Abstract
Previous studies have implicated CXCL12 in the neuropathogenesis of HIV infection. Proteolysis of CXCL12 generates a neurotoxic molecule, CXCL12(5-67), which engages and activates CXCR3, in addition to exhibiting increased expression in the brains of patients with HIV-associated dementia (HAD). Herein, we investigated CXCR3-mediated neuronal injury, particularly, its contribution to autophagy suppression and the concomitant effects of antiretroviral therapy using human brain samples and models of HIV neuropathogenesis. Neurons in the brains of HAD patients and feline immunodeficiency virus (FIV)-infected animals, as well as cultured human neurons, expressed CXCR3, which was modulated in a ligand-specific manner. Exposure of human neurons to CXCL12(5-67) caused a reduction in the autophagy-associated molecule LC3 (P<0.05) and neuronal survival (P<0.05), which recapitulated findings in FIV- and HIV-infected brains (P<0.05). Oral didanosine (ddI) treatment of FIV-infected animals reduced neurobehavioral abnormalities in conjunction with diminished plasma viral load (P<0.05). F4/80 transcript abundance and CXCL12(5-67) immunoreactivity were reduced with restored neuronal LC3 expression in the brains of FIV-infected animals after ddI treatment (P<0.05). ddI treatment also prevented microglial activation and depletion of synaptic proteins in the cortex of FIV-infected animals (P<0.05). These findings indicate that the beneficial effects of ddI might be a consequence of a reduced systemic viral burden and concurrent leukocyte activation, leading to diminished neuroinflammation with preservation of neuronal autophagy by regulating CXCR3 activation.—Zhu, Y., Vergote, D., Pardo, C., Noorbakhsh, F., McArthur, J. C., Hollenberg, M. D., Overall, C. M., Power, C. CXCR3 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy.
Keywords: HIV, FIV, neuroinflammation, CXCL12
The lentiviruses, including human (HIV), simian (SIV), and feline (FIV) immunodeficiency viruses, cause a central nervous system (CNS) disease that involves concurrent neuroinflammatory and neurodegenerative mechanisms, similar to other neurological disorders defined by complex pathogenic processes (1, 2, 3). The mechanisms underlying the development of lentivirus-induced CNS disease are incompletely understood, although host (and perhaps virus)-derived molecules secreted by CNS-infiltrating and activated leukocytes have been posited to exert neuroimmune and neurotoxic properties, thereby contributing to both neuroinflammation and neurodegeneration, respectively (4). These neuropathogenic events likely involve neuronal injury caused by apoptosis and other cytotoxic mechanisms, which are in part driven by systemic viral replication, suppression of adaptive immunity, and concomitant (innate) immunological perturbations leading to leukocyte activation, which can then traverse the blood-brain barrier (5). Because neuroinflammation is a key component of the neuropathology of lentivirus infection, there is intense interest in the inflammatory mechanisms by which neural cells undergo neurodegeneration (6). Autophagy’s promotion of neuronal survival is a recently recognized mechanism, but the effects of inflammation on autophagy in the CNS remain uncertain (7, 8). Recent studies (9) point to autophagy inhibition as a putative mechanism leading to neuronal cell death in lentivirus infections. The effects of antiretroviral therapies on lentivirus neuropathogenesis are beneficial in terms of improved neurocognitive performance, albeit often with incomplete resolution of neurological deficits (10). This partial improvement in neurological status has been attributed to limited penetration of antiretroviral agents into the CNS (11). That said, the benefits of antiretroviral drugs in terms of maintaining overall neuronal viability and function remain uncertain, although neurodegeneration during lentivirus infections is clearly related to the extent of past and concurrent suppression of adaptive immunity (12).
Proteolysis of host and likely viral proteins is an integral feature of neurodegeneration, with the cleavage of amyloid precursor protein to yield β-amyloid representing the most prominent example (13). Previous studies (14) from our group indicate that the chemokine CXCL12 [also termed stromal cell-derived factor-1 (SDF-1)], when processed by matrix metalloproteinase (MMP)-2 to remove four N-terminal amino acids, generates a neurotoxic molecule CXCL12(5-67), which causes neuronal death and concomitant neuroimmune activation during HIV infection and in models thereof. These neuropathogenic effects are in keeping with the increased abundance of CXCL12(5-67) in the brains of patients with HIV-associated dementia and with the ability of CXCL12(5-67) to activate the chemokine receptor CXCR3 (15). CXCR3 activation triggers death-signaling pathways in human neurons and up-regulates inflammatory gene induction in glial cells (15). However, the effect of this pathobiological process on neurodegeneration in other lentivirus infections and the effects of systemic immunosuppression on this neurodegenerative process are unknown.
Earlier studies (16) reveal that a number of chemokines including CXCL4, CXCL9, CXCL10, and CXCL11, acting via CXCR3, can promote both cell survival as well as apoptosis, depending on the cell type and/or the experimental conditions. These divergent effects may be mediated by splice variant-based isoforms of CXCR3. Indeed, like CXCR4 and CX3CR1 (17, 18), CXCR3 is alternatively spliced, generating at least three functional isoforms differing in either their N or C terminus. The best studied CXCR3 isoform, initially cloned in 1996, and later renamed CXCR3A, binds CXCL9, CXCL10, and CXCL11. A second CXCR3 isoform named CXCR3B, encoded by the same gene and characterized by a longer amino-terminal domain, was described in 2003 (19). Interestingly, CXCR3B is to date the only high-affinity receptor shown to bind CXCL4 in addition to the three ligands mentioned above. These two receptor splice variants have been shown to be functionally distinct. While CXCR3A appears to mediate proliferative, chemotactic, and prosurvival effects, CXCR3B, which exhibits a lower affinity for the several ligands mentioned, appears to mediate the antiproliferative, angiostatic, and proapoptotic effects of the CXCR3 ligands. A third splice variant was described by Ehlert et al. (20). This last isoform, named CXCR3-alt, differing from CXCR3A in its carboxyl terminus, is missing several transmembrane domains characteristic of 7-transmembrane G protein-coupled receptors but is still able to mediate CXCL11 but not CXCL9- or CXCL10-dependent chemotactic activity. While the cellular outcomes of engagement of the different CXCR3 splice variant isoforms have received some attention, little is known about the mechanisms that govern the choice of splicing sites in a given cell system.
FIV is a member of the lentivirus subfamily that uses CXCR4 and likely also CD134 and CCR5 for infection to cause persistent disease in domestic cats (21). Indeed, FIV pathogenesis shares many immunological and neurological aspects with HIV and SIV in their respective hosts (22, 23), together with similar viral structural and biochemical properties. FIV causes neurological abnormalities in 20–40% of naturally infected cats by entering the CNS and infecting parenchymal microglia and perivascular macrophages, resulting in neuroinflammation and neurodegeneration (24, 25). The neurological phenotype induced by FIV infection is diverse, including psychomotor retardation, seizures, ataxia, and behavioral abnormalities such as aggressivity, disrupted sleep patterns, and stereotypic motor behavior (26), similar to behavioral and neurological abnormalities observed in patients with advanced HIV infection and immunosuppression (27). Neuroimmune activation in FIV infection is accompanied by neuronal injury that involves select neuronal populations (28). Induction of the MMP-2 in the brain is evident in FIV infection at the levels of both transcription and translation, similar to HIV infection of the CNS (29). Like HIV and SIV infections, immunosuppression and neurological disease caused by FIV are ameliorated by antiretroviral therapy, particularly with nucleoside analog reverse transcriptase inhibitors such as didanosine (ddI) and zidovudine despite limited CNS penetration of the drugs (30). For the studies we describe here, we hypothesized that CXCR3 expression and engagement by CXCL12(5-67) contributed to neuronal process retraction and eventual death, possibly through suppression of autophagy. Moreover, we hypothesized that antiretroviral therapy might exert its beneficial effects on lentivirus-induced neurological disease by suppressing viral replication and the associated pathogenic consequences including CXCR3 activation. We selected ddI because it exhibits limited CNS penetration but with efficient suppression of circulating viral burden and is effective against multiple lentiviruses (31, 32). Our results demonstrate that antiretroviral drug treatment effectively prevented neurodegeneration caused by lentivirus infections by reducing neuroinflammation and restoring neuronal autophagy.
MATERIALS AND METHODS
Human brain samples
Human CNS tissue (frontal lobe) was collected at autopsy from HIV-1 sero-negative or -positive patients with consent and stored at −80°C. HIV-1-seropositive patients were diagnosed premortem as having HIV-associated dementia (HAD) or nondemented (ND), although all patients were AIDS-defined, as described previously (33). Non-HIV-infected controls were comprised of other neurological diseases including Alzheimer’s disease, multiple sclerosis, and stroke. Tissue sections were kindly provided by Dr. Arthur Clark (Department of Laboratory Medicine, University of Calgary, Calgary, AB, Canada).
Human fetal neuron cultures
To establish human neuronal cultures, fetal brain tissues were dissected, meninges were removed, and a single cell suspension was prepared by trituration through serological pipettes, followed by digestion for 30 min with 0.25% trypsin (Life Technologies, Burlington, ON, Canada) and 0.2 mg/ml DNase I (Roche Diagnostics, Mannheim, Germany) and passage through a 70-μm cell strainer (BD Biosciences, Mississauga, ON, Canada). Brain tissue from 14- to 21-wk old fetuses was obtained according to Institutional Ethics Review Board Guidelines at the University of Alberta (Edmonton, AB, Canada). Cells were washed 2 times with fresh medium and plated in T-75 flasks coated with poly-l-ornithine (Sigma-Aldrich, Oakville, ON, Canada) at 6–8 × 107 cells/flask in MEM supplemented with 10% FBS (Life Technologies), 2 mM l-glutamine (Life Technologies), 1 mM sodium pyruvate (Life Technologies), 1× MEM nonessential amino acids (Life Technologies), 0.1% dextrose (Sigma-Aldrich), 100 U/ml Penicillin (Life Technologies), 100 μg/ml streptomycin (Life Technologies), 0.5 μg/ml amphotericin B (Life Technologies), and 20 μg/ml gentamicin (Life Technologies). Cultures of neurons were additionally supplemented with 25 μM cytosine arabinoside (Sigma-Aldrich) to prevent astrocyte growth. After 14 days of culture, the neurons were exposed to CXCL12, CXCL12(5-67), CXCL10, CXCL4, or IL-1β (R&D Systems, Minneapolis, MN, USA) for 6 h before lysis for RNA or protein extraction. Human neuroblastoma cells (LAN-2 and SK-N-SH) were grown in MEM supplemented with 10% FBS and 1% P/S and were differentiated as described previously (34).
Virus preparation
The FIV strain used in this study was an infectious neurovirulent recombinant molecular clone, FIV-Ch, derived by transfection of CrFK cells and amplification in feline peripheral blood mononuclear cells (PBMCs), as described previously (29). Culture supernatants from FIV-infected feline PBMCs, which served as sources of infectious virus for these experiments, were cleared of cellular debris by centrifugation and titered by limiting dilution, as described previously (24).
Experimental animals and tissue collection
Specific pathogen-free neonatal (day 1) cats were infected with 0.2 ml of infectious (104 TCID50/ml) or heat-inactivated virus (mock-infected cats) in accordance with Canadian Council on Animal Care guidelines, as described previously (24). FIV-infected and mock-infected animals were treated with ddI (33 mg/kg daily; Bristol-Myers Squibb Pharmaceutical Group, Montreal, QC, Canada) by oral gavage starting at 6 wk postinfection until 12 wk postinfection. Animals were weaned at 6 wk and monitored until 12 wk of age, at which time all animals were euthanized, as described previously (24, 35). Sera were collected and stored at −80°C immediately. The left cerebral hemisphere was collected from each animal and fixed in 4% PBS-buffered paraformaldehyde for at least 1 wk at 4°C. The right cerebral hemisphere was stored −80°C immediately.
Behavioral studies
To determine the neurobehavioral features associated with FIV infection, the animals were examined weekly and weighed by an animal care staffer who was unaware of the infection status. The age (wk) was recorded at which each developmental milestone was manifested; these included play interaction, walking, running, air righting, ability to walk along a plank, and blink reflex (24). In addition, the height to which an animal jumped and pursued a moving light on a wall was measured. Five different parameters, including activity level, play interaction, motor ability, inquisitiveness, and general health, were scored by using a feline behavioral scale (24), from which a mean deficit score (MPS) was calculated for each group.
Real-time RT-PCR
Total RNA was extracted from CXCL12- or CXCL12(5-67)-exposed cells or from the right cerebral cortex of FIV-infected and uninfected with or without ddI treatment animals using TRIzol, according to the manufacturer’s guidelines. First-strand cDNA was synthesized by using aliquots of 1 μg of total RNA, reverse transcriptase, and random primers. A real-time PCR protocol using primers that detect the FIV pol gene was used to determine the number of copies of viral RNA/ml in plasma with or without ddI treatment, as previously reported (35). Specific host genes were quantified by real-time PCR using i-Cycler IQ system (Bio-Rad, Mississauga, ON, Canada). cDNA prepared from total RNA of frontal cortex of brain was diluted 1:1 with sterile water, and 5 μl was used per PCR reaction. The primers used in the real time PCR are shown in Table 1. Semiquantitative analysis was performed by monitoring in real-time the increase in fluorescence of SYBR-Green dye. All data were normalized to the GAPDH mRNA threshold cycle level and expressed as mRNA relative fold change.
TABLE 1.
PCR oligonucleotide primers
| Target gene | Sense and antisense primer sequences | Annealing temperature (°C) | Amplicon size (bp) |
|---|---|---|---|
| GAPDH | 5′-AGC CTT CTC CAT GGT GGT GAA-3′; 5′-CGG AGT CAA CGG ATT TGG TCG-3′ | 50–58 | 308 |
| p62 | 5′-GAA AGT CCC GGT ATC CAA AG-3′; 5′-CCA GCC AAT TCT CTT TTT-3′ | 58 | 161 |
| CXCL12 | 5′-GCC AGA GCC AAC GTC AAG C-3′; 5′-CAA TTT CGG GTC AAT GCA CAC-3′ | 56 | 110 |
| CXCR3A | 5′-ACC CAG CAG CCA GAG CAC C-3′; 5′-TCA TAG GAA GAG CTG AAG TTC TCC A-3′ | 60 | 110 |
| CXCR3B | 5′-TGC CAG GCC TTT ACA CAG C-3′; 5′-TCG GCG TCA TTT AGC ACT TG-3′ | 60 | 154 |
| MMP-2 | 5′-GAT GGA TAC CCG TTT GAT TGG-3′; 5′-CCA TCA GCG TTC CCA TAC TT-3′ | 56 | 152 |
| F4/80 | 5′-GGC AAA CTG GAA GAA AAA AGG-3′; 5′-ATT TCC ACC AAT AGA GAG AC-3′ | 50 | 123 |
| atg 5 | 5′-GGA CAA TTG CAC ACA CTA GGT G-3′; and 5′-GTT CAC TCA GCC ACT GCA GAG-3′ | 52 | 141 |
Immunofluorescence detection and confocal microscopy analysis
Four percent of PBS-buffered paraformaldehyde fixed brains (left hemisphere) were embedded in paraffin, and sections (5.0 μm thick) were cut with a microtome (Leica, Nussloch, Germany). The section slides were deparaffinized in 2 changes of xylene for 5 min each, followed by rehydration using a series of graded alcohols. Antigen retrieval was performed by boiling the sections in sodium citrate (0.01 M, pH 6.0) for 10 min. After application of blocking buffer for 1 h, the sections were immunostained with antibodies to MAP-2 (clone HM-2; 1:200 dilution; Pharmingen, Franklin Lakes, NJ, USA) or neuronal-specific nuclear protein (NeuN; 1:200; Chemicon, Billerica, MA, USA) for neuron detection, Iba-1 (1:100; Wako Chemicals, Neuss, Germany) or CD18 (1:10) for macrophage and microglia detection, GFAP (1:200; Pharmingen) for astrocyte detection, and CXCL12(5-67) (1:25) and microtubule-associated protein 1A/1B-light chain 3 (LC3; 1:200; Novus Biologicals, Littleton, CO, USA) at 4°C overnight. Immunostaining protocols for single and double labeling were performed (36). After being washed in PBS, the sections were incubated with either Cy3 conjugated goat anti-mouse or Alexa 488 conjugated goat anti-rabbit IgG (1:1000; Molecular Probes, Eugene, OR, USA) for 2 h at room temperature in the dark followed by repeated washing in PBS. The slides were mounted with Gelvatol. The specificity of staining was confirmed by omitting the primary antibody. Images were captured on an LSM510 META (Carl Zeiss MicroImaging, Thornwood, NY, USA) confocal laser-scanning microscope and analyzed using LSM 5 Image Browser software (Carl Zeiss MicroImaging).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections of human brain tissue (frontal lobe) were deparaffinized and rehydrated using decreasing concentrations of ethanol. Antigen retrieval was performed by boiling the slides in 0.01 M trisodium citrate buffer, pH 6, for 10 min followed by incubation with Levamisole to block endogenous alkaline phosphatase. Sections were then preincubated with 10% normal goat serum and 0.2% Triton X-100 overnight at 4°C to block nonspecific binding. To detect CXCR3 or LC3 immunoreactivity, slides were incubated overnight at 4°C with a mouse monoclonal antibody CXCR3 (1:25; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or a rabbit polyclonal antibody LC3 (1:200; Novus Biologicals) diluted in 5% normal goat serum and 0.2% Triton X-100 (47). A secondary alkaline phosphatase-conjugated goat anti-mouse or anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) followed by NBT/BCIP substrate (Vector Laboratories, Burlingame, CA, USA) were used for single labeling. For double labeling with NeuN (1:200; Chemicon), sections pretreated with 0.3% hydrogen peroxide to block endogenous peroxidases were incubated with NeuN antibody, followed by biotinylated goat anti-rabbit antibody by avidin-biotin-peroxidase complexes (Vector Laboratories), and then 3,3′-diaminobenzidine tetrachloride (Vector Laboratories) was applied.
Immunoblot
Protein extracts were prepared from human or feline frontal cortex or cells by homogenization in cell lysis buffer (34), and concentrations were determined using a Bio-Rad protein assay kit. Western blots were probed with polyclonal anti-CXCL12(5-67) neoepitope antibodies [1:100, preadsorbed for 30 min with full-length CXCL12 to optimize its specificity against CXCL12(5-67)] or anti-CXCL12 (1:250; R&D Systems) monoclonal antibodies, monoclonal antibodies to β-arrestin-1 (1:250; BD Transduction Laboratories; Mississauga, ON, Canada), vesicular acetylcholine transferase (VAChT; 1:1000; Sigma), LC3 (1:1000; Novus), MMP-2 (1:200; Santa Cruz), or anti-CXCR3 and anti-CXCR4 polyclonal antibodies (1:250; R&D Systems) or HRP-conjugated anti-β-actin antibody (1:200; Chemicon). Detection of the primary antibodies was performed using either chemiluminescence or fluorescence (HRP- or Alexa680-/IRDye800-conjugated secondary antibodies, respectively) and quantified using Quantity One (Bio-Rad) or Odyssey 1.2 (LI-COR Biosciences, Lincoln, NE, USA), respectively.
Cellular viability assay
The abundance of βIII-tubulin and CXCR3 was assessed by infrared immunofluorescence (LI-COR Biosciences) with anti-βIII-tubulin or anti-CXCR3 monoclonal antibodies (1:800; Sigma and 1:50; R&D Systems, respectively) according to the manufacturer’s guidelines (34). To assess the cell surface CXCR3 relative to the overall CXCR3 abundance, the antibody was added to either nonpermeabilized or Triton X-100 permeabilized cells. Alexa680- and IRDye800-conjugated secondary antibodies were used to detect the immunoreactivity, and fluorescence intensities were quantified using the Odyssey 1.2 software.
Peptides and other reagents
CXCL12, CXCL12(5-67), and CXCL11(5-73) were chemically synthesized and purified as described previously (37). CXCL4 and CXCL10 used in this study were from R&D Systems.
Statistical analysis
Statistical analyses were performed using GraphPad InStat version 3.0 (GraphPad Software, San Diego, CA, USA), using ANOVA, for mRNA alteration, viral load, and protein expression. Values of P < 0.05 were considered significant. Unless otherwise stated, all post hoc significant comparisons indicate differences between the control or ddI and individual treatment groups, i.e., FIV infection or FIV infection with ddI treatment alone.
RESULTS
CXCR3 and LC3 expression in human brain
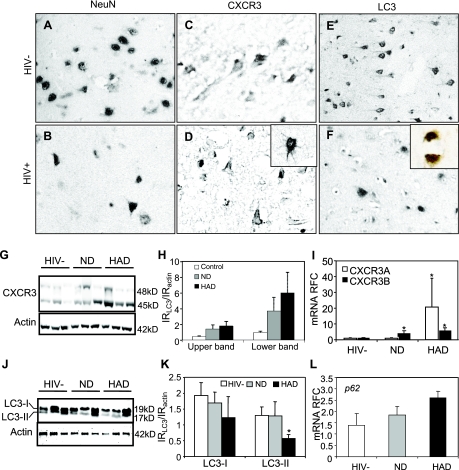
Previous studies (15) have shown that the MMP-2-mediated processing of CXCL12 (formerly SDF-1α) results in the cleavage product CXCL12(5-67), which is increased in the brains of patients with HAD. Moreover, proteolytic processing of CXCL12 results in a change in receptor preference for the chemokine ligand, from CXCR4 to CXCR3, associated with both neuroinflammatory and neurotoxic effects (15). To examine the relation between neuronal viability and CXCR3 expression, we monitored the mature neuronal marker NeuN and CXCR3 abundance in human brain sections from patients with (HIV+) and without HIV infection (HIV−) (Fig. 1). These studies revealed that NeuN immunoreactivity was evident in numerous nuclei in tissue from an HIV− brain (Fig. 1A) compared with fewer immunopositive nuclei in the HIV+ brain (from a patient with HAD) (Fig. 1B). CXCR3 was detected on neurons from HIV− (Fig. 1C) and HIV+ (Fig. 1D) brains, although immunoreactive neurons appeared more abundant in the HIV+ brain including detection in the basal ganglion (Fig. 1D, inset). Likewise, a marker of autophagosome formation, LC3, was widely expressed in the HIV− brain (Fig. 1E) in the cytoplasm of cells resembling neurons. Conversely, LC3 immunoreactivity was reduced in the HIV+ brain (Fig. 1F), particularly in cells coexpressing NeuN (Fig. 1F; brown, LC3; blue, NeuN). Western blot analysis of brain extracts revealed two CXCR3-immunoreactive bands with molecular masses corresponding to CXCR3A and CXCR3B (Fig. 1G), indicating that both isoforms were present in human brains and were induced in the HIV+ brains (Fig. 1H). Transcripts for the two principal CXCR3 isoforms, CXCR3A and CXCR3B, were detected in the brains of HIV+ patients, with and without HAD. CXCR3A was selectively increased in the brains of HAD patients, whereas CXCR3B expression was increased in both HIV-infected groups (ND and HAD) compared with the HIV-uninfected group (Fig. 1I). In contrast, CXCR4 transcript levels did not differ among the three groups of patients (data not shown). Western blot analysis of brain lysates, using anti-LC3 antibodies, disclosed two bands corresponding to cytosolic LC3-I and its phosphatidylethanolamine-conjugated form, LC3-II (Fig. 1J). The delipidated LC3-II isoform was reduced in HAD brains (Fig. 1K). The transcript level of the autophagy-related gene p62 was increased in the brains of HIV-infected patients (Fig. 1M), implying inhibition of autophagy. Thus, increased expression and ensuing activation of CXCR3 could in principle contribute to the neurological deficits observed in HAD, possibly by suppressing autophagy.
Figure 1.
CXCR3 isoforms and autophagy regulation in HIV-infected human brains. Neuronal nuclear maker NeuN was readily detected in non-HIV-infected (HIV−) brain tissue (A), compared with fewer immunopositive neurons in the HIV-infected (HIV+) brain (B). CXCR3 immunoreactivity was detected in neurons of brains from HIV− persons (C), but there was more abundant CXCR3 immunoreactivity in HIV+ samples (D), including basal ganglia (D, inset). LC3-immunoreactive cells were evident in the HIV− brain (E), but fewer cells were observed in the HIV+ brain (F), although LC3 immunoreactivity (brown; F, inset) colocalized with NeuN (blue; F, inset). CXCR3 immunoreactivity in human brain lysates in Western blot revealed two bands (G), showing an increase in intensity confirmed by quantification (H). CXCR3A transcript expression was higher in the brains of patients diagnosed with HAD compared with HIV− brains or ND; CXCR3B expression was higher in both demented and nondemented HIV+ brains compared with HIV− brains (I). LC3 immunoreactivity of brain lysates on Western blot also showed two bands (J), although the bottom (lipidated) band was suppressed in HAD brains (K). p62 transcript levels showed a trend toward increased expression in HIV+ brains (L). *P < 0.05; ANOVA. Original view: ×400 (A–D; insets); ×200 (E, F).
CXCR3 regulation in neural cells
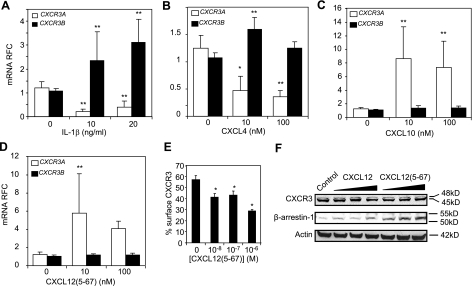
HIV infection of the brain is defined by local inflammation resulting from infection and activation of macrophages, microglia, and astrocytes (38). To investigate the effect of HIV-mediated inflammation on CXCR3 expression in human neurons, human fetal neuronal cell (HFN) cultures were exposed to IL-1β, which has been shown to be up regulated in HAD (39). IL-1β exposure resulted in a marked suppression of CXCR3A mRNA abundance whereas there was a >2-fold increase in CXCR3B mRNA levels (Fig. 2A). To examine the transcriptional regulation of both CXCR3 isoforms by different CXCR3 ligands in neuronal cultures, HFN cultures were exposed to CXCL12(5-67), CXCL10, or CXCL4. Interestingly, like IL-1β, CXCL4 exposure increased the expression of CXCR3B, its cognate receptor but in contrast suppressed CXCR3A expression (Fig. 2B). Unlike CXCL4 and IL-1β, CXCL10 and CXCL12(5-67) both increased CXCR3A transcript levels with no significant effects on CXCR3B expression (Fig. 2C). Of note, the three CXCR3 ligands examined here exerted no effects on CXCR4 expression (data not shown). Thus, the CXCR3 mRNA splicing pattern appeared to be differentially regulated by the different inflammation-associated CXCR3 ligands.
Figure 2.
CXCR3 isoforms are differentially regulated by inflammatory mediators. A) Expression of CXCR3A and CXCR3B in HFN treated with 10 or 20 ng/ml of IL-1β. B–D) Relative fold change (RFC) in transcript abundance of CXCR3A and CXCR3B in human fetal neuronal cultures treated with 10 or 100 nM of either CXCL12(5-67) (D), CXCL10 (C), or CXCL4 (B). E) Percentage of cell surface CXCR3 on neuronal (LAN-2) cells, expressed as ratio of CXCR3 immunoreactivity in nonpermeabilized to permeabilized cells, measured by In Cell Western blot, showed a concentration-dependent decrease after CXCL12(5-67) exposure for 8 h. F) Western blots revealed a concentration-dependent increase in β-arrestin-1 immunoreactivity in neuronal cells on exposure to 10, 100, or 1000 nM of CXCL12(5-67) compared with control; exposure to full-length CXCL12 showed only a modest increase in β-arrestin-1 abundance. As controls, Western blots did not show any significant modulation of CXCR3 or β-actin after the same treatment. *P < 0.05, **P < 0.01 vs. no treatment; ANOVA.
When neuronal cell cultures were exposed to CXCL12(5-67), there was suppression of cell surface relative to total CXCR3 (signal in permeabilized cells) expression, as indicated by CXCR3 immunoreactivity (Fig. 2E). Approximately 30% of total CXCR3 immunoreactivity was present at the cell membrane after 8 h of exposure to CXCL12(5-67), compared with 60% of total CXCR3 immunoreactivity in the absence of CXCL12(5-67) (data not shown). Interestingly, CXCL12(5-67)-induced internalization of CXCR3 was associated with an increase in β-arrestin-1 abundance within the cells (Fig. 2E). Of note, the CXCL12(5-67) precursor, CXCL12, demonstrated lower induction of β-arrestin-1 than CXCL12(5-67) (Fig. 2E). These observations emphasized the effects of different ligands on CXCR3 expression and highlighted potentially diverse outcomes associated with this ligand-receptor interaction.
ddI-mediated changes in neurobehavioral performance, viral burden, and neuroinflammation
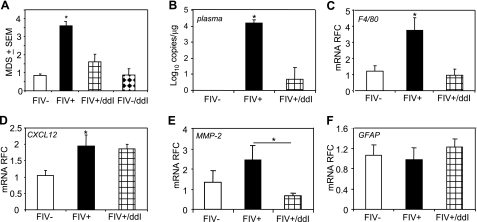
To investigate the in vivo effects of lentivirus infection and its treatment on CXCR3 and CXCL12 expression, we studied FIV-infected cats treated with ddI for 6 wk. Neurobehavioral performance was assessed in animals with or without concurrent FIV infection and ddI treatment. FIV-infected animals exhibited significant impairment in their ability to perform multiple tasks compared with mock-infected animals over the 12-wk period, particularly after 6 wk postinfection, based on the cumulative mean deficient score (Fig. 3A). From 6 to 12 wk postinfection, ddI treatment improved neurobehavioral performance among those animals infected with FIV. ddI treatment alone did not affect neurobehavioral performance among mock-infected animals (Fig. 3A). We then investigated whether ddI influenced viral replication in plasma using a quantitative PCR assay for viral genome copy number (Supplemental Fig. 1). ddI treatment suppressed plasma virus levels, reflected by a reduced mean in plasma viral load of FIV-infected animals treated with ddI at wk 12 postinfection (Fig. 3B). We then examined expression of host genes specifically associated with lentivirus-induced neuroinflammation and degeneration in the cortex, a brain region that is particularly vulnerable to injury caused by lentiviruses (40). FIV infection was associated with the induction of the marker of macrophage and microglia activation, F4/80 (Fig. 3C), as well as MMP-2 (Fig. 3E). ddI treatment was associated with an inhibition of both F4/80 and MMP-2 in the brains of FIV-infected animals. FIV infection also up-regulated CXCL12 transcript abundance compared with mock-infected animals, but ddI treatment reduced CXCL12 levels in FIV-infected animals (Fig. 3D). For all of the above host mRNAs, ddI treatment of mock-infected animals exerted no effect on their relative abundance in the brain (data not shown); likewise, GFAP expression was unaffected by FIV infection or ddI treatment (Fig. 3F). These data implied that treatment of FIV infection with ddI reduced plasma viral burden associated with substantial effects on the transcript levels of genes involved in lentivirus-induced neuroinflammation.
Figure 3.
Neurobehavioral performance, viral burden, and neuroinflammation in FIV infection. A) FIV-infected animals (FIV+) exhibit greater neurobehavioral deficits than mock-infected controls (FIV−), while ddI treatment reversed neurobehavioral deficits in FIV-infected animals (FIV+/ddI) and had no effects on mock-infected animals. B) ddI treatment suppressed viral load in plasma of FIV-infected animals. C) Glycoprotein, F4/80, expressed in activated myeloid cells showed increased transcript abundance in FIV-infected animals, which was reversed by ddI treatment. D) CXCL12 transcript levels were higher in FIV-infected animals but were not reduced by ddI treatment. E) MMP-2 transcripts were unregulated in FIV-infected animals but were suppressed with ddI treatment. F) GFAP transcriptional activity was not affected by FIV infection or ddI treatment. *P < 0.05; Dunnet multiple comparison test.
CXCL12 and MMP-2 expression in the brain
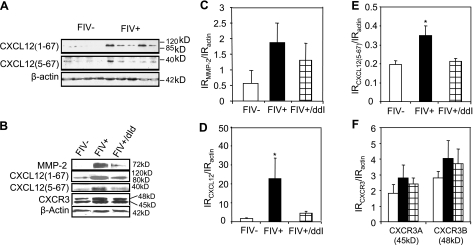
Since CXCL12 can be converted to the neuroinflammatory and neurotoxic peptide CXCL12(5-67) by precise proteolytic processing mediated by active MMP-2 (41), we investigated the relative protein expression of CXCL12, CXCL12(5-67), MMP-2, and CXCR3 in cortical tissue. FIV infection increased both CXCL12(5-67) and CXCL12 immunoreactivity compared with mock-infected animals (Fig. 4A). MMP-2 immunoreactivity was also increased in the brains of FIV-infected animals, while there was no significant change in expression of CXCR3 (Fig. 4B). FIV-infected cortex showed greater MMP-2 immunoreactivity compared with mock-infected animals (Fig. 4C), but there was a nonsignificant MMP-2 reduction in FIV-infected animals treated with ddI. Both CXCL12 (Fig. 4D) and CXCL12(5-67) (Fig. 4E) were significantly increased in the brains of FIV-infected animals, but ddI treatment reduced the immunoreactivity of both chemokines. CXCR3 immunoreactivity was not significantly affected by FIV infection or treatment of animals with ddI (Fig. 4F). ddI treatment of mock-infected animals exerted no significant effects on MMP-2 or CXCL12 expression (data not shown). Since FIV infection resulted in an increase of both MMP-2 and its substrate, CXCL12, concomitant with an increase in the neuropathogenic chemokine, CXCR12 (5-67), similar to data obtained with brain samples from patients with HIV infection (14), these findings supported our hypothesis that CXCL12(5-67) induction, mitigated by ddI treatment, might represent a neuropathogenic mechanism amenable to antiviral therapy.
Figure 4.
Brain expression of CXCL12, MMP-2, and CXCR3 in FIV infection. A) Immunoblotting showed increased full-length (1-67) and cleaved (5-67) CXCL12 in brains of FIV− compared with mock-infected animals. B) CXCL12, CXCL12(5-67), MMP-2, and CXCR3 immunoreactivities were increased in brains of FIV+ animals but were suppressed by concurrent ddI treatment. C) MMP-2 immunoreactivity (IR) showed minimal induction in FIV+ animals with and without ddI treatment. D, E) CXCL12(1-67) (D) and CXCL12(5-67) (E) were induced by FIV infection, but these changes were reversed by ddI treatment. F) CXCR3 showed minimal induction of both immunoreactive bands in FIV infection and was not affected by ddI treatment. *P < 0.05; Dunnet multiple comparison test.
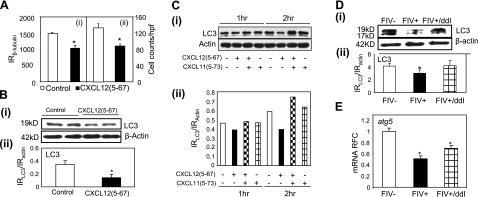
Suppression of autophagy in neural cells
Autophagy plays an integral prosurvival role during neuronal cell starvation, stress, and infection. Several studies (42) indicate that neurodegeneration might be a consequence of compromised autophagy. CXCL12(5-67) exerted neurotoxic effects in terms of both neuronal process retraction (reduction in cellular β-tubulin; Fig. 5Ai) and neuronal death (reduction in cell numbers; Fig. 5Aii). Thus, we investigated the effects of CXCL12(5-67) on autophagy in human neurons. Analyses of immunoblots (Fig. 5. Bi) showed a reduction in LC3 abundance of >50% in CXCL12(5-67)-exposed neurons compared with unexposed neurons (Fig. 5Bii), implying that CXCL12(5-67) might mediate autophagy suppression in neurons. In addition, the suppression of autophagy in neurons caused by CXCL12(5-67) was blocked by pretreatment with the CXCR3 antagonist CXCL11(5-73) (Fig. 5Ci) at 1 and 2 h postexposure pointing to a receptor-mediated mechanism (Fig. 5Ci). To investigate the potential contribution of autophagy to lentivirus-mediated neurodegeneration in vivo, LC3 immunoreactivity was determined in FIV-infected brains (Fig. 5Di). The immunohistochemistry revealed a suppression of both LC3-I and LC3-II in the cortex during FIV infection (Fig. 5Dii), and this reduction was reversed with ddI treatment (Fig. 5Dii). Furthermore, expression of the autophagy-related gene transcript atg5 (Fig. 5E) was decreased in the cortex during FIV infection, although atg6 expression was not affected either by FIV infection or ddI treatment (data not shown), compared with mock-infected animals. These results implied that suppression of autophagy was associated with lentivirus-mediated neurodegeneration but was prevented by ddI treatment.
Figure 5.
Suppression of autophagy in neurons by CXCL12(5-67) and FIV infection. A) CXCL12(5-67) reduced human neuronal viability in terms of β-tubulin immunoreactivity (i) and cell counts/hpf (ii). B) CXCL12(5-67) exposure reduced LC3 immunoreactivity (i) by >50% in human primary neurons (ii). C) LC3 immunoreactivity, including its lipidated form, was detectable in human neuronal (LAN-2) cells, but CXCL12(5-67) exposure reduced LC3 immunoreactivity, which was blocked by concomitant CXCL11(5-73) treatment (i), although CXCL11(5-73) alone did not affect LC3 expression (ii). D) LC3 immunoreactivity (i) was suppressed in the brains of FIV-infected animals but was rescued by ddI treatment (ii). E) The autophagy-associated gene atg5 was suppressed in brains of FIV+ animals, although this change was partially reversed by ddI treatment. *P < 0.05; Dunnet multiple comparison test.
Neuropathological changes in FIV infection with ddI treatment
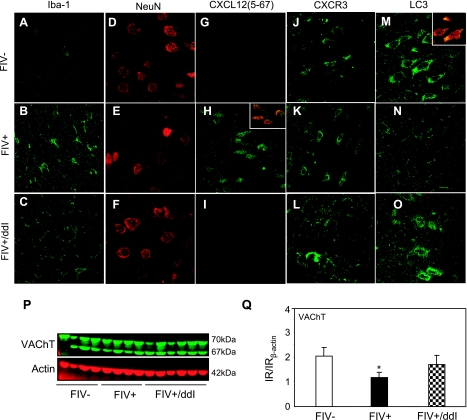
Lentivirus infections result in infiltration of leukocytes into the brain with concurrent infection and immune activation of astrocytes and microglia, while also secreting neurotoxins leading to neuronal injury. To determine whether ddI affected immune activation in brain after FIV infection, we compared Iba-1 immunoreactivity in the cortex among mock-infected and FIV-infected animals with or without ddI treatment. Although Iba-1 immunoreactivity was detected in mock-infected animals (Fig. 6A), its expression was increased in FIV-infected animals (Fig. 6B), while ddI treatment substantially suppressed Iba-1 abundance (Fig. 6C). Moreover, fewer neurons were evident in FIV-infected cortex (Fig. 6E) compared with mock-infected cortex (Fig. 6D), as evidenced by NeuN immunoreactivity on tissue sections, although neurons were comparatively preserved by ddI treatment (Fig. 6F). More CXCL12(5-67)-immunoreactive cells were present in the cortex of FIV-infected animals (Fig. 6H) compared with mock-infected animals (Fig. 6G) and ddI-treated/FIV-infected animals (Fig. 6I), confirming our findings from Western blot analyses (Fig. 5). The CXCL12(5-67)-immunoreactive cells were colocalized with NeuN (Fig. 6H, top inset). CXCR3-immunoreactive cells were more apparent in FIV-infected animals (Fig. 6K) compared with mock-infected animals (Fig. 6J). ddI treatment reversed the effect of lentivirus infection on CXCR3 immunoreactivity (Fig. 6L). FIV infection also reduced the LC3 immunoreactivity (Fig. 6N) compared with mock-infected animals (Fig. 6M). However, LC3 immunoreactivity was restored by ddI treatment (Fig. 6O). LC3-immunoreactive cells were also NeuN-immunopositive cells (Fig. 6M, inset). These findings indicated that suppression of autophagy in neurons accompanied FIV infection of the brain but could be rescued with antiretroviral therapy.
Figure 6.
Neuropathological changes associated with FIV infection and ddI treatment. Iba-1-immunoreactive microglia were detected in brains of mock-infected FIV− animals (A) but were more abundant and hypertrophied in FIV+ brains (B), and ddI treatment reversed this activation of microglia effect (C). NeuN-immunopositive neurons were detected in the parietal cortex of mock-infected controls (D), but were reduced in frequency in FIV+ animals (E), although ddI treatment appeared to protect neurons (F). CXCL12(5-67)-positive cells were not detected in mock-infected controls (G); in contrast CXCL12 immunoreactivity was observed in FIV-infected brains (H) but minimally detected in FIV-infected and ddI-treated animals (I). Inset shows colocalization of CXCL12(5-67) and NeuN in neurons. CXCR3 immunopositive cells resembling neurons were detected in mock-infected (J), FIV+ (K), and FIV+ animals receiving ddI (L). LC3-immunopositive cells colabeled with NeuN were readily found in mock-infected brains (M), but were reduced in number in FIV+ animals (N); ddI treatment appeared to reverse LC3 immunoreactivity in FIV infection (O). Synaptic protein VAChT was detected in brains of all groups (P). However, VAChT was reduced in FIV infection, but ddI treatment prevented the suppression of this synaptic protein (Q). Original view: ×630. *P < 0.05; Dunnet multiple comparison test.
Synaptodendritic injury and neuronal death are the cellular underpinnings of lentivirus-associated neurodegeneration (43). We examined the immunoreactivity of the neuronal protein VAChT in each experimental group (Fig. 6P). Reduced immunoreactivity for VAChT was evident in the cortex of FIV-infected animals compared with mock-infected animals, which was restored by ddI treatment (Fig. 6Q). These results suggested that ddI treatment might protect neurons against FIV-induced neuronal injury, mediated by CXCR3 activation and ensuing suppression of autophagy.
DISCUSSION
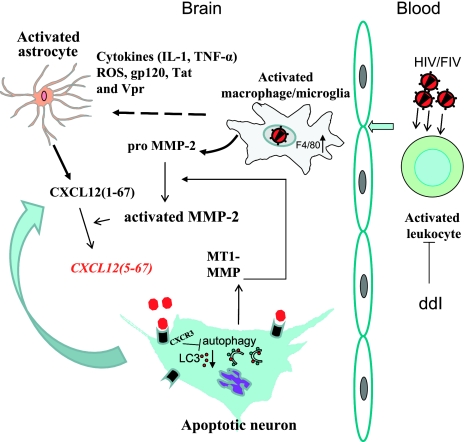
Herein, we show that exposure of neurons to the proteolytically cleaved chemokine CXCL12(5-67) and other CXCR3-specific chemokines influenced CXCR3 expression but also suppressed autophagy leading to reduced neuronal viability. Antiretroviral therapy with ddI reduced plasma viral load in FIV-infected animals together with attenuating neuroinflammation, thereby preventing induction of CXCL12(5-67) and protecting neurons by preservation of autophagy (Fig. 7). Moreover, these ddI-mediated actions restored neuronal function and survival, in keeping with the improved behavioral indices resulting from ddI treatment (Fig. 3A). FIV infection induced brain MMP-2 and its substrate, CXCL12, which extends our finding of increased CXCL12(5-67) in the brain from HAD patients (15). In the feline model, although the total abundance of CXCR3 was not significantly increased (Fig. 5), there was an increase observed for cell membrane-associated CXCR3 in histological sections (Fig. 6K), similar to increased CXCR3 observed in brains of HAD patients. Thus, overall, these data support the hypothesis that an increased abundance of CXCL12(5-67) and its actions via CXCR3 engagement contribute to lentivirus neuropathogenesis. This proposed mechanism appears to be a target for antiretroviral treatment, principally through suppression blood viral load and the associated activation of circulating leukocytes but also appears to diminish neuroinflammation and ensuing neurotoxicity (Fig. 7).
Figure 7.
Suppression of neuronal autophagy and its regulation by antiretroviral therapy. ddI suppressed viral burden in the peripheral circulation, diminishing leukocyte activation and CNS entry with an ensuing reduction in MMP-2 production and its actions, thereby preventing the induction of CXCL12(5-12) with its adverse effects on neuronal autophagy and survival.
Since the initial description of a splicing alternative for the CXCR3 chemokine receptor, named CXCR3B (19), this alternative isoform has been described in T cells as well as myeloma, epithelial, and endothelial cells, but appeared to be absent from monocytes, macrophages, neutrophils, and brain tissues. However, the results obtained in the present study showed that CXCR3B was also detected in the human brain. Moreover, the immunoreactive doublet observed in the Western blot with an antibody targeting the region common to both isoforms also suggested the presence of both CXCR3A and CXCR3B in the cortex of human and feline brains. These data are at odds with the study of Lasagni et al. (19), which described an absence of the CXCR3B transcript in human brains. This discrepancy in results is likely due to our use of a more sensitive technique (RT-PCR) to detect CXCR3B mRNA, compared with Northern blotting for CXCR3B RNA detection.
Previous reports focusing on CXCR3 splicing isoforms reported that CXCR3A appeared to be chemotactic, antiapoptotic, and proproliferative, while CXCR3B appeared to be angiostatic, proapoptotic, and antiproliferative. To date, only two studies have reported regulation of CXCR3 splicing by cell physiology or signaling pathways. Indeed, abundance of CXCR3B relative to CXCR3A appeared to be cell cycle and Ras dependent (44, 45). In the present study, we have shown that the expression of the two main splicing isoforms of the chemokine receptor CXCR3 can be specifically and/or differentially altered on lentivirus-induced neuroinflammation. Our results demonstrated that several inflammatory molecules [CXCL12(5-67), CXCL10, CXCL4, IL-1β, and TNF-α; data not shown] exert different effects on the regulation of CXCR3 isoforms in neuronal cultures, thus suggesting that the pattern of CXCR3 isoforms expression may vary in vivo between different neuroinflammatory conditions according to the pool of inflammatory molecules present within the CNS.
Infection by HIV-1 leads to infection of the brain and subsequent neuropsychological impairment, including HAD (46, 47). The introduction of a highly active antiretroviral therapy has led to a marked decline in the overall death rate and incidence rate of HAD, together with improvement of neurocognitive performance. However, the mechanisms by which antiretroviral therapy improve neurocognitive performance in HAD are unclear, as viral burden in the brain parenchyma is relatively unaffected by antiretroviral therapy (48). In the FIV model, ddI, a nucleotide reverse transcriptase inhibitor, significantly improved FIV-infected animals’ neurobehavioral performance, resembling observations in treated HIV-infected humans. ddI suppressed viral replication in the peripheral circulation of FIV-infected animals, but conversely, it shows limited CNS penetration (49). Hence, ddI treatment might decrease neuroinflammatory and neurotoxic molecules derived from activated/infiltrating leukocytes, which contributed to the improved neurobehavioral performance. The present study also showed a reduction in microglial activation in vivo based on Iba-1 immunoreactivity as well as suppression of the monocytoid activation maker, F4/80, which supports the notion that regulation neuroimmune activation might be an effective strategy for treating HAD.
The neuropathology of HAD is characterized by extensive leukocyte infiltration into the brain, multinucleated giant cells, activated microglia, astrogliosis, white matter pallor, reduced synaptic density, and neuronal loss in the cortex and basal ganglia (50). Studies of patients with HAD and milder forms of HIV-associated neurocognitive impairment, as well as unimpaired HIV-seropositive controls, found that global ratings of neuropsychological function derived from an extensive battery of tests correlated significantly with synaptic density (51, 52), and loss of synapses was not simply a result of loss of neurons (51, 53, 54), suggesting that synaptic injury before neuronal death is the major determinant of HIV-associated neurological disease (43). The present study found that the production of the neurotransmitter transport and synaptic transmission related protein VAChT was decreased in the cortex from FIV-infected animals compared with untreated animals but restored with ddI treatment. These findings suggest that ddI treatment improved neurological function in FIV-infected animals through the prevention of synaptic injury from FIV infection in the cortex. The activated myeloid cells in the CNS likely release neuroinflammatory and neurotoxic molecules leading to neuronal injury in terms of synaptic injury and neuronal loss. As a component of the neuroinflammatory response in HIV-infected patients, MMP-2 is markedly induced in the brain and cerebrospinal fluid (29, 55). Pro-MMP-2 is induced and secreted from HIV-infected macrophages and microglia, which are subsequently activated on contact with neurons, and then converts CXCL12, a chemokine expressed by astrocytes and neurons (56), into the neurotoxic protein CXCL12(5-67), after precise proteolytic processing, which removed the N-tetrapetide (14). Our previous study showed that CXCL12(5-67) utilized CXCR3 as its receptor, which expressed on neurons, astrocyte, and microglia, to exert neurotoxic activity on neurons (15). In the present study, ddI reduced expression of MMP-2 in FIV-infected animals, together with a decrease in CXCL12(5-67) and CXCR3 production mainly expressed on neurons in FIV-infected animals. Interestingly, while CXCR3 splicing isoforms have only been described in humans, the immunoreactive doublet on Western blot in feline brain also suggested the presence of at least two CXCR3 isoforms in cats.
Programmed cell death is increasingly recognized as a fate of neurons during different neurodegenerative processes. Indeed, apoptosis is the best understood programmed cell death mechanism in neurons, although the pathways to apoptosis are diverse. Autophagy has been associated with neurodegeneration and might be an antecedent process culminating in neuronal apoptosis (57), although it also contributes to the fate of leukocytes in HIV infection (58) (59). Autophagy is a fundamental cellular homeostatic process that enables cells to clean up, in a regulated manner, portions of their own cytoplasm and degrade their constituents (60). In the absence of autophagy, the turnover of cytosolic proteins is impaired, increasing their propensity to become damaged and misfolded and subsequently ubiquitinated and aggregated, thus leading to the accumulation of potentially neurotoxic proteins that are discarded by lysosomal degradation in healthy conditions (61). The formation of autophagosome in mammalian cells is dependent on a complex pathway involving activation of a series of autophagy-related genes (atg) as well as several prototypic proteins including Beclin-1, p62, and LC3 (62, 63). Atg7 deletion in hepatocytes, Atg5 and Atg7 deletion in neurons, and Atg5 deletion in cardiomyocytes result in the accumulation of ubiquitin-positive protein aggregates in inclusion bodies that are associated with cellular degeneration (63). The contribution of autophagy to neurodegeneration during HIV infection remains unknown, although a recent study (9) showed that disruption of neuronal autophagy by infected microglia resulted in neurodegeneration. In our study, FIV infection reduced atg5 transcript abundance and LC3 protein production in the cortex, while p62 was induced, all pointing to a role for autophagy suppression in lentiviral infections, especially affecting neurons. Indeed, there was little evidence of LC3 expression in astrocytes (data not shown). These effects were reversed by ddI treatment, implying that ddI restored FIV-induced autophagy impairment. Of importance, CXCL12(5-67) impaired autophagy in human fetal neurons thus identifying CXCL12(5-67) and the subsequent CXCR3 activation as the missing link between infection by lentiviruses and autophagy impairment associated with synaptic injury and neuronal loss. Interestingly, ddI in the present work prevented neuronal injury through inhibition of CXCL12(5-67) production and CXCR3 activation and subsequently reversing autophagy suppression. The pathway from CXCR3 activation to autophagy inhibition remains uncertain, but the activation of MAPK and mTOR in the regulation of autophagy (64) implies these signaling pathways might be important therapeutic targets for future interventions.
Supplementary Material
Acknowledgments
We thank Leah DeBlock for assistance with manuscript preparation. Y.Z. was a Canadian Institutes for Health Research (CIHR) fellow. D.V. was supported by a Toupin Chair fellowship. F.N. is supported by CIHR and Alberta Heritage Foundation for Medical Research fellowships. C.M.O. and C.P. hold Canada Research Chairs (tier 1) in metalloproteinases and systems biology and neurological infection and immunity, respectively. C.P. is an Alberta Heritage Foundation for Medical Research senior scholar. These studies were supported by the U.S. National Institutes of Health–National Institute of Mental Health and CIHR.
References
- Rock R B, Peterson P K. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroimmune Pharmacol. 2006;1:117–126. doi: 10.1007/s11481-006-9012-8. [DOI] [PubMed] [Google Scholar]
- Fehder W P, Douglas S D. Interactions between the nervous and immune systems. Semin Clin Neuropsychiatry. 2001;6:229–240. doi: 10.1053/scnp.2001.26994. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Overall C M, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Ryan L A, Cotter R L, Zink W E, 2nd, Gendelman H E, Zheng J. Macrophages, chemokines and neuronal injury in HIV-1-associated dementia. Cell Mol Biol (Noisy-le-grand) 2002;48:137–150. [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton S A. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Yang D S, Kumar A, Stavrides P, Peterson J, Peterhoff C M, Pawlik M, Levy E, Cataldo A M, Nixon R A. Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer’s disease. Am J Pathol. 2008;173:665–681. doi: 10.2353/ajpath.2008.071176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses W B, Flynn C T, Brady N R, Fox H S. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R W, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197:S294–S306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, McCutchan J A, Ellis R J. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2008;16:15–22. [PubMed] [Google Scholar]
- Rumbaugh J A, Nath A. Developments in HIV neuropathogenesis. Curr Pharm Des. 2006;12:1023–1044. doi: 10.2174/138161206776055877. [DOI] [PubMed] [Google Scholar]
- Muller T, Meyer H E, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog Neurobiol. 2008;85:393–406. doi: 10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban G A, Silva C, Butler G S, Johnston J B, Holden J, Clark-Lewis I, Overall C M, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler G S, Ooms M, Cox J H, Silva C, Hollenberg M D, Jhamandas J H, Overall C M, Power C. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–19187. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman E D, Weinreich D M, Carroll N M, Burness M L, Feldman A L, Turner E, Xu H, Alexander H R., Jr Interferon gamma-inducible protein 10 selectively inhibits proliferation and induces apoptosis in endothelial cells. Ann Surg Oncol. 2006;13:125–133. doi: 10.1245/ASO.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Garin A, Tarantino N, Faure S, Daoudi M, Lecureuil C, Bourdais A, Debre P, Deterre P, Combadiere C. Two novel fully functional isoforms of CX3CR1 are potent HIV coreceptors. J Immunol. 2003;171:5305–5312. doi: 10.4049/jimmunol.171.10.5305. [DOI] [PubMed] [Google Scholar]
- Gupta S K, Pillarisetti K, Lysko P G. Modulation of CXCR4 expression and SDF-1alpha functional activity during differentiation of human monocytes and macrophages. J Leukoc Biol. 1999;66:135–143. doi: 10.1002/jlb.66.1.135. [DOI] [PubMed] [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert J E, Addison C A, Burdick M D, Kunkel S L, Strieter R M. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. 2004;173:6234–6240. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- Elder J H, Sundstrom M, de Rozieres S, de Parseval A, Grant C K, Lin Y C. Molecular mechanisms of FIV infection. Vet Immunol Immunopathol. 2008;123:3–13. doi: 10.1016/j.vetimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J, Pancino G, Merat R, Leste-Lasserre T, Moraillon A, Schneider-Mergener J, Alizon M, Sonigo P, Heveker N. Shared usage of the chemokine receptor CXCR4 by primary and laboratory-adapted strains of feline immunodeficiency virus. J Virol. 1999;73:3661–3671. doi: 10.1128/jvi.73.5.3661-3671.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H, Fuller F J, Tompkins W A. Mechanism of feline immunodeficiency virus envelope glycoprotein-mediated fusion. Virology. 2004;321:274–286. doi: 10.1016/j.virol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Power C, Buist R, Johnston J B, Del Bigio M R, Ni W, Dawood M R, Peeling J. Neurovirulence in feline immunodeficiency virus-infected neonatal cats is viral strain specific and dependent on systemic immune suppression. J Virol. 1998;72:9109–9115. doi: 10.1128/jvi.72.11.9109-9115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher N F, Brayden D J, Brankin B, Callanan J J. Feline immunodeficiency virus infection: a valuable model to study HIV-1 associated encephalitis. Vet Immunol Immunopathol. 2008;123:134–137. doi: 10.1016/j.vetimm.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Zink M C, Laast V A, Helke K L, Brice A K, Barber S A, Clements J E, Mankowski J L. From mice to macaques–animal models of HIV nervous system disease. Curr HIV Res. 2006;4:293–305. doi: 10.2174/157016206777709410. [DOI] [PubMed] [Google Scholar]
- Boisse L, Gill M J, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Power C, Zhang K, van Marle G. Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases. J Neurovirol. 2004;10:113–117. doi: 10.1080/753312762. [DOI] [PubMed] [Google Scholar]
- Johnston J B, Jiang Y, van Marle G, Mayne M B, Ni W, Holden J, McArthur J C, Power C. Lentivirus infection in the brain induces matrix metalloproteinase expression: role of envelope diversity. J Virol. 2000;74:7211–7220. doi: 10.1128/jvi.74.16.7211-7220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K, Donath A, Beer B, Egberink H F, Horzinek M C, Lutz H, Hoffmann-Fezer G, Thum I, Thefeld S. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol. 1992;35:167–175. doi: 10.1016/0165-2427(92)90129-e. [DOI] [PubMed] [Google Scholar]
- Roth J S, McCully C M, Balis F M, Poplack D G, Kelley J A. 2′-beta-fluoro-2′,3′-dideoxyadenosine, lodenosine, in rhesus monkeys: plasma and cerebrospinal fluid pharmacokinetics and urinary disposition. Drug Metab Dispos. 1999;27:1128–1132. [PubMed] [Google Scholar]
- Antinori A, Perno C F, Giancola M L, Forbici F, Ippolito G, Hoetelmans R M, Piscitelli S C. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–1793. doi: 10.1086/498310. [DOI] [PubMed] [Google Scholar]
- St Hillaire C, Vargas D, Pardo C A, Gincel D, Mann J, Rothstein J D, McArthur J C, Conant K. Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J Neurovirol. 2005;11:535–543. doi: 10.1080/13550280500385203. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, McArthur J C, Silva C, Vodjgani M, Andrade-Gordon P, Hollenberg M D, Power C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- Kennedy J M, Hoke A, Zhu Y, Johnston J B, van Marle G, Silva C, Zochodne D W, Power C. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS. 2004;18:1241–1250. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Tsutsui S, Vergnolle N, Boven L A, Shariat N, Vodjgani M, Warren K G, Andrade-Gordon P, Hollenberg M D, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Vo L, Owen P, Anderson J. Chemical synthesis, purification, and folding of C-X-C and C-C chemokines. Methods Enzymol. 1997;287:233–250. doi: 10.1016/s0076-6879(97)87018-8. [DOI] [PubMed] [Google Scholar]
- Koenig R E, Gautier T, Levy J A. Unusual intrafamilial transmission of human immunodeficiency virus. Lancet. 1986;2:627. doi: 10.1016/s0140-6736(86)92448-7. [DOI] [PubMed] [Google Scholar]
- Boven L A, van der Bruggen T, van Asbeck B S, Marx J J, Nottet H S. Potential role of CCR5 polymorphism in the development of AIDS dementia complex. FEMS Immunol Med Microbiol. 1999;26:243–247. doi: 10.1111/j.1574-695X.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Anthony I C, Bell J E. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- McQuibban G A, Butler G S, Gong J H, Bendall L, Power C, Clark-Lewis I, Overall C M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Rubinsztein D C. Small molecule enhancers of autophagy for neurodegenerative diseases. Mol Biosyst. 2008;4:895–901. doi: 10.1039/b804606a. [DOI] [PubMed] [Google Scholar]
- Bellizzi M J, Lu S M, Gelbard H A. Protecting the synapse: evidence for a rational strategy to treat HIV-1 associated neurologic disease. J Neuroimmune Pharmacol. 2006;1:20–31. doi: 10.1007/s11481-005-9006-y. [DOI] [PubMed] [Google Scholar]
- Datta D, Flaxenburg J A, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser A M, Briscoe D M, Pal S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- Aksoy M O, Yang Y, Ji R, Reddy P J, Shahabuddin S, Litvin J, Rogers T J, Kelsen S G. CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L909–918. doi: 10.1152/ajplung.00430.2005. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8:115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Janssen R S, Nwanyanwu O C, Selik R M, Stehr-Green J K. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- Langford D, Marquie-Beck J, de Almeida S, Lazzaretto D, Letendre S, Grant I, McCutchan J A, Masliah E, Ellis R J. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirol. 2006;12:100–107. doi: 10.1080/13550280600713932. [DOI] [PubMed] [Google Scholar]
- Wynn H E, Brundage R C, Fletcher C V. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16:595–609. doi: 10.2165/00023210-200216090-00002. [DOI] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Everall I P, Heaton R K, Marcotte T D, Ellis R J, McCutchan J A, Atkinson J H, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton R K, Marcotte T D, Ellis R J, Wiley C A, Mallory M, Achim C L, McCutchan J A, Nelson J A, Atkinson J H, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25:123–133. doi: 10.1046/j.1365-2990.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- Everall I P, Glass J D, McArthur J, Spargo E, Lantos P. Neuronal density in the superior frontal and temporal gyri does not correlate with the degree of human immunodeficiency virus-associated dementia. Acta Neuropathol. 1994;88:538–544. doi: 10.1007/BF00296490. [DOI] [PubMed] [Google Scholar]
- Conant K, McArthur J C, Griffin D E, Sjulson L, Wahl L M, Irani D N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249:163–166. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- Ventruti A, Cuervo A M. Autophagy and neurodegeneration. Curr Neurol Neurosci Rep. 2007;7:443–451. doi: 10.1007/s11910-007-0068-5. [DOI] [PubMed] [Google Scholar]
- Zhou D, Spector S A. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Sodora D L. HIV and CXCR4 in a kiss of autophagic death. J Clin Invest. 2006;116:2078–2080. doi: 10.1172/JCI29447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D J, Emr S D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky D J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- Tang G, Yue Z, Talloczy Z, Hagemann T, Cho W, Messing A, Sulzer D L, Goldman J E. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum Mol Genet. 2008;17:1540–1555. doi: 10.1093/hmg/ddn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.