Abstract
Targeting of numerous transmembrane proteins to the cell surface is thought to depend on their recognition by cargo receptors that interact with the adaptor machinery for anterograde traffic at the distal end of the Golgi complex. We report here on consortin, a novel integral membrane protein that is predicted to be intrinsically disordered, i.e. that contains large segments whose native state is unstructured. We identified consortin as a binding partner of connexins, the building blocks of gap junctions. Consortin is located at the trans-Golgi network (TGN), in tubulovesicular transport organelles, and at the plasma membrane. It directly interacts with the TGN clathrin adaptors GGA1 and GGA2, and disruption of this interaction by expression of a consortin mutant lacking the acidic cluster–dileucine (DXXLL) GGA interaction motif causes an intracellular accumulation of several connexins. RNA interference-mediated silencing of consortin expression in HeLa cells blocks the cell surface targeting of these connexins, which accumulate intracellularly, whereas partial depletion and redistribution of the consortin pool slows down the intracellular degradation of gap junction plaques. Altogether, our results show that, by studying connexin trafficking, we have identified the first TGN cargo receptor for the targeting of transmembrane proteins to the plasma membrane. The identification of consortin provides in addition a potential target for therapies aimed at diseases in which connexin traffic is altered, including cardiac ischemia, peripheral neuropathies, cataracts and hearing impairment.
Sequence accession numbers. GenBank: Human CNST cDNA, NM_152609; mouse Cnst cDNA, NM_146105.
INTRODUCTION
Protein traffic along the secretory pathway involves the recognition of specific sorting signals by specialized machinery at multiple steps, from the endoplasmic reticulum to other intracellular compartments or the plasma membrane (1). Many sorting signals (e.g. peptide motifs, signal patches, post-translational modifications…) mediate the direct interaction of the protein to be sorted with the adaptor proteins required for the formation of vesicular or tubular transport carriers (2,3). Sorting of proteins lacking such signals relies instead on the binding of the protein to a specific cargo receptor, i.e. a transmembrane protein that simultaneously interacts with the protein to be sorted and with the adaptor machinery. The best-characterized example of cargo receptors is provided by the sorting of lysosomal acidic hydrolases. These soluble enzymes, labeled with mannose-6-phosphate (M6P) groups, are recognized at the trans-Golgi network (TGN) by transmembrane M6P receptors, which interact on the cytosolic side of the membrane with the adaptor protein 1 (AP1) complex (4) and the Golgi-localized, gamma-ear containing, ADP-ribosylation-factor binding (GGA) adaptors (5,6) to promote transport out of the TGN. After releasing their cargo in endosomes, the M6P receptors are recycled back to the TGN for further rounds of trafficking (6).
Although the sorting signals for targeting of transmembrane proteins to the plasma membrane have begun to be elucidated (1,3), the identities of the cargo receptors required for the trafficking of such proteins remain unknown (7). In this work, we focused on the plasma membrane targeting of connexins, the major constituents of the intercellular channels that compose vertebrate gap junctions. Connexins are non-glycosylated, integral membrane proteins with four membrane-spanning segments, one cytosolic and two extracellular loops, and N- and C-termini exposed to the cytosol (8,9). The connexin family comprises 22 members in humans and 19 in mice, with molecular weight from 25 to 62 kDa (10–12). All connexins enter the secretory pathway and are delivered to the plasma membrane by way of the endoplasmic reticulum and the Golgi complex. Along this route, connexin monomers assemble into hexameric connexons. There is also evidence for an alternative, Golgi-independent pathway for the delivery of connexin 26 to the plasma membrane (9,13).
Members of the connexin family underlie human pathological conditions as diverse as hearing impairment, cataracts, peripheral neuropathies, genodermatoses, oculodentodigital dysplasia or Pelizaeus–Merzbacher-like disease. In fact, the most prevalent form of inherited non-syndromic hearing impairment, DFNB1, is caused by mutations affecting the genes that encode connexins 26 (Cx26) and 30 (Cx30). To better understand the pathologic process in DFNB1 hearing impairment, we tried to identify ligands shared by these two connexins. By using a yeast two-hybrid approach, we identified consortin, a novel ubiquitously-expressed, connexin-binding protein. Our results show that consortin, named after its role in the intracellular sorting of connexins, acts as a TGN receptor involved in connexin targeting to the plasma membrane and recycling from the cell surface.
RESULTS
Identification of consortin, a predicted intrinsically disordered protein
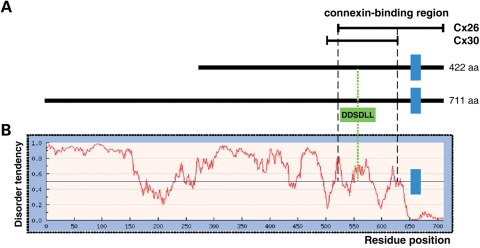
We sought to identify ligands common to Cx26 and Cx30, the major connexins expressed in the inner ear, by using the yeast two-hybrid technique. All previous attempts to identify connexin-binding proteins by means of such technique had relied on using either the cytosolic loop or the C-terminal tail of connexins as baits, instead of the full-length protein (14–16). Indeed, it was felt that the transmembrane segments of connexins would prevent the translocation of the full-length bait polypeptide to the yeast nucleus (14), resulting in the failure of the screen. Since the cytosolic loops and the C-terminal tails are the most variable segments of connexins (9,10), such strategy resulted in the identification of ligands specific of a single connexin species. Instead, we used full-length Cx26 and Cx30 as baits to screen a mouse cDNA library obtained from the sensory areas of the vestibule, the balance organ of the inner ear (17). In the Cx26 and Cx30 assays we identified, respectively, 18 and 6 independent positive clones that corresponded to the product of 9630058J23Rik, an 11-exon gene. RT–PCR analyses indicated that this gene was expressed in all mouse tissues we tested (inner ear, eye, brain, heart, lung, liver, kidney, testis and muscle), which suggests that the cognate protein, which we termed consortin, is ubiquitous. 5'-RACE experiments indicated that the gene encodes at least two different isoforms: a short isoform (422 amino acids, 45.3 kDa), encoded by exons 8–11; and a long isoform (711 amino acids, 76.8 kDa), encoded by exons 2–11, which includes the entire short isoform (Fig. 1A).
Figure 1.
Consortin is a disordered integral membrane protein. (A) Structure of mouse consortin short and long isoforms, showing the position of the predicted transmembrane segment (blue bar, residues 652–669 in the long isoform) and of the DDSDLL sequence (green, residues 557–562 in the long isoform) for interaction with the monomeric GGA (consensus DXXLL) and tetrameric AP-1, AP-2 and AP-3 adaptors [consensus (D/E)XXXL(L/I)]. The regions for binding to Cx26 (residues 521–711 in the long isoform) and Cx30 (residues 503–631 in the long isoform), deduced from the overlap of consortin yeast two-hybrid clones interacting with the connexin baits, are indicated. (B) Prediction of disordered regions in consortin by the IUPred algorithm, showing the positions of the transmembrane domain and connexin-interaction region (residues 521–631). The short C-terminal tail of consortin is well structured and likely to form a globular domain. In contrast, the putatively cytosolic, N-terminal segment of consortin, which includes the connexin-interacting region, is highly disordered. This structural arrangement is conserved in all full-length consortin orthologs known.
BLAST database searches showed the existence of consortin gene orthologs in several vertebrate genomes from fish to mammals, but not in any of the fully sequenced, non-vertebrate eukaryotic genomes, which suggests that consortin is limited to vertebrates, as is the connexin family of proteins.
Computer-assisted analysis of the deduced polypeptide showed that the two consortin isoforms share the same characteristics: (1) a high content of acidic residues (19%), which results in a substantial negative net charge at physiological pH; (2) a lack of an amino-terminal signal peptide; and (3) the existence of a hydrophobic stretch long enough to be a membrane-spanning segment (reliability 0.86), located near the C-terminus (Fig. 1A). Topology analyses indicated that consortin is a type-II membrane protein with a long N-terminal cytosolic fragment, and a short extracellular (luminal) C-terminal tail. Consortin bears no significant similarity to any other protein in the sequence databases. Moreover, screening of PFAM and SMART databases did not detect any known sequence motifs that would allow classification of consortin into any of the already identified protein families. Remarkably, different algorithms predicted that 65–76% of consortin residues are disordered (Fig. 1B). This indicates that consortin should be considered an intrinsically disordered protein, i.e. a protein that contains large segments that do not fold spontaneously into well-organized globular structures in the absence of stabilizing interactions.
Consortin is a connexin-binding protein
We tested the ability of consortin to bind Cx26 and Cx30 by means of glutathione-S-transferase (GST) pulldown assays. We defined the minimal region of the consortin amino acid sequence that was required for the interaction with Cx26 and Cx30 by analyzing the overlap of all independent clones identified in the 2 yeast two-hybrid assays. Of note, the minimal interaction region did not include the putative transmembrane segment of consortin (Fig. 1A). Since this minimal interaction region was contained within the consortin short isoform (Fig. 1A), we used a GST-consortin short isoform fusion protein as bait in pulldown assays.
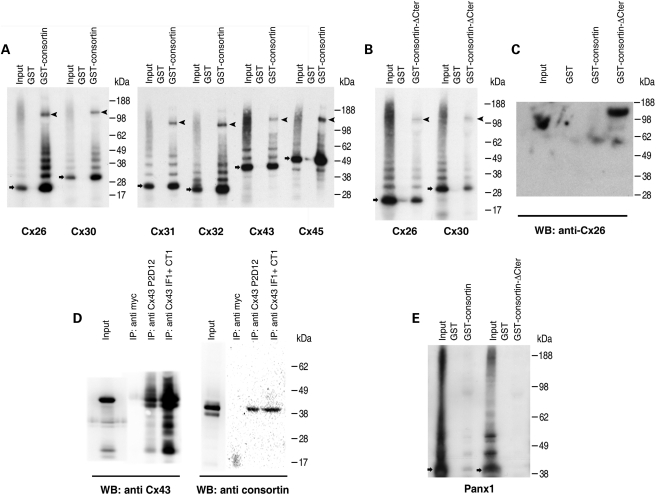
The recombinant GST-consortin fusion protein purified from Escherichia coli was able to precipitate all the in vitro-synthesized mouse connexins that we tested, namely Cx26, Cx30, Cx31, Cx32, Cx43 and Cx45 (Fig. 2A). To rule out the possibility that binding occurred by unspecific hydrophobic interactions between the transmembrane domain of consortin and those of connexins, we generated a truncated GST-consortin fusion protein (GST-consortin-ΔCter, corresponding to residues 1–362 of the short isoform) that lacked the putative transmembrane domain and C-terminal segment. GST-consortin-ΔCter interacted in pulldown assays with all in vitro-synthesized connexins previously tested (Fig. 2B). GST-consortin-ΔCter was also able to pulldown rat Cx26 from detergent-free lysates of a stably transfected Cx26 HeLa cell line (18) (Fig. 2C). Moreover, we observed that different monoclonal antibodies directed against Cx43 co-precipitated consortin and Cx43 from extracts of HeLa cells transiently expressing this connexin (Fig. 2D). Altogether, the data show that consortin is a binding partner for all groups of connexins.
Figure 2.
Consortin interacts with connexins both in vitro and in vivo. (A) Pulldown experiments of different in vitro synthesized and radioactively labeled connexins (Cx26, Cx30, Cx31, Cx32, Cx43 and Cx45) by a batch-purified GST-consortin (short isoform) chimera. For each pulldown assay, three lanes are shown, corresponding to the relevant in vitro translation reaction (input), a control assay with batch-purified GST (GST), and the assay with the appropriate GST chimera (GST-consortin). Bands corresponding to putative connexin monomers and hexamers are indicated by arrows and arrowheads, respectively. Note that the hexamer band is consistently enriched in the GST-consortin pulldown lane, compared with the input lane. (B) In vitro synthesized, radioactively-labeled Cx26 and Cx30 were pulled down by batch-purified GST-consortin-ΔCter synthesized in Escherichia coli, which indicates that connexin binding does not occur through hydrophobic interactions with the putative transmembrane domain of consortin. (C) Pulldown assay of Cx26 from detergent-free lysates of HeLa-Cx26 cells with GST-consortin (short isoform) and GST-consortin-ΔCter chimeras synthesized in E. coli. A band corresponding to putative Cx26 hexamers, detected with an anti-Cx26 antibody, is observed in the GST-consortin-ΔCter lane. In contrast, no band was detected in the GST-consortin lane, indicating that membrane-inserted Cx26 does not interact with the short isoform of consortin. This may be due to a blockade by the transmembrane segment and C-terminal tail of non-membrane-inserted consortin, which are not part of the minimal connexin-interaction region of consortin. (D) Immunoprecipitation of Cx43 from extracts of HeLa cells transiently transfected with expression plasmid pNTCx43. Extracts were immunoprecipitated with an irrelevant monoclonal antibody (anti-myc), anti-Cx43 monoclonal antibody P2D12, or a mixture of anti-Cx43 monoclonal antibodies IF1 and CT1. Bands corresponding to Cx43 and the short isoform of consortin are detected by immunoblotting in the immunoprecipitates obtained with the anti-Cx43 antibodies, but not in those obtained with the irrelevant antibody, indicating that consortin and Cx43 co-precipitate and may interact in vivo. (E) Escherichia coli-synthesized, batch-purified, GST-consortin and GST-consortin-ΔCter do not pulldown in vitro synthesized pannexin 1 (Panx1).
We next assayed whether consortin would also bind pannexins, which are gap junction proteins with the same topological arrangement than connexins but no amino acid sequence similarity with them (19,20) and, unlike connexins, are found in both vertebrates and invertebrates (21). We did not detect any interaction between in vitro synthesized pannexin 1 and either GST-consortin or GST-consortin-ΔCter (Fig. 2E).
Endogenous consortin localizes in the TGN
RT–PCR experiments showed that HeLa cells expressed the consortin gene (HUGO approved symbol: CNST). To detect the cognate, endogenously synthesized protein, we raised rabbit polyclonal antibodies against peptides of human consortin and verified the specificity of the affinity-purified anti-consortin antibodies by immunoblotting (Fig. 3A), as well as by immunostaining of HeLa cells that synthesized myc- or GFP-tagged human consortin, and antigen absorption assays (our unpublished data).
Figure 3.
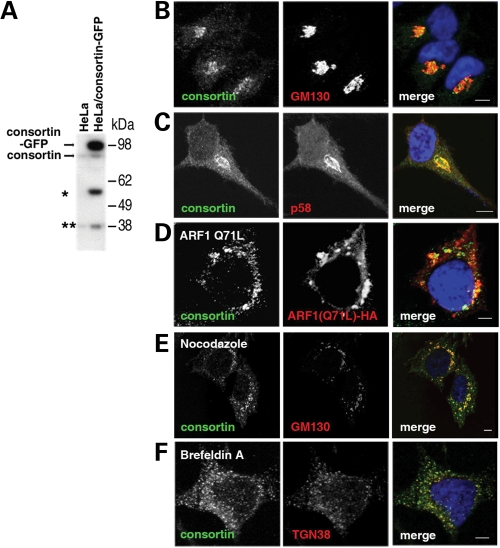
Subcellular localization of consortin in the Golgi apparatus. (A) Detection of consortin by an affinity-purified polyclonal antibody in lysates of untransfected HeLa cells (HeLa) or HeLa cells transiently transfected with a GFP-tagged human consortin construct (HeLa/consortin-GFP). Exogenously expressed consortin-GFP and endogenously expressed consortin are indicated by arrows. The lower molecular mass bands detected by the antibody (asterisks) may correspond to isoforms generated by translation started from a hypothetical internal ribosomal entry site or by post-translational processing. (B, C) Confocal microscopy images of HeLa cells stained for endogenous consortin (green) and the Golgi apparatus markers (red) GM130 (B) and p58 (C). (D) Modification of the distribution of consortin (green) in HeLa cells transiently transfected with the dominant negative Q71L mutant of human ARF1 (red). (E, F) Modification of the distribution of consortin in HeLa cells treated with Golgi-disrupting agents nocodazole (15 µm for 2 h) (E) and brefeldin A (5 µm for 45 min) (F); cells were stained for consortin (green) and for the Golgi markers (red) GM130 (E) or TGN48 (F). Bars, 5 µm.
Immunofluorescence microscopy of untransfected HeLa cells showed that most endogenous consortin was concentrated in a perinuclear region, largely overlapping with the Golgi complex markers GM130 and p58 (Fig. 3B and C). The remaining consortin labeling was scattered in a punctate pattern throughout the cytoplasm and up to the cell membrane (Fig. 3B and C). We tested the association of endogenous consortin with the Golgi complex by treating HeLa cells with Golgi-disrupting agents. Expression of the dominant activating Q71L mutant of human ADP ribosylation factor 1 (ARF1), which enlarges and vesiculates the Golgi apparatus (22), caused expansion and disaggregation of the perinuclear consortin staining, overlapping in part with ARF1 Q71L (Fig. 3D). Furthermore, incubation of HeLa cells with nocodazole or brefeldin A resulted in the loss of the perinuclear localization of consortin (Fig. 3E and F). Remarkably, after brefeldin A treatment the immunostaining for the TGN integral membrane protein TGN38 and for consortin entirely overlap (Fig. 3F), strongly arguing for the TGN localization (23) of consortin. In contrast, we did not observe co-localization of consortin with Golgi cisternal markers (GM130, p58) in brefeldin A-treated cells.
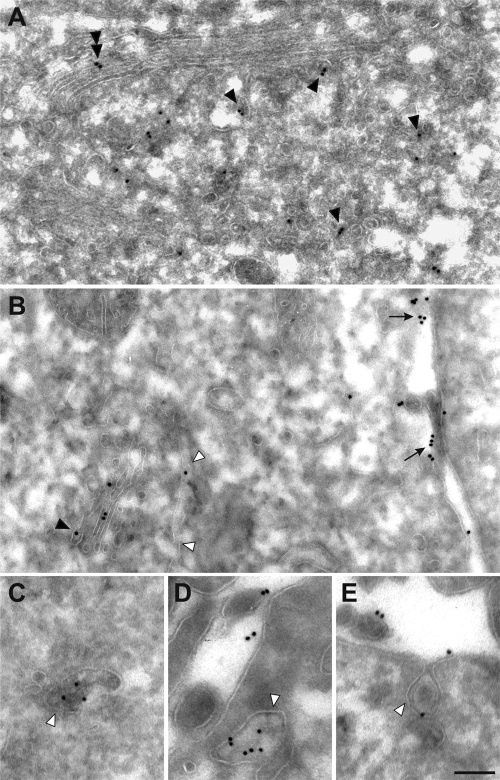
Immunoelectron transmission microscopy detected endogenous consortin in tubulo-vesicular structures on the trans side of the Golgi apparatus (Fig. 4A and B) as well as in tubulo-vesicular organelles throughout the cytoplasm of HeLa cells (Fig. 4B–D), consistently with the immunofluorescence data. Occasional vesicles were seen to fuse with the plasma membrane (Fig. 4E), where sizable levels of consortin were immunolabeled (Fig. 4B, D and E). These results suggest that consortin may traffic between the TGN and the plasma membrane, along the secretory pathway.
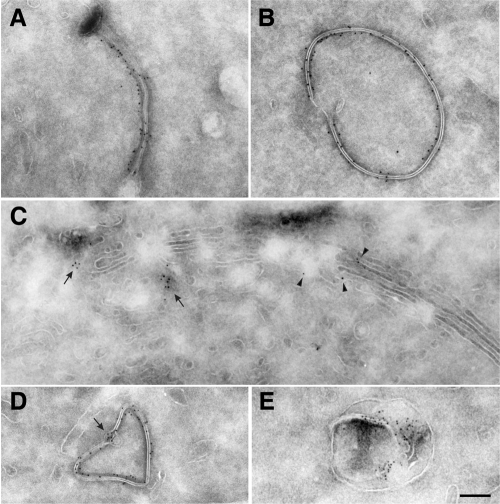
Figure 4.
Endogenous consortin localizes to the Golgi apparatus and the cell membrane. (A) Immunogold labeling of HeLa cells reveals most consortin in tubulo-vesicular structures (some pointed by arrowheads) of the TGN, and rarely in the cisternae of Golgi stacks (double arrowhead). (B) Consortin is also immunolabeled in tubulo-vesicular structures (white arrowheads) at a distance from the TGN (black arrowhead), as well as at the plasma membrane (arrows), mostly at microvilli. (C–E) Consortin-containing tubulo-vesicular structures (arrowheads) are observed throughout the cytoplasm (C), close to the plasma membrane (D) and fusing with it (E). Bars, 300 nm (A, B) and 200 nm (C–E).
Consortin binds to the TGN adaptors GGA1 and GGA2
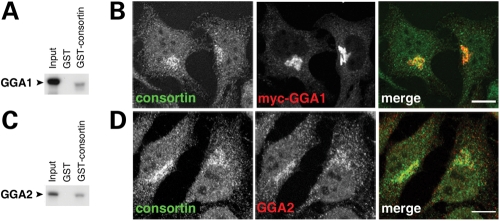
To assess the cellular role of consortin, we searched for its binding partners by means of a yeast two-hybrid screening of our inner ear cDNA library. We used the short isoform of consortin as bait in order to find ligands that were common to both the long and short consortin isoforms. In our screening we identified single clones of the TGN adaptors GGA1 and GGA2. GST-pulldown experiments confirmed that GGA1 and GGA2 indeed bind to consortin in vitro (Fig. 5A and C). Moreover, consortin and the two GGA adaptors are located in the same subcellular compartments (Fig. 5B and D).
Figure 5.
Consortin interacts with the TGN adaptors GGA1 and GGA2. (A) GST-pulldown assay of in vitro synthesized GGA1 with the GST-consortin chimera. (B) Co-localization of consortin (green) with GGA1-myc (red), in transfected HeLa cells synthesizing this chimeric protein. (C) GST-pulldown assay of in vitro synthesized GGA2 with GST-consortin. (D) Co-localization of consortin (green) with endogenous GGA2 (red) in HeLa cells. Bars, 10 µm.
The GGA proteins are monomeric clathrin adaptors that mediate cargo sorting and vesicle formation at the TGN (24–27). GGAs consist of four domains arranged in tandem: a VHS domain, which interacts with cargo proteins or cargo receptors (e.g. M6P receptors, sortilin) to be sorted; a GAT domain, which associates with the GTP-bound forms of ARF1 and ARF3 mediating the recruitment of the GGAs to TGN membranes; a flexible hinge region that binds to clathrin; and a GAE domain that recognizes accessory proteins of the trafficking machinery (28). In our two-hybrid assay, the consortin-interacting fragments of GGA1 (residues 1–260) and GGA2 (residues 1–278) spanned the full-length VHS domain. This domain binds to an ‘acidic cluster–dileucine’ peptide motif (consensus DXXLL, close to a phosphorylatable serine residue) located in the cytoplasmic tails of proteins sorted by the GGA adaptors (5,29–31). Examination of the consortin sequence with different motif-screening algorithms detected one DDSDLL sequence (residues 571–576) matching this consensus (Fig. 1A), and conserved in all consortin orthologs. Notably, the same site also matches the consensus sequence (D/E)XXXL(L/I) recognized by the tetrameric AP1, AP2 and AP3 adaptor complexes (2) (Fig. 1A).
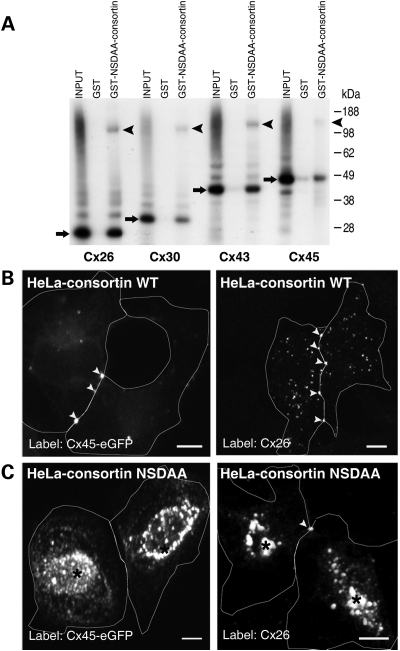
In view of the TGN and plasma membrane localization of consortin, of its ability to bind to connexins, and of its interactions with the GGA1 and GGA2 adaptors, we hypothesized that consortin might play a role in the targeting of connexins to the plasma membrane. To test this hypothesis, we generated by site-directed mutagenesis an expression plasmid coding for a short consortin isoform in which the DSDLL sequence had been replaced with NSDAA. This substitution is known to inactivate the DXXLL sorting signal (2). NSDAA-consortin retained the ability to bind connexins in GST-pulldown experiments (Fig. 6A). Transfection of HeLa cells producing Cx26 or Cx45-eGFP with a control expression plasmid coding for wild-type consortin had no effect on the plasma membrane targeting of either connexin, which formed immunostained plaques at cell–cell contacts (Fig. 6B). In contrast, transfection of connexin-expressing HeLa cells with the plasmid encoding NSDAA-consortin resulted in a striking intracellular accumulation of both Cx26 and Cx45 (Fig. 6C). These results imply that consortin recruits TGN adaptors that are required for the intracellular trafficking of connexins.
Figure 6.
Mutant NSDAA-consortin alters the intracellular traffic of connexins. (A) Pulldown of Cx26, Cx30, Cx43 and Cx45 by GST-NSDAA-consortin. Bands corresponding to the monomeric form of the indicated connexin and its putative hexamer are indicated by arrows and arrowheads, respectively. (B) In HeLa cells expressing either Cx45-eGFP or Cx26, co-expression of wild-type consortin has no effect on the cell surface display of either connexin, which formed plaques at intercellular contacts consistent with the presence of gap junctions (arrowheads). (C) In contrast, expression of the NSDAA-consortin mutant caused the intracellular accumulation (asterisks) of both Cx45-eGFP and Cx26. Thus, NSDAA-consortin behaves as a dominant-negative mutant. White lines outline cell contours, as observed in differential interference contrast (DIC) confocal images. Bars, 5 µm.
We did not observe any intracellular accumulation of connexins when Cx26- or Cx45-eGFP-expressing cells were transfected with plasmids that encode either of the dominant negative GGA mutants GGA1 VHS-GAT and GGA2 VHS-GAT, which do not recruit clathrin and thus inhibit GGA-dependent coat assembly (5,32). This result suggests that, in addition to the GGAs, consortin may recruit other adaptors to promote connexin traffic.
Knockdown of consortin expression blocks targeting of Cx32, Cx43 and Cx45 to the plasma membrane
To confirm the role of consortin in connexin trafficking, we tested the effect of RNAi-mediated consortin knockdown on the membrane targeting of different connexins. We used two complementary approaches to reduce consortin expression: transfection of synthetic short interfering RNAs (siRNAs), and generation of HeLa cell clones stably expressing microRNA-based short hairpin RNAs (shRNAs). Both approaches resulted in significant reduction of the protein levels of consortin and, when combined, silenced consortin expression (Fig. 7A).
Figure 7.
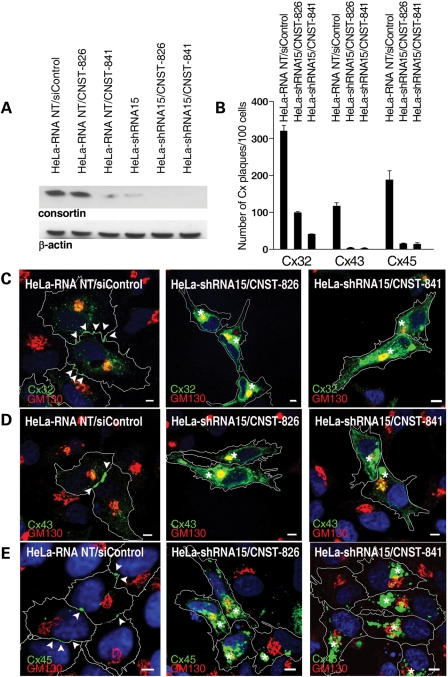
Consortin silencing alters the plasma membrane targeting of connexins 32, 43 and 45. (A) Immunoblot of consortin in lysates of HeLa-RNA-NT (stably expressing a non-silencing control shRNA) and HeLa-shRNA15 cells (stably expressing a consortin-targeting shRNA), after 72 h of siRNA-mediated consortin knockdown. Ten microgram total cellular protein extract were loaded per lane and β-actin was used as loading control. Maximum consortin silencing was obtained when combining shRNA stable knockdown with transfection of either CNST-826 or CNST-841 siRNAs. (B) Effect of consorting silencing on the number of connexin immunolabeled plaques in connexin-expressing HeLa-RNA-NT and HeLa-shRNA15 cells after 72 h of siRNA-mediated consortin knockdown. In all cases, differences between control and consortin-silenced cells were statistically significant (Supplementary Material, Table S1). (C–E) Effects of consortin knockdown on connexin distribution in HeLa/RNA-NT and HeLa-shRNA15 cells expressing Cx32 (C), Cx43 (D) or Cx45-eGFP (E) after 72 h of siRNA transfection. Cells were stained for the appropriate connexin (green) and the Golgi marker GM130 (red), and counterstained with DAPI (blue). Cell contours are drawn as white lines. Arrowheads indicate immunostained connexin plaques at intercellular contacts. Note the absence of plaques and the intracellular connexin accumulation (asterisks) in cells undergoing consortin silencing. Bars, 5 µm.
In HeLa cells expressing Cx32, Cx43 or Cx45-eGFP, consortin knockdown by means of different siRNA/shRNA combinations induced a dramatic reduction (up to 90%) in the number of immunostained connexin plaques at cell–cell contacts, compared with control cells treated with non-targeting siRNAs/shRNAs (Fig. 7B and Supplementary Material, Table S1). Consortin knockdown also induced a massive intracellular accumulation of Cx32, Cx43 or Cx45-eGFP (Fig. 7C–E), reminiscent of that observed in cells transfected with a plasmid coding for NSDAA-consortin (Fig. 6C). Only a portion of the intracellular connexin localized at the Golgi complex, as indicated by a partial overlap of connexin and GM130 stainings (Fig. 7C–E). Consortin knockdown in HeLa cells expressing Cx26 also led to the intracellular accumulation of this connexin, but did not significantly alter the number of connexin plaques at cell–cell contacts (Supplementary Material, Fig. S1 and Table S1).
Partial depletion and redistribution of the cellular consortin pool increases the size of membrane gap junction plaques and the number of cytoplasmic annular gap junctions
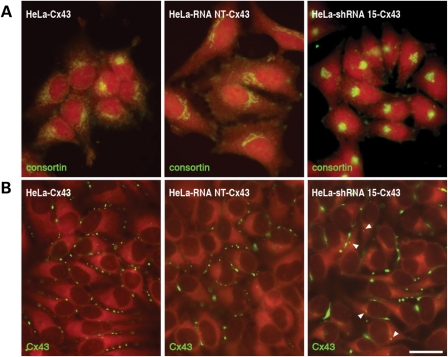
We further explored the effects of consortin down-regulation mediated by shRNA by using immunoelectron microscopy. In agreement with previous reports (33), no labeling for Cx43 was detected in untransfected HeLa cells. However, consortin was observed in the Golgi apparatus, in cytoplasmic vesicles and at the plasma membrane (Supplementary Material, Table S2). After stable transfection of a Cx43 expression plasmid, HeLa cells featured a similar pattern of consortin immunostaining, and further showed Cx43 immunolabeling, largely predominating as gap junction plaques at cell–cell contacts (Fig. 8 and Supplementary Material, Table S2). Analogous observations were made in HeLa-RNA NT-Cx43 cells, which were stably co-transfected with expression plasmids coding for Cx43 and non-targeting (NT) shRNA (Figs 8 and 9, and Supplementary Material, Table S2). In contrast, HeLa-shRNA 15-Cx43 cells, stably co-transfected with expression plasmids coding for Cx43 and CNST-targeting shRNA—which substantially decreases consortin protein levels (Fig. 7A), featured a different distribution of both consortin and Cx43. The residual consortin immunolabeling was essentially restricted to the Golgi apparatus (Fig. 8 and Supplementary Material, Table S2), and Cx43 immunolabeling was seen both at gap junction plaques on the cell membrane, which were often larger than in HeLa-Cx43 and HeLa-RNA NT-Cx43 cells and at cytoplasmic spots (Figs 8 and 9, and Supplementary Material, Table S2). Immunoelectron microscopy revealed that these spots corresponded to numerous, internalized annular gap junctions (Fig. 9 and Supplementary Material, Table S2). Cx43 was also immunolocalized, at much lower levels, within small vesicles and TGN elements (Fig. 9 and Supplementary Material, Table S2), alike those labeled for consortin.
Figure 8.
The distribution of Cx43 in transfected HeLa cells changes with that of consortin. (A) Immunofluorescence labeling of consortin shows a loose distribution throughout most of the cytoplasm in HeLa-Cx43 and HeLa-RNA NT-Cx43 cells. In contrast, consortin is mostly restricted to the perinuclear Golgi apparatus in HeLa-shRNA 15-Cx43 cells (right panel). (B) Immunostaining of Cx43 detects this connexin at small plaques along the membrane interfaces of HeLa-Cx43 and HeLa-RNA NT-Cx43 cells. In HeLa-shRNA 15-Cx43 cells, Cx43 forms larger plaques at the cell surface and also appears as discrete spots within the cytoplasm (arrowheads). Bar, 10 µm.
Figure 9.
The intracellular trafficking of gap junction plaques is affected by consortin redistribution. (A) Immunogold labeling of Cx43 in a typical gap junction plaque at the membrane interface of two adjacent HeLa-RNA NT cells stably expressing Cx43. (B) In HeLa-shRNA15 cells stably expressing Cx43, many annular gap junction plaques are found within the cytoplasm. (C) In the same cells, much lower levels of Cx43 are detected in Golgi-related vesicles (arrow) and tubular structures (arrowheads). (D, E) Several of the annular, Cx43-containing gap junctions found in the cytoplasm of HeLa-shRNA15 cells show a partial splitting of the two interacting membranes (D, arrow) or lie within lysosomal structures (E). Bar, 170 nm.
DISCUSSION
We have identified consortin as the first potential TGN cargo receptor for the sorting and cell surface targeting of transmembrane proteins. Consortin interacts both with cargo proteins (connexins) and with elements of the budding machinery (GGA adaptor proteins) required for assembly of clathrin-coated vesicles. Its predominant subcellular localization at the TGN, as well as in transport vesicles dispersed throughout the cytoplasm, and the occurrence of consortin-containing vesicles fused with the plasma membrane, are consistent with a role of consortin in TGN-to-plasma membrane secretory traffic. Moreover, targeted silencing of consortin expression reduced the number of connexin plaques at the plasma membrane and caused a massive intracellular accumulation of connexins. These alterations are strongly reminiscent of those observed after down-regulation of proteins involved in microtubule-dependent transport of connexins to the plasma membrane (34), and essentially distinct from the disappearance of gap junctions and dramatic reduction in the total cellular amount of connexin caused by knocking down the gap junction-stabilizing protein drebrin (35), leading us to conclude that consortin knockdown blocks the delivery of connexins to the plasma membrane. Consortin thus satisfies all the requirements for being a dedicated TGN cargo receptor.
It has been postulated that TGN receptors for multipass transmembrane proteins should detect their oligomeric state, incorporating only those proteins that are properly folded and assembled into TGN-derived carriers for delivery at the cell surface (36). It is thus possible that the interaction between consortin and connexins actually involves the recognition of their oligomeric state as part of a quality-control mechanism. The molecular basis of such an interaction may lie in the intrinsically disordered structure of consortin. The plasticity of the interaction surfaces of disordered proteins confers them the ability to adapt to many different partners. This ‘binding promiscuity’ is a hallmark of proteins such as chaperones, adaptors and complex assemblers (37). The finding that consortin interacts with connexins of all classes (α-, β-, γ- or δ) is consistent with this view. It is also conceivable that as yet unidentified ‘quality-control’ chaperones may prevent the binding of consortin before connexons are properly assembled.
Although all connexins reach the plasma membrane by traveling along the secretory pathway, some experiments suggest that Cx26 may also reach the cell surface via a route bypassing the Golgi complex (9), a property that can be conferred on Cx32 by a single amino acid substitution that makes Cx32 resemble Cx26 more closely (38). Our results are consistent with the existence of this parallel route, since knockdown of consortin expression did not decrease the number of Cx26 plaques at the plasma membrane, unlike what we observed for Cx32, Cx43 and Cx45. Still, the intracellular accumulation of Cx26 which we observed after disruption of consortin function suggests that a substantial proportion of Cx26 travels through the canonical secretory pathway, as reported (13,39). A route for plasma membrane targeting of integrins that bypasses the Golgi complex has been reported in Drosophila at specific developmental stages (40), indicating that alternative, non-canonical routes may be used for delivering specific subsets of proteins to the plasma membrane under certain situations.
Whereas silencing of consortin expression blocked delivery of connexins to the plasma membrane, partial depletion and redistribution of the cellular consortin pool unveiled an additional role for this protein. Gap junction plaques at HeLa cell interfaces were larger in consortin-depleted cells than in either wild-type or NT shRNA-expressing control cells. Under those conditions, cells featured increased numbers of internalized annular gap junction plaques, which were seen to undergo progressive degradation. These results suggest that consortin is implicated in the internalization and/or intracellular degradation of gap junction plaques. In cells lacking consortin, the dramatic reduction in these membrane plaques likely masked this effect, although it may have contributed to the intracellular connexin accumulation we observed.
To fulfill its proposed role as a cargo receptor, consortin must interact simultaneously with its target substrates (connexins) and with coated-vesicle adaptors (Supplementary Material, Fig. S2). Although the connexin- and GGA-binding sites lie in the same region of the protein, the observation that NSDAA-consortin retains the ability to interact with connexins indicates that the two sites are different. It is thus likely that the two interactions are not mutually exclusive.
The interaction of consortin with the GGA adaptors has two remarkable features. First, the unusual location of the DXXLL motif. All previously described type I transmembrane proteins that bind to the VHS domain of GGAs have their DXXLL motifs located 1–2 residues away from their C-termini (2,25). In consortin, a predicted type-II transmembrane protein, this motif is located within a disordered region in the middle of the protein, as reported for the autoinhibitory DXXLL motifs in the unstructured hinge domains of the GGAs (41). The second feature is the relationship between consortin-mediated traffic and GGA function. Expression of dominant-negative GGA mutants lacking the hinge and GAE domains, unable to recruit clathrin, results in the perinuclear accumulation of M6P receptors (5,29) and blocks secretion of soluble proteins whose traffic depends on the GGA adaptors, like adiponectin (42). However, such truncated GGAs had no detectable effect on the expression of connexins at the surface of HeLa cells, whereas expression of a consortin mutant lacking the motif recognized by the GGAs did induce an intracellular accumulation of connexins. An attractive explanation for these apparently discordant results is that consortin may interact with multiple adaptors to promote traffic, and not exclusively with the GGAs. Accordingly, the mutation that inactivates the DXXLL sequence of consortin also destroys an overlapping motif [consensus (D/E)XXXL(L/I)] recognized by the AP1, AP2 and AP3 adaptors. An internal, bifunctional sorting signal analogous to that observed in consortin has been reported recently for another transmembrane GGA ligand, the lipoprotein receptor-related protein LRP9 (43,44). The existence of such bifunctional sorting signals may be part of a common mechanism for sequential sorting of cargoes at successive compartments.
Current models of the secretory pathway envision the existence of a post-TGN, trans-endosomal route to the plasma membrane, mediated by clathrin-coated vesicles (1). Because of the interaction of consortin with the GGAs, it is likely that consortin targets its cargoes to this route. However, this does not exclude the possibility that consortin might also participate in the generation of tubular carriers for direct trafficking of its cargoes from the TGN to the plasma membrane (45,46). Alternatively, since the GGA adaptors participate in recycling the GLUT4 transporter from the plasma membrane (47) and in the traffic of ubiquitinated cargo (48), the interaction of consortin with the GGAs might also take place at steps of the connexin recycling pathway, hence contributing to both anterograde and retrograde connexin transport.
The emergence of specialized TGN sorting mechanisms for polarized transport and regulated secretion was essential in the evolution of multicellular animals (7). It is plausible that the appearance in vertebrates of connexin-mediated intercellular communication occurred in parallel with that of consortin, their sorting protein. However, since consortin binds to several other transmembrane proteins in yeast two-hybrid assays (our unpublished results), a broader role for consortin in TGN transport pathways should be envisaged.
Mutations that impair the intracellular trafficking of cell surface integral membrane proteins, such as receptors, transporters and channels, have been described in many human inherited disorders. In some instances, the mutation simply causes an aberrantly folded protein that is retained in the endoplasmic reticulum and targeted for degradation. However, other mutations are known to interfere with later stages of trafficking at the Golgi apparatus or the TGN, as reported for Cx26 (39), by mechanisms poorly understood. The identification of consortin as the first cargo receptor implicated in the traffic of integral membrane proteins provides a starting point for unraveling those mechanisms and for designing novel therapeutic approaches.
MATERIALS AND METHODS
Yeast two-hybrid assays
Screenings of a mouse vestibular cDNA library (17) with either full-length mouse Cx26, full-length mouse Cx30 or the mouse short consortin isoform were performed by Hybrigenics.
5′ RACE assays
Nested PCR amplification of the 5′ end of the mouse consortin cDNA was performed on a cDNA library generated by the Marathon method (Clontech) with an oligo-dT primer. The reverse oligonucleotides used for the specific amplification of the consortin cDNA were: (1312–1291) 5′-CGCCTGCCACGCTTACTGCTA-3′ (first PCR) and (1111–1091) 5′-GCAGGAACCACCACCAACCTC-3′ (nested PCR). PCR products were cloned into pCR2.1-TOPO (Invitrogen) and sequenced.
Sequence analyses
Prediction of transmembrane domains by different algorithms and prediction of membrane topology was performed by the PSORTII (http://psort.nibb.ac.jp) and PredictProtein (http://www.predictprotein.org) software packages. Search for structural patterns was carried out with the SMART web server (http://smart.embl-heidelberg.de). Protein disorder was analyzed with the IUPred (http://iupred.enzim.hu) (49), DisEMBL (http://dis.embl.de) (50) and PDisorder (http://www.softberry.com/cgi-bin/programs/propt/pdisorder.pl) (Softberry Inc.) web servers. Search for sequence motifs was performed with the MnM (http://sms.engr.uconn.edu/servlet/SMSSearchServlet) (51) and ELM (http://elm.eu.org/) (52) algorithms.
Expression constructs
Expression plasmids coding for the GFP-tagged, dominant negative VHS-GAT GGA1 and GGA2 were provided by J.S. Bonifacino. The plasmids coding for Cx45-eGFP and rat pannexin 1 were obtained from R. Bruzzone. Mouse cDNAs encoding the short and long consortin isoforms, the truncated consortin-ΔCter isoform, and the GGA1 and GGA2 adaptors were obtained by RT–PCR of total cochlear mRNA. The full-length human consortin cDNA was RT–PCR-amplified from total HeLa cell mRNA. The Cx26, Cx30, Cx31, Cx32, Cx43 and Cx45 coding sequences were amplified by PCR on mouse 129Sv genomic DNA, since each of them is contained in a single exon. The sequences of all PCR products were verified on both strands. cDNAs were cloned into pcDNA3.1V5-His (Invitrogen; pNT plasmid series, for expression of untagged proteins and in vitro coupled transcription-translation), pCMV-tag3B (Stratagene; for fusion to N-terminal myc tag), pSGC1 (a pCS2+ derivative for fusion to C-terminal eGFP) and pGEX4T1 (Amersham; for fusion to N-terminal GST). Mutation of the consortin sequence coding for the DSDLL motif to NSDAA was carried out with the QuikChange XL site-directed mutagenesis kit (Stratagene).
Antibodies
Polyclonal antibodies against a mixture of two synthetic peptides of human consortin were raised in rabbits (Covalab). Peptide sequences were: CNST1 (449–462): NH2-EGKYSQAQRKELRL-COOH, and CNST2 (541–555): NH2-NKETEDYLNSLLEGC-COOH. The specificity of the affinity-purified antibodies was verified by immunoblotting of total HeLa cell lysates, immunostaining of HeLa cells that expressed consortin-myc and consortin-GFP chimeras and antigen absorption assays. Primary antibodies used in immunofluorescence experiments were: mouse monoclonals anti-myc epitope (9E10; Santa Cruz), anti-hemagglutinin epitope (HA-7; Sigma), anti-Cx26 (CX12H10; Zymed), anti-Cx30 (CX30-8E8; Zymed), anti-Golgi p58 antigen (58K-9; Sigma), anti-GM130 (BD transduction), anti-GGA2 (BD transduction), anti pan-cytokeratin (C-11; Sigma); rabbit polyclonals anti-Cx26 (UM214; Zymed), anti-Cx30 (Z-PP9; Zymed), anti-Cx32 (ZMD.193; Zymed), anti-Cx43 (71–0700; Zymed); and goat polyclonal anti-TGN38 (Santa Cruz). All primary antibodies were used at 1/100 dilutions, except for the anti-hemagglutinin, anti-GM130 and anti-TGN38 antibodies, which were used at 1/50 dilutions. For immunofluorescence detection, we used as secondary antibodies Alexa Fluor 488-conjugated, goat anti-rabbit F(ab’)2 IgG fragment (Molecular Probes), Cy3-conjugated, sheep anti-mouse IgG (Amersham) and Alexa Fluor 568-conjugated, donkey anti-goat IgG (Molecular Probes).
GST pulldown assays of cell-free expressed proteins
In vitro synthesis of proteins was carried out with TnT T7 Quick coupled transcription-translation mixtures (Promega) containing 35S-l-methionine (Amersham). GST-pulldown assays were performed as described (53).
GST pulldown assay of HeLa cell-synthesized Cx26
Detergent-free lysates of HeLa cells stably expressing rat Cx26 (HeLa-Cx26, a gift from K. Willecke) (18) were prepared as reported (54) and cleared of nuclei by centrifugation (24). The resulting cytoplasmic fractions were incubated for 3 h at 4°C in binding buffer (phosphate-buffered saline with 5% glycerol, 5 mm MgCl2 and 0.1% Triton X-100) with GST-consortin-ΔCter fusion protein, bound to glutathione-sepharose beads. After five washes with binding buffer containing 150 mm NaCl, proteins were separated by denaturing electrophoresis in NuPAGE 4–12% SDS-polyacrylamide gradient gels (Invitrogen) and electrotransferred to nitrocellulose membranes. Cx26 was detected with rabbit polyclonal anti-Cx26 (Zymed; 0.25 µg ml−1) and horseradish-peroxidase-conjugated goat anti-rabbit antibodies (Amersham), by using the SuperSignal West Pico enhanced chemiluminescence system (Pierce).
Co-immunoprecipitation
HeLa cells transiently transfected with a Cx43-expressing plasmid pNTCx43 were grown to confluence, scraped off the plates, harvested by centrifugation and resuspended in lysis buffer (20 mm Tris–HCl pH 8.0, 150 mm NaCl, 0.2% Nonidet P-40, 1 mm DTT, 1X EDTA-free Complete Protease Inhibitor from Roche). Cell lysates were clarified by a 20-min ultracentrifugation step at 100 000g. Immunoprecipitation was performed by incubating the cleared lysates with any of three (IF1, CT1, P2D12) anti-Cx43 monoclonal antibodies (55) bound to Dynabeads Protein G (Invitrogen), as per the manufacturer's instructions. After three washes with lysis buffer, beads were resuspended in NuPAGE LDS sample buffer (Invitrogen) to release immunoprecipitates. Samples were separated by denaturing electrophoresis in NuPAGE 4–12% SDS-polyacrylamide gradient gels (Invitrogen) and electrotransferred to nitrocellulose membranes. Consortin and Cx43 were detected with rabbit polyclonal antibodies (anti-consortin, 0.2 µg ml−1; anti-Cx43, C6219 Sigma, 0.25 µg ml−1) and horseradish-peroxidase-conjugated goat anti-rabbit antibodies (Amersham), by using the SuperSignal West Pico enhanced chemiluminescence system (Pierce).
Cell culture, transfection and drug treatments
HeLa and HeLa-Cx26 cells were grown on Dulbecco-modified Eagle medium supplemented with 10% fetal calf serum, in 5% CO2 at 37°C. Transient transfections were carried out with Lipofectamine 2000 (Invitrogen). For Golgi-disrupting treatments, cells were incubated at 37°C with either 5 µM brefeldin A (Sigma) for 45 min, or 15 µM nocodazole (Sigma) for 2 h.
Immunofluorescence
HeLa cells were grown on coverslips, fixed in 4% phosphate-buffered paraformaldehyde for 15 min at room temperature, processed for immunofluorescence as described (56), and imaged in a LSM 510 Meta confocal microscope (Zeiss).
siRNAs and shRNAs
siRNAs targeted the CNST sequences: CNST-826 siRNA (826–844): 5′-CTACGAGAAAGCAATGAAA-3′ and CNST-841 siRNA (841–859): 5′-GAAATTCATTCAGCTAGAA-3′. We used siCONTROL (Dharmacon) as non-targeting control for siRNA experiments. Transfection of 50 nM siRNAs was carried out with Lipofectamine 2000. Plasmid V2HS_44615 (Open Biosystems), coding for shRNA HP_44254 (57), targeted CNST sequence 5′-GAGGAACTCTGTTAGAATA-3′ (2538–2546). Control plasmid encoding a non-silencing shRNA was also from Open Biosystems. These plasmids were used to obtain the stable HeLa cell clones HeLa-shRNA-15 and HeLa RNA-NT, respectively, by transfection and selection in 1 µg ml−1 puromycin.
RNA interference assays
RNAi assays were performed in duplicate to assess consortin protein levels (by immunoblotting) and the effect of consortin silencing on connexin trafficking (by immunofluorescence). Transfection of low-passage number (<20) HeLa-shRNA-15 and HeLa RNA-NT cells with either CNST-826, CNST-841 or non-interfering siCONTROL siRNAs was considered the start point (0 h) of the assay. At 48 h, cells were transfected with expression plasmids encoding one of the following connexins: Cx26, Cx30 or Cx45-eGFP. Cells were then incubated for a further 24 h before being processed for either immunoblotting or immunofluorescence.
Evaluation of connexin plaques
Cultures of cells transiently expressing different connexins were grown on coverslips, immunostained for the appropriate connexin and for Golgi marker GM130, counterstained with DAPI, and imaged in a LSM 510 Meta confocal microscope (Zeiss). Bright dots at contacts between adjacent connexin-expressing cells, as observed in DIC images, were scored as connexin plaques. Connexin-expressing cells undergoing division (as shown by DAPI counterstaining) or having a disorganized Golgi apparatus (as indicated by GM130 staining) were excluded from the counts. The statistical significance of the results was evaluated with a two-tailed, unpaired t-test with Welch's correction (InStat 3.0 software package, GraphPad).
Generation of HeLa cells stably expressing Cx43
Wild-type HeLa cells (33) and the stable HeLa cell clones HeLa-shRNA-15 and HeLa RNA-NT that were maintained under 1 µg ml−1 puromycin (InvivoGen) selection pressure, were transfected with the rat Cx43-encoding plasmid G2A (58) by using either Lipofectamine 2000 (Invitrogen), PolyFect Transfection Reagent (Qiagen) or an MP-100 MicroPorator system (Digital Bio), as per the manufacturers’ instructions. Stable transfectants were selected with 350 µg ml−1 neomycin (Invitrogen), plus 1 µg ml−1 puromycin for double transfectants. These experiments resulted in the selection of 11, 6 and 10 clones of wild-type, HeLa RNA-NT and HeLa-shRNA-15 clones, respectively. In each clone, 60–80% of the cells expressed Cx43, as determined by a 2 h immunolabeling at room temperature of cells permeabilized for 10 min with −20°C acetone and stained with rabbit polyclonal antibodies against Cx43 (71–0700; Zymed), diluted 1:100, and sheep antibodies against rabbit IgGs (1238833, Boehringer Mannheim), diluted 1:500 (59). Most of the cells were also positive for consortin, as evaluated by immmunolabeling with anti-consortin rabbit polyclonal antibody, diluted 1:50 (overnight incubation at 4°C of cells fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100) and reacted first with a biotin conjugated goat serum against rabbit IgGs (111-066-045, Jackson ImmunoResearch), diluted 1:200, and then with fluorescein-conjugated streptavidin (016-090-084, Jackson ImmunoResearch), diluted 1:600. To minimize the influence of clone-dependent differences, further experiments were carried out on cell populations made in equal parts of three to four different clones (one per transfection procedure) that were pooled for each type of Cx43-expressing cell.
Immunoelectron microscopy of consortin
HeLa cells were pelleted by centrifugation and fixed in 4% paraformaldehyde plus 0.1% glutaraldehyde for 5 min at room temperature, followed by 60 min fixation in 4% paraformaldehyde (all fixatives diluted in 0.1 M phosphate buffer, pH 7.4). Cells were washed three times in 0.1 M phosphate buffer, embedded in 12% gelatin, and cooled on ice. Small blocks of gelatin-embedded cells were infused with 2.3 M sucrose, frozen in liquid nitrogen, and sectioned with a EMFCS cryo-ultramicrotome (Leica). Ultrathin sections were mounted on Parlodion-coated copper grids. Sections were processed and immunolabeled as previously described (60–62), which included incubations at room temperature with rabbit anti-consortin polyclonal antibody (diluted 1:1–1:10) for 1–12 h, and with goat anti-rabbit IgGs antibodies (diluted 1:10) conjugated to 15-nm gold particles, for 20 min. Labeled sections were screened and photographed in a CM10 electron microscope (Philips). Negative controls were run by exposing the sections only to the gold-conjugated anti-rabbit IgGs antibodies, which did not result in a consistent staining of the cells.
Immunoelectron microscopy of Cx43
Control and Cx43-expressing HeLa cells were pelleted, fixed, embedded and mounted as indicated in the previous paragraph. Ultrathin sections were processed and immunolabeled as previously described (60–62) which, in these experiments, included a 1 h exposure at room temperature to either the rabbit anticonsortin polyclonal antibody (diluted 1:20), or a rabbit antiCx43 antibody (C6219, Sigma), diluted 1:40 (59). This incubation was followed by a 20 min exposure at room temperature to either Protein A-coated 15 nm diameter gold particles (diluted 1:150) or goat anti rabbit serum (EM.GAR10, British Biocell International). Cryosections were screened as mentioned above. Negative controls were run by exposing the sections only to the Protein A-coated particles. These incubations did not result in a consistent staining of the cells (not shown). For evaluation of the labeling distribution, at least 30 cells were screened per group at the original magnification of ×21 000, and the presence of gold particles on different organelles scored on prints, at the final magnification of ×63 000. Data on the distribution of gold particles between compartments were analyzed by chi square tests, as provided by Statistical Package for Social Sciences (SPSS Inc.).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Integrated Project EuroHear of the Sixth Framework Program of the European Union (LSHG-CT-2004-512063 to C.P.); the Swiss National Science Foundation (310000-122430 to P.M.); the Juvenile Diabetes Research Foundation International (1-2007-158 to P.M.); the Seventh Framework Program of the European Union (project BETAIMAGE, 222980 to P.M.); Novo Nordisk (to P.M.); the USA National Institutes of Health (GM55632 to P.D.L.); and the Spanish Fondo de Investigaciones Sanitarias (CP06/00050 to F.J.d.C.). F.J.d.C. was supported by a Marie Curie postdoctoral fellowship.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J.S. Bonifacino, R. Bruzzone and K. Willecke for gifts of plasmids and cell lines; P. Roux and E. Perret for help with confocal microscopy; V. Yuste and S. Susín for advice on RNAi; B. Goud, J.-P. Hardelin and I. del Castillo for critical reading of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Rodríguez-Boulan E., Müsch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta. 2005;1744:455–464. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino J.S., Traub L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Boulan E., Kreitzer G., Müsch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell. Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh P., Dahms N.M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell. Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 5.Puertollano R., Aguilar R.C., Gorshkova I., Crouch R.J., Bonifacino J.S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh P., Griffith J., Geuze H.J., Kornfeld S. Mammalian GGAs act together to sort mannose 6-phosphate receptors. J. Cell. Biol. 2003;163:755–766. doi: 10.1083/jcb.200308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bard F., Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell. Dev. Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N.M., Gilula N.B. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 9.Evans W.H., Martin P.E.M. Gap junctions: structure and function. Mol. Memb. Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 10.Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Güldenagel M., Deutsch U., Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 11.Cruciani V., Mikalsen S.O. The vertebrate connexin family. Cel. Mol. Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scemes E., Spray D.C., Meda P. Connexins, pannexins, innexins: novel roles of ‘hemi-channels. Pflugers Arch. - Eur. J. Physiol. 2009;457:1207–1226. doi: 10.1007/s00424-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laird D.W. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C., Lau A.F., Martyn K.D. Identification of connexin-interacting proteins: application of the yeast two-hybrid screen. Methods. 2000;20:219–231. doi: 10.1006/meth.1999.0939. [DOI] [PubMed] [Google Scholar]
- 15.Lan Z., Kurata W.E., Martyn K.D., Jin C., Lau A.F. Novel Rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry. 2005;44:2385–2396. doi: 10.1021/bi048306w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama M., Ishida N., Ogawa T., Yogo K., Takeya T. Molecular cloning and functional analysis of a novel Cx43 partner protein CIP150. Biochem. Biophys. Res. Comm. 2005;335:1264–1271. doi: 10.1016/j.bbrc.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Boëda B., El-Amraoui A., Bahloul A., Goodyear R., Daviet L., Blanchard S., Perfettini I., Fath K.R., Shorte S., Reiners J., et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R.A., Hülser D.F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelan P., Starich T.A. Innexins get into the gap. BioEssays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- 20.Panchin Y. Evolution of gap junction proteins–the pannexin alternative. J. Exp. Biol. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 21.Panchin Y., Kelmanson I., Matz M., Lukyanov K., Usman N., Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr. Biol. 2000;10:R473–R474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C.J., Rosenwald A.G., Willingham M.C., Skuntz S., Clark J., Kalm R.A. Expression of a dominant allele of human ARF1 inhibits membrane traffic in vivo. J. Cell Biol. 1994;124:289–300. doi: 10.1083/jcb.124.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Bio.l. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell'Angelica E.C., Puertollano R., Mullins C., Aguilar R.C., Vargas J.D., Hartnell L.M., Bonifacino J.S. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boman A.L., Zhang C.J., Zhu X., Kahn R.A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirst J., Lui W.W., Bright N.A., Totty N., Seaman M.N., Robinson M.S. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poussu A., Lohi O., Lehto V.P. Vear, a novel Golgi-associated protein with VHS and gamma-adaptin ‘ear’ domains. J. Biol. Chem. 2000;275:7176–7183. doi: 10.1074/jbc.275.10.7176. [DOI] [PubMed] [Google Scholar]
- 28.Bonifacino J.S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. doi: 10.1038/nrm1279. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y., Doray B., Poussu A., Lehto V.P., Kornfeld S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 2001;292:1716–1718. doi: 10.1126/science.1060896. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen M.S., Madsen P., Christensen E.I., Nykjaer A., Gliemann J., Kasper D., Pohlmann R., Petersen C.M. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takatsu H., Katoh Y., Shiba Y., Nakayama K. Golgi-localizing, gamma-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J. Biol. Chem. 2001;276:28541–28545. doi: 10.1074/jbc.C100218200. [DOI] [PubMed] [Google Scholar]
- 32.Puertollano R., Randazzo P.A., Presley J.F., Hartnell L.M., Bonifacino J.S. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- 33.Charpantier E., Cancela J., Meda P. Beta cells preferentially exchange cationic molecules via connexin 36 gap junction channels. Diabetologia. 2007;50:2332–2341. doi: 10.1007/s00125-007-0807-9. [DOI] [PubMed] [Google Scholar]
- 34.Shaw R.M., Fay A.J., Puthenveedu M.A., von Zastrow M., Jan Y.N., Jan L.Y. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butkevich E., Hülsmann S., Wenzel D., Shirao T., Duden R., Majoul I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 2004;14:650–658. doi: 10.1016/j.cub.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 36.Ponnambalam S., Baldwin S.A. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol. Membr. Bio.l. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- 37.Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005;579:3346–3354. doi: 10.1016/j.febslet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 38.Martin P.E.M., Blundell G., Ahmad S., Errington R.J., Evans W.H. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- 39.Thomas T., Jordan K., Simek J., Shao Q., Jedeszko C., Walton P., Laird D.W. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005;118:4451–4462. doi: 10.1242/jcs.02569. [DOI] [PubMed] [Google Scholar]
- 40.Schotman H., Karhinen L., Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev. Cell. 2008;14:171–182. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Doray B., Bruns K., Ghosh P., Kornfeld S.A. Autoinhibition of the ligand-binding site of GGA1/3 VHS domains by an internal acidic cluster–dileucine motif. Proc. Natl. Acad. Sci. USA. 2002;99:8072–8077. doi: 10.1073/pnas.082235699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie L., Boyle D., Sanford D., Scherer P.E., Pessin J.E., Mora S. Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J. Biol. Chem. 2006;281:7253–7259. doi: 10.1074/jbc.M511313200. [DOI] [PubMed] [Google Scholar]
- 43.Doray B., Knisely J.M., Wartman L., Bu G., Kornfeld S. Identification of acidic dileucine signals in LRP9 that interact with both GGAs and AP-1/AP-2. Traffic. 2008;9:1551–1562. doi: 10.1111/j.1600-0854.2008.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boucher R., Larkin H., Brodeur J., Gagnon H., Thériault C., Lavoie C. Intracellular trafficking of LRP9 is dependent on two acidic cluster/dileucine motifs. Histochem. Cell Biol. 2008;130:315–327. doi: 10.1007/s00418-008-0436-5. [DOI] [PubMed] [Google Scholar]
- 45.Fölsch H., Pypaert M., Maday S., Pelletier L., Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ang A.L., Taguchi T., Francis S., Fölsch H., Murrells L.J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L.V., Kandror K.V. Golgi-localized, gamma-ear-containing, Arf-binding protein adaptors mediate insulin-responsive trafficking of glucose transporter 4 in 3T3-L1 adipocytes. Mol. Endocrinol. 2005;19:2145–2153. doi: 10.1210/me.2005-0032. [DOI] [PubMed] [Google Scholar]
- 48.Puertollano R., Bonifacino J.S. Interactions of GGA3 with the ubiquitin sorting machinery. Nat. Cell Biol. 2004;6:244–251. doi: 10.1038/ncb1106. [DOI] [PubMed] [Google Scholar]
- 49.Dosztányi Z., Csizmok V., Tompa P., Simon I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics. 2005;21:3433–3434. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 50.Linding R., Jensen L.J., Diella F., Bork P., Gibson T.J., Russell R.B. Protein disorder prediction: implications for structural proteomics. Structure. 2003;11:1453–1459. doi: 10.1016/j.str.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Balla S., Thapar V., Verma S., Luong T.B., Faghri T., Hsuang C.H., Rajasekaran S., del Campo J.J., Shinn J.H., Mohler W.A., et al. Minimotif Miner: a tool for investigating protein function. Nat. Methods. 2006;3:175–177. doi: 10.1038/nmeth856. [DOI] [PubMed] [Google Scholar]
- 52.Puntervoll P., Linding R., Gemünd C., Chabanis-Davidson S., Mattingsdal M., Cameron S., Martin D.M.A., Ausiello G., Brannetti B., Costantini A., et al. ELM server: A new resource for investigating short functional sites in modular eukaryotic proteins. Nucl. Acids Res. 2003;31:3625–3630. doi: 10.1093/nar/gkg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adato A., Lefèvre G., Delprat B., Michel V., Michalski N., Chardenoux S., Weil D., El-Amraoui A., Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 2005;14:3921–3932. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- 54.Dell'Angelica E.C., Ohno H., Ooi C.E., Rabinovich E., Roche K.W., Bonifacino J.S. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;15:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sosinsky G.E., Solan J.L., Gaietta G.M., Ngan L., Lee G.J., Mackey M.R., Lampe P.D. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem. J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Küssel-Andermann P., El-Amraoui A., Safieddine S., Nouaille S., Perfettini I., Lecuit M., Cossart P., Wolfrim U., Petit C. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva J.M., Li M.Z., Chang K., Ge W., Golding M.C., Rickles R.J., Siolas D., Hu G., Paddison P.J., Schlabach M.R., et al. Second-generation shRNA libraries covering the mouse and human genomes. Nat. Genet. 2005;37:1281–1288. doi: 10.1038/ng1650. [DOI] [PubMed] [Google Scholar]
- 58.Vozzi C., Ullrich S., Charollais A., Philippe J., Orci L., Meda P. Adequate connexin-mediated coupling is required for proper insulin production. J. Cell Biol. 1995;131:1561–1572. doi: 10.1083/jcb.131.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmerli S.C., Masson F., Cancela J., Meda P., Hauser C. Lack of evidence for connexin-43 expression in human epidermal Langerhans cells. J. Immunol. 2007;179:4318–4321. doi: 10.4049/jimmunol.179.7.4318. [DOI] [PubMed] [Google Scholar]
- 60.Tokuyasu K.T. Immuno-cytochemistry on ultrathin cryosections. In: Spector D.L., Goodman R.D., Leinwand L.A., editors. Cells, A Laboratory Manual. Cold Spring Harbor, USA: CSHL Press; 1997. pp. 131.1–131.27. [Google Scholar]
- 61.Haefliger J.A., Krattinger N., Martin D., Pedrazzini T., Capponi A., Doring B., Plum A., Charollais A., Willecke K., Meda P. Connexin43-dependent mechanism modulates renin secretion and hypertension. J. Clin. Invest. 2006;116:405–413. doi: 10.1172/JCI23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheiermann C., Meda P., Aurrand-Lions M., Madani R., Yiangou Y., Coffey P., Salt T.E., Ducrest-Gay D., Caille D., Howell O., et al. Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science. 2007;318:1472–1475. doi: 10.1126/science.1149276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.