Abstract
The disruption of the growth hormone (GH) axis in mice promotes insulin sensitivity and is strongly correlated with extended longevity. Ames dwarf (Prop1df, df/df) mice are GH, prolactin (PRL), and thyrotropin (TSH) deficient and live approximately 50% longer than their normal siblings. To investigate the effects of GH on insulin and GH signaling pathways, we subjected these dwarf mice to twice-daily GH injections (6 μg/g/d) starting at the age of 2 weeks and continuing for 6 weeks. This produced the expected activation of the GH signaling pathway and stimulated somatic growth of the Ames dwarf mice. However, concomitantly with increased growth and increased production of insulinlike growth factor-1, the GH treatment strongly inhibited the insulin signaling pathway by decreasing insulin sensitivity of the dwarf mice. This suggests that improving growth of these animals may negatively affect both their healthspan and longevity by causing insulin resistance.
Keywords: Ames dwarf, Aging, Insulin, Growth hormone
THE incidence of insulin resistance and diabetes mellitus is rapidly increasing in industrialized countries, causing serious public health and economic concerns. More importantly, the occurrence of these conditions increases with age. The first report of impaired glucose metabolism in humans older than 60 years was published in 1920 (1). A progressive increase in glucose intolerance with advancing age has been observed by scientists and health care professionals and studied throughout most of the 20th century. It is well documented that glucose tolerance generally decreases with age, starting in the third decade (1,2). However, centenarians exhibit remarkably low insulin resistance (3). Data derived from human studies, together with results obtained in different vertebrate and invertebrate species, identify insulin and homologous signaling as a major mechanism that controls aging and longevity (4,5).
Ames dwarf (df/df) mice used in the present study are homozygous for a recessive loss-of-function mutation at the Prop1 locus (Prop1df). This mutation causes deficiencies of growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone (TSH), with secondary suppression of circulating levels of insulinlike growth factor 1 (IGF-1), thyroid hormones, insulin, and glucose (6–8). Ames dwarf mice live approximately 50% longer than their normal (N) siblings (6). Calorie restriction (CR) is an intervention known to delay aging and to increase lifespan in a number of organisms ranging from worms to mammals (9,10). CR reduces growth, body weight, and plasma levels of insulin, IGF-1, glucose, and thyroid hormones. In Ames dwarf mice, CR further extends lifespan, similar to its effects in their normal siblings (11). As mentioned previously, studies in humans and animals indicated that the insulin signaling pathway plays an important role in longevity. Our earlier studies suggested that altered insulin signaling may have a vital role in extending the longevity of Ames dwarf mice and in their responses to CR (8).
Initially, it was unclear whether GH deficiency is the main factor responsible for improved insulin action and extended longevity in these animals and to what extent a deficiency of other hormones may be involved. However, subsequent studies in other laboratories demonstrated that GH receptor and binding protein knockout (GHRKO) mice produced in Dr J. J. Kopchick's laboratory are also insulin sensitive and long-lived (12,13). Moreover, transgenic dwarf rats with suppressed GH and IGF-1 axis caused by overexpression of antisense GH transgene are characterized by reduced body weight and decreased circulating IGF-1, blood glucose, and insulin levels, which cause increased insulin sensitivity (14,15). Similar to Ames dwarf and GHRKO mice, this reduction in GH signaling in dwarf rats is also associated with extended longevity (14,15).
The purpose of this study was to examine effects of GH replacement therapy on insulin signaling in long-lived mutant mice. Toward this aim, we have selected mice, which, in contrast to GHRKO animals, have functional GH receptors and therefore can normally respond to GH.
METHODS
Animals and Tissue Collection
Ames dwarf (Prop1df) homozygous mice (df/df) were produced by mating heterozygous females and homozygous mutant males in our breeding colony. In this colony, the Prop1df mutation is maintained on a heterogeneous genetic background. All animal protocols were approved by the Southern Illinois University Laboratory Animal Care Committee. Animals were maintained under temperature- and light-controlled conditions (20°C–23°C, 12-hour light and 12-hour dark cycle). Groups of 12–14 Ames dwarf males were subjected to twice-daily porcine-GH injections starting at the age of 2 weeks and continuing for 6 weeks (df/df-GH). In previous preliminary experiments, 2-week-old Ames dwarf mice injected with saline experienced body weight loss, difficulties in wound healing, and in some cases mortality. Hence, untreated dwarfs (df/df) and normal (N) littermates of the same age were used as a control in these studies. After 6 weeks of GH treatment, the animals were fasted overnight (food removed at 7 PM). The last GH injection was delivered in the morning, approximately 1 hour before blood glucose measurment (about 8 AM). Then, blood was collected, plasma isolated and liver removed and immediately frozen on dry ice, and stored at −80°C until analysis.
GH Preparation and Treatment
For stability, porcine-GH (Alpharma, Victoria, Australia) was dissolved in 0.1 M NaHCO3 (pH 8.3) and then 0.9% saline was added to obtain the concentration of 21 μg/50 μl for a single injection of the average animal weight of 7 g (3 μg/g of body weight). Animals were injected twice daily, in the morning about 9 AM and second injection about 4 PM (∼6 μg/g/d total GH treatment) On Saturdays and Sundays, animals were injected only once with a full dosage (6 μg/g/d). The dosage was evaluated based on previous studies, which utilized 2–8 μg of GH per gram of body weight at early age in mice and rats (16–18). Using this information, final dosage for treatment was assessed by preliminary experiments in our Ames dwarf colony.
Assessment of Blood Chemistry
Fasting glucose levels were measured in blood collected via tail vein using OneTouch Ultra glucose meter (Life Scan, Inc. Milpitas, CA). Blood plasma was used for assessment of the levels of insulin using Rat/Mouse Insulin ELISA, adiponectin using Mouse Adiponectin ELISA, and leptin using Mouse Leptin ELISA following manufacturer's protocols (Linco Research Inc, St Charles, MO, and IDS, Inc, Fountain Hills, AZ). Plasma IGF-1 was measured using ELISA kits from IDS, Inc (Fountain Hills, AZ).
Relative Insulin Sensitivity Index
Fasted glucose and insulin was used to calculate relative insulin sensitivity index (RISI) using the formula:
Insulin Tolerance Test
After 5 weeks of GH treatment, and 1 week before termination of the study, all animals were fasted by removing food on the morning of the test date. Basal glucose was checked using glucometer ONE Touch Ultra, Life Scan, Inc in blood from the tip of the tail. Then, the mice were injected with porcine insulin (Sigma, St Louis, MO) at 0.75 IU/kg of body weight. The blood glucose was checked after 15, 30, and 60 minutes.
Western Blot and ELISA
The level of total and phosphorylated pY1158 insulin receptor (IR) was analyzed by commercially available ELISA kit according to provided protocol. The levels of protein kinase B 1 (AKT1), AKT2, pAKT-Ser473, phosphorylated mammalian target of rapamycin (p-mTOR), forkhead box O1 (FOXO1), janus kinase 2 (JAK2), Signal Transducers and Activator of Transcription 3 (STAT3), p-STAT3, STAT5a, and STAT5b were measured using Western blot with specific antibodies as described previously (19).
Statistical Analysis
Data were analyzed using analysis of variance (ANOVA) followed by Fisher’s PLSD test to compare individual means in Figures 1 and 3–5. Results of insulin tolerance tests (ITTs) were presented and analyzed as mean percentage change from baseline within experimental groups and repeated measurement. ANOVA was used to determine the interaction of the main effects variables Figure 2. α was set at .05 for determination of significance, and all values are reported as mean ± SEM throughout the figures and text.
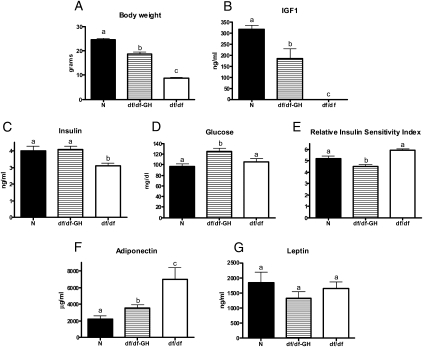
Figure 1.
The effects of growth hormone (GH) treatment in Ames dwarf mice (df/df-GH) in comparison to normal (N) and df/df mice on: (A) body weight, (B) plasma IGF-1, (C) plasma insulin, (D) plasma glucose, (E) relative insulin sensitivity index, (F) plasma adiponectin, and (G) plasma leptin. Means ± SEM. a, b, c: values that do not share the same letter in the superscript are statistically significant (p < .05).
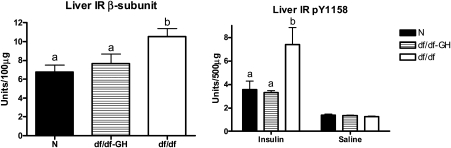
Figure 3.
The level of proteins related to insulin signaling in liver tissue of normal (N) and growth hormone (GH)–treated Ames dwarf (df/df-GH) and df/df mice. The level of total and pY1158 IR was measured using ELISA kit. Means ± SEM. a, b: values that do not share the same letter in the superscript are statistically significant (p < .05).
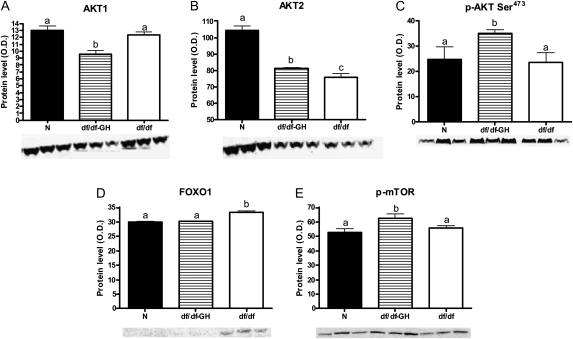
Figure 4.
The level of proteins related to insulin signaling in liver tissue of normal (N) and growth hormone (GH)–treated Ames dwarf (df/df-GH) and df/df mice. Means ± SEM. a, b, c: values that do not share the same letter in the superscript are statistically significant (p < .05). (A) AKT1, (B) AKT2, (C) p-AKT Ser473, (D) FOXO1, and (E) p-mTOR.
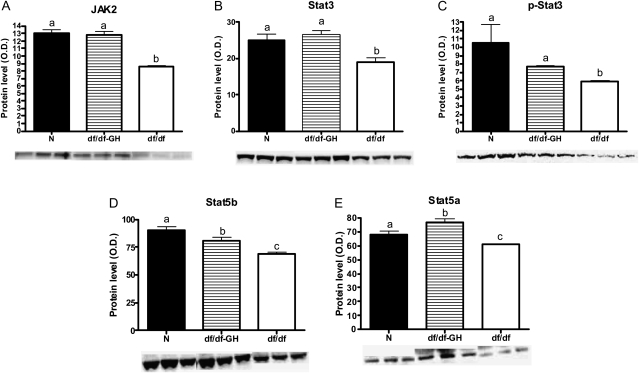
Figure 5.
The level of proteins related to growth hormone (GH) signaling in liver tissue of normal (N), GH-treated Ames dwarf (df/df-GH) and df/df mice. Means ± SEM. a, b, c: values that do not share the same letter in the superscript are statistically significant (p < .05). (A) JAK2, (B) STAT3, (C) p-STAT3, (D) STAT5b, and (E) STAT5a.
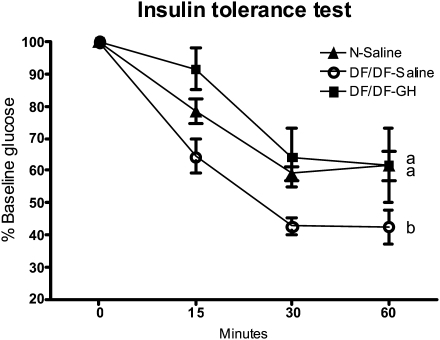
Figure 2.
Results of insulin tolerance test in normal growth hormone (GH)–treated Ames dwarf (df/df-GH) and df/df mice. All groups were randomly fed overnight. Mice were injected intraperitoneal injection with insulin (0.75 U/kg of body weight). Glucose was measured in samples collected from the tail vein at specified time points (see Methods). a, b: values that do not share the same letter in the superscript are statistically significant (p < .05).
RESULTS
Ames dwarfs (df/df) are characterized by a severe reduction of body weight when compared with their normal siblings (p < .0001; Figure 1A). Early treatment with GH initiated at the young age increased body weight of Ames dwarf mice in comparison to their untreated df/df littermates (p < .0001). However, the treatment did not normalize the growth completely (p < .0001; Figure 1A). Consistent with GH deficiency, the level of IGF-1 in plasma of df/df mice was severely reduced and was undetectable by employed ELISA (Figure 1B). GH therapy increased IGF-1 in df/df-GH mice, but the level did not reach the levels measured in N mice (p < .0016; Figure 1B).
Ames dwarf mice have decreased levels of plasma insulin in comparison to N mice (p < .0117). Treatment with GH increased the level of insulin in df/df-GH mice in comparison to untreated df/df mice (p < .0068; Figure 1C). At 8 weeks of age, there was no difference in glucose levels between df/df and N mice, but GH treatment increased glucose levels in df/df-GH mice in comparison to untreated df/df and N mice (p < .0277 and p < .0025, respectively; Figure 1D). RISI indicated that GH treatment decreased insulin sensitivity in df/df-GH mice in comparison to both df/df and N mice (p < .0004 and p < .0234, respectively). Surprisingly, RISI did not reveal significant differences in insulin sensitivity between untreated df/df and N mice (p < .08; Figure 1E).
Consistent with our earlier findings, df/df mice had markedly elevated levels of plasma adiponectin (p < .0008; Figure 1F). Correlating with the changes of insulin sensitivity, the level of adiponectin was significantly decreased by GH treatment in df/df mice (p < .0118; Figure 1F). The levels of leptin were not affected by genotype or treatment (Figure 1G).
ITTs indicated increased sensitivity to injected insulin in Ames dwarf mice when compared with their N siblings (p < .0001; Figure 2). GH treatment decreased sensitivity to injected insulin in df/df mice (p < .0001), bringing it down to the level measured in N mice (Figure 2).
Total hepatic IR levels as measured by ELISA were higher in df/df than in N mice (p < .005). Treatment of Ames dwarfs with GH decreased the level of total hepatic IR (p < .029), normalizing it to the level maintained by N mice (Figure 3). The level of phosphorylated pY1158 IR was not affected by genotype or treatment at basal level. However, in response to insulin stimulation, the level of pY1158 of IR was significantly higher in df/df mice than in N or df/df-GH mice (p < .0128 and p < .0087, respectively; Figure 3).
Total AKT1 protein was decreased by GH treatment in df/df mice when compared with N and control df/df mice (p < .0007 and p < .0039, respectively; Figure 4A). The level of total AKT2 was downregulated in df/df mice in comparison to N animals (p < .0001), and GH treatment increased protein level of AKT2 in df/df mice (p < .0487). However, AKT2 levels in GH-treated dwarfs were still significantly below the levels in N mice (p < .0001; Figure 4B). The level of p-AKT Ser473 was no different between the phenotypes, but GH treatment upregulated the level of this phosphoprotein in df/df mice in comparison to both N and untreated df/df mice (p < .0148 and p < .026, respectively; Figure 4C). The level of total FOXO1 in the liver of df/df was increased in comparison to the levels measured in N and df/df-GH mice (p < .0001 and p < .0001, respectively; Figure 4D). The level of phosphorylated mTOR (p-mTOR) protein was increased in df/df-GH mice in comparison to N mice and df/df controls (p < .0062 and p < .0431, respectively; Figure 4E).
The analysis of proteins involved in the GH signaling pathway indicated that JAK2 protein was decreased in the df/df when compared with N mice (p < .0001) and GH treatment normalized the level of JAK2 in df/df mice (Figure 5A). The level of total STAT3 and p-STAT3 was also downregulated in df/df when compared with N animals (p < .0081 and p < .0319, respectively) and the levels were normalized by GH treatment (Figure 5B and C). Similarly to JAK and STAT3 proteins, STAT5b and STAT5a were downregulated in df/df mice when compared with N mice (p < .0001 and p < .0376, respectively; Figure 5D and E). GH treatment increased the level of STAT5b in df/df mice (p < .0129) but failed to normalize it (p < .0365; Figure 5D). Moreover, the treatment not only increased STAT5a in df/df-GH mice in comparison to df/df mice (p < .0001) but also upregulated the level of this protein when compared with N animals (p < .0118; Figure 5E).
DISCUSSION
In this study, we subjected long-lived insulin-sensitive Ames dwarf mice to GH treatment at a young age. We analyzed the effects of this regimen on the insulin, IGF-1, and GH signaling pathways to determine whether GH can reverse the characteristics of Ames dwarf mice that are believed to be important contributors to the extended longevity of these mutants. As mentioned in the methodology, the dosages for this treatment were calculated using previously published data from other laboratories and following our own preliminary trials. The expected increase in body weight in df/df-GH mice followed treatment and the lack of complete normalization of body weight in these animals indicated that the animals were not overdosed. Some early age studies in mice and rats used similar dosages. Presumably, more frequent injections, higher doses, and/or longer period of treatment would be necessary to attain growth rate and body weight characteristics of N animals. Deficiencies of other hormones could have also contributed to the failure to reach normal body weights in the df/df-GH group. As expected from the lack of GH and previous publications, circulating IGF-1 was severely reduced in the Ames dwarfs. Similarly to what was observed for body weight, the IGF-1 increase in the df/df-GH group did not reach the level measured in N mice, which adds to the evidence that the GH treatment was not supraphysiological.
The GH treatment administered to these hypoinsulinemic mutants caused elevation of insulin levels. Similarly to previous studies conducted in older animals, df/df mice had reduced levels of glucose. However, at a very young age, there is usually no difference in glucose levels between these two phenotypes. GH treatment caused an increase of glucose levels that together with the elevated insulin levels indicated decreased insulin sensitivity, confirmed by calculating RISI. These findings correlate with the effects observed in studies in humans (20–22), which indicate that treatment with GH in children or adults alters the insulin levels and decreases insulin sensitivity. Additionally, the high level of adiponectin in df/df mice was greatly reduced by GH treatment. Adiponectin is released by adipose tissue into the circulation, and a high level of this adipo-protein is correlated with increased insulin sensitivity and extended longevity in humans (23). Conversely, patients with acromegaly have decreased adiponectin levels, which can be normalized by correction of GH levels (24). In addition to affecting insulin and glucose levels, GH treatment reduced tolerance to injected insulin, agreeing with observations of decreased insulin sensitivity measured by euglycemic hyperinsulinemic clamp in GH-deficient patients subjected to GH treatment (22). In df/df-GH mice, the sensitivity to injected insulin was reduced to the sensitivity of N mice (Figure 2). These critical changes in blood chemistry and insulin action suggest that replacement of GH in the Ames dwarf mouse throughout the animal's lifespan could reverse many characteristics of this long-living phenotype. The insulin signaling pathway and, more importantly, heightened insulin sensitivity positively correlate with longevity (8). One could speculate that decreasing insulin sensitivity in Ames dwarf mice may also negatively affect their longevity (8).
After finding that insulin, glucose, adiponectin, and whole-body insulin action were affected by the GH treatment, we decided to investigate the insulin signaling pathway in the liver. In response to insulin binding to its receptor, the β-subunit of IR is autophosphorylated at tyrosine (Tyr) residue 1158 [pY1158] located within the catalytic loop of the tyrosine kinase domain, which activates the insulin signaling cascade. The phosphorylation site of IR is monitored in studies of type II diabetes.
Based on this information and plasma parameters showing changes in insulin sensitivity, it was important to investigate the phosphorylation status of IR. Additionally, we detected a reduction in total IR protein in the liver in response to GH treatment. The decreased insulin-induced pY1158 phosphorylation of IR in df/df-GH mice probably contributes to the reduction in whole-body insulin sensitivity in response to this treatment. Decreasing the level of the pY1158 of IR after insulin stimulation could silence the whole insulin signaling cascade and result in suppressed insulin signaling throughout the body. The decrease of IR protein levels and its binding ability was also detected previously in short-lived insulin-resistant transgenic mice overexpressing GH (25–27). Furthermore, the suppression of IR binding was previously found in the livers of rats with upregulated GH levels caused by GH-secreting tumors (28). This could explain the mechanism of GH action on whole-body insulin sensitivity; however, there are several studies that contradict this finding (29).
The changes observed in AKT1, AKT2, and p-AKT Ser473 in df/df-GH mice in comparison to df/df mice confirm that GH affects the insulin signaling pathway downstream from the IR. FOXO1 is known to be one of the regulators through which insulin and its opponents such as glucocorticoids and glucagon regulate gluconeogenesis. In response to insulin stimulation, FOXO1 was reported to bind PGC1α and as a complex can further activate PEPCK and G6Pase. This suggests that elevated level of total FOXO1 in df/df mice accelerates hepatic glucose production and alters lipid metabolism, and these changes can be reversed by GH treatment of these mutants.
The protein p-mTOR is involved in cell growth, stress responses, and, more importantly in the context of the presented study, the insulin signaling pathway. The activation of mTOR is known to be responsible for inhibiting insulin action. It was previously reported that mTOR signaling was reduced in the skeletal muscle of insulin-sensitive Ames dwarf and GHRKO mice (30,31). However, at the age of 8 weeks, the level of hepatic p-mTOR protein was unaffected in this genotype. This lack of difference could be due to the very young age of the animals in this experiment. This may correspond to the lack of changes at the young age in the expression of several insulin signaling pathway genes in the liver of long-living GHRKO mice, with the differences appearing when the animals get older (32). However, GH treatment increased p-mTOR protein level in df/df-GH mice in comparison to both N and df/df mice. This activation of p-mTOR could be responsible for decreased efficiency of whole-body insulin action presumably by promoting inhibitory (serine) phosphorylation of IRS-1. Additionally, most recent finding indicates that feeding mice with rapamycin, a known mTOR inhibitor increases their longevity (33). This suggests that enhancing the activation of mTOR pathway in Ames dwarf mice via GH treatment could be detrimental to their lifespan.
The levels of proteins involved in GH signaling, JAK2, STAT3, STAT5a, and STAT5b, were decreased in df/df mice as expected from a lack of GH. Moreover, GH treatment either normalized the levels of these proteins or increased them in df/df-GH mice in comparison to df/df mice, which correlates with the growth response of these GH-deficient mutants.
Summary
The present results indicate that GH treatment strongly affected the insulin signaling pathway in df/df mutants. Low levels of insulin, together with high insulin sensitivity, are suspected of representing an important mechanism that allows these mutants to live longer in comparison to their normal siblings (8). Results of the present study suggest that replacing GH throughout the entire lifespan of Ames dwarf mice would most likely shorten their lifespan. However, in a study of GH effects in Snell dwarf mice, longevity was not affected, perhaps due to the short-term GH treatment (18). There is evidence that some of the negative effects of GH (e.g., reduced glucose tolerance in humans and insulin tolerance in mice) return to normal after discontinuation of GH administration (8,20). This could suggest that only lifelong GH replacement would have a negative impact on longevity in Ames or Snell dwarf mice. However, the age at the beginning of the treatment can be also critical. In a study with Snell dwarfs, Vergara and colleagues started the treatment at the age of 4 weeks. Using once-daily injection, these investigators did not observe any lifespan alterations. However, in our ongoing study of GH treatment, twice-daily GH treatment started at 2 weeks of age appears to decrease the lifespan of Ames dwarf mice (unpublished data). Increasing the dosages and/or frequency of the GH treatment could also severely affect the insulin and IGF-1 signaling pathway, causing serious insulin resistance and/or developing diabetes. Finally, reversing the alterations in insulin and IGF-1 signaling in Ames dwarf by GH treatment alone suggests that in df/df mice, it is most likely the GH deficiency rather than a TSH or PRL deficiency that is responsible for the phenotypic characteristics related to the remarkably increased longevity of these animals.
FUNDING
National Institute on Aging, AG 19899, and U19 AG023122, The Ellison Medical Foundation, Central Research Committee of Southern Illinois University School of Medicine, Southern Illinois University Excellence in Academic Medicine Howard.
Acknowledgments
The authors would like to thank Dr Andrzej Bartke for his support of this study. We would also like to thank Steve Sandstrom for editorial assistance.
References
- 1.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 2.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri M, Rizzo MR, Manzella D, et al. Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol. 2003;38(1–2):137–143. doi: 10.1016/s0531-5565(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 4.Bartke A. Insulin and aging. Cell Cycle. 2008;7(21):3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- 5.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 7.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173(1):81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 8.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Thomas; 1988. [Google Scholar]
- 10.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 12.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 13.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa I, Higami Y, Utsuyama M, et al. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol. 2002;160(6):2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimokawa I, Chiba T, Yamaza H, Komatsu T. Longevity genes: insights from calorie restriction and genetic longevity models. Mol Cells. 2008;26(5):427–435. [PubMed] [Google Scholar]
- 16.Carter CS, Ramsey MM, Ingram RL, et al. Models of growth hormone and IGF-1 deficiency: applications to studies of aging processes and life-span determination. J Gerontol A Biol Sci Med Sci. 2002;57(5):B177–B188. doi: 10.1093/gerona/57.5.b177. [DOI] [PubMed] [Google Scholar]
- 17.Fintini D, Alba M, Salvatori R. Influence of estrogen administration on the growth response to growth hormone (GH) in GH-deficient mice. Exp Biol Med (Maywood) 2005;230(10):715–720. doi: 10.1177/153537020523001004. [DOI] [PubMed] [Google Scholar]
- 18.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59(12):1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146(2):851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 20.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 21.de ZF, Ong K, van HM, Mohn A, Woods K, Dunger D. High-dose growth hormone (GH) treatment in non-GH-deficient children born small for gestational age induces growth responses related to pretreatment GH secretion and associated with a reversible decrease in insulin sensitivity. J Clin Endocrinol Metab. 2002;87(1):148–151. doi: 10.1210/jcem.87.1.8293. [DOI] [PubMed] [Google Scholar]
- 22.Norrelund H, Djurhuus C, Jorgensen JO, et al. Effects of GH on urea, glucose and lipid metabolism, and insulin sensitivity during fasting in GH-deficient patients. Am J Physiol Endocrinol Metab. 2003;285(4):E737–E743. doi: 10.1152/ajpendo.00092.2003. [DOI] [PubMed] [Google Scholar]
- 23.Atzmon G, Pollin TI, Crandall J, et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci. 2008;63(5):447–453. doi: 10.1093/gerona/63.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam KS, Xu A, Tan KC, Wong LC, Tiu SC, Tam S. Serum adiponectin is reduced in acromegaly and normalized after correction of growth hormone excess. J Clin Endocrinol Metab. 2004;89(11):5448–5453. doi: 10.1210/jc.2003-032023. [DOI] [PubMed] [Google Scholar]
- 25.Balbis A, Dellacha JM, Calandra RS, Bartke A, Turyn D. Down regulation of masked and unmasked insulin receptors in the liver of transgenic mice expressing bovine growth hormone gene. Life Sci. 1992;51(10):771–778. doi: 10.1016/0024-3205(92)90487-a. [DOI] [PubMed] [Google Scholar]
- 26.Balbis A, Bartke A, Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity. Life Sci. 1996;59(16):1363–1371. doi: 10.1016/0024-3205(96)00462-6. [DOI] [PubMed] [Google Scholar]
- 27.Dominici FP, Cifone D, Bartke A, Turyn D. Alterations in the early steps of the insulin-signaling system in skeletal muscle of GH-transgenic mice. Am J Physiol. 1999;277(3, pt 1):E447–E454. doi: 10.1152/ajpendo.1999.277.3.E447. [DOI] [PubMed] [Google Scholar]
- 28.Davidson MB, Melmed S. Hepatocyte insulin binding and action in rats with somatomammotrophic tumours. Diabetologia. 1983;25(1):60–65. doi: 10.1007/BF00251899. [DOI] [PubMed] [Google Scholar]
- 29.Dominici FP, Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med (Maywood) 2002;227(3):149–157. doi: 10.1177/153537020222700301. [DOI] [PubMed] [Google Scholar]
- 30.Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60(3):293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- 32.Panici JA, Wang F, Bonkowski MS, et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64A:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]