Abstract
Background
The prevalence of overweight and obesity has increased in all age groups, including older adults. However, it is not known whether higher body weight is maintained in the very old and in the years prior to death. The present study examines whether there are secular trends in body weight in old age among three birth cohorts.
Methods
The study population includes 1,364 Caucasian men born between 1877 and 1941 from the Baltimore Longitudinal Study of Aging who were followed until death. Four hundred and seventy-seven men had body weight measured during the last 5 years prior to death. Body weight was measured biannually with the last visit occurring between 1959 and 2008. Differences in body weight at the last visit and body weight trajectories across birth cohorts were examined with linear regression and linear mixed-effect regression models.
Results
Men born between 1920 and 1941 had significantly higher body weight over the entire follow-up time compared with men born between 1900 and 1919 (p < .001) and 1877 and 1899 (p = .001), and the difference was also significant between the two earlier birth cohorts (p < .001). A significant increasing trend in body weight across birth cohorts was also observed in the few years prior to death.
Conclusions
In generally healthy men, there is a significant secular increase in body weight over the adult life span and in the few years prior to death. This study confirms that the obesity epidemic also extends into late life in the current elderly population.
Keywords: Obesity, BSLA, Body weight, Old age
OBESITY is a reigning public health concern because of its established association with cardiovascular disease, type 2 diabetes, disability, and mortality (1). Over the past four decades, the United States and other countries have experienced a dramatic increase in the prevalence of overweight and obesity across the full age spectrum, including older adults (2). Based on the most recent National Health and Nutrition Examination Surveys (NHANES), in 2005–2006 (3), more than two thirds of persons aged 65 years or older are either overweight or obese.
Data on secular trends in body weight, overweight, and obesity in the oldest old adults are scarce (3–7). Based on the NHANES, the prevalence of overweight or obesity in men aged 75 or older increased between 1988 and 2006 from 56.5% to 65.8% and the prevalence of obesity from 13.2% to 24.0% (3). In women, the corresponding increments were 52.3%–62.6% and 19.2%–24.2%. Trends of increasing body weight and fat mass with later birth cohorts have also been reported for Americans and Swedes (5,6,8). Thus, it appears that the classic image of older persons as thin and frail no longer applies.
Examining secular trends in body weight in older adults can be somewhat challenging because body weight is not stable throughout the life span. Previous studies suggest that body weight tends to increase with advancing aging and peak around age 60–65 years and decrease thereafter (9–13). It has been suggested that the increase in body weight stems from a progressive decline in total energy expenditure due to decreased resting metabolic rate and physical activity with aging (14).
The decline in body weight that occurs in late life has been attributed to health deterioration. Thus, it may be hypothesized that in the few years prior to death, when health deterioration occurs, the trend toward overweight and obesity will desist. It is not known, however, whether the increased prevalence of obesity in the population modifies the entire trajectory of weight change during adult and late life or only affects the portion of life characterized by good health and function. This question can be addressed by comparing longitudinal series of weight measures obtained in individuals from different age cohorts. To date, only one study has used this approach. Eiben and colleagues (5) compared women born in 1922 and in 1930 and found that at age 70 and in younger ages women in the later birth cohort were heavier than those in the earlier birth cohort.
Using data from the Baltimore Longitudinal Study of Aging (BLSA), we examined secular trends in body weight in older men born between 1877 and 1941 and compared the longitudinal trajectories in body weight between subsequent birth cohorts. We also tested the hypothesis that higher weight in the later cohorts persists even in the years prior to death when individuals may have substantial morbidity.
PARTICIPANTS AND METHODS
Study Design and Participants
The BLSA is an ongoing longitudinal study of community-dwelling volunteers with above average education, income, and access to medical care as well as general health consciousness. Volunteers have been continually recruited in the beginning of 1958, primarily from the Baltimore—Washington, DC area. From 1958 through 1978 participants were exclusively men. Women were enrolled after 1978. Participants underwent extensive evaluations approximately every 2 years at the Gerontology Research Center in Baltimore, Maryland. Each visit lasted 2–3 days and included medical evaluations and physiological and cognitive testing. A general description of the sample and the recruitment criteria of the BLSA has been reported previously (15,16).
Due to the limited number of women and minorities in the sample born prior to 1900, the present study uses data from white men only. Men included in the analysis were born between 1877 and 1941 and had at least two weight measurements (n = 1,364). To address the second aim of this study, a subgroup of men who had died before 2008 were identified. Of men aged 60–90 years at their last follow-up visit prior to death, 477 men had measured body weight within 5 years prior to death and 257 within 1 year prior to death.
The BLSA protocol is approved by a combined institutional review board of the Gerontology Research Center and Johns Hopkins Bayview Medical Center, which complied with the principles stated in the Declaration of Helsinki. All participants signed institutional review board–approved informed consents.
Body Weight and Body Mass Index
At each visit, body weight in kilograms and height in centimeters were measured after overnight fasting with participants wearing a hospital gown, using a standard physician’s scale and stadiometer, respectively. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters.
Mortality
Mortality status was ascertained through intermittent contact with participants and their relatives, supplemented by annual searches of the National Death Index. Cause of death was classified as “cardiovascular,” “cancer,” or “other or unknown.” Before 1999, the International Classification of Diseases (ICD)-9 was used to classify cause of death and after that the ICD-10 was used.
Statistical Methods
Differences in age-associated longitudinal body weight trajectories across birth cohorts were estimated using linear mixed-effect regression models (MIXED procedure in SAS) (17). Mixed-effect regression models were used to analyze longitudinal change in body weight because these models take into account the correlation between serial measures obtained from the same participants and do not assume balanced observations. By using mixed-effects models, we were able to decompose longitudinal information on weight into between-person effects and within-person effects. Age-related changes in body weight were examined in the model as random effects, assuming that change in body weight is person specific with a normal distribution. Based on the mixed-effect model estimates, we plotted age-related change in body weight according to birth cohorts.
Differences in body weight at the last visit across birth cohorts were examined by generalized linear regression models adjusting for age and height, time to death, and cause of death. An interaction term Birth Cohort × Age was included to evaluate whether differences in weight between birth cohorts varied with respect to participant age. Because this interaction term was nonsignificant, it was not included in the final models. Based on the regression model estimates, the average body weights at age 60, 70, 80, and 90 years were calculated for each birth cohort. Results are presented based on participants who had their last follow-up within 5 years prior to death. The statistical analyses presented in this study are limited to men who were aged 60–90 years at their last follow-up visit (n = 477). However, the descriptive plot (Figure 2) includes data from all deceased men born between 1877 and 1941 (n = 577) in order to show overall trends in body weight across age groups. The SAS 9.1 Statistical Package was used for all analyses (SAS Institute Inc., Cary, NC).
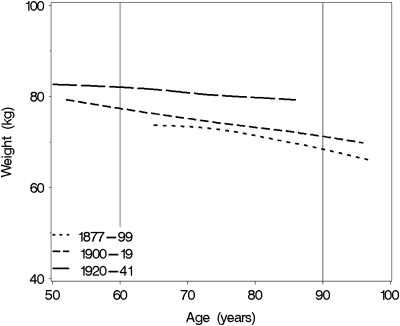
Figure 2.
Cross-sectional association of body weight in the 5 years prior to death according to age at measurement and birth cohort.
RESULTS
The average age at last visit was 81.1 years (SD 7.0) for those born between 1877 and 1899, 76.3 years (SD 11.3) for those born between 1900 and 1919, and 59.8 years (SD 16.8) for those born between 1920 and 1941 (Table 1). Mean follow-up time was 17.6 years (SD 12.3) ranging from 1 to 49 years and mean number of visits was 9.4 (SD 6.2) ranging from 2 to 32.
Table 1.
Number of Participants and Basic Characteristics at Last Study Visit According to the Birth Cohorts
| All (n = 1,364) |
Deceased (n = 477)* |
|||||
| 1877–1899 | 1900–1919 | 1920–1943 | 1877–1899 | 1900–1919 | 1920–1943 | |
| n | 214 | 498 | 652 | 144 | 252 | 81 |
| Age at last visit, y | 81.1 (7.0) | 76.3 (11.3) | 59.8 (16.8) | 80.6 (5.5) | 78.8 (7.7) | 72.0 (6.8) |
| Weight at last visit, kg | 71.6 (10.4) | 75.0 (11.3) | 86.4 (15.0) | 71.7 (10.9) | 74.2 (11.4) | 84.5 (14.3) |
| Height at last visit, cm | 171.9 (6.3) | 172.4 (6.6) | 177.2 (7.1) | 172.0 (6.6) | 171.8 (6.2) | 174.8 (6.4) |
| BMI at last visit, kg/m2 | 24.2 (3.0) | 25.2 (3.2) | 27.5 (4.3) | 24.2 (3.2) | 25.1 (3.4) | 27.7 (4.5) |
| Years of education, y | 16.3 (2.7) | 16.8 (2.6) | 16.7 (2.5) | 16.4 (2.6) | 16.8 (2.7) | 16.6 (2.6) |
| Cause of death, % | ||||||
| Cardiovascular | 56.9 | 33.5 | 24.2 | |||
| Cancer | 18.1 | 21.8 | 25.8 | |||
| Other or unknown | 25.0 | 44.8 | 50.0 | |||
Including persons who had their last visit within 5 years prior to their death and were 60–90 years old at last visit.
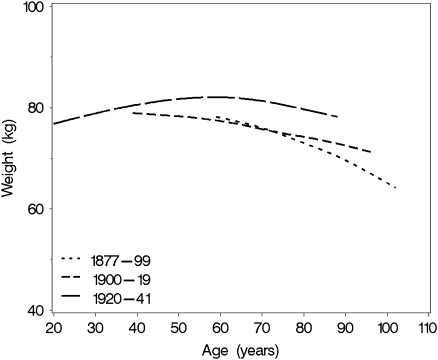
We used all available weight data in the study population (n = 1,364), including data from participants who died and those who survived over the follow-up to evaluate secular trends in age-associated longitudinal body weight trajectories. Age-related longitudinal trajectories in body weight by birth cohort estimated for this population are depicted in Figure 1. Men born between 1920 and 1941 had higher body weight throughout the adult life span than those in either of the earlier birth cohorts. It also appears that body weight peaked slightly later in men born between 1920 and 1941 than those born before 1920. The linear mixed-effect model with birth cohort, age, and follow-up time effects adjusted for body height (Table 2) confirms these observations. The significant Age × Birth Cohort interaction indicates that men born between 1920 and 1941 had higher body weight across age groups than those born between 1877 and 1899 (p = .001) and those born between 1900 and 1919 (p < .001). Furthermore, the significant Time × Birth Cohort effect indicates that men born between 1920 and 1947 experienced less decline in weight over time compared with those born between 1877 and 1899 (p = .002) and between 1900 and 1919 (p < .001). The decline was not statistically different between two earlier birth cohorts (p = .26).
Figure 1.
Longitudinal trajectory in body weight across adult life span by birth cohort.
Table 2.
Linear Mixed-Effect Regression Model Estimates of Body Weight Over Time According to the Birth Cohorts
| Beta estimate | SE | p | |
| Intercept | 80.87 | 9.24 | <.001 |
| Age | 0.17 | 0.03 | <.001 |
| Time | 0.74 | 0.06 | <.001 |
| 1877–1899 | −18.86 | 7.50 | .012 |
| 1900–1919 | −10.63 | 2.31 | <.001 |
| 1920–1943 | 0 | ||
| Age × Time | −0.01 | 0.00 | <.001 |
| Age × 1877–1899 | −0.37 | 0.11 | .001 |
| Age × 1900–1919 | −0.26 | 0.04 | <.001 |
| Age × 1920–1941 | 0.00 | ||
| Time × 1877–1899 | −0.21 | 0.07 | .002 |
| Time × 1900–1919 | −0.15 | 0.03 | <.001 |
| Time × 1920–1941 | 0 |
Note: Analysis adjusted for height. Age = age at first visit; Time = duration of follow-up.
Next, we examined whether body weight in the last years prior to death differed by birth cohort. Only men who had their last follow-up visit within 5 years prior to death and were aged 60–90 years at their last visit were included (n = 477). The average age at the last visit was 80.6 years (SD 5.5) for those men born in 1877–1899, 78.8 years (SD 7.7) for those born in 1900–1919, and 72.0 years (SD 6.8) for those born in 1920–1941. The most common cause of death for men born between 1877 and 1899 and 1900 and 1919 was cardiovascular disease. Men born between 1920 and 1941 died equally from cardiovascular disease and cancer. Other study population characteristics by birth cohort are presented in Table 1.
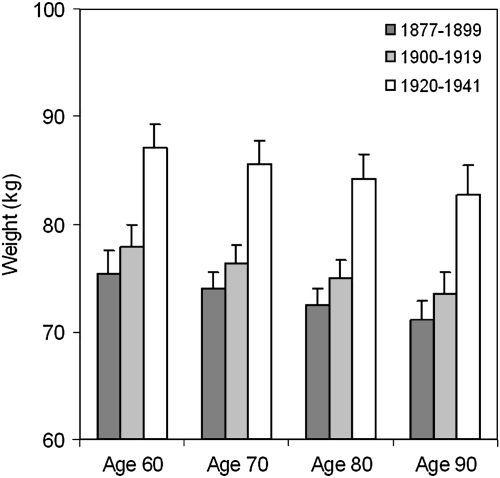
Figure 2 illustrates secular trends in body weight by age and birth cohort within the 5 years prior to death. With increasing age body weight prior to death decreased in men in all three birth cohorts. Men born between 1920 and 1941 showed higher body weight at all ages compared with earlier birth cohorts. In particular, body weight within the 5 years prior to death was significantly higher in men born in 1920–1943 than those born in 1877–1899 (p < .001) and 1900–1919 (p < .001), after adjusting for height, cause of death, and time to death (Table 3). Body weight within the 5 years prior to death was also different between men born in 1877–1899 and those born in 1900–1919 (p = .02). Body height was positively associated with weight (p < .001). Regression model–based estimates for body weights at age 60, 70, 80, and 90 years for participants who died within 5 years from their last visit are shown in Figure 3. For example, the average body weight in men at age of 80 years who died within 5 years was 72.5 kg (SD 1.5) for those born in 1877–1899, 75.0 kg (SD 1.6) for those born in 1900–1919, and 84.2 kg (SD 2.3) for those born in 1920–1941.
Table 3.
Association Between Birth Cohort and Body Weight at Last Visit Among Men Aged 60–90 Years
| Beta estimate | SE | p | |
| 5 y prior to death* | |||
| Intercept | 59.63 | 15.75 | <.001 |
| 1877–1899 | −10.80 | 1.80 | <.001 |
| 1900–1919 | −8.02 | 1.59 | <.001 |
| 1920–1941 | 0 | ||
| Age | −0.04 | 0.08 | .615 |
| Height | 0.85 | 0.08 | <.001 |
| 1 y prior to death† | |||
| Intercept | 63.68 | 20.61 | .002 |
| 1877–1899 | −10.19 | 2.33 | <.001 |
| 1900–1919 | −7.21 | 2.06 | <.001 |
| 1920–1941 | 0 | ||
| Age | −0.01 | 0.10 | .889 |
| Height | 0.87 | 0.10 | <.001 |
Notes: Analysis adjusted for height, cause of death, and time to death. Age = age at last visit.
Including men who had body weight measured within 5 years prior to death (n = 477).
Including men who had body weight measured during the last year prior to death (n = 257).
Figure 3.
Estimated body weight in the 5 years prior to death according to age at measurement and birth cohort.
Results were comparable for participants whose body weight was measured during the last year prior to death (n = 257) with men born between 1920 and 1941 having higher body weight than men from either of the earlier birth cohorts (p for both comparisons < .001; Table 3). Body weight within 1 year of death was different between men born in 1877–1899 and those born in 1900–1919 (p = .05).
DISCUSSION
In white, largely well-educated men who participated in the BLSA, we observed a consistent secular increase in body weight throughout the adult life span across three birth cohorts—1877–1899, 1900–1919, and 1920–1941 independent of body height. This higher average weight persisted to within 1 year of death. It appears that not only did the most recent cohort have higher weight up through death but also experienced less decline in weight in the years immediately preceding death.
The secular increases in body weight observed in this study generally agree with recent nationally representative data from the United States (3) and Sweden (5,18), but extend the observations to men aged 80 years and older and men born before 1920 as well as men within 1 and 5 years of death. The only previous study that reported increasing levels of obesity throughout more than 30 years of follow-up was limited to women born in 1922 and 1930 (5). Our results clearly indicate that the average body weight throughout the adult life span is higher among men born after 1920 than earlier birth cohorts, and differences in body weight are apparent in midlife (Figure 1). Interestingly, men born between 1877 and 1899 and 1900 and 1919 showed relatively similar body weights at midlife, and the age-related weight decline did not also differ in height-adjusted models.
To summarize, our findings confirm that the recent “obesity epidemic” affects even the oldest old and persists into the last remaining years of life, which stands in contrast to the traditional view of thin and frail older persons. In fact, one hallmark indicator of frailty, unintentional weight loss and underweight (19,20), appears to be a diminishing feature of men near death in the most recent birth cohort. However, although frailty is clearly associated with adverse health outcomes, need for long-term care, and mortality (19), we want to emphasize that our results should not be interpreted that the observed secular increase in body weight in the old age has a favorable effect on health. Further studies are needed to examine the health consequences of obesity among the oldest old population.
The adverse health consequences of increasing body weight in adult population are many. Obesity is associated with increased risk of type 2 diabetes, cardiovascular disease, cancer, and osteoarthritis (1,21). In addition, obesity increases the risk of functional limitation and disability in older adults thus potentially limiting their quality of life (22–25). The association between body weight and mortality in older adults is complex and under dispute (26). In most previous studies carried out in older populations, the association between BMI and mortality follows a U-shaped curve and the BMI associated with the lowest mortality increases with age (27). Underweight (BMI < 18.5 kg/m2) is consistently associated with an increased risk of mortality, but at the other end of the BMI, spectrum increased mortality risk is often observed only among the most severely obese persons (BMI ≥ 35 kg/m2) (27–32). The reasons for the diluted mortality risk among the obese is not fully understood, but factors such as cause-specific mortality, weight history, smoking, weight loss, short expected life span, and survival effect may confound the association (26,32,33).
Another perplexing aspect to secular increases in body weight is that it has occurred simultaneously with increasing life expectancy. This paradox may be a function of improved secondary and tertiary treatments of obesity-related conditions, which permits more obese people to live longer, and thus, we may witness increasing numbers of older obese persons in the years to come. An alternative scenario is that with the rising prevalence and severity of obesity in the middle-age population coupled with the life-shortening consequences of obesity-related conditions, such as type 2 diabetes and cardiovascular diseases (34), the life expectancy may start to level off and possibly decline in the older population (35).
The secular body weight differences observed among the oldest old may originate from different social, economic, and environmental conditions earlier in life. Indeed, men participating to this study were born 64 years apart. When men from the 1877 to 1899 birth cohort entered the BLSA in 1958 at the age of near 60–80 years, men from the latest birth cohort (1920–1941) were only about 10 to 20 years old. Living environment and lifestyles have changed dramatically during the 20th century due to urbanization, industrialization, and automation. Dietary habits and physical activity behavior are likely to provide some explanation for the increasing secular trends in body weight (8). Changes in dietary habits, such as eating calorie-rich and processed food, drinking soda, and consuming fast food as well as replacing regular meal times with snacking, have contributed to increased caloric consumption. Moreover, leisure-time and work-related physical activity has decreased over decades thus decreasing energy consumption.
Some limitations of the present study should be recognized. First, BLSA participants were predominantly well educated and health conscious and may have had a more health-promoting lifestyle than the general older population (36), thus limiting the generalizability of our findings. For example, the prevalence of obesity (BMI ≥ 30 kg/m2) among persons aged 75 years in this study population was only 8.2%, whereas it was 24.0% in the most recent NHANES (3). Thus, the secular increase in body weight observed in the BLSA sample may greatly underestimate trends for the general older American population.
Second, the cross-sectional analysis was limited to participants who had died. This decision reflects our interest in creating a sample of older men with information on body weight close to death and our focus on “biologic age” as opposed to “chronological age.” However, our findings are necessarily influenced by selective survival because only the healthiest persons born between 1877 and 1899 were participating in the BLSA (average age at the beginning of the study was 69.5 years), whereas men in the latest birth cohort (1920–1941) entered the study at mean age of 48 years thus comprising more heterogeneous sample. Finally, in contrast to other studies examining secular trends in obesity, we decided to use body weight instead of BMI as decreasing height with age may overestimate the amount of increase in BMI with aging (37). However, our analyses were adjusted for body height to take into account the cohort differences in body size. Study strengths include biannual and comparably collected body weight data and an extensive follow-up amounting to several decades for many participants.
In conclusion, in generally healthy men, there has been a significant increasing secular trend in body weight across the adult life span that persists until death. Furthermore, age-associated weight loss in the oldest old appears to have diminished over time. Additional studies with representative populations are needed to confirm these findings and examine the consequences of obesity among the oldest old. To tackle the increasing body weight among the oldest old, development of appropriate interventions that target healthy weight throughout the life span are needed.
FUNDING
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. This work was also supported by grant from the Finnish Academy (no. 125494 to S.S.).
Acknowledgments
Data for these analyses were obtained from the BLSA, a study performed by the National Institute on Aging.
References
- 1.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva, Switzerland: 2000. World Health Organization, WHO Technical Report Series 894; [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Health, United States, 2008 With Chartbook. Hyattsville, MD: 2009. [Google Scholar]
- 4.Liese AD, Doring A, Hense HW, Keil U. Five year changes in waist circumference, body mass index and obesity in Augsburg. Germany Eur J Nutr. 2001;40:282–288. doi: 10.1007/s394-001-8357-0. [DOI] [PubMed] [Google Scholar]
- 5.Eiben G, Dey DK, Rothenberg E, et al. Obesity in 70-year-old Swedes: Secular changes over 30 years. Int J Obes. 2005;29:810–817. doi: 10.1038/sj.ijo.0802940. [DOI] [PubMed] [Google Scholar]
- 6.Ding J, Kritchevsky SB, Newman AB, et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am J Clin Nutr. 2007;85:405–410. doi: 10.1093/ajcn/85.2.405. [DOI] [PubMed] [Google Scholar]
- 7.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–1912. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 8.Dey DK, Rothenberg E, Sundh V, Bosaeus I, Steen B. Height and body weight in elderly adults: a 21-year population study on secular trends and related factors in 70-year-olds. J Gerontol A Biol Sci Med Sci. 2001;56:M780–M784. doi: 10.1093/gerona/56.12.m780. [DOI] [PubMed] [Google Scholar]
- 9.Noppa H, Andersson M, Bengtsson C, Bruce Å, Isaksson B. Longitudinal studies of anthropometric data and body composition. The population study of women in Gotenberg, Sweden. Am J Clin Nutr. 1980;33:155–162. doi: 10.1093/ajcn/33.1.155. [DOI] [PubMed] [Google Scholar]
- 10.Rissanen A, Heliövaara M, Aromaa A. Overweight and anthropometric changes in adulthood: A prospective study of 17,000 Finns. Int J Obes. 1988;12:391–401. [PubMed] [Google Scholar]
- 11.Droyvold WB, Nilsen TI, Kruger O, et al. Change in height, weight and body mass index: Longitudinal data from the HUNT Study in Norway. Int J Obes. 2006;30:935–939. doi: 10.1038/sj.ijo.0803178. [DOI] [PubMed] [Google Scholar]
- 12.Lissner L, Sjostrom L, Bengtsson C, Bouchard C, Larsson B. The natural history of obesity in an obese population and associations with metabolic aberrations. Int J Obes Relat Metab Disord. 1994;18:441–447. [PubMed] [Google Scholar]
- 13.Seidell JC, Visscher TLS. Body weight and weight change and their health implications for the elderly. Eur J Clin Nutr. 2000;54:S33–S39. doi: 10.1038/sj.ejcn.1601023. [DOI] [PubMed] [Google Scholar]
- 14.Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr. 2000;54:S92–S103. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- 15.Shock NW, Greulich R, Andres R, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Govt. Printing Office; 1984. NIH Publication 84-2450. 1984. [Google Scholar]
- 16.Lissner L, Andres R, Muller DC, Shimokata H. Body weight variability in men: Metabolic rate, health and longevity. Int J Obes. 1990;14:373–383. [PubMed] [Google Scholar]
- 17.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. 2nd ed. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 18.Lissner L, Johansson SE, Qvist J, Rossner S, Wolk A. Social mapping of the obesity epidemic in Sweden. Int J Obes Relat Metab Disord. 2000;24:801–805. doi: 10.1038/sj.ijo.0801237. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 20.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 21.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 22.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes. 2006;30:364–373. doi: 10.1038/sj.ijo.0803130. [DOI] [PubMed] [Google Scholar]
- 23.LaCroix AZ, Guralnik JM, Berkman LF, Wallace RB, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 24.Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci. 2007;62:859–865. doi: 10.1093/gerona/62.8.859. [DOI] [PubMed] [Google Scholar]
- 25.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: The Health, Aging and Body Composition Study. Am J Epidemiol. 2009;169:927–936. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: A review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 28.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 29.Allison DB, Gallagher D, Heo M, Pi-Sunyer FX, Heymsfield SB. Body mass index and all-cause mortality among people age 70 and over: The Longitudinal Study of Aging. Int J Obes Relat Metab Disord. 1997;21:424–431. doi: 10.1038/sj.ijo.0800423. [DOI] [PubMed] [Google Scholar]
- 30.Al Snih S, Ottenbacher KJ, Markides KS, Kuo Y-F, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med. 2007;167:774–780. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30:822–829. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 32.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 33.Strandberg TE, Strandberg AY, Salomaa VV, et al. Explaining the obesity paradox: Cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30:1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- 34.Gregg EW, Cheng YJ, Cadwell BI, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 35.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 36.Talbot LA, Fleg JL, Metter EJ. Secular trends in leisure-time physical activity in men and women across four decades. Prev Med. 2003;37:52–60. doi: 10.1016/s0091-7435(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 37.Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index. The Baltimore Longitudinal Study of Aging. Am J Epidemiol. 1999;150:969–977. doi: 10.1093/oxfordjournals.aje.a010106. [DOI] [PubMed] [Google Scholar]