Abstract
Background. End-stage renal disease (ESRD) results in increased susceptibility to infections, impaired response to vaccination and diffuse B-cell lymphopenia. However, the precise nature and mechanism of ESRD-induced B-cell lymphopenia remains unclear. Therefore, we studied the distribution of major B-cell subsets, B-cell growth, differentiation and survival factors, IL-7 and BAFF, and their receptors in 21 haemodialysis patients and 21 controls.
Methods. Innate B1 cells (CD19+, CD5+), conventional B2 cells (CD19+, CD5−), newly formed transitional B cells (CD19+, CD10+, CD27−), naïve B cells (CD19+, CD27−) and memory B cells (CD19+, CD27+) and BAFF receptor were quantified by flow cytometry. Plasma IL-7, BAFF, IL-6, TNF-α and IL-10 were measured by ELISA.
Results. The ESRD group exhibited significant reductions of all B-cell subpopulations except for transitional B cells that were less severely affected. No significant difference was found in B-cell apoptosis between the ESRD and control groups. Moreover, plasma IL-7 and BAFF levels were elevated in ESRD patients, therefore excluding their deficiencies as a possible culprit. However, BAFF receptor expression was significantly reduced in transitional but not mature B cells in the ESRD group. Interestingly, B-cell activation with the TLR9 agonist resulted in significantly greater production of IL-6 and TNF alpha but not IL-10 in the ESRD group.
Conclusions. Thus, despite elevation of B-cell growth, differentiation and survival factors, ESRD patients exhibited diffuse reduction of B-cell subpopulations. This was associated with the down-regulation of BAFF receptor in transitional B cells. The latter can, in part, contribute to B-cell lymphopenia by promoting resistance to the biological actions of BAFF that is a potent B-cell differentiation and survival factor.
Keywords: infection, immune system, inflammation, vaccination, antibody production
Introduction
While bacterial infections have diminished as a cause of death in the general population, they remain the second most common cause of death in the end-stage renal disease (ESRD) population [1–3]. This is thought to be largely due to the impaired host immune response in uraemia [1,4,5]. Reported immunological abnormalities in ESRD patients include decreased granulocyte and monocyte/macrophage phagocytic function [4,6,7], defective antigen presentation by monocyte/macrophages [4,8,9], reduced antibody production by B lymphocytes [4,10,11] and impaired T-cell-mediated immunity [4,12,13]. The exact mechanisms responsible for these derangements are not fully understood. We recently reported that naïve and central memory, but not effector memory, CD 4+ and CD8+ T cells are depleted in ESRD patients [14].
B lymphocytes (CD19+) are precursors of plasma cells that are responsible for antibody production. Upon contact with the antigen, naïve B cells undergo clonal expansion/differentiation that leads to formation of memory B cells and antibody-secreting plasma cells. Peripheral blood B cells (CD19+) are comprised of distinct phenotypical/functional subpopulations including innate B1 cells (CD19+, CD5+), conventional B2 cells (CD19+, CD5−), newly formed transitional B cells (CD19+, CD10+,CD27−), naïve B cells (CD19+, CD27−) and memory B cells (CD19+, CD27+).
Earlier studies have documented a significant reduction in the peripheral blood total B-cell count in ESRD. However, little is known about the effect of ESRD on B-cell subpopulations. To address the phenotypic changes in the B cells, we studied distribution of the major B-cell subsets in peripheral blood of 21 haemodialysis-dependent ESRD patients and 21 control individuals.
Materials and methods
Patients
The study protocol was approved by Human Subjects Institutional Review Board of the University of California Irvine and completed with the assistance of the University of California General Clinical Research Center.
Twenty-one stable patients with ESRD maintained on haemodialysis for an average of 38.5 months (3–113 months) were recruited for the study. Individuals with evidence of acute or chronic infection, acute intercurrent illnesses and those receiving immunosuppressive drugs were excluded. Medical history, systolic and diastolic blood pressures, body weight, routine monthly laboratory data and medications were recorded. Haemodialysis therapy was performed thrice weekly using cellulose triacetate dialysers.
A group of 21 normal age-matched control subjects served as controls. Control subjects exhibiting acute or chronic infection, acute intercurrent illnesses, chronic illnesses such as hypertension, diabetes, malignancy, psychiatric disorders or those requiring chronic medications were excluded. All participants provided informed consent prior to enrolment in the study. Blood haemoglobin, total leukocyte and differential counts and plasma biochemical measurements were obtained using standard laboratory methods.
Blood collection
In all ESRD patients, whole blood was collected from the vascular access prior to the initiation of dialysis. The blood samples were obtained by a syringe, applying gentle aspiration to minimize shear stress. Blood samples form the control individuals were collected from a peripheral vein in the same manner.
Immunostaining
B-lymphocyte subsets were analysed by triple-colour flow cytometry using PerCP-conjugated anti-CD19 monoclonal antibodies (mAb), FITC-conjugated anti-CD 27 mAb and PE-conjugated anti-CD5, anti-CD10 or isotype control mAb. Antibodies and isotype controls were purchased from B.D. Biosciences, San Diego, CA, USA. A total of 100 μL of whole blood was incubated with 10 μL of each of the above antibodies for 15 min at room temperature in the dark. The cells were then incubated in 2 mL of the FACS lysing solution (Becton-Dickinson, San Jose, CA, USA) for 15 min to lyse red blood cells. The cells were then washed twice with phosphate-buffered saline (1X PBS) to remove red blood cell remnants, and lymphocytes were resuspended, in 0.5 mL of 1% paraformaldehyde, and used for flow cytometry.
Flow cytometry
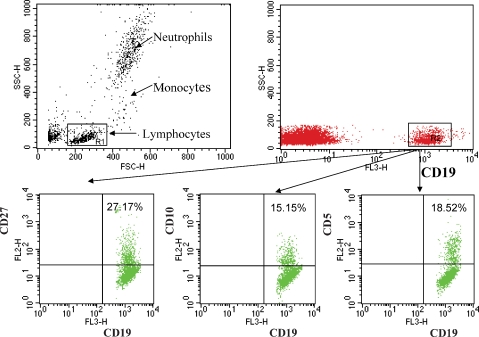
Lymphocyte phenotyping was carried out by three-colour analysis using a FACSort flow cytometer and CellQuest software (Becton-Dickinson, San Jose, CA, USA). For each sample, data from 10 000 cells were collected and analysed. Forward and side scatters were used to gate and exclude cellular debris, and FL3 channels were used to gate CD19+ B cells. During analysis, an electronic gate was placed on CD19+ B cells. The associated expression of CD27 versus CD10 and CD5 was then used to obtain the percentages of cells identifying (CD19+, CD27+), (CD19+, CD10+), (CD19+, CD5+) and subsets of CD 19+ B cells.
Detection of apoptosis
Apoptosis was measured by the Annexin V-FITC binding assay according to the manufacturer's instructions (BD Biosciences). Annexin staining was performed both in whole blood and isolated lymphocytes. Briefly, 100 μL of whole blood or isolated peripheral blood mononuclear cells (0.5 × 106) were stained with 10 μL of PerCP-conjugated anti-CD19 antibody, washed twice with 2 mL of PBS and RBC were lysed with the FACS lysing buffer. The cells were washed and resuspended in 100 μL of the Annexin V-conjugate binding buffer to which 5 μL of FITC-conjugated Annexin V was added. The mixture was incubated in a dark at room temperature for 15 min, after which 400 μL of the binding buffer was added and 5000 cells were acquired and analysed by FACS. FL3 channels were used to gate CD19+ B cells and the FL1 channel was used to detect the population of cells positive for Annexin V-FITC.
Measurement of IL-7 and BAFF
Plasma IL-7 and B-cell-activating factor of the TNF family (BAFF) were measured by ELISA using kits purchased from R & D systems (Minneapolis, MN). Briefly, 96-well microtiter plates pre-coated with IL-7 and BAFF antibody were incubated with suitable dilutions of the plasma. Bound cytokines were detected using enzyme-linked detection antibodies. After washing and addition of substrate and an amplifier, the OD in the wells was measured at 490 nm, and background values were subtracted. The average of duplicate measurements was taken. Cytokine concentrations in the samples were derived from a standard curve using purified IL-7 and BAFF.
Measurement of BAFF and IL-7 receptors
Triple colour Flow cytometry was used to assess BAFF and IL7 receptor expression on B cells. Briefly, whole blood was stained using PE-Cy5-conjugated anti-CD19, PE-conjugated anti-CD10, FITC conjugated anti-human BAFF receptor or Alexa Fluor 488-conjugated anti-human IL7-Rα. Isotype controls were used to set positive and negative gates for BAFF-R, IL-7-Rα, CD19 and CD10. Receptor antibodies as well as appropriate isotype controls were purchased from BioLegend, San Diego, CA, USA. A total of 20 000 CD19+ cells were acquired, and the proportion of CD19+CD10+ and CD19+ CD10− cells expressing receptors and the mean fluorescence intensity (MFC number) of staining (an indicator of receptor density) were analysed using the CellQuest software.
Cytokine production by B cells
Human peripheral blood mononuclear cells were isolated, counted and re-suspended to equal cell concentration. They were then incubated with 2 μg/mL of endotoxin-free un-methylated CpG oligodeoxynucleotides (CpG ODN 2006, InvivoGen, San Diego, CA, USA) for 24 h. Supernatants were collected and stored at −70°C until analysed. TNF-α, IL-6 and IL-10 were measured by ELISA kits purchased from BD Pharmingen, San Diego, CA, USA as per the manufacturer's protocol.
Data analysis
Quadrant statistics were used in the dot plot of the CellQuest software. The relative proportion and calculated absolute numbers of B lymphocytes and their subsets were analysed with independent sample t-tests in SPSS. Data were expressed as mean ± SEM, unless otherwise specified. P-values < 0.05 were considered significant.
Results
General data
The data are summarized in Table 1. The underlying cause of renal disease in the ESRD group included diabetic nephropathy in nine, hypertension in three, chronic glomerulonephritis in seven and ESRD of unknown aetiology in two patients. The types of vascular access included A-V fistulas in 12, A-V grafts in 8 and tunnelled central catheter in one of the patients. As expected, serum creatinine, urea nitrogen, phosphorus and triglyceride concentrations were significantly higher in the ESRD patients compared to the control group. Blood haemoglobin was significantly lower, whereas, serum PTH and ferritin levels and transferrin saturation were higher, and serum albumin and calcium concentrations were unchanged in the ESRD patients when compared with the corresponding values found in the control group. The mean Kt/V value in the ESRD patients was 1.62, reflecting adequacy of dialysis regimen in the study participants.
Table 1.
Clinical and biochemical parameters in normal control and ESRD groups (mean ± SD)
| Control | ESRD | ||
|---|---|---|---|
| (n = 21) | (n = 21) | P-value | |
| Age (years) | 47 ± 13 | 52 ± 16 | NS |
| Gender (male/female) | 10/11 | 11/10 | |
| BUN (mg/dL) | 13.3 ± 1.02 | 73.5 ± 25.3 | <0.001 |
| Creatinine (mg/dL) | 0.85 ± 0.18 | 10.9 ± 3.3 | <0.001 |
| Calcium (mg/dL) | 9.1 ± 0.24 | 8.9 ± 0.6 | NS |
| Phosphorus (mg/dL) | 3.2 ± 0.16 | 5.9 ± 1.8 | <0.001 |
| iPTH (pg/mL) | 40.0 ± 3.84 | 334.1 ± 268.3 | <0.001 |
| Haemoglobin (g/dL) | 14.6 ± 1.1 | 11.9 ± 1.2 | <0.001 |
| Ferritin (ng/mL) | 32.63 ± 6.26 | 309.1 ± 211.3 | <0.001 |
| Transferrin saturation (%) | 18.66 ± 2.12 | 24.8 ± 8.0 | 0.012 |
| Albumin (g/dL) | 3.8 ± 0.14 | 3.8 ± 0.3 | NS |
| Cholesterol (mg/dL) | 159.5 ± 5.47 | 141.4 ± 36.4 | 0.031 |
| Triglyceride (mg/dL) | 100.2 ± 15.5 | 167.5 ± 140.8 | 0.052 |
| Kt/V | – | 1.62 ± 0.74 |
Peripheral blood leukocyte data
The data are shown in Table 2. Compared to the normal control group, the ESRD group exhibited a significant elevation of the number of total white blood cells and polymorphonuclear leukocytes and marked reduction of circulating lymphocytes. No significant difference was found in the numbers of circulating monocytes between the two groups.
Table 2.
Blood leukocyte counts given as mean ± SD in normal control and ESRD groups
| Control | ESRD | P-value | |
|---|---|---|---|
| Total leukocytes | 6100 ± 1300 | 7400 ± 1900 | 0.009 |
| Neutrophils | 3500 ± 900 | 4700 ± 1500 | 0.004 |
| Monocytes | 400 ± 100 | 500 ± 200 | 0.053 |
| Lymphocytes | 1900 ± 600 | 1500 ± 600 | 0.019 |
B-lymphocyte subset data
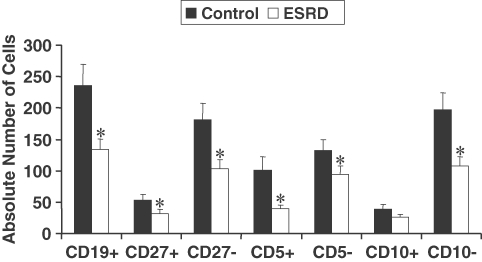
Data are shown in Figures 1 and 2. The number of transitional B cells in the ESRD group was not significantly different from that found in the control group. However, the populations of innate B1 and conventional B2 cell subsets were markedly reduced in the ESRD patients as compared to the corresponding values in the normal control group. Likewise, the numbers of naïve and memory B-cell subsets were reduced in the ESRD group. Comparison of the data obtained from diabetic and non-diabetic ESRD patients revealed no differences in either total B-lymphocyte count or the B-cell subpopulations (Table 3).
Fig. 1.
Flow cytometric analysis of B-cell subsets. Whole blood was stained with anti-CD19 PerCP, anti-CD27 FITC, anti-CD5 PE or anti-CD10 PE. CD19+ cells were gated for further analysis. B cells were divided into subpopulations according to the surface expression CD5+, CD27 and CD10; CD5+ (innate B cells), CD5−(conventional B cells), CD27+ (memory B cells), CD27− (mature B cells) CD10+ (transitional B cells).
Fig. 2.
The absolute numbers of B cells in control (N = 21) and ESRD patients (N = 21). B-cell subset percentages were analysed by flow cytometry, and the absolute numbers were calculated from CBC data. The asterisks on top of the bars represent significant difference from the control subjects. P ≤ 0.05.
Table 3.
Comparison of total B cells and B-cell subsets among diabetic and non-diabetic ESRD patients
| Diabetic | Non-diabetic | ||
|---|---|---|---|
| (cells/μL) | (cells/μL) | P-value | |
| Total B cells (CD19+) | 140 | 127 | 0.69 |
| Memory B cells (CD19+/CD27+) | 36 | 25 | 0.37 |
| Naïve B cells (CD19+/CD27−) | 104 | 102 | 0.93 |
| Transitional B cells (CD19+/CD10+) | 27 | 27 | 0.98 |
| Innate B1 cells (CD19+/CD5+) | 38 | 39 | 0.92 |
| Conventional B2 cells (CD19+/CD5−) | 102 | 87 | 0.59 |
No significant correlation was found between Kt/v and either total B-lymphocyte count or any of the B-cell subsets. Likewise, no significant correlation was found between Ferritin, which is a marker of inflammation, and iron stores with either total B-lymphocyte count or the B-cell subsets. However, a significant inverse correlation was found between the duration of dialysis treatment and the percentage of CD19±/CD27± memory B cells (r = −0.52, P = 0.04).
Apoptosis data
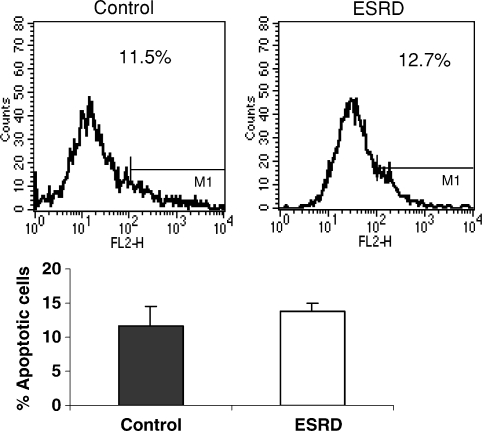
We next sought to determine whether increased apoptosis may account for the B-cell deficiency in ESRD patients. To this end, whole blood and isolated lymphocytes were stained with annexin V and anti-CD19 mAb (B-cell marker). As demonstrated in Figure 3, no significant difference was found in spontaneous B-cell apoptosis between the ESRD patients and the normal control subjects, thus excluding heightened apoptosis as the main cause of B-cell lymphopenia.
Fig. 3.
Flow cytometric analysis of B-cell apoptosis. Peripheral blood lymphocytes were stained with anti-CD19 PerCP and Annexin V–PE. CD19+ cells were gated and annexin+ cells were analysed.
Plasma levels of interleukin-7 (IL-7) and BAFF
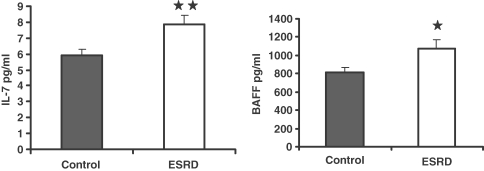
To further explore the potential mechanism(s) of B-cell lymphopenia in ESRD patients, we measured plasma levels of the main B-cell differentiation factor, IL-7 and B-cell survival factor, BAFF, in the study populations. Surprisingly, plasma IL-7 and BAFF levels were higher in ESRD patients than those found in the control subjects (Figure 4). It is of note that inflammation and lymphopenia are known to promote production and release of BAFF [15–17]. The observed elevation of BAFF in our ESRD patients may be a consequence of lymphopenia and inflammation that are common features of ESRD.
Fig. 4.
Measurement of serum concentrations of interleukin (IL)-7, and B-cell-activating factor (BAFF). The differences between normal control and ESRD patient groups were assessed by t-test. *P < 0.05 and **P < 0.01.
BAFF and IL-7 receptor data
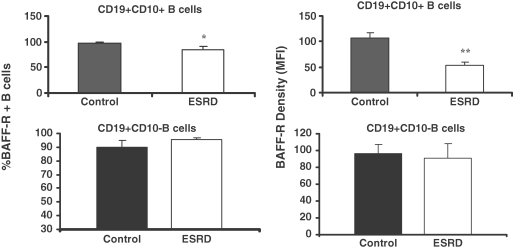
To address the potential mechanism of B-cell lymphopenia despite elevated IL-7 and BAFF levels, we next examined expression of IL-7 and BAFF receptors on CD19+ CD10+ transitional B cells. Data are illustrated in Figure 5. The study revealed a significant reduction of BAFF receptor density on the transitional B cells from the ESRD group as compared with that found in the controls. In addition, the percentage of transitional B cells with detectable BAFF receptor was mildly but significantly reduced in the ESRD group. In contrast, the BAFF receptor density in the mature B cells (CD19+ CD10− cells) and the percentage of mature B cells with detectable BAFF receptor were unchanged in the ESRD patients when compared with those found in controls. As expected IL-7 receptor expression was not found in the circulating transitional B cells in either group. This is not surprising since IL-7-responsive pre-B cells are normally confined to the bone marrow.
Fig. 5.
Bar graphs depicting proportion of transitional (CD19+ CD10+) and mature (CD19+ CD10−) B lymphocytes expressing BAFF receptor (BAFF-R in upper panel) as well as density of BAFF-R in the ESRD and normal control groups. *P < 0.05 and **P < 0.01.
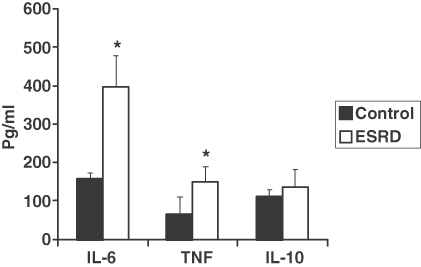
The B-cell response to TLR9 activation
Results are shown in Figure 6. Previous in vitro studies have shown that the production of IgM, IgA and IgG is impaired in ESRD patients [11,18]. However, little is known about the effect of ESRD on cytokine production [19,20] that represents another function of B cells. Human B cells express TLR9 [21,22] whose activation with un-methylated DNA triggers the production of pro- and anti-inflammatory cytokines. Activation of peripheral blood lymphocytes with the TLR9 ligand, CpG ODN, resulted in significantly greater production of pro-inflammatory cytokines, IL-6 and TNF alpha in the ESRD patients than that in the control subjects. However, no significant difference was found in the production of the anti-inflammatory cytokine, IL-10, between the ESRD and the control groups.
Fig. 6.
Bar graph depicting cytokine production by B lymphocytes in response to stimulation with TLR-9 agonist, CpG ODN 2006, in the ESRD and normal control groups. *P < 0.05.
Discussion
The ESRD patients included in the present study exhibited a marked reduction of all B-cell subpopulations except transitional cells that were less severely affected. No difference was observed in the magnitude of B-cell deficiency among diabetic and non-diabetic ESRD subgroups, pointing to the dominant role of ESRD in this process. These findings point to the impact of uraemia on B-cell differentiation and survival as a major cause of B-cell lymphopenia and impaired humoral immunity in ESRD.
B lymphocytes are generated from haematopoietic stem cells in the bone marrow throughout life. They contribute to the immune system by producing antigen-specific antibodies. IL-7, is a pleiotropic cytokine that plays a major part in B lymphopoiesis by promoting the maturation of pre-B cells to B cells in the bone marrow [23,24]. After differentiation and selection in the bone marrow, newly emerging B lymphocytes (termed transitional B cells, CD19+ CD10+) migrate to the spleen. In humans, CD10 is highly expressed on B-cell progenitors in the bone marrow and progressively disappears with maturation. A majority of CD10+ B cells in the peripheral blood represent immature transitional B cells [25,26]. Further differentiation of transitional B cells into mature long-lived lymphocytes is critically dependent on BAFF [27–29].
The diversity of the B-lymphocyte pool determines the individual's capacity to mount a protective immune response. In adults, innate B1 cells (CD5+ B cells) account for 25–27% of peripheral blood B lymphocytes. Innate B1 cells produce mainly IgM antibodies that have low-affinity and high cross-reactivity properties [30,31]. These antibodies constitute a readily available pool of immunoglobulins that fight a variety of infections prior to the production of high-affinity-specific antibodies. On the other hand, conventional B cells, otherwise known as B2 cells (CD5− B cells), produce a more diverse array of antibodies with high-affinity interactions, and account for 75–80% of peripheral blood B lymphocytes [31,32]. When the naive mature B lymphocytes recognize antigen with their specific Ig receptors, they undergo clonal expansion and differentiation into long-lived memory B cells (CD27+) and plasma cells that produce and secrete antigen-specific antibodies. Memory cells that can survive decades actively circulate from blood to lymph nodes and populate mucosal tissues. Upon a subsequent encounter with the antigen, memory B cells respond rapidly by producing Ig isotypes with a high affinity for the given antigen. In adult humans, ∼40% of all circulating B cells are memory B cells [33,34].
B-cell lymphopenia has been previously reported in ESRD patients [18,35–41]. In addition, a diminished population of CD5+ innate B cells and CD27+ memory B cells has been demonstrated in children with chronic renal failure [35]. The present study extends those findings by demonstrating that ESRD causes depletion of several other B-cell subtypes in adults and by showing that B-cell lymphopenia is unlikely a consequence of either increased peripheral apoptosis or deficiencies in IL-7 or BAFF, two key B-cell differentiation and survival factors.
In this study, we found that transitional B cells were not significantly reduced in ESRD patients suggesting that decreased output of B cells from bone marrow may not be the main cause of the B-cell lymphopenia in ESRD patients. This view is supported by the finding that plasma levels of IL-7, a cytokine that facilitates conversion of pre-B cells to B cells, were increased in ESRD patients. Two alternative mechanisms can be proposed to account for B lymphopenia in ESRD. First, the uraemic milieu may increase susceptibility of B cells to apoptosis in ESRD patients. Second, the uraemic environment may render transitional B cells resistant to differentiation and survival signals. The above two possibilities are not mutually exclusive.
In the present study, we found no difference in the proportions of apoptotic B cells among the ESRD patients and control subjects. In contrast, Fernández-Fresnedo et al. reported increased apoptosis of B cells obtained from patients with chronic renal failure [39]. The cause of this discrepancy may be related to the techniques used to detect apoptosis. Fernández-Fresnedo et al. cultured the peripheral blood cells for 96 h prior to assessing apoptosis, whereas we assessed apoptosis on freshly isolated cells. It should be noted that the inability to detect increased apoptosis in freshly isolated cells could be due to loss of apoptotic cells during the cell isolation procedure. This is unlikely, however, since we found no increase in the apoptotic B cells with the whole blood staining technique. However, we cannot exclude the possibility that increased apoptosis of B cells might have occurred in other compartments of the immune system.
The growth and differentiation of B cells from stem cell to mature plasma cell are governed, among other factors, by the level of cytokines in the microenvironment and the expression of their respective receptors on the cells. The presence of severe B lymphocytopenia in the face of elevation of IL-7 and BAFF that are the major B-cell differentiation and survival factors is indicative of uraemia-induced B cell resistance to the action of these cytokines. Since biological actions of IL-7 and BAFF depend on activation of their receptors and down-stream signal transduction pathways, we next examined expression of IL-7 and BAFF receptors on transitional (CD19+ CD10+) and mature (CD19+ CD10−) B cells to determine whether B-cell lymphopenia in ESRD may be a consequence of a defect in B-cell differentiation from transitional cells to mature B cells or decreased survival of mature B cells. BAFF binds with similarly high affinity to three receptors: BCMA (B-cell maturation antigen), TACI (transmembrane activator and CAML-interactor) and BAFF receptor [16]. Since studies in BCMA, TACI and BAFF receptor-deficient mice indicated that the BAFF differentiation and survival signal in transitional and mature B cells are mediated by the BAFF-receptor and not through BCMA and TACI [16], we examined the expression of BAFF receptor on transitional and mature B cells. We found a significant reduction of BAFF receptor density on transitional B cells and not on mature B cells from the ESRD group as compared with that found in the controls. In addition, the percentage of transitional B cells with detectable BAFF receptor was mildly but significantly reduced in the ESRD group. These results would suggest that uraemia impacts the differentiation of transitional B cells rather than the survival of mature B cells. Furthermore, the elevation of the circulating BAFF levels and the normality of BAFF receptor expression in mature B cells preclude the deficiency of either as the primary cause of the observed reduction of mature circulating B cells (CD19+ CD10−) in ESRD. Down-regulation of BAFF receptor expression on transitional B cells can constitute resistance to the biological actions of BAFF and as such may, in part, contribute to the pathogenesis of B-cell lymphopenia in ESRD patients. It should be noted that the observed down-regulation of the BAFF receptor in transitional B cells from ESRD patients may be a response of these cells to the elevated BAFF concentrations.
Further studies are needed to explore the effects of uraemia on B-cell precursors in the bone-marrow and down-stream signal transduction pathways involved in B-cell growth, differentiation and survival. Regardless of the cause, uraemia-induced naïve and memory B-cell lymphopenia is, in part, responsible for the defective humoral response to infections, vaccination and recall antigens and increased incidence of infection in ESRD patients.
Earlier studies have shown that B-cell antibody production capacity is impaired in ESRD patients [11,18]. In addition to their well-known function as antibody-producing cells, B cells significantly contribute to the production of pro- and anti-inflammatory cytokines such as TNF-α, IL-6 and IL-10 [19,20]. In this study, we sought to examine the effect of ESRD on the cytokine-producing capacity of B cells. The study revealed that stimulation of lymphocytes with the TLR9 agonist elicited significantly greater IL-6 and TNF production in the ESRD group as compared to the control group. It should be mentioned that in humans TLR9 is primarily expressed by B cells, but is also expressed by plasmacytoid dendritic cells [21,22]. Given the small size of the plasmacytoid dendritic cell population (∼0.06% of circulating leukocytes) and relatively large population of B cells (8–11%), the observed increase in cytokine production in response to the TLR9 activation must be largely derived from the B-cell population. The mechanism(s) responsible for heightened production of inflammatory cytokines by uraemic B cells in response to the TLR9 agonist is not known. However, it reflects the participation of B cells in the prevailing inflammatory milieu in uraemia.
The present study did not include as yet un-dialysed ESRD patients or ESRD patients maintained on peritoneal dialysis, the available data do not allow conclusions as to the potential contribution of dialysis modalities to the observed B-cell lymphopenia. However, earlier studies [13] have found an equal reduction of total B cells (CD19±) in as yet un-dialysed ESRD patients and those maintained on either haemodialysis or peritoneal dialysis modalities. These observations tend to illustrate the dominant role of ESRD as opposed to the dialysis modalities in the pathogenesis of the associated B-cell lymphopenia.
In conclusion, despite elevation of B-cell growth/ differentiation factors, ESRD patients exhibited diffuse reduction of B-cell subpopulations. This was associated with down-regulation of the BAFF receptor in transitional B cells. The latter can, in part, contribute to B-cell lymphopenia by promoting resistance to the biological actions of BAFF that is a potent B-cell survival factor. Despite their reduced numbers, the uraemic B cells had a heightened capacity to produce inflammatory cytokines suggesting their possible participation in the prevailing inflammatory milieu in uraemia.
Conflict of interest statement. None declared.
References
- 1.Girndt M, Sester U, Sester M, et al. Impaired cellular immunity in patients with end-stage renal failure. Nephrol Dial Transplant. 1999;14:2807–2810. doi: 10.1093/ndt/14.12.2807. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System: USRDS Annual Data Report. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD: 1998.
- 3.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Girndt M, Sester M, Sester U, et al. Molecular aspects of T- and B-cell function in uremia. Kidney Int. 2001;78:S206–S211. doi: 10.1046/j.1523-1755.2001.59780206.x. [DOI] [PubMed] [Google Scholar]
- 5.Meier P, Dayer E, Blanc E, et al. Early T cell activation correlates with expression of apoptosis markers in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:204–212. doi: 10.1681/ASN.V131204. [DOI] [PubMed] [Google Scholar]
- 6.Alexiewicz JM, Smogorzewski M, Fadda GZ, et al. Impaired phagocytosis in dialysis patients: studies on mechanisms. Am J Nephrol. 1991;11:102–111. doi: 10.1159/000168284. [DOI] [PubMed] [Google Scholar]
- 7.Massry S, Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: role of parathyroid hormone. Kidney Int. 2001;78:S195–S196. doi: 10.1046/j.1523-1755.2001.59780195.x. [DOI] [PubMed] [Google Scholar]
- 8.Sester U, Sester M, Hauk M, et al. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transplant. 2000;15:1217–1223. doi: 10.1093/ndt/15.8.1217. [DOI] [PubMed] [Google Scholar]
- 9.Meuer SC, Hauer M, Kurz P, et al. Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J Clin Invest. 1987;80:743–749. doi: 10.1172/JCI113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaman M, Michael J, MacLennan IC, et al. T-cell-independent and T-cell-dependent antibody responses in patients with chronic renal failure. Nephrol Dial Transplant. 1989;4:216–221. doi: 10.1093/oxfordjournals.ndt.a091858. [DOI] [PubMed] [Google Scholar]
- 11.Smogorzewski M, Massry SG. Defects in B-cell function and metabolism in uremia: role of parathyroid hormone. Kidney Int. 2001;78:S186–S189. doi: 10.1046/j.1523-1755.2001.59780186.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto Y, Shinzato T, Amano I, et al. Relationship between susceptibility to apoptosis and Fas expression in peripheral blood T cells from uremic patients: a possible mechanism for lymphopenia in chronic renal failure. Biochem Biophys Res Commun. 1995;215:98–105. doi: 10.1006/bbrc.1995.2438. [DOI] [PubMed] [Google Scholar]
- 13.Moser B, Roth G, Brunner M, et al. Aberrant T cell activation and heightened apoptotic turnover in end-stage renal failure patients: a comparative evaluation between non-dialysis, haemodialysis, and peritoneal dialysis. Biochem Biophys Res Commun. 2003;308:581–585. doi: 10.1016/s0006-291x(03)01389-5. [DOI] [PubMed] [Google Scholar]
- 14.Joon J, Gollapudi S, Pahl M, et al. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70:371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 15.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay F, Schneider P, Rennert R, et al. BAFF and April: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 17.Mayne C, Amanna I, Nashold F, et al. Systemic autoimmunity in BAFF-R-mutant A/WySnJ strain mice. Eur J Immunol. 2008;38:587–598. doi: 10.1002/eji.200737817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raskova J, Ghobrial I, Czerwinski DK, et al. B-cell activation and immunoregulation in end-stage renal disease patients receiving hemodialysis. Arch Intern Med. 1987;147:89–93. [PubMed] [Google Scholar]
- 19.Balin SJ, Platt JL, Cascalho M. Noncognate function of B cells in transplantation. Transplantation. 2009;22:593–598. doi: 10.1111/j.1432-2277.2008.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 21.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-Like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 22.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–450. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Shah N, Panoskaltsis-Mortari A, et al. Murine and human IL-7 activate STAT5 and induce proliferation of normal human pro-B cells. J Immunol. 2005;175:7325–7331. doi: 10.4049/jimmunol.175.11.7325. [DOI] [PubMed] [Google Scholar]
- 24.Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18:20–30. doi: 10.1016/j.smim.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Sims G, Ettinger R, Shirota Y, et al. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 27.Waldschmidt T, Noelle R. Long live the mature B cell, a BAFFling mystery resolved. Science. 2001;293:2012–2013. doi: 10.1126/science.1065591. [DOI] [PubMed] [Google Scholar]
- 28.Kalled L. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Woodland R, Schmidt M, Thompson C. BLyS and B cell homeostasis. Semin Immunol. 2006;18:318–326. doi: 10.1016/j.smim.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Kipps T. The CD5 B cell. Adv Immunol. 1989;47:117–185. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- 31.Herzenberg L, Haughton G, Rajewsky K. CD5 B cells in development and disease. Ann NY Acad Sci. 1992;651:591–601. [Google Scholar]
- 32.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 33.Uckun F. Regulation of human B-cell ontogeny. Blood. 1990;76:1908–1923. [PubMed] [Google Scholar]
- 34.Gray D. Immunological memory: a function of antigen persistence. Trends Microbiol. 1993;1:39–41. doi: 10.1016/0966-842x(93)90026-n. [DOI] [PubMed] [Google Scholar]
- 35.Bouts A, Davin J, Krediet R, et al. Children with chronic renal failure have reduced numbers of memory B cells. Clin Exp Immunol. 2004;137:589–594. doi: 10.1111/j.1365-2249.2004.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Descamps-Latscha B, Chatenoud L. T cells and B cells in chronic renal failure. Semin Nephrol. 1996;16:183–191. [PubMed] [Google Scholar]
- 37.Raska K, Jr, Raskova J, Shea SM, et al. T cell subsets and cellular immunity in end-stage renal disease. Am J Med. 1983;75:734–740. doi: 10.1016/0002-9343(83)90401-1. [DOI] [PubMed] [Google Scholar]
- 38.Degiannis D, Mowat AM, Galloway E, et al. In-vitro analysis of B lymphocyte function in uraemia. Clin Exp Immunol. 1987;70:463–470. [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Fresnedo G, Ramos MA, González-Pardo MC, et al. B lymphopenia in uremia is related to an accelerated in vitro apoptosis and dysregulation of Bcl-2. Nephrol Dial Transplant. 2000;15:502–510. doi: 10.1093/ndt/15.4.502. [DOI] [PubMed] [Google Scholar]
- 40.Hoy WE, Cestero RV, Freeman RB. Deficiency of T and B lymphocytes in uremic subjects and partial improvement with maintenance hemodialysis. Nephron. 1978;20:182–188. doi: 10.1159/000181220. [DOI] [PubMed] [Google Scholar]
- 41.Deenitchina SS, Ando T, Okuda S, et al. Cellular immunity in hemodialysis patients: a quatitative analysis of immune cell subsets by flow cytometry. Am J Nephrol. 1995;15:57–65. doi: 10.1159/000168802. [DOI] [PubMed] [Google Scholar]