Abstract
The ability of cells to respond to external mechanical stimulation is a complex and robust process involving a diversity of molecular interactions. Although mechanotransduction has been heavily studied, many questions remain regarding the link between physical stimulation and biochemical response. Of significant interest has been the contribution of the transmembrane proteins involved, and integrins in particular, because of their connectivity to both the extracellular matrix and the cytoskeleton. Here, we demonstrate the existence of a mechanically based initiation molecule, syndecan-4. We first demonstrate the ability of syndecan-4 molecules to support cell attachment and spreading without the direct extracellular binding of integrins. We also examine the distribution of focal adhesion-associated proteins through controlling surface interactions of beads with molecular specificity in binding to living cells. Furthermore, after adhering cells to elastomeric membranes via syndecan-4-specific attachments we mechanically strained the cells via our mechanical stimulation and polymer surface chemical modification approach. We found ERK phosphorylation similar to that shown for mechanotransductive response for integrin-based cell attachments through our elastomeric membrane-based approach and optical magnetic twisting cytometry for syndecan-4. Finally, through the use of cytoskeletal disruption agents, this mechanical signaling was shown to be actin cytoskeleton dependent. We believe that these results will be of interest to a wide range of fields, including mechanotransduction, syndecan biology, and cell–material interactions.
Keywords: cytoskeleton, ERK phosphorylation, fibroblast, mechanobiology, cell–material interaction
The biochemical response of cells to external mechanical stimulation has generated tremendous interest over the past decade. Of particular interest are the causative contributions of mechanotransductive transmembrane integrin molecules that link the extracellular matrix (ECM) to the cell interior and play a role in physiological response and molecular signaling within the cell. Recently, numerous exciting discoveries have evolved from these studies including the role of mechanotransduction in vascular physiology and atherogenesis, Src activation (Src phosphorylation/activation under local mechanical stimulation), and the effect of matrix stiffness on stem cell differentiation (1–3). Mechanical stimulation has been shown to produce a wide range of cellular responses including the proteomic activation of the mitogen-activated protein kinase (MAPK) pathways in extracellular signal-regulated kinases (ERKs), alterations of genomic expression profiles, and control of cell morphology, differentiation, and proliferation (4, 5). However, such research has focused primarily on the integrins as the mechanical signal initiator/transmembrane protein. Although other strain-sensitive proteins, such as mechanosensitive ion channels and protein kinase C, are known to be altered by mechanical stimulation, these responses are thought to occur downstream or be directly linked to the transmembrane protein-initiating events at the cell surface (6). Identifying another class of mechanotransduction-initiating transmembrane proteins would be an intriguing finding that would be in line with well-known redundancy principles; cells exhibit many signaling pathways for critical functional behavior to avoid reliance on one alone. The impact of furthering our understanding of the mechanotransductive process is essential, because the effects are correlated to sensory responses such as sensorineural hearing loss, gravity sensation, baroreception, and even organism development (7–9).
When examining integrin characteristics, it is important to note their link from the extracellular to the intracellular complexes (6, 10), and their location within the focal adhesion complex (FAC). FACs are a heterocomplex of proteins including paxillin, vinculin, and talin that form a structural signaling connection from the ECM to the cytoskeleton. These cell surface receptors are not only involved in mechanical responses, they can also control cell structure and function and affect cell migration and adhesion. In the mechanotransductive response, the downstream regulation of complexes, such as ERK phosphorylation as part of the MAPK pathway, demonstrates parallel signaling responses when activated with both biochemical and mechanical stimulation, implying that independent modes of activation (mechanical and chemical) result in similar biochemical responses. Mechanically stimulating living cells and assaying their response has been investigated through a variety of techniques (2, 11). These techniques enable mechanotransduction theories to be tested and focus on both single-cell mechanical stimulation approaches such as magnetic beads, atomic force microscopes, and local elastomeric membrane deformation, and multiple-cell stimulation approaches such as Flexcell technology (11–14). Developing a technique with the capacity to attach cells to flexible membranes with molecular specificity while simultaneously providing for the mechanical stimulation of numerous cells is necessary to allow for larger-scale biochemical assays that might help identify new initiator proteins.
Based on the characteristics of and responses to integrins, proteins that exhibit similar structural characteristics to the integrins could be candidates as the novel transducers of mechanical stimuli. Syndecan-4, for example, although different from integrin in regard to specific structural organization, has similar location and linkage characteristics. Syndecans are heparan sulfate proteoglycans positioned at the cell surface. Most importantly, they exhibit a membrane spanning distribution (15). Because syndecans, like integrins, are known to have binding affinities for both the ECM and the actin cytoskeleton (16), they likely provide connectivity between the extracellular and intracellular domains. Furthermore, syndecan-4 molecules are also known to be a component of the FAC (17), which is very important for communication across the cell membrane during mechanotransduction. Syndecan-4 molecules have been shown to indirectly attach to primary protein components of the FAC, including paxillin, that is linked to the actin cytoskeleton (16). In addition, studies have already indicated that heparin sulfate-linked proteins could have a role in mechanical signaling (15) and have been implicated in numerous processes from cell signaling to adhesion (18). Furthermore, in tests of mechanotransduction using integrin-based adhesion, syndecans are known to cluster at the FAC, potentially implicating them in this pathway and reinforcing their interrelationship to the process of mechanical activation (19). From a biochemical activation standpoint, syndecans are regarded as integrators of extracellular signals. They have been linked with local domain protein distribution and binding to cytoplasmic proteins and are involved in intracellular phosphorylation (20). Furthermore, syndecan binding has been shown to play a role in a wide variety of functional areas including the activation of the Rho and Rac pathways (16, 21). This finding indicates the possibility of a progressive sequence that could lead to the activation of ERK phosphorylations, because both Rho and Rac are part of the integrin mechanotransduction pathway as well. In addition, syndecans are present in a variety of tissues, including mesenchymal and arterial tissues, and have been demonstrated to be essential in a diversity of physiologically relevant areas such as wound healing, neointima formation, liver disease, and cancer (22, 23). The ability to control the surface interactions of cells with their interface (e.g., polymer membrane surface) is critical for investigating mechanotransduction related to types of transmembrane proteins. Here, we demonstrate that syndecan-4 is a transmembrane protein that can recruit FAC proteins to sites of syndecan-specific cellular attachments. Furthermore, mechanical stimulation through syndecan-4 increases ERK phosphorylation in a similar manner to that observed for integrin-based attachments, and, like integrins, demonstrates a dependence on the cytoskeleton. To impose specific mechanical stimulation and examine the response, this research used our own method of elastomeric stretching and chemical surface modification and optical magnetic twisting cytometry, which allowed us to create specific attachments through antibody binding.
Results and Discussion
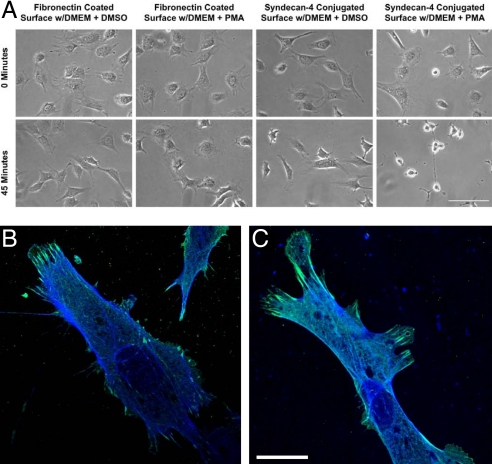
As the existing body of research on transmembrane mechanotransduction has largely focused on integrins, our goal was to use integrin-based knowledge as a comparative approach to develop a system through which we could explore the potential role of syndecan-4 in mechanotransduction independent of integrin-based attachments. We first investigated the attachment and spreading of NIH 3T3 fibroblasts through antibody-based syndecan-4-specific cell adhesion (Fig. 1). Cell attachment and spreading was investigated by incubating cultures with anti-syndecan-4 KY8.2 antibodies on glass coverslip surfaces. These antibodies are known to be highly specific for syndecan-4 and previous studies have provided support for syndecan-specific cell adhesion (24). The cells were then fixed with paraformaldahyde, permeabilized with Triton-X, and stained for vinculin (blue) and actin (green) as shown in Fig. 1 B and C. Although natural syndecan ligands would normally be considered most useful for probing these interactions, and there are generalized ligands for syndecans (for example, those derived from fibronectin), unfortunately, there are currently no known ligands that can be used to specifically probe syndecan-4. However, initiation of response using antibodies to examine specific linkages to receptor proteins has been successfully demonstrated in previous integrin-based mechanotransduction research (6). Furthermore, recent studies have shown that syndecan molecules indeed form attachments through their protein chains (25, 26). Glass substrate surfaces in our research were treated with a 1% BSA (or, alternately, pluronics F-127; Invitrogen) in double-distilled H2O (ddH2O) solution for 30 min to inhibit nonspecific attachments. To investigate the dependence of syndecan-4 binding to the antibody for cell attachment and spreading, syndecan shedding was induced in adherent cells by using phorbol 12-myristate 13-acetate (PMA). PMA induces the activation of protein kinase C, which results in a wide array of cellular responses, including the shedding of syndecans but not integrins (27). The known lack of integrin shedding in response to PMA indicates the specificity of syndecan-4 binding to the surfaces over 45 min of stimulation when we compared PMA-treated cells on anti-syndecan-4 antibody-coated surface with PMA-treated cells on fibronectin-coated surface as shown in Fig. 1A. The timing of the spreading of NIH 3T3 fibroblasts on fibronectin, anti-syndecan-4 antibodies, glass, and nonspecific rat IgG through 8.5 h was investigated as well (Fig. S1). The cells were found to spread on fibronectin and anti-syndecan-4 antibody-coated surfaces, and untreated glass. The negative controls, which used rat IgG antibodies in place of anti-syndecan-4 antibodies, did not enable cell attachment and spreading.
Fig. 1.
NIH 3T3 fibroblasts grown on fibronectin or anti-syndecan-4 antibody-coated glass surfaces. (A) After growing cells on fibronectin or anti-syndecan-4 antibodies, cells were treated with PMA or DMSO as a control. PMA induces the enzyme-driven shedding of syndecans, but not integrins. The rounding and detachment of cells on anti-syndecan-4 antibody surfaces, but not fibronectin surfaces, indicates that integrins are not directly involved in the binding to anti-syndecan-4 antibody-coated surfaces. (Bar: 200 μm.) (B and C) Colocalization of vinculin and actin for NIH 3T3 fibroblasts spread on modified glass surfaces for attachment through transmembrane proteins. NIH 3T3 cells on surfaces modified with fibronectin (B) and anti-syndecan-4 antibodies (C) were immunofluorescently labeled for vinculin (green) and phalloidin for actin (blue). Note that cells attached exclusively through syndecan-4 recruit vinculin to sites of focal contact. (Bar: 25 μm.)
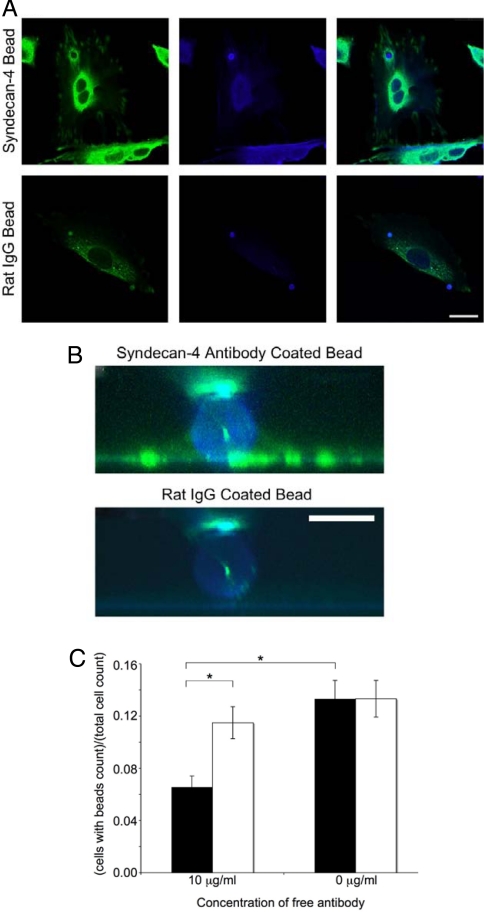
We also wanted to investigate microdomain responses of the NIH 3T3 fibroblasts to local attachments via syndecan-4 (Fig. 2). We coated carboxyl functionalized beads with anti-syndecan-4 antibodies, applied them to cells, and then stained the cells for vinculin, a primary structural component of the FAC. We observed a spatial colocalization at the periphery of the anti-syndecan-4 antibody-coated beads based on the fluorescent intensity of the vinculin (Fig. 2 A and B). This response was compared with beads coated with nonspecific rat IgG molecules, which showed no vinculin recruitment at sites of bead localization on cells. Bead binding was also shown to be specific for syndecan-4-based attachments by conducting competition studies between conjugated beads and free antibodies in solution. When we added free antibodies together with antibody-coated beads, the number of beads attached on cells decreased (Fig. 2C). Furthermore, beads coated with fibronectin and β-1 integrin antibodies showed similar vinculin distribution patterns to the anti-syndecan-4 antibody-coated beads (Fig. S2). These bead attachment and cellular spreading experiments indicate that syndecan-4 plays a crucial role in recruiting vinculin to FAC sites without requiring integrin binding to an extracellular ligand. Because syndecan-4 is able to begin the assembly of FACs on its own, the role of syndecan-4 in a mechanotransduction cascade on its own is structurally plausible.
Fig. 2.
Localization of structural proteins with syndecan-4 through bead binding. (A) NIH 3T3 cells were grown on glass and incubated for 45 min at 37 °C with syndecan-4 antibody or rat IgG-conjugated beads. Cells were fixed and labeled for vinculin (green) and actin (blue). Note the vinculin halo structure and increased actin labeling surrounding syndecan-4 antibody beads and the lack of such labeling surrounding rat IgG bead. (Bar: 25 μm.) (B) An 8-μm side view of bead attachments from A created by reconstruction of 100 × 0.08-μm confocal slices. (Bar: 5 μm.) (C) Free anti-syndecan-4 antibodies (filled bars) inhibited anti-syndecan-4 conjugated beads from binding to NIH 3T3 cells, whereas free rat IgG antibodies (empty bars) did not (*, P < 0.05).
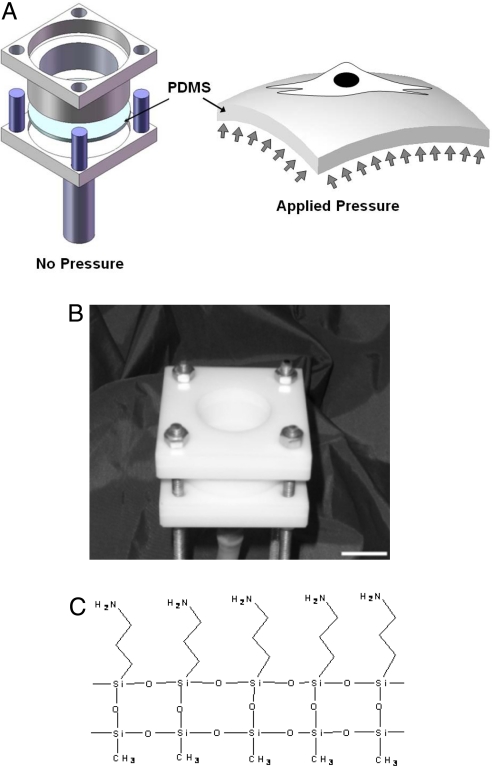
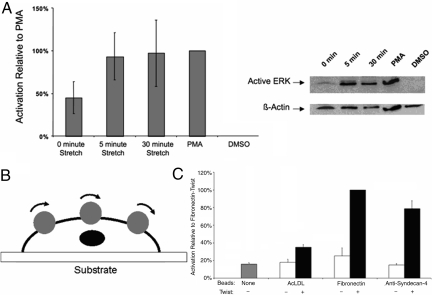
We next probed the response of NIH 3T3 fibroblasts to mechanical stimulation through syndecan-4-based binding. We developed a technique to control the mechanical stimulation of living cells while simultaneously controlling the surface chemistry of an elastomeric membrane for molecular specificity under stimulation. This approach allowed for mechanically straining numerous living cells while simultaneously controlling their molecular surface interactions during adherence and spreading. We used the pressure-driven cell-stretching (PreCS) device (28) that was able to impose controlled mechanical stimulation on single cells and cell populations through stretching of an elastomeric membrane (Fig. 3 A and B). This system constrains a polydimethylsiloxane (PDMS) membrane to dictate the mechanical stimulation of cells through their basal domains by controlling the polymer-to-cell surface interactions. A regulator applied a pressure of 5 lb/in2 to the bottom surface of the membrane to induce the deformation of the PDMS. Importantly, control over the surface chemistry is critical for this mechanical stimulation, because unmodified PDMS is hydrophobic and challenging for incorporating molecular coatings (29). The PDMS membranes were chemically modified to make them conducive to specific protein conjugation before the cells were cultured on the membranes (Fig. 3C). To accomplish this, PDMS surfaces were treated with 1 min of air plasma (Harrick Plasma PDC-32G), followed by 24 h of 2% 3-aminopropyltriethoxysilane (AMEO) in ethanol. The membranes were then heat-treated in an 80 °C oven for 8 h, followed by a thorough ethanol and PBS rinse. To probe the specific role of syndecan-4 in mechanical signaling, we linked anti-syndecan-4 antibodies to the modified PDMS. Cells were attached through syndecan-4-specific adhesion to the antibody-conjugated membranes and introduced into the PreCS device. We then increased the pressure on the lower side of PDMS membrane to create an equibiaxial strain (28). The strain was transferred to the cells through their basal attachments to the anti-syndecan-4 antibody-coated PDMS surface. Through examining the levels of ERK activation in cells adhered to membranes coated with anti-syndecan-4 antibody, we could examine one mechanotransduction indicator (Fig. 4); ERK activation is a commonly used measure to assess a mechanotransduction pathway (6, 30). NIH 3T3 fibroblast cells were cultured on the anti-sydecan-4 antibody-coated membranes to induce cell attachment and spreading in preparation for mechanical stimulation. When a 10% strain was introduced for 5 min, an increase in the activation of ERK was observed (Fig. 4A). This increase continued under the static strain at 30 min of stretching. By comparing these activation levels to unstretched cells (0 min), these findings indicated that the activation is not just attachment based, but that mechanical stimulation is required. These ERK phosphorylation responses after syndecan-4-based mechanical stimulation were similar to integrin-based responses under mechanical stimulation (31). The surface strain of the PDMS substrate was an average of 10%. This average was calculated based on the strain distribution across the polymer surface. We documented this strain distribution through developing a model of our system using ANSYS, as shown in Fig. S3. A total of 8,640 shell elements were used in ANSYS to model the PDMS membrane, which was constrained at its periphery and deformed on the opposite surface by an uniform pressure as depicted in Fig. 3A. The material properties such as the elastic modulus of PDMS were assumed to be homogeneous (32). The resulting deformation was not homogeneous across the substrate as expected and experimentally observed because of the pressure and the periphery constraints, but produced a consistent average strain of 10% when deformed. Mesh independence was verified by examining higher-density meshes. We then compared the stretching results to BSA-only-coated membranes subjected to a strain of 10%; these membranes had minimal cell attachment (<5% of the cells attached that were introduced into the cell culture dish for attachment to the membrane) relative to anti-syndecan-4 antibody-coated membranes. These samples revealed no detectable ERK phosphorylation in our Western blots. We further demonstrated the effects of syndecan-4 in mechanotransduction through an alternate independent technique, optical magnetic twisting cytometry (OMTC), to mechanically stimulate NIH 3T3 fibroblasts through twisting beads coated with anti-syndecan-4 antibodies that were attached to the cells. We twisted ferromagnetic beads [4.5-μm diameter, ≈5–15 beads per cell; Spherotech (7)] attached to NIH 3T3 cells by using OMTC (33, 34); a schematic of this OMTC system is shown in Fig. 4B. Beads were coated following the manufacturer's instructions with fibronectin, antibodies to syndecan-4, and acetylated LDL (AcLDL) (Biomedical Technologies) as one control. The NIH 3T3s were seeded in 35-mm Petri dishes without prior surface modification or coating and allowed to attach. After ≈2–3 days to allow them to attach and spread, the beads with the proteins on them were introduced into the media and allowed to attach to the cells for 30 min. A twisting stress (25 Pa) was then applied for 10 min with cyclic twisting; these parameters were based on conventional twisting studies conducted previously (33–35). After bead twisting, cell lysates were collected and analyzed for the activation of ERK through an ELISA using the Surveyor IC Human/Mouse/Phospho-ERK1/ERK2 Immunoassay kit (R&D Systems) following the manufacture's instructions. With the addition of mechanical stimuluation through magnetic beads coated with both fibronectin and anti-syndecan-4 antibodies, we observed similar significant increases in p-ERK (Fig. 4C). It is noted that because of the lack of specific ligands for syndecan-4, the presence of multiple activating integrin types, and the redundancy observed for syndecan-4 with respect to integrin function, the use of siRNA approaches was limited in our experiments. As a result, we developed an approach that used specific binding of cells to anti-syndecan-4 antibodies; if we alternatively use RNA interference strategies to knock down the syndecan-4 expression, there is no cell attachment on PDMS surfaces with anti-syndecan-4 antibody treatment. When using other types of ECMs (e.g., fibronectin), knocking down a specific syndecan (i.e., syndecan-4), does not completely inhibit a response because there are other initiating molecules such as integrins that allow attachments to occur and in parallel can activate the mechanotransduction pathway. These results suggest that, although syndecan-4 is able to activate the mechanotransduction pathway, it is likely a complementary activation pathway that parallels integrin activation. In addition, we have demonstrated that these experiments block the binding of integrins and not that integrins are not activated. Integrins may still be participating in this process, but the initial activator is syndecan-4 in our experiments. Such multiple paths in the activation of signaling cascades are a hallmark of robust cell activities that have been observed in numerous cell behaviors (36, 37).
Fig. 3.
Mechanical stimulation of NIH 3T3 fibroblasts used to examine syndecan-4-based mechanotransduction. (A and B) Schematics (A) and image (B) of the PreCS device for mechanical stimulation of individual cells on their basal domains through stretching a PDMS membrane. (Scale bar: 2 cm.) (C) Surface modification method for attaching living cells to the elastomeric membranes with molecular specificity. The molecular attachment was accomplished by using an AMEO modification of PDMS, which then enabled the direct attachment of anti-syndecan-4 antibodies to the polymer surface.
Fig. 4.
Activation of ERK under mechanical stimulation through syndecan-4. (A) Western blot and densitometry analysis for mechanical strain that was applied to the NIH 3T3 cells attached through anti-syndecan-4 antibodies for 0, 5, and 30 min. PMA-treated cells are a positive control for ERK phosphorylations, and the resulting level of relative activation was defined as 100% for comparison between experimental trials. DMSO-treated cells were a negative control for chemical activation of ERK phosphorylation. The results for a 0-min strain were significantly different from the 5-min strain (P < 0.025) and the 30-min strain (P < 0.025) based on t tests of log10-transformed data with a Bonforroni correction (α = 0.05/2) to treat for repeated testing. Each bar represents the average of at least three independent experiments. (B and C) Using an OMTC device (B) we obtained ERK activation measurements (C) with ELISA-based analysis of lysates of NIH 3T3 cells subjected to strain by twisting coated beads at 1 Hz for 30 min. The resulting ELISA data were normalized based on lysate protein concentration, and then the resulting values were scaled based on defining the twist treatment of fibronectin beads as 100% activation. The resulting ELISA T tests of selected comparisons among the resulting data show that the twist treatment of anti-syndecan-4-coated beads was significantly different with the no-bead sample (P < 0.0167), the no-twist anti-syndecan-4-coated bead sample (P < 0.0167), and the twist AcLDL-coated bead sample (P < 0.0167), using a Bonferroni correction (α = 0.05/3) to treat for repeated testing. Each bar represents the average of three independent experiments.
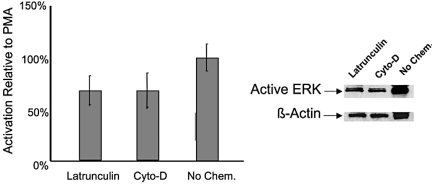
A critical component of cell mechanics is the link to the cytoskeletal structure, which is connected to the FACs. To test whether the pathway of syndecan-4 mechanoactivation was linked to cell structure, we chemically disrupted the actin cytoskeleton and assayed the ERK phosphorylation state after mechanical stimulation. The structural link in nonsyndecan-based mechanical stimulation has provided previous insight into the mechanotransduction cascade (38, 39). Because syndecan-4 is linked to the actin cytoskeleton (16), we used both latrunculin-B and cytochalasin-D to modify the actin microfilaments. Latrunculin-B (0.4 μg/mL) or cytochalasin-D (0.1 μg/mL; Sigma L5288, Sigma 30385) was added to the cells before the mechanical stimulation. After actin cytoskeleton disruption, the cells were exposed to 30 min of 10% equibiaxial mechanical strain, as previously described, to study ERK phosphorylation. When the actin cytoskeleton was disrupted with latrunculin-B or and cytochalasin-D, the ERK activation decreased by ≈30% (Fig. 5). Previous studies have shown both the attachment of cells and the level of ERK phosphorylation has a limited effect under the addition of actin cytoskeleton disruption agents (40). In addition, other researchers have found molecules that are important in the mechanotransduction cascade, including platelet–endothelial cell adhesion molecule 1 and vascular cell adhesion molecule 1, that also have a direct corelation with cytoskeletal integrity (41, 42). We found a similar response in the syndecan mechanotransduction pathway. Syndecan-4 may play a role in mechanical activation and the associated signaling is affected by cell structure.
Fig. 5.
Effects of disruption of the actin cytoskeleton with mechanical stimulation through syndecan-4. Shown are Western blot and densitometry analysis of ERK phosphorylation with latrunculin-B and cytochalasin-D treatment under 30 min of strain. The level of ERK phosphorylation in cells that were not chemically treated but mechanically stimulated was statistically higher than both the latrunculin-treated cells (P < 0.025) and the cytochalasin-D treated cells (P < 0.025) based on t tests with a Bonforroni correction (α = 0.05/2) to treat for repeated testing. Each bar represents the average of at least four independent experiments.
Our studies indicate that syndecan-4 is an initiator of cellular mechanotransduction. We found that anti-syndecan-4 antibodies enabled the attachment and spreading of NIH 3T3 cells and that the attachment of cells to these antibodies was affected by the shedding of syndecans. In addition, vinculin and actin have similar distributions with syndecan-4 binding as those associated with integrin binding. One of the key findings was that ERK phosphorylation was increased with mechanical activation in cells attached solely through syndecan-4 through both elastomeric membrane stretching and OMTC. Furthermore, the presence of actin filaments was important as there was a decrease in mechanical signaling in the presence of actin cytoskeletal disrupting agents. Over the past decade, integrins have received considerable research attention as the primary mechanosensitive transmembrane protein initiating signaling events. We have similarly demonstrated that syndecan-4 is important in mechanotransduction and syndecan-4 has analogous organization in terms of the FACs and the cell structure found with integrins. The identification of syndecan-4 as a mechanosensitive transmembrane protein therefore opens a myriad of directions of research and has implications in areas including mechanotransduction, tissue engineering, and ECM–cell interactions.
Materials and Methods
Cell Line.
NIH 3T3 fibroblast cells were cultured in growth media including DMEM with 10% calf serum, glutamine (0.3 mg/mL), penicillin (100 units/mL), streptomycin (100 mg/mL), and 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, at pH 7.4, under 5% carbon dioxide.
Antibody Conjugation to Glass Coverslips.
Coverslips were treated with 2% AMEO and then maleimide-activated with Sulfo-SMCC. Antibodies were treated with Traut's reagent and then conjugated to the maleimide-activated glass surface by a 4-h incubation on the glass surface. Coverslips were then blocked in 4% BSA in PBS-EDTA + 0.05% Tween-20 before sterilization by treatment with 70% ethanol.
Fibronectin Coating of Glass Coverslips.
Fibronectin was coated onto glass coverslips by preparing human plasma fibronectin as a 100 μg/mL solution in DMEM. A total of 250 μL of the solution was pooled on cover slips and allowed to incubate for 30 min at room temperature. The solution was removed and cover slips were rinsed in DMEM. Cover slips were stored in DMEM at 4 °C until use.
Antibody and Fibronectin Conjugation to M-450 to Sylactivated Beads.
A suspension of beads (Dynal) in a carbonate buffer was washed and then resuspended in fresh carbonate buffer containing antibody or fibronectin. Typically, 25 mg of protein was used to conjugate an ≈25-mL volume of packed beads. Beads were incubated for 16–24 h at 37 °C with gentle tilting and rotation. Conjugated beads were washed with carbonate buffer and stored at 4 °C until use.
Antibody Conjugation to PDMS Surfaces.
PDMS surfaces were treated with 1 min of air plasma, followed by 24 h of 2% AMEO in ethanol. The membranes were then heat-treated in an 80 °C oven for 8 h, followed by a thorough ethanol and PBS rinse. The membranes were coated with 0.16 mg/mL of anti-syndecan-4 KY8.2 antibodies in PBS for 24 h at 4 °C. Finally, the PDMS was treated with a 1% BSA in ddH2O solution for 30 min to inhibit nonspecific attachments.
ERK Western Blot Analysis.
To determine the normalized levels of ERK activation in stretched cells, lysates were prepared in ice-cold lysis buffer [20 mM Tris·HCl (pH 7.3), 1 mM EGTA, 50 mM NaF, 2 mM sodium orthovanadate, 10 μg/mL leupeptin, 10 μg/mL aprotinin], and subjected to Western blot analysis. Cell lysate samples were mixed with 6× loading dye and heated at 65 °C for 10 min. Samples were separated on precast Tris·HCl 4–20% linear gradient gels (BioRad 161-1105) and transferred to nitrocellulose membranes. For Western blot analysis, membranes were blocked at room temperature for 1 h with TBS-T [100 mM Tris (pH 7.5), 0.9% (wt/vol) NaCl, 0.1% Tween-20] containing 1% (wt/vol) BSA. Primary antibody probing for active ERK was conducted overnight at 4 °C using a 1:1,250 dilution of anti-active MAPK antibody (Promega V8031), followed by three 15-min washes in TBS-T. Secondary antibody incubation was conducted for 1 h at room temperature using a 1:5,000 dilution of donkey anti-rabbit HRP secondary antibody (Promega V7951), followed by three 15-min washes in TBS-T, and then two rinses with Tris-buffered saline before detection using ECL (Amersham) and exposure to film. After probing for active ERK, membranes were stripped of antibodies in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris·HCl, pH 6.7) at 50 °C for 30 min in a shaking incubator. Membranes were then washed twice in high volumes of TBS-T for 10 min before probing for β-actin using a 1:1,000 dilution of anti-β-actin (A2066; Sigma). Scans of film exposures were done with a MagicScan scanner (UMAX) and Photoshop CS software (Adobe Systems). Densitometry analysis for each film was performed in triplicate using ImageJ software (National Institutes of Health, Bethesda). Averages of each active ERK signal were normalized by using the corresponding β-actin averages from the corresponding lane on the reprobed blot to control for loading differences between lanes. These values were normalized to the active ERK/β-actin ratio of a positive control lysate of NIH 3T3 cells treated for 30 min with 2.5 μM PMA run on the same blot, which was defined as 100% to generate a relative activation value that could be accurately compared between different experimental trials. In this case, the treatment of cells with PMA is to activate ERK through via protein kinase C activation; the shedding response related to PMA does not affect this lysate generation because cells grown for PMA-induced lysate generation were allowed to grow on a nonsyndecan-4-specific grown surface (i.e., tissue culture plastic).
Microscopy.
Phase-contrast images of cells for the adhesion/spreading, bead competition, and PMA experiments were taken with a Nikon Eclipse TS100 inverted microscope with a SPOT-RT camera (Diagnostic Instruments). All fluorescence images were taken with a Leica TCS SP5 laser scanning confocal microscope. XZ reconstructions were generated from Z-stacks of images using the Leica LAS AF software.
Supplementary Material
Acknowledgments.
We thank Robert Molt and Bin Li for assistance with the PDMS membrane conjugation method and Mark Marzinke, Marshall McKenna, and Shruti Sreepathi for work on the development of methods related to the studies described herein. This work was supported in part by the National Science Foundation (R.M.B. and P.R.L), the National Academies Keck Foundation Futures Initiative (P.R.L), the Office of Naval Research (P.R.L), and the Beckman Young Investigators Program (P.R.L).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902639106/DCSupplemental.
References
- 1.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 3.Engler A, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer I, et al. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson's disease and dementia with Lewy bodies. J Neural Transm. 2001;108:1383–1396. doi: 10.1007/s007020100015. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Hu Y, Mayr M, Xu Q. Cyclic strain stress-induced mitogen-activated protein kinase (MAPK) phosphatase 1 expression in vascular smooth muscle cells is regulated by Ras/Rac-MAPK pathways. J Biol Chem. 1999;274:25273–25280. doi: 10.1074/jbc.274.36.25273. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 7.Gaboyard S, Sans A, Lehouelleur J. Differential impact of hypergravity on maturating innervation in vestibular epithelia during rat development. Dev Brain Res. 2003;143:15–23. doi: 10.1016/s0165-3806(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 8.Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol. 2009;10:44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 9.Wozniak MA, Chen CS. Mechanotransduction in development: A growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens. 1998;16:1921–1929. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y-W, Cheng C-M, LeDuc PR, Chen C-C. Understanding sensory nerve mechanotransduction through localized elastomeric matrix control. PLoS One. 2009;4:e4293. doi: 10.1371/journal.pone.0004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannix RJ, et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat Nanotechnol. 2008;3:36–40. doi: 10.1038/nnano.2007.418. [DOI] [PubMed] [Google Scholar]
- 13.Charras GT, Horton MA. Single-cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys J. 2002;82:2970–2981. doi: 10.1016/S0006-3495(02)75638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, LeDuc PR. Dissecting the molecular basis of the mechanics of living cells. Exp Mech. 2009;49:11–23. [Google Scholar]
- 15.Moon J, et al. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203:166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 16.Denhez F, et al. Syndesmos, a syndecan-4 cytoplasmic domain interactor, binds to the focal adhesion adaptor proteins paxillin and hic-5. J Biol Chem. 2002;277:12270–12274. doi: 10.1074/jbc.M110291200. [DOI] [PubMed] [Google Scholar]
- 17.Woods A, Longley R, Tumova S, Couchman J. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 18.LeDuc PR, Bellin RM. Nanoscale intracellular organization and functional architecture mediating cellular behavior. Ann Biomed Eng. 2006;34:102–113. doi: 10.1007/s10439-005-9008-1. [DOI] [PubMed] [Google Scholar]
- 19.Xu CW. High-density cell microarrays for parallel functional determinations. Genome Res. 2002;12:482–486. doi: 10.1101/gr.213002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann P, David G. The syndecans, tuners of transmembrane signaling. FASEB J. 1999;13:91–100. doi: 10.1096/fasebj.13.9001.s91. [DOI] [PubMed] [Google Scholar]
- 21.Bass MD, Humphries MJ. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signaling. Biochem J. 2002;368:1–15. doi: 10.1042/BJ20021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, et al. Regulation of syndecan-4 expression with mechanical stress during the development of angioplasty-induced intimal thickening. J Vasc Surg. 2002;36:361–370. doi: 10.1067/mva.2002.124364. [DOI] [PubMed] [Google Scholar]
- 23.Echtermeyer F, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita Y, et al. Syndecan-4 is expressed by B lineage lymphocytes and can transmit a signal for formation of dendritic processes. J Immunol. 1999;162:5940–5948. [PubMed] [Google Scholar]
- 25.Saoncella S, et al. Syndecan-4 signals cooperatively with integrins in a Rhodependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFall AJ, Rapraeger AC. Identification of an adhesion site within the syndecan-4 extracellular protein domain. J Biol Chem. 1997;272:12901–12904. doi: 10.1074/jbc.272.20.12901. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 28.Kubicek JD, Brelsford S, Ahluwalia P, LeDuc PR. Integrated lithographic membranes and surface adhesion chemistry for three-dimensional cellular stimulation. Langmuir. 2004;20:11552–11556. doi: 10.1021/la0487646. [DOI] [PubMed] [Google Scholar]
- 29.Li B, Chen J, Wang JH-C. RGD peptide-conjugated poly(dimethylsiloxane) promotes adhesion, proliferation, and collagen secretion of human fibroblasts. J Biomed Mater Res A. 2006;79:989–998. doi: 10.1002/jbm.a.30847. [DOI] [PubMed] [Google Scholar]
- 30.Jansen J, et al. Stretch-induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J Cell Biochem. 2004;93:542–551. doi: 10.1002/jcb.20162. [DOI] [PubMed] [Google Scholar]
- 31.Lei L, Chaikof EL. Mechanical stress regulates syndecan-4 expression and redistribution in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:61–68. doi: 10.1161/hq0102.100314. [DOI] [PubMed] [Google Scholar]
- 32.Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffold with physiologically relevant elastic moduli: Interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26:3123–3129. doi: 10.1016/j.biomaterials.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Fabry B, et al. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102-1–148102-4. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 34.Trepat X, et al. Universal physical responses to stretch in the living cell. Nature. 2007;447:592–595. doi: 10.1038/nature05824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer CJ, et al. Mechanical control of cyclic amp signaling and gene transcription through integrins. Nat Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- 36.Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;22:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 37.Kholodenko BN. Cell signaling dynamics in time and space. Nat Rev Mol Cell Biol. 2006;7:165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Numaguchi K, Eguchi S, Yamakawa T, Motley E, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Li S, et al. Fluid shear stress activation of focal adhesion kinase. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 40.Lallemand D, et al. Stress-activated protein kinases are negatively regulated by cell density. EMBO J. 1998;17:5615–5626. doi: 10.1093/emboj/17.19.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell respponse to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 42.Traub O, Berk BC. Mechanisms by which endothelial cells transduce an artheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:667–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.