Abstract
Background
The influence of long-term adult weight history on metabolic risk independent of attained body mass index (BMI) is unknown.
Methods
Using nationally representative data on adults aged 50–64 years from the 1999–2006 National Health and Nutrition Examination Surveys, we examined weight change for two periods of adulthood: prime age (age 25–10 years ago) and midlife (the last 10 years). Weight changes in each period were categorized as stable (gain <10 kg) or gain (gain ≥10 kg) to create weight history comparison groups: stable-stable, gain-stable (prime age gain), stable-gain (midlife gain), and gain-gain (continuous gain). Persons who lost weight were excluded. Logistic regression predicted odds of metabolic syndrome and its subcomponents based on weight history, adjusting for current BMI and covariates.
Results
Participants in the gain-stable group had 89% elevated odds of metabolic syndrome (odds ratio = 1.89, 95% CI: 1.19–3.01) relative to the stable-stable group, even after adjustment for current BMI. All weight gain groups had increased odds of low HDL and high triglycerides relative to participants with continuously stable weights. No significant associations were found between weight history and hypertension or high glucose.
Conclusions
Weight history confers information about metabolic risk factors above and beyond attained weight status. In particular, adult weight gain is related to risk of low HDL and high triglycerides. Weight history may contribute to our understanding of why some obese older persons are metabolically healthy but others are not.
Keywords: Metabolic syndrome, Weight history, Body mass index, Lipids
OVERWEIGHT and obesity are clearly associated with the metabolic syndrome, an increasingly prevalent cluster of cardiometabolic risk factors that predicts diabetes, cardiovascular disease, and mortality (1–3). Yet, recent research has identified subgroups of metabolically healthy obese persons and high-risk normal-weight persons, highlighting the variability in cardiometabolic health across a range of body mass index (BMI) groups (4,5). Although most attention has focused on current weight, research suggests that weight history can independently influence metabolic and cardiovascular outcomes (6–8).
The vast majority of research on weight gain and cardiovascular risk controls for weight in early adulthood and examines the influence of subsequent weight gain (9–11). Hence, a positive association between weight gain and cardiovascular risk may occur simply because those who have gained more weight also have higher current weight (12). In contrast, we focus on whether weight history contributes to metabolic risk controlling for current BMI. From a clinical standpoint, it is important to know whether information on a patient’s weight history has relevance above and beyond the patient’s current weight status.
The age at which weight gain occurs (i.e., the timing of weight gain) may also modify the risk of metabolic syndrome (2). Research has shown that relative risk of hypertension, high cholesterol, and diabetes associated with a given BMI is higher at younger ages (13,14). Additionally, earlier adult weight gain appears to confer a greater risk for coronary heart disease than later life weight gain (15). The health consequences of weight change may differ by age for a variety of reasons, including age differences in the interrelationships between body weight, body composition, and health behaviors.
The aim of this analysis was to determine whether long-term adult weight history predicts metabolic syndrome independent of current weight.
METHODS
Analyses used participants from the National Health and Nutrition Examination Survey (NHANES) conducted between 1999 and 2006. NHANES is an ongoing survey conducted in 2-year cycles (16). Each survey consists of an interview and a medical examination. Eight years of the most recently available data are combined here to create an adequate sample size. Eligible participants included persons aged 50–64 years who participated in the fasting subsample. Analyses used STATA 10.0 survey commands to produce weighted estimates and account for the complex survey design.
The NHANES interview component included a weight history questionnaire with the following questions: “How much did you weigh 10 years ago?” and “How much did you weigh at age 25?” Recalled weight has been found to be highly correlated with measured weight (Pearson correlations of .73–.87) for men and women with recall periods as long as 37 years (17–20). Participants’ current height and weight were measured as part of the medical examination.
Analysis was restricted to participants aged 50–64 years for three reasons: (a) Based on typical weight trajectories, participants’ current weights should be near each individual’s highest BMI, providing the opportunity to collect a complete weight history before significant age-associated weight loss has occurred (21,22); (b) This age group is of particular interest because it represents a period when the prevalence of metabolic syndrome increases (23); and (c) Because the weight history questionnaire asked about weight at age 25 and 10 years prior to the interview, the sample age range must be narrow enough to allow for reasonably comparable weight change intervals across participants.
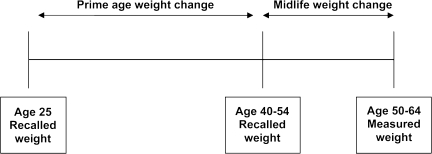
Figure 1 depicts observed periods of weight change. Two measures of weight change were computed. Prime age weight change was computed as the difference between self-reported weight 10 years ago and weight at age 25 years, and midlife weight change was computed as the difference between current measured weight and self-reported weight 10 years ago. We modeled weight change, rather than BMI change, because information about height is incorporated into analysis by controlling for baseline BMI and a measure of absolute weight change may better reflect changes in fat in adulthood (24).
Figure 1.
Weight history measure summary.
Weight change for each interval was categorized as loss (any weight loss), stable (a gain of less than 10 kg/22 lb), or gain (gain of ≥10 kg/22 lb). Weight gains of 10 kg or more are clinically meaningful (9), and slight variations in this cut point did not meaningfully alter our results. Because intentionality of weight loss was unknown and unintentional weight loss may confound associations between weight change and health (25), participants who lost weight were excluded. Thus, our analysis of patterns of weight change focused on participants with four possible patterns of weight change across two age periods: stable-stable (stable weight), gain-stable (prime age gain), stable-gain (midlife gain), and gain-gain (continuous gain).
In accordance with guidelines of the American Heart Association and the National Heart, Lung, and Blood Institute (26), metabolic syndrome was defined as having three or more of the following: (a) triglycerides greater than or equal to 150 mg and the dL or treatment for elevated triglycerides; (b) HDL cholesterol less than 40 mg/dL in men and less than 50 mg/dL in women or treatment for low HDL; (c) blood pressure greater than or equal to 130/85 mmHg or use of antihypertensive medication; (d) fasting glucose greater than or equal to 100 mg/dL or drug treatment for elevated glucose; or (e) waist circumference greater than or equal to 102 cm in men or 88 cm in women. Up to four blood pressure readings were obtained, and all readings after the first were averaged to compute systolic and diastolic blood pressure. Waist circumference was measured to the nearest millimeter. Serum lipid and glucose measures were analyzed in the fasting subsample. Triglycerides were measured enzymatically using a series of reactions in which triglycerides are hydrolyzed to produce glycerol. From 1999 to 2002, HDL was measured using a heparin–manganese precipitation method. In 2003–2006, HDL was measured directly in serum using the Roche/Boehringer-Mannheim Diagnostics (Branchburg, NJ) direct HDL method. Glucose concentration was determined by a hexokinase method. Participants reported whether they were currently taking medication to lower their blood pressure or medication for diabetes. Current use of fibrates and nicotinic acid were considered indicators of treatment for elevated triglycerides and low HDL (26).
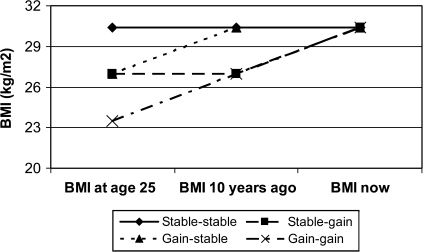
Using logistic regression, we predicted the relative odds of metabolic syndrome and its subcomponents at age 50–64 years based on current measured BMI and weight history pattern. The stable-stable category is used as the reference category in all analysis. Hence, given the same current BMI, we compare persons who have roughly maintained weight (within 20 kg) since age 25 years with those who experienced gains during prime age (gain-stable), gains during midlife (stable-gain), and continuous weight gain (gain-gain). Controlling for BMI at the time of the exam allows us to examine whether weight history exerts an effect on metabolic syndrome and its components that is independent of current weight status. Figure 2 depicts a simplified schematic. Models compare those with a stable BMI (shown at the sample mean of 30.4 kg/m2) with those who gained weight (shown as 10 kg of weight change) during the prime age period, the midlife period, or both.
Figure 2.
Weight history comparison groups.
If length of exposure to excess weight is the primary determinant of metabolic syndrome risk, those with stable weights should have the highest risk of metabolic syndrome and its subcomponents and those who gained weight most recently, in the midlife period, should have lower risk of metabolic syndrome. However, if weight gain during the prime age or midlife period has unique negative metabolic consequences, participants who gained weight during that period may have higher relative risk. The effect of weight gain may also differ by current weight status. For example, weight stability may be protective among those who are not obese, but harmful among obese persons. Hence, we also test for interactions between weight change categories and obesity status.
All models additionally controlled for current age, sex, race/ethnicity (African American, Mexican American, and other compared with non-Hispanic white), education (less than high school and more than high school compared with high school graduate), income (using the poverty income ratio), smoking history (current smoking and former smoking compared with never smoking), and exercise (any vigorous activity in the past 30 days vs no activity). Because waist circumference is strongly associated with BMI and may have an overwhelming influence on any association between weight change and metabolic syndrome, additional models excluded waist circumference from the definition of metabolic syndrome and predict modified metabolic syndrome (MMS), defined as having at least three of the remaining four risk factors (27).
Of the 1751 participants aged 50–64 years who participated in the fasting exam components, 18 were missing measured height or weight data, 65 were missing weight history items, 97 were missing metabolic syndrome data, and 121 were missing additional covariates. Additionally, 535 participants with weight loss or a history of underweight were excluded. Underweight has long-term health effects thought to reflect unmeasured disease (28), and associations between weight gain and health are likely to be significantly different in this group, where weight gain may indicate recovery from disease (e.g., cancer). This yielded a final analytic sample of 915 participants.
RESULTS
Table 1 provides sample characteristics by weight history. Stable-stable trajectories were observed in 38.1% of the sample, and 19%–23% of participants were in each of the weight gain groups. Age varied across weight history groups; participants in the stable-gain group were younger than those with continuously stable weights but those in the gain-gain group were older. Women were more likely to be in the gain-gain group. Participants in the stable-gain and gain-stable groups had lower incomes than those with continuously stable weights. Participants with a gain-gain pattern were less likely to have been engaged in vigorous exercise in the past 30 days relative to those with stable weights. Participants in all three gain-related weight history groups had higher current mean BMIs and a higher prevalence of obesity than those with stable weights. Participants in the stable-gain and gain-gain groups had higher BMIs at age 25 years than those with stable weights. The prevalence of metabolic syndrome was significantly higher in all weight gain groups relative to those with stable weights.
Table 1.
Sample Characteristics By Weight History: Mean (SD) or %
| Stable-Stable | Stable-Gain | p | Gain-Stable | p | Gain-Gain | p | |
| N | 325 | 217 | 189 | 184 | |||
| Weighted % | 38.1 | 23.3 | 19.7 | 19.0 | |||
| Age, y | 55.9 (3.3) | 55.1 (3.2) | 0.047 | 56.8 (3.6) | 0.089 | 57.3 (3.4) | 0.003 |
| Female, % | 48.2 | 52.2 | 0.421 | 46.4 | 0.715 | 63.0 | 0.010 |
| Race, % | 0.151 | 0.717 | 0.325 | ||||
| White | 82.3 | 80.2 | 80.6 | 81.6 | |||
| Black | 5.1 | 9.1 | 6.3 | 8.5 | |||
| Mexican American | 3.7 | 4.4 | 5.2 | 3.8 | |||
| Other | 9.0 | 6.3 | 8.0 | 6.2 | |||
| Education, % | 0.172 | 0.321 | 0.265 | ||||
| Less than high school | 11.6 | 17.0 | 16.4 | 16.6 | |||
| High school | 25.5 | 28.1 | 27.6 | 28.0 | |||
| More than high school | 62.9 | 55.0 | 56.0 | 55.4 | |||
| Poverty income ratio (0–5) | 3.8 (1.1) | 3.5 (1.3) | 0.021 | 3.5 (1.2) | 0.025 | 3.6 (1.2) | 0.088 |
| Exercise in last 30 d, % | 36.9 | 32.8 | 0.407 | 28.6 | 0.099 | 18.3 | <.001 |
| Smoking, % | 0.508 | 0.346 | 0.117 | ||||
| Never | 50.9 | 45.0 | 51.4 | 50.2 | |||
| Former | 30.4 | 34.2 | 35.2 | 37.8 | |||
| Current | 18.6 | 20.8 | 13.5 | 12.0 | |||
| Current BMI, kg/m2 | 25.8(2.8) | 32.0(3.8) | <.001 | 30.9 (3.0) | <.001 | 37.3 (4.6) | <.001 |
| Obese, % | 10.9 | 58.2 | <.001 | 55.1 | <.001 | 92.5 | <.001 |
| BMI 10 y ago, kg/m2 | 24.3 (2.7) | 25.6 (3.0) | <.001 | 29.1 (3.0) | <.001 | 30.3 (3.7) | <.001 |
| BMI at age 25 y, kg/m2 | 22.7 (2.6) | 23.9 (3.0) | 0.002 | 22.9 (2.4) | 0.664 | 23.7 (2.9) | 0.016 |
| Metabolic syndrome, % | 32.3 | 58.5 | <.001 | 66.2 | <.001 | 73.0 | <.001 |
| High blood pressure, % | 50.8 | 57.9 | 0.148 | 68.9 | <.001 | 69.9 | <.001 |
| High fasting glucose, % | 42.8 | 52.9 | 0.041 | 61.5 | 0.001 | 63.1 | <.001 |
| Low HDL cholesterol, % | 20.4 | 33.8 | <.001 | 39.4 | <.001 | 40.4 | <.001 |
| High triglycerides, % | 29.9 | 45.0 | <.001 | 48.8 | 0.006 | 53.9 | <.001 |
| High waist circumference, % | 37.5 | 89.4 | <.001 | 83.3 | <.001 | 98.6 | <.001 |
Note: p values indicate difference from stable-stable group using a t-test or chi-square test. BMI = body mass index.
*p < .05;**p < .01; ***p < .001.
Table 2 provides odds ratios (ORs) from a logistic regression predicting the odds of metabolic syndrome and its subcomponents. Model 1 predicts metabolic syndrome based only on weight history and demographic characteristics, without controlling for current BMI. In this model, all three gain weight history patterns were associated with elevated risk of metabolic syndrome relative to continuously stable weight. As expected, the highest risk was associated with gain in both age periods (gain-gain); participants in this group had more than five times the odds of metabolic syndrome relative to participants in the stable-stable group (OR = 5.44, 95% CI: 3.40–8.71). After controlling for current BMI (Model 2), participants in the gain-stable (prime age gain) group had 89% increased odds of metabolic syndrome (OR = 1.89, 95% CI: 1.19–3.01), but other weight history patterns were not significantly associated with metabolic syndrome after BMI adjustment.
Table 2.
Relative Odds of Metabolic Syndrome Based on Current BMI, Weight Change, and Covariates (N = 915)*
| Metabolic Syndrome |
MMS† |
Hypertension |
High Glucose |
Low HDL |
High Triglycerides |
|||||||||
| Model 1 |
Model 2 |
|||||||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Current BMI | 1.18 (1.12–1.25) | <.001 | 1.05 (1.01–1.09) | .019 | 1.08 (1.03–1.14) | .002 | 1.11 (1.06–1.16) | <.001 | 1.02 (0.99–1.05) | 0.236 | 1.01 (0.97–1.04) | 0.767 | ||
| Stable-stable | — | — | — | — | — | — | — | |||||||
| Stable-gain | 3.15 (2.17–4.57) | <.001 | 1.29 (0.76–2.19) | .344 | 1.54 (0.85–2.79) | .154 | 0.89 (0.51–1.54) | .664 | 0.88 (0.55–1.42) | .597 | 1.75 (1.08–2.82) | 0.023 | 1.95 (1.25–3.04) | 0.004 |
| Gain-stable | 3.90 (2.49–6.10) | <.001 | 1.89 (1.19–3.01) | .008 | 2.53 (1.42–4.50) | .002 | 1.35 (0.85–2.15) | .194 | 1.30 (0.79–2.12) | .298 | 2.39 (1.45–3.94) | 0.001 | 2.09 (1.23–3.58) | 0.008 |
| Gain-gain | 5.44 (3.40–8.71) | <.001 | 1.04 (0.54–2.02) | .899 | 1.98 (1.03–3.79) | .040 | 0.78 (0.40–1.52) | .460 | 0.85 (0.46–1.60) | .617 | 2.18 (1.15–4.13) | 0.018 | 2.56 (1.52–4.31) | 0.001 |
Note: BMI = body mass index; MMS = modified metabolic syndrome; OR = odds ratio.
All models control for age, sex, race/ethnicity, poverty income ratio, education, vigorous exercise, and smoking history in addition to variables shown.
MMS, excluding waist circumference from definition and requiring three of the four remaining risk factors.
High waist circumference may have an overwhelming influence on any association between weight change and metabolic syndrome. To address this issue, Table 2 also provides ORs from a logistic regression predicting the odds of MMS by weight pattern. MMS excludes waist circumference, so that metabolic syndrome is defined by having three or more of the remaining four risk factors (27). Using this definition, participants with gain-stable (OR = 2.53, 95% CI: 1.42–4.50) and gain-gain (OR = 1.98, 95% CI: 1.03–3.79) weight histories had elevated odds of metabolic syndrome relative to the stable-stable group.
Further analysis examined the association between weight history pattern and the components of metabolic syndrome, excluding waist circumference. Weight history pattern was not associated with hypertension or high glucose, though current BMI was significantly and positively associated with these risk factors. Participants in all three weight gain groups had elevated odds of low HDL, with more than twice the odds of low HDL observed for the gain-stable group (OR = 2.39, 95% CI: 1.45–3.94) and gain-gain groups (OR = 2.18, 95% CI: 1.15–4.13) relative to continuously weight stable participants. Participants in all three weight gain groups also had elevated odds of high triglycerides, ranging from 95% increased odds for the stable-gain (midlife gain) group (OR = 1.95, 95% CI: 1.25–3.04) to more than 2.5 times elevated odds for the gain-gain group (OR = 2.56, 95% CI: 1.52–4.31).
The advantage of weight stability may be protective among those who are currently not obese but harmful among obese persons. Interactions between obesity status and the weight change categories, however, were not statistically significant in any of our models. Indeed, the strength of the estimated influence of weight gain on MMS, low HDL, and higher triglycerides appears large and positive among obese persons.
DISCUSSION
The aim of this analysis was to determine whether long-term adult weight history predicts metabolic syndrome independent of attained BMI. Previous analysis has shown that duration of overweight predicts metabolic syndrome in U.S. adults (8). This article builds on prior analysis by examining weight gain itself across a broader span of adulthood. Compared with participants in the stable-stable group, those in the gain-stable group, who experienced weight gains during the prime age period (age 25 years to a mean age of 47 years), had elevated odds of metabolic syndrome at age 50–64 years, even after accounting for current BMI. This relationship was particularly strong after excluding waist circumference from the definition of metabolic syndrome; participants who gained 10 kg or more during the prime age period had approximately twice the odds of MMS at ages 50–64 years, regardless of whether prime age weight gain was followed by weight stability (gain-stable group, OR = 2.53, 95% CI: 1.42–4.50) or additional weight gain (gain-gain group, OR = 1.98, 95% CI: 1.03–3.79).
These findings suggest that weight gain during adulthood may have a unique effect on metabolic risk. Further analysis suggested that this effect may occur through an association between weight gain, low HDL, and high triglycerides. These results point to a potential association between weight gain during adulthood and atherogenic dyslipidemia, a primary mechanism through which obesity is thought to be associated with cardiovascular disease (29).
These relationships did not appear to vary by current weight status. Even among participants who were obese at midlife, weight gain during adulthood appeared to confer additional risk relative to weight stability. Recent research has highlighted the heterogeneity of metabolic risk among those who are obese (4,5). These results suggest that weight gain in adulthood may be one risk factor that could help us understand why some obese persons are metabolically healthier than others.
There are several potential explanations for the independent effect of weight gain on lipid profiles. The pathways that lead to obesity may differ by age and these varied pathways may exert unique effects on risk factors, such that individuals with the same current weight but different weight histories have different metabolic profiles. For example, weight gain in adulthood may be more a product of health behaviors, but weight gains earlier in life may be more heavily influenced by genetic disposition (30). The health effects of obesity may be different for those with early-onset obesity than for those who became obese related to factors in adulthood. Previous research has identified a group of metabolically healthy obese individuals, characterized by an earlier onset of obesity (before age 20 years) and lower levels of visceral adipose tissue, as well as lower levels of triglycerides and higher levels of HDL relative to their metabolically unhealthy counterparts with later-onset obesity (31). Weight gain in adulthood may be more likely to result in visceral fat accumulation, which accords a higher risk for high triglycerides than fat accumulation in other areas (32). Thus, participants who became obese in adulthood may display particular behavioral or body composition characteristics that alter risk profiles relative to those who became obese at an earlier age.
In contrast to findings for HDL and triglycerides, none of the measures of weight history used here was associated with high glucose, consistent with the hypothesis that attained weight may be the most dominant risk factor for diabetes, regardless of weight history (33). The same may be true of hypertension.
Previous research on the association between weight change and cardiovascular risk factors has typically examined the effect of weight change independent of baseline or early-life weight, rather than current or attained weight (9). This research has yielded critical public health insights: a 2001 meta-analysis concluded that every kilogram of weight gain after high school increased the risk of coronary heart disease by 5.7% for women and 3.1% for men (9). However, this approach reveals little about whether prior weight information is useful in the clinical setting, in which physicians typically use current weight to assess cardiovascular disease risk. Associations between weight gain and current health status that control for baseline weight may simply reflect the fact that those who have gained the most weight have higher current weights. This analysis suggests that much of the effect of previous weight change on metabolic syndrome and its subcomponents is accounted for by controlling for current BMI. However, even after controlling for current BMI, we found that participants who gained weight in adulthood had substantially elevated odds of low HDL and high triglycerides (relative to stable weight persons). Future research should examine these associations using longitudinal data that allows for more specific comparisons of the effects of weight gain during different age periods.
There are important limitations to this analysis. First, any attempt to categorize weight history is likely to be oversimplified and may obscure differences within weight history groups. For instance, by coding all participants who gained less than 10 kg as having stable weights, we include participants who could have gained as much as 19.8 kg since age 25 years in the stable-stable category. If anything, however, this is likely to result in an underestimate of the effects of weight gain, because participants in the reference group have not had truly stable weights. Previous studies have suggested that slight weight gain in late adulthood may be associated with improved health (relative to stable weight, declining weight, or larger gains), but it is not possible to distinguish these potential differences in the coding scheme used here (34). We experimented with modeling weight history using several different methods. These included the use of multiple categories for each age period (e.g., stable, weight loss, gain of 10–19 kg, gain ≥20 kg); the use of categories based on quintiles of weight change for each period; classifying persons based on the percentage of total weight gain that can be attributed to each period; simply modeling continuous BMI in each period; and modeling total weight gain without separating by age period. In each case, results suggested that larger weight gains were associated with MMS, low HDL, and high triglyceride levels. We elected to use the method presented in this article because we find it to be the most straightforward to interpret. Future research using more complex measures and categorizations of weight history is needed.
Another limitation was our use of self-reported weight history. Although recalled weight has been found to be highly correlated with measured weight, those who most seriously misreport weight gain may bias results, particularly if participants with metabolic syndrome were more likely to underreport previous weights. An additional limitation of this self-reported weight history questionnaire is that the period of weight change differed based on current age, resulting in overlap between the prime age and midlife periods. Alternate models that excluded participants aged 60–64 years to create a more narrow current age range yielded generally similar results, but had less power due to reduced sample size. Finally, our analysis was based on adults aged 50–64 years in 1999–2006. Given the rapid secular trend of increasing weight across all ages (35), it is difficult to know whether these results are generalizable to other cohorts with different weight histories.
Despite these limitations, this research has yielded important findings. Weight history confers information about metabolic risk factors above and beyond attained BMI. In particular, adult weight gain is related to risk of low HDL and high triglycerides. Weight history may contribute to our understanding of variability in cardiometabolic health within categories of current weight status (e.g., why some obese older persons are metabolically healthy but others are not).
FUNDING
Robert Wood Johnson Foundation Health and Society Scholars program and National Institute of Child Health and Human Development (K12-HD043459).
Acknowledgments
We thank Carolyn Cannuscio for helpful suggestions on previous drafts of the article. Preliminary results were presented at the Gerontological Society of America Annual Meeting on November 22, 2008.
References
- 1.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 2.Lakka H-M, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering. Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 6.Black E, Holst C, Astrup A, et al. Long-term influences of body-weight changes, independent of the attained weight, on risk of impaired glucose tolerance and Type 2 diabetes. Diabetes Care. 2005;22:1199–1205. doi: 10.1111/j.1464-5491.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- 7.Sonne-Holm S, Sorensen TIA, Jensen G, Schnohr P. Independent effects of weight change and attained body weight on prevalence of arterial hypertension in obese and non-obese men. BMJ. 1989;299:767–770. doi: 10.1136/bmj.299.6702.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obesity. 2001;9:S326–S334. doi: 10.1038/oby.2001.138. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Arch Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Truesdale KP, Stevens J, Lewis CE, Schreiner PJ, Loria CM, Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes. 2006;30:1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowman TS, Kurth T, Sesso HD, Manson JE, Gaziano JM. Eight-year change in body mass index and subsequent risk of cardiovascular disease among healthy non-smoking men. Prev Med. 2007;45:436–441. doi: 10.1016/j.ypmed.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 14.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 15.Galanis DJ, Harris T, Sharp DS, Petrovitch H. Relative weight, weight change, and risk of coronary heart disease in the Honolulu Heart Program. Am J Epidemiol. 1998;147:379–386. doi: 10.1093/oxfordjournals.aje.a009460. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. NHANES 1999–2006 public data release file documentation. http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. Accessed January 10, 2008. [Google Scholar]
- 17.Stevens J, Keil J, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;130:1156–1163. doi: 10.1093/oxfordjournals.aje.a115758. [DOI] [PubMed] [Google Scholar]
- 18.Norgan NG, Cameron N. The accuracy of body weight and height recall in middle-aged men. Int J Obes. 2000;24:1695–1698. doi: 10.1038/sj.ijo.0801463. [DOI] [PubMed] [Google Scholar]
- 19.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 21.Alley DE, Ferrucci L, Barbagallo M, Studenski SA, Harris TB. A research agenda: the changing relationship between body weight and health in aging. J Gerontol A Biol Sci Med Sci. 2008;63:1257–1259. doi: 10.1093/gerona/63.11.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidell JC, Visscher TLS. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr. 2000;54:S33–S39. doi: 10.1038/sj.ejcn.1601023. [DOI] [PubMed] [Google Scholar]
- 23.Ford E, Giles W, Dietz W. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 24.Colditz G, Willett W, Rotnitzky A, Manson J. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Gregg E, Gerzoff R, Thompson T, Williamson D. Trying to lose weight, losing weight, and 9-year mortality in overweight U.S. adults with diabetes. Diabetes Care. 2004;27:657–662. doi: 10.2337/diacare.27.3.657. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 27.St-Onge M-P, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans. Diabetes Care. 2004;27:2222–2228. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 28.Ferraro KF, Su Y, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Pub Health. 2002;92:834–840. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–2190. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Stunkard AJ, Sorensen TI, Hanis C, et al. An adoption study of human obesity. N Engl J Med. 1986;314:193–198. doi: 10.1056/NEJM198601233140401. [DOI] [PubMed] [Google Scholar]
- 31.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 32.Despres J, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 33.Brancati FL, Wang NY, Mead LA, Liang KY, Klag MJ. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus. Arch Intern Med. 1999;159:957–963. doi: 10.1001/archinte.159.9.957. [DOI] [PubMed] [Google Scholar]
- 34.Andres R, Muller DC, Sorkin JD. National institutes of health technology assessment conference: long-term effects of change in body weight on all-cause mortality: a review. Ann Intern Med. 1993;119:737–743. doi: 10.7326/0003-4819-119-7_part_2-199310011-00022. [DOI] [PubMed] [Google Scholar]
- 35.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]