruskal–Wallis test for skewed continuous variables, and t test for normally distributed continuous variables.
Over 6.5 years of follow-up, 55% of the women and 44% of the men developed mobility limitation. Participants excluded from analyses, due to missing data, were more likely to be women (p = .03) and current smokers (p < .001) and less likely to have the MetS (p < .001). They did not differ with respect to obesity (p = .70) or incident mobility limitation (p = .34).
Associations of obesity and the MetS on the risk of developing mobility limitation are explored in Table 3. In women, obesity and the MetS independently predicted mobility limitation after adjusting for demographics, lifestyle factors, and prevalent disease (obesity: HR = 1.62, 95% confidence interval [CI] = 1.37–1.91; MetS: HR = 1.35, 95% CI = 1.16–1.58). In men, obesity was independently associated with incident mobility limitation (HR = 1.43, 95% CI = 1.17–1.75), but the MetS was not (HR = 1.02, 95% CI = 0.85–1.22). Adjustment for CRP, IL-6, and TNF-α attenuated the associations especially between the MetS and the mobility limitation. The proportional reductions in HRs from Model 2 to Model 3 were 4% in women and 5% in men for obesity and 14% in women and 10% in men for the MetS.
Table 3.
The Independent and Interaction Effects of Obesity and the MetS on Developing Mobility Limitation
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Women | ||||||||
| Obesity (BMI ≥30 kg/m2) | 1.76 | 1.51–2.05 | 1.62 | 1.37–1.91 | 1.55 | 1.29–1.85 | 2.01 | 1.28–3.17 |
| MetS, ≥3 criteria | 1.40 | 1.21–1.61 | 1.35 | 1.16–1.58 | 1.16 | 0.98–1.37 | 1.95 | 1.51–2.53 |
| Obesity × MetS | 0.74 | 0.54–0.99 | ||||||
| Men | ||||||||
| Obesity (BMI ≥30 kg/m2) | 1.44 | 1.19–1.74 | 1.43 | 1.17–1.75 | 1.36 | 1.10–1.69 | 1.64 | 1.19–2.25 |
| MetS, ≥3 criteria | 1.22 | 1.03–1.45 | 1.02 | 0.85–1.22 | 0.91 | 0.75–1.11 | 1.33 | 0.77–2.29 |
| Obesity × MetS | 0.81 | 0.54–1.21 | ||||||
Notes: BMI = body mass index; CI = confidence interval; HR = hazard ratio; MetS = metabolic syndrome.
Model 1: Adjusted for age, race, study site, and education. Model 2: Model 1 + adjusted for physical activity, smoking, alcohol use, lung disease, heart disease, peripheral arterial disease, cerebrovascular disease, osteoarthritis, and depression. Model 3: Model 2 + adjusted for C-reactive protein, interleukin-6, and tumor necrosis factor-α. Model 4: Model 2 + interaction term Obesity × MetS.
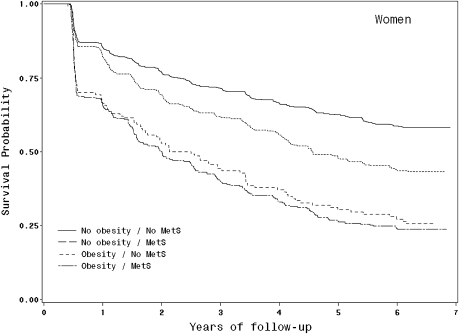
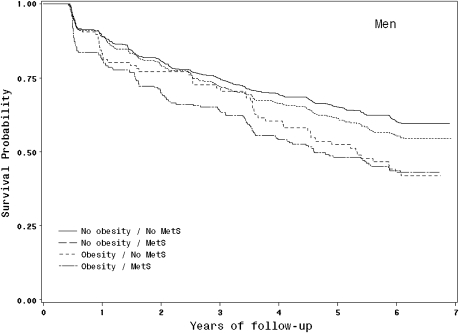
We found a significant interaction between the obesity and the MetS on risk of developing mobility limitation in women (p = .046) but not in men (p = .23), which led us to conduct further analyses considering separately the association of different combinations of obesity and the MetS on the risk of developing mobility limitation. Figures 1 and 2 provide Kaplan–Meier survival curves to illustrate the different patterns of risk of developing mobility limitation among nonobese and obese participants with and without the MetS.
Figure 1.
Kaplan–Meier survival curves for mobility limitation based on obesity and the metabolic syndrome (MetS) status in women.
Figure 2.
Kaplan–Meier survival curves for mobility limitation based on obesity and the metabolic syndrome (MetS) status in men.
In Table 4, risk of incident mobility limitation according to obesity and the MetS status is studied in more detail. In women, the adjusted risk of incident mobility limitation (Model 2) was progressively greater in nonobese persons with the MetS (HR = 1.49, 95% CI = 1.24–1.80), obese persons without the MetS (HR = 1.95, 95% CI = 1.51–2.53), and obese persons with the MetS (HR = 2.16, 95% CI = 1.78–2.63) relatively to nonobese participants without the MetS. However, obese women did not significantly differ in their risk of developing mobility limitation according to presence of the MetS. In men, obesity and the MetS separately and together were associated with greater risk of incident mobility limitation compared with no obesity and no MetS in models adjusting for demographics. After adjusting for lifestyle factors and prevalent diseases, nonobese men with the MetS no longer had increased risk of developing mobility limitation compared with nonobese men without the MetS. In addition, the adjusted risks for incident mobility limitation were 1.64 (95% CI = 1.19–2.25) for obese without the MetS and 1.41 (95% CI = 1.12–1.78) for obese with the MetS.
Finally, the role of inflammatory markers on the association between combination of obesity and the MetS and mobility limitation risk was examined in Model 3. The adjustment for CRP, IL-6, and TNF-α, in addition to other covariates, attenuated the risk of incident mobility limitation especially among nonobese and obese persons with the MetS. The proportional reductions in HRs from Model 2 to Model 3 in nonobese with the MetS, obese without the MetS, and obese with the MetS were 15%, 6%, and 17% for women and 10%, 6%, and 14% for men, respectively.
DISCUSSION
In this study, the individual and joint associations of obesity and the MetS on incident mobility limitation were examined in initially well-functioning older adults for more than 6.5 years. Both the obesity and the MetS independently predicted the risk of developing mobility limitation in women, but obesity only and not the MetS predicted the development of mobility limitation in men. Furthermore, having the MetS increased the risk of developing mobility limitation in nonobese women, but in obese women and men, the MetS did not significantly increase the risk of mobility limitation beyond the effects of obesity.
These results confirm earlier findings on the association between obesity and mobility limitation in old age (4–7) and further support the notion that both the obesity (4,28,29) and the MetS seem to expose women to greater risk of mobility limitation than men (10,29). As far as we know, this is the first study to report that obesity per se independent of its metabolic consequences is a stronger risk factor for mobility limitation in older obese adults. Thus, we cannot compare our findings with past work.
It has been suggested that obese persons are not a homogeneous group and that the effect of obesity on health may be substantially different when obesity is associated with metabolic dysregulation (15,16). Previous studies have shown a higher risk of cardiovascular events and mortality among obese persons with metabolic alterations (30–32). Interestingly, although we identified a subgroup of obese persons who did not have the MetS, sometimes referred to as “metabolically healthy obese,” their adjusted risk for mobility limitation was similar as the risk of obese persons who additionally had the MetS. A possible interpretation of our findings is that obesity-related factors other than metabolic consequences are more important in increasing the risk of mobility limitation. For example, excess body weight can cause biomechanical stress on the lower extremity joints leading to pain, osteoarthritis, reduced physical activity, and impaired muscle strength, all of which can predispose an individual to mobility limitation (33,34). In addition, in older obese persons, the lower extremity muscle strength (35) or cardiorespiratory fitness may be inadequate to perform weight-bearing activities without difficulties.
However, when interpreting our findings, it must be emphasized that the prevalence of metabolically healthy obese was relatively low (9% of women and 7% of men) in the present study population; thus, we may lack power to show significant difference in the risk of mobility limitation between obese persons with and without the MetS. Another explanation for the nonsignificant effect of the MetS among obese participants is selective survival. Although there was no difference in survival among those who entered the study, it is possibly that due to the strict inclusion criteria of the Health ABC Study and the relatively older age of the study participants, obese persons with more serious obesity-related consequences, including the MetS and related cardiovascular conditions, were excluded from this study. Thus, the effect of obesity and MetS on incident mobility limitation may have been underestimated in this study. Future studies in a general population, including younger participants, are needed to confirm our findings.
Although the presence of the MetS did not present additional risk of mobility limitation in obese participants, nonobese women with the MetS had 1.5 times higher risk of developing mobility limitation compared with those without the MetS. In nonobese men, the MetS did not increase risk of mobility limitation. Previous studies have shown that the MetS is associated with poorer physical functioning and predicts the development of mobility limitation (10–12), but the effect of general obesity (measured with BMI) on the association of the MetS and mobility limitation was not addressed in these studies. Potential explanation for the sex difference in the present study is that women and men exhibit a different pattern of factors that constitutes the MetS. Nearly 90% of nonobese women with the MetS have high waist circumference, whereas in men, the corresponding proportion is 59%. As abdominal obesity, independent of general obesity, is a known risk factor for mobility limitation (4,36) and because mobility limitation is more prevalent in women, this may explain the found differences between men and women.
Finally, our study suggests that elevated inflammatory markers partly explain the association between obesity, the MetS, and mobility limitation. The role of heightened inflammatory state was especially clear in explaining the additional mobility limitation risk related to the MetS. This is in accordance with current knowledge about the association of chronic subclinical inflammation with both the MetS (20,37,38) and the mobility limitation (21). The role of inflammation as a risk factor of functional decline has proven to be very important. Increasing evidence suggests that proinflammatory cytokines have catabolic effects on muscle, thus decreasing muscle mass and strength (21,23,39) and further predisposing older people to functional decline (21,40).
In conclusion, this prospective study provides evidence that obesity itself, independent of its metabolic consequences, is a risk factor for mobility limitation among obese older adults. In addition, having the MetS increases the risk of functional decline only in nonobese women. Our study implies that it is important to recognize the MetS in the nonobese population, especially in women. Furthermore, in addition to lifelong control of healthy body weight, interventions targeting nonmetabolic consequences of obesity, such as reduction of pain, treatment of lower extremity joint problems, improvement of muscle strength, and cardiorespiratory fitness, may be useful in preventing and delaying mobility decline in older obese adults.
FUNDING
This work was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. This research was supported in part by the Intramural Research Program of the National Institutes of Health, NIA. This work was also supported by grant from the Finnish Academy (No. 125494 SS).
CONFLICT OF INTEREST
None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here.
Acknowledgments
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. All authors have approved the final version.
References
- 1.Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring) 2007;15:2855–2865. doi: 10.1038/oby.2007.339. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 4.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes. 2006;30:364–373. doi: 10.1038/sj.ijo.0803130. [DOI] [PubMed] [Google Scholar]
- 5.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 6.Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci. 2007;62:859–865. doi: 10.1093/gerona/62.8.859. [DOI] [PubMed] [Google Scholar]
- 7.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the Health, Aging and Body Composition Study. Am J Epidemiol. 2009;169:927–936. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:581–601. doi: 10.1016/j.ecl.2008.06.005. vii–viii. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg O, Tilvis RS. Does the metabolic syndrome predict mobility impairment in the elderly? Arch Gerontol Geriatr. 1998;26:131–139. doi: 10.1016/s0167-4943(97)00037-x. [DOI] [PubMed] [Google Scholar]
- 10.Penninx BWJH, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64A:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. 2007;62:766–773. doi: 10.1093/gerona/62.7.766. [DOI] [PubMed] [Google Scholar]
- 12.Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54:502–506. doi: 10.1111/j.1532-5415.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 13.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower-extremity disability in older women with diabetes: the Women's Health and Aging Study. Diabetes Care. 2003;26:70–75. doi: 10.2337/diacare.26.1.70. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 16.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 17.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Prevalence of uncomplicated obesity in an Italian obese population. Obes Res. 2005;13:1116–1122. doi: 10.1038/oby.2005.130. [DOI] [PubMed] [Google Scholar]
- 18.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 19.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 20.You T, Nicklas BJ, Ding J, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrucci L, Penninx BWJH, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 22.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; for the Health Aging and Body Composition Study Research Group. The association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 28.Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res. 2007;19:277–283. doi: 10.1007/BF03324702. [DOI] [PubMed] [Google Scholar]
- 29.Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc. 2001;49:398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- 30.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 31.St-Pierre AC, Cantin B, Mauriege P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–1315. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 33.Ling SM, Fried LP, Garrett ES, Fan MY, Rantanen T, Bathon JM. Knee osteoarthritis compromises early mobility function: the Women's Health and Aging Study II. J Rheumatol. 2003;30:114–120. [PubMed] [Google Scholar]
- 34.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 35.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI Study. Int J Obes (Lond) 2009;33:635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houston DK, Stevens J, Cai J. Abdominal fat distribution and functional limitations and disability in a biracial cohort: the Atherosclerosis Risk in Communities Study. Int J Obes. 2005;29:1457–1463. doi: 10.1038/sj.ijo.0803043. [DOI] [PubMed] [Google Scholar]
- 37.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008;32:772–779. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 38.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 39.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 40.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]