Abstract
Anhedonia, the loss of pleasure or interest in previously rewarding stimuli, is a core feature of major depression. While theorists have argued that anhedonia reflects a reduced capacity to experience pleasure, evidence is mixed as to whether anhedonia is caused by a reduction in hedonic capacity. An alternative explanation is that anhedonia is due to the inability to sustain positive affect across time. Using positive images, we used an emotion regulation task to test whether individuals with depression are unable to sustain activation in neural circuits underlying positive affect and reward. While up-regulating positive affect, depressed individuals failed to sustain nucleus accumbens activity over time compared with controls. This decreased capacity was related to individual differences in self-reported positive affect. Connectivity analyses further implicated the fronto-striatal network in anhedonia. These findings support the hypothesis that anhedonia in depressed patients reflects the inability to sustain engagement of structures involved in positive affect and reward.
Keywords: anhedonia, emotion regulation, nucleus accumbens
Anhedonia, the loss of pleasure or interest in previously rewarding stimuli, is a hallmark of clinical depression (1). Anhedonia and/or a persistently depressed mood are required by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) for the diagnosis of major depressive disorder (MDD). While the exact biological bases of anhedonia are not known, research suggests that anhedonia may result, in part, from disruption of systems implicated in reward and motivation, which likely include the nucleus accumbens (NAcc) (2–4) and the frontostriatal network (5). Furthermore, human and nonhuman work suggest that the prefrontal cortex (PFC) influences activity in the striatum and can modulate reward-related firing in the NAcc in a top-down manner (5–8). Indeed, a recent review of the rodent literature by DelArco and Mora (5) makes a strong case for modulation of NAcc activity by efferent PFC neurons. This network has been proposed as one pathway by which anhedonia is instantiated (2, 3).
One hypothesis regarding the etiology of anhedonia is that depressed patients suffer from reduced hedonic capacity, defined as the total amount of positive affect that is possible for one to experience (9, 10). While many current depression researchers subscribe to the notion of reduced hedonic capacity, behavioral and physiological studies of individuals with anhedonia have been mixed as to whether anhedonic individuals actually show a reduction in their capacity to experience pleasure. Many studies have shown that individuals with depression or non-depressed individuals with the trait of anhedonia have decreased facial electromyography (EMG) zygomatic responses (11), reduced startle attenuation (12), and reduced self-reported experience of positive affect to positive stimuli (13). Other studies, however, have been unable to replicate these findings (14–16). Neuroimaging studies of individuals with anhedonia are also inconclusive. For instance, some studies show that depressed individuals or those with trait-like anhedonia display a lack of increase in NAcc activity when presented with pleasurable stimuli (17, 18). Yet, other studies have not found group differences in NAcc activity in MDD (19–22).
Such inconsistencies suggest the possibility that MDD reflects more than a simple reduction in the capacity to experience pleasure. As was proposed by Myerson (9), anhedonia may not be solely due to a tonic reduction in the capacity to experience pleasure, but an inability to sustain positive affect and reward responsiveness over time. The notion of sustainability of positive affect was more recently discussed by Tomarken and Keener (23) who hypothesized that the inability to sustain positive affect in depression may result from disordered positive emotion regulation. They suggested that effective up-regulation and down-regulation of positive emotion is necessary to experience positive affect over time. Indeed, in healthy individuals there is evidence suggesting that the regulation of positive affect involves biasing signals directed from PFC to the NAcc (6, 7). Collectively, this suggests the hypothesis that depressed individuals will exhibit difficulties using PFC to sustain NAcc activity over time, particularly when that activity occurs in the context of attempts to up-regulate or enhance positive affect.
Accordingly, we used functional magnetic resonance imaging (fMRI) data acquired from a sample of depressed (n = 27) and control (n = 19) individuals during an instructed emotion regulation paradigm to test whether depression reflects a deficit in the ability to sustain activity in structures involved in reward, motivation and positive affect over a 37-min scan session. Participants were instructed to use cognitive appraisal to “enhance” or “suppress” their emotional response to positive and negative images, or to simply “attend” to the stimuli in the absence of cognitive reappraisal. To investigate changes across the scan session, we modeled time in two ways. In our primary analyses, we split the 37-min scan session into two halves and examined the change in activation across those two halves. Secondary analyses treated time continuously across the six scan runs and examined the slope of change in activity across time. Thus, we were able to test several specific predictions. We first predicted that depressed patients will fail to sustain activity in the striatum (including the NAcc) when up-regulating affect in response to positive stimuli. To this end, we examined the weighted Group × Time interaction for the “enhance” vs. “suppress” contrast. Our initial analysis contrasted the “enhance” vs. “suppress” conditions for two reasons. First, this contrast compares changes in activity across time in the condition which putatively causes the most positive affect with the condition which causes the least positive affect. Second, contrasting the two active regulatory conditions accounts for the cognitive load produced by volitional emotion regulation (24). Our second prediction was that the deficit in sustaining engagement of the NAcc will be more pronounced when depressed patients were required to repeatedly regulate (or increase) their positive affect. To do this, we conducted a similar Group × Time analysis using the “enhance” vs. “attend” contrast. The third prediction was that individual differences in the ability to sustain activity in reward related regions would predict overall self-reported positive affect acquired outside the scanner. The fourth prediction was that the inability to sustain engagement of the NAcc would be related to attenuated connectivity between the NAcc and PFC.
Results
Depressed Individuals Fail to Sustain NAcc Activation When Amplifying Positive Affect.
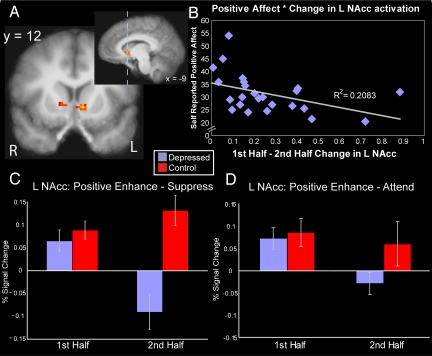
We first examined whether individuals with depression showed an inability to sustain activity in reward-related regions across the scan session when attempting to up-regulate positive affect. Supporting the failure to sustain positive affect hypothesis of depression, we observed a significant weighted group–by-time interaction (P < 0.05, corrected for multiple comparisons) in the NAcc (Fig. 1A; peak x, y, z: −9, 12, 0), such that the depressed group showed a decrease in the “enhance” vs. “suppress” contrast during the second half of the scan session only; the control group showed sustained activity in this region (Fig. 1C; Fig. 2). Follow-up pairwise contrasts supported this. The groups differed during the second half [t(43) = 4.22; P < 0.001], but not during the first half [t(43) = 0.717; P = 0.48]. In addition, the depressed group showed a reduction in activity from first to second half [t(25) = 3.09; P = 0.005], whereas the controls showed no change [t(18) = −1.37; P = 0.18]. Other regions showing an effect included the left insula/transverse temporal gyrus and right thalamus (see Table 1).*
Fig. 1.
Activation in nucleus accumbens (NAcc) shows specific decrease for individuals with depression. (A) Depressed show a specific decrease from first to second half of scan session (P < 0.05 corrected; k > 50 voxels) in the NAcc. (B) For depressed, less change in left NAcc activity is associated with greater self-reported positive affect. (C) Depressed patients show a decrease in NAcc activity, across time, in the Enhance vs. Suppress contrast. (D) Depressed patients show a decrease in NAcc activity, across time, in the Enhance vs. Attend contrast (error bars, standard error of mean).
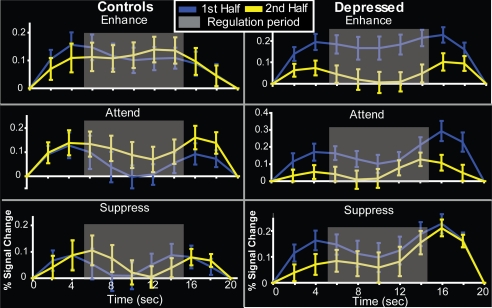
Fig. 2.
Time courses of activation in nucleus accumbens ROI for controls and depressed, first and second half of scan session for the “enhance,” “attend,” and “suppress” conditions. Gray box denotes regulation period.
Table 1.
Significant activations at P < 0.05 (corrected) for the weighted Group × Time interaction for enhance vs. suppress
| Location | Cluster maximum |
No. of voxels | Cluster volume, mm3 | F value | P value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L insula/transverse temporal gyrus | −53 | −17 | 14 | 99 | 792 | 16.62 | 9.8 × 10−5 |
| R thalamus | 1 | −13 | 9 | 70 | 560 | 17.31 | 7.2 × 10−5 |
| L nucleus accumbens | −9 | 12 | 0 | 65 | 520 | 18.78 | 3.8 × 10−5 |
Data correspond to a P value threshold of 0.005 (k > 50 voxels). L, left; R, right; the x, y, z coordinates use the Talaraich system. F and P values correspond to the peak.
Second, we examined the hypothesis that the deficit in sustaining engagement of the NAcc will be more pronounced when depressed patients were required to repeatedly up-regulate their positive affect. To do this, we assessed whether changes in the NAcc cluster found in the first step showed a similar weighted Group × Time effect for the more conventional “enhance” vs. “attend” contrast. Indeed, this test was significant [F(1, 88) = 8.56; P = 0.004], and follow up tests indicated a trend for the groups differing during the second half [t(43) = 1.74; P = 0.089], but not during the first half [t(43) = 0.324; p = 0.747]. In addition, the depressed group showed a reduction in activity from first to second half [t(25) = 2.60; P = 0.015], whereas the controls showed no change [t(18) = 4.64; P = 0.65]. This indicates that this result was not specific to our choice of control condition. We also performed this contrast in a map-wise fashion; for other significant regions, see Table S1. In addition, we performed the above two analyses in which time was modeled continuously (as a function of run) with an amplitude modulator. Within the NAcc cluster found in the first step and using the “enhance” vs. “suppress” contrast, the depressed group showed a significantly greater linear reduction across time than the controls (at P < 0.005) in the NAcc, providing further evidence that NAcc activity habituates in a linear fashion across time in depressed patients. In an analysis using the “enhance” vs. “attend” contrast, a similar pattern emerged, albeit at a lower threshold (P < 0.025). These results corroborate our findings based on splitting the session into halves and suggest that the decreased engagement of NAcc by the depressed patients occurred in a linear fashion.
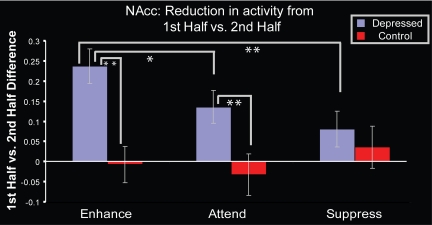
As a way of integrating these results, we performed a follow-up trend analysis using the NAcc cluster from the first analysis to examine whether the reduction in NAcc activity across time was linearly related to the amount of positive affect putatively produced by condition (defined as the greatest decrease across scan session for the “enhance,” followed by the “attend” condition, and the least decrease during the “suppress” condition). Using change in activity from first half to second half as the dependent variable, the Group × Condition (“enhance,” “attend,” “suppress”) interaction was significant [F(2, 42) = 5.01; P = 0.01]. Simple effects tests showed that for the MDD group, change in NAcc activity was linearly related to regulation instruction [F(1, 76) = 6.77; P = 0.01] such that the greatest change in NAcc activity occurred in the condition which putatively engendered the most positive affect; for the control group, change in NAcc activity was not linearly related to regulation instruction [F(1, 55) < 1; P = 0.54; see Fig. 3].
Fig. 3.
For depressed patients, the greatest decrease in NAcc activity across time occurs to the enhance condition. Greater values indicate a greater decrase in activation from first to second half. Across the three conditions, the Group × Condition interaction was significant [F(2, 42) = 5.01; P = 0.01]; the MDD group also showed a significant linear trend across conditions [F(1, 76) = 6.77; P = 0.01]; controls did not (F < 1). Pairwise t tests for the MDD group were significant in the Enhance vs. Attend [t(25) = 2.60; P = 0.015] and Enhance vs. Suppress [t(25) = 3.09; P = 0.005] contrasts, there were no significant pairwise contrasts for the controls. Significant group differences were also found in the Enhance [t (43) = −3.86; P < 0.001] and Attend [t(43) = −2.59; P = 0.01] conditions such that the MDD group showed a greater decrease in NAcc activity from first to second half than controls. Error bars indicate SEM. *, P < 0.05; **, P < 0.01.
To examine change in NAcc activity across time in the absence of effortful emotion regulation), we investigated whether the mean activity in the NAcc cluster defined in the first analysis also evinced a significant Group × Half (first and second half) interaction in the “attend” vs. baseline contrast. The NAcc cluster did show a significant interaction [F(1, 43) = 6.71; P = 0.013] and follow up t tests showed that the depressed group showed a specific reduction in NAcc activity in the second half of the experiment [t(25) = 3.325; P = 0.003]; controls showed no change [t(18) = −0.63; P = 0.536; see Table S2 for whole brain effects].
We further tested the hypothesis that sustainability of activity in the NAcc during the enhance condition can reliably differentiate between depressed patients and healthy controls. Given recent discussions in the neuroimaging literature (e.g., 25), we wish to emphasize that this analysis is meant to be descriptive in nature, with the intention of quantifying the consistency of this finding across participants, and not as a separate novel test. To do this, we performed a linear discriminant analysis to show that the ability to sustain NAcc activation was able to significantly classify depressed patients [81% of MDD group, and 68% of controls were correctly classified; F(1, 44) = 13.22; P = 0.001]. By contrast, NAcc activation averaged across time was not predictive of group, [F(1, 44) < 1; P = 0.434].
Deficits in Sustaining Activity in the NAcc Is Specific to Positive Emotion.
We further examined whether the changes in NAcc activity in the depressed group were specific to trials on which positive stimuli were presented. To test this, we created an “enhance” vs. “suppress” and “enhance” vs. “attend” contrast map for each subject (for both the first and second half of the scan session) for trials on which negative stimuli were presented. We then performed a weighted ANOVA to examine whether decreased NAcc activity in the second half of the scan session occurred only during up-regulation of positive and not negative affect in the MDD group. As predicted, a cluster in the NAcc, overlapping the cluster found in the previous analysis was significant at P < 0.005, indicating that reductions found in NAcc across the scan session are specific to positive stimuli.
In addition, taking the mean percent signal change across the entire NAcc cluster found in the first analysis, we tested whether changes in NAcc activity across the scan session during the positive “attend” condition was also present during the “attend” condition when negative stimuli were presented. For the MDD group only, there was a significant Valence × Half (first and second half) interaction [F(1, 25) = 6.55; P = 0.017]. Follow-up paired t tests revealed that NAcc activity decreased across the scan session during the positive “attend” [t(25) = 3.325; P = 0.003], but not the negative “attend” [t(25) = 0.597; P = 0.57] condition.
Patients Who Fail to Sustain NAcc Activity Report Less Intense Positive Emotion.
We next used a highly reliable and well-validated measure of self-reported positive affect to assess whether the failure to sustain ventral striatal activity in depression is related to the conscious experience of positive emotion. To do this, we examined relations between overall, self-reported positive affect [as assessed by the PANAS (26)] with activity changes from the NAcc cluster across time. For the depressed group only, less decrease in NAcc activity across time in the enhance condition predicted greater overall self-reported positive affect (r = −0.46; P = 0.019; Fig. 1B). In the positive “enhance” condition, those depressed individuals with a sharper decline of activity in the NAcc across the two scan session halves reported experiencing less overall positive affect. This relationship was not driven by any outliers as confirmed by a Spearman's rho test (ρ = −0.538; P = 0.005). These relations were valence-specific, as similar relations were not found for self-reported negative affect (r = 0.05; P = 0.81). These relations were also specific to the sustainability of activity in the NAcc, as aggregated activity across the scan session to the positive enhance condition was not related to self-reported positive affect (r = 0.05; P = 0.82). In fact, the correlation between self-reported positive affect and sustainability of NAcc activity was significantly greater than the correlation between self-reported positive affect and aggregated NAcc activity [t(25) = −2.095; P = 0.047]. This provides further evidence that it is the specific ability to sustain activity in the NAcc across time that is related to general levels of self-reported positive affect.
Difficulties Sustaining NAcc Activation Reflect Reduced Prefrontal Connectivity.
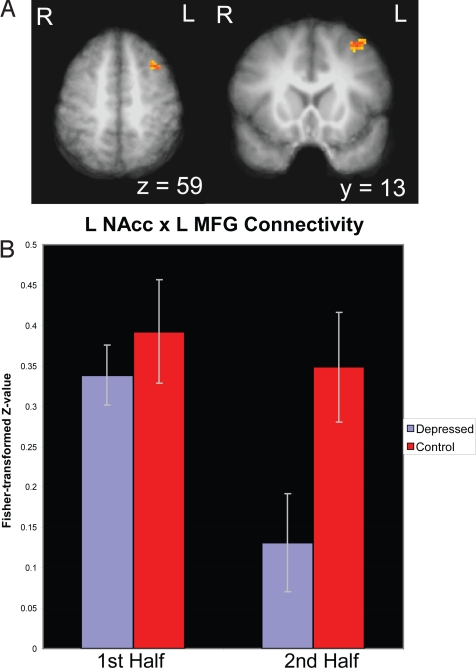
We then tested whether depressed individuals' inability to sustain NAcc activity during the positive enhance condition reflected a failure to engage regions of PFC implicated in emotion regulation. To this end, we performed a connectivity analysis using the significant cluster in left NAcc as the seed region. Consistent with our prediction, a prefrontal cluster [BA 8; peak x, y, z: −37, 13, 59; in the left middle frontal gyrus (MFG)] (Fig. 4 and Table S1) showed a significant Group × Time interaction (P < 0.05; small volume corrected), such that a reduction in connectivity with the NAcc across time was specific to the depressed group. This suggests that the reductions in activity in the NAcc may be driven, in part, by disordered connectivity with the left MFG.
Fig. 4.
Connectivity analysis indicating reduction of LNAcc/L MFG connectivity as a function of time (P < 0.05, small volume corrected; k > 15 voxels). Error bars indicate SEM.
Analyses Controlling for Task Engagement.
It was possible that depressed individuals' inability to sustain positive affect simply reflects nonspecific group differences in task engagement or motivation to regulate. If so, depressed individuals would be expected to show parallel differences in measures sensitive to motivation and workload, such as reaction time (RT) or pupil dilation. In particular, over the scan session, one would predict that depressed individuals would be slower to indicate the valence of the image, reflecting their diminished engagement or greater fatigue. A two-way Group × Time (first vs. second half) ANOVA of RT to the positive images revealed no significant interaction (F < 1). There was also no Group × Time interaction when RT to all images was used (F < 1). Another objective measure of motivation is that of changes in pupil dilation, which reflect total cognitive effort (27, 28). A two-way Group × Time (first vs. second half) ANOVA of pupil dilation occurring during the enhance condition revealed no significant interaction (F < 1), suggesting that changes in NAcc activity in the depressed group are specific to affect and not to motivation or attention.
Discussion
Anhedonia is a hallmark symptom of MDD and elucidating the core neural signatures and processes of anhedonia is necessary for a more complete understanding and treatment of the disorder (29). Given that the NAcc and fronto-striatal network have been implicated in reward processing (30–32) and positive emotion regulation (7), we hypothesized these networks to be involved in the disordered regulation of positive affect characteristic of anhedonia. In addition, due to inconsistencies in the depression literature, we tested the hypothesis that anhedonia reflects, in part, an inability to sustain positive affect across time.
FMRI results supported our hypotheses. First, while attempting to up-regulate positive emotion, individuals with MDD showed a specific decrease in activation in the NAcc across time, while control subjects maintained their level of activation. Depressed participants were also unable to sustain elicitation of NAcc engagement when simply attending to their emotional response to positive images. Furthermore, depressed patients displayed the greatest decreases in NAcc activity across time in those conditions which putatively engendered the most positive affect. The implication of this finding is that it is more difficult for depressed patients to sustain NAcc engagement in contexts during which the maintenance and/or up-regulation of positive affect is expected. Second, we demonstrated that the amount of decrease in NAcc activity across time predicted overall self-reported positive affect. Lastly, a connectivity analysis revealed that the inability to sustain activity of NAcc may result from a breakdown in connectivity with left MFG. Collectively, these results suggest that disordered positive emotion regulation may result from an inability to sustain activity in the fronto-striatal network which leads to abnormalities in reward processing and reductions in positive affect.
Our laboratory previously published data from these same patients examining the neural correlates of cognitive emotion regulation in response to negatively valenced stimuli (33). In comparison to controls, the MDD patients displayed abnormalities in distinct networks when regulating negative as opposed to positive affect. However, both studies found abnormalities in the PFC. The fact that in depression, the PFC appears to be abnormally engaged in both positive and negative emotion regulation contributes to a growing body of work that suggests that depressed patients may have difficulty recruiting prefrontal resources to regulate subcortical structures involved in affect.
Researchers working at the nonhuman level work have found that the NAcc responds differentially to the anticipation vs. consumption of reward (34), suggesting that differentiating these phases of reward processing in depression is theoretically important. A recent publication by Pizzagalli and colleagues (35) sheds light on this issue. Patients engaged in a monetary incentive delay task in which they pressed a button in response to a target stimulus. Group differences in basal ganglia activity were weak during the anticipation period, but robust group differences were found in the caudate and NAcc during the consummatory phase of the trial. While a rich nonhuman literature underscores the complexity of the NAcc in reward processing (34), the findings of Pizzagalli and colleagues suggest that the inability to sustain NAcc activity found here may result from specific deficits in the consummatory phase of reward processing—which rely heavily on the ventral striatum. Indeed, previous research suggests that NAcc activity tracks the hedonic value of outcomes (36, 37). It may be the case, therefore, that depressed patients have difficulty sustaining the engagement of the NAcc in response to tasks that require both the effortful heightening and maintaining of positive affect.
The clinical implications of our findings suggest that a treatment regimen which attempts to increase the depressed patient's ability to sustain engagement of the NAcc may ameliorate anhedonic symptoms. Because NAcc activation has been linked to reward and motivation, training depressed individuals to sustain engagement with tasks which may activate the NAcc might be able to be used in clinical practice. Indeed, the behavioral therapy model for depression explicitly instructs depressed patients to increase the length of time spent performing rewarding activities (38). Because outcome studies have supported the clinical efficacy of the behavioral model (39), we would predict that one component of recovery from depression may be the ability to sustain engagement of the brain circuits implicated in reward and motivation.
Additionally, because both positive and negative stimuli were presented, it is important to be clear about the implications of these findings. Our study examines the ability of depressed patients to sustain engagement of the NAcc while enhancing positive affect in response to positive images embedded within a stream of stimuli that included both positive and negative images. This is a very important point, particularly with regard to the ecological validity of these findings. Namely, in everyday life, individuals do not generally encounter uninterrupted positive stimuli. Negative experiences often intermix with positive ones, and the ability of individuals to heighten and maintain positive affect in the face of negative stimuli is vitally important for health and well-being.
Lastly, an important issue that requires further delineation is distinguishing the neural substrates underlying the symptoms of anhedonia versus those of decreased motivation and psychomotor retardation seen in depression. A large nonhuman literature has suggested that the NAcc is involved in motivated, goal-directed behavior in addition to its contribution to reward processing (40, 41). Yet it is not entirely clear to what degree disordered firing in the NAcc could also be relevant to other MDD symptoms of psychomotor retardation and reduced motivation. An important challenge for future work will be to disentangle the role of the NAcc in specific symptomatology associated with MDD including anhedonia, psychomotor retardation, and decreased motivation.
In conclusion, our findings suggest that individuals with depression suffer from an inability to sustain reward-related activity that is reflected in the fronto-striatal network across time, and that this deficit is associated with reduced positive affect. These findings are consistent with the hypothesis that the hallmark symptoms of anhedonia in MDD are based on an inability to sustain positive affect. These data offer an interpretation for the symptom of anhedonia in depression. The findings also underscore the need for future studies to investigate the temporal dynamics of positive affect in depression and underscore the important role of affective chronometry in understanding the mechanistic bases of affective style (42).
Materials and Methods
Participants.
We examined a group of 27 medication-free, right handed adults satisfying the DSM-IV (1) criteria for unipolar major depressive disorder (age range, 19–53; mean age, 31.48 years; SD, 11.58; 12 males). These depressed individuals were compared with an age and sex-matched group of 19 right-handed controls (age range, 20–60 years; mean age, 31.84 years; SD, 14.65; 9 males; age difference between groups not significant; F < 1). All subjects were recruited via the use of flyers posted in public places around the Madison, Wisconsin area. Depressed participants had depressive symptoms for at least 1 month before their screening visit and a score of at least 18 on the Hamilton Rating Scale for Depression (43) (HAM-D) at screen and the fMRI scan (mean HAM-D ± SD depressed, 20.6 ± 2.39; controls, 1.2 ± 1.6). In addition to standard MRI compatibility criteria, subjects were screened for and excluded if they met DSM-IV criteria for alcohol or drug abuse or dependence, other DSM-IV Axis I or Axis II diagnoses, had a personal or family history of bipolar disorder or were using any medications that affect CNS function. Participants were also excluded if they had an anxiety disorder, ensuring that the sample represent a relatively “pure” MDD group. In addition to the HAM-D, both before and after the scan session, all participants except one depressed subject completed the extended version (48 total items) of the Positive Affect/Negative Affect scales (26) (PANAS-X) and was therefore removed from the analysis. The pre- and post-scan PANAS scores were then averaged to ensure reliable self-reported affect scores. Subjects participating in this study are the same as those who participated in (33). This research was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board, and all participants provided written informed consent.
Task.
The experimental task was a variant of that used previously in our laboratory with normal subjects (27) and similar to the tasks used in other recent studies (44–46). Subjects were scanned while viewing a sequence of 72 emotionally positive and 72 negative pictures taken from the International Affective Picture System (IAPS) (47). A 1-s fixation cross coupled with a tone oriented subjects to the upcoming trial, after which each image was presented for 10 s, followed by a 6-s blank screen. To ensure participants remained attentive to the task, at the onset of each picture, subjects had to judge whether the image was positive or negative and respond with an appropriate button press on a two-button response pad. Four seconds into the presentation of each picture, an audio prompt instructed the participant to either increase (“enhance”) or decrease (“suppress”) their emotional response to the picture or to continue to “attend” to the picture. Participants were trained during a previous session while positioned inside a mock scanner on the use of cognitive re-appraisal strategies to re-evaluate the images as more or less emotional (27, 44). For the enhance condition, participants were trained to either imagine themselves or a loved one experiencing the situation being depicted or imagine a more extreme outcome than the one depicted (e.g., in response to a picture of a stunning natural scene, a participant might imagine being in that scene or one of their own choosing). Conversely, for the suppress condition, individuals were trained to either view the situation as fake or unreal or imagine that the situation being depicted had a different outcome than the one suggested (e.g., a couple in love were just actors and did not feel the way depicted in the image). Alternatively, on attend trials, participants were instructed to maintain their attention to the picture without changing their affective experience. Simulated scanner sounds and task instructions were presented using earbud headphones during this training session. The training was succeeded by follow-up queries to ensure that participants were using the strategies as instructed and reported being able to perform the task. See SI Text for additional detail.
In the fMRI session, there were 24 repetitions of each regulation condition and 12 repetitions of the attend condition for each picture valence, evenly distributed over six scans, each lasting 380 s. The order of positive and negative images and the three regulation instructions was pseudorandomized such that each scan run contained at least one trial of each condition type. With regard to order of positive and negative trials, positive trials were preceded by other positive trials approximately as often as by negative trials (42% of the positive trials were preceded by a positive trial and 57% of the positive trials were preceded by a negative trial; χ2 = 1.37; P = 0.241), suggesting that the results are not due to the lingering effects of negative stimuli on BOLD response to positive stimuli.
Behavioral Measures.
See SI Text.
Image Analysis.
See SI Text for additional details. Individual subject data were slice-time corrected, motion corrected, and analyzed in AFNI (48). Our GLM included covariates intended to model each of the six trial types (positive/negative stimulus; enhance, attend, and suppress reappraisal instruction), and for both the early and late phases of the scanning session (early: runs 1–3; late: runs 4–6) as well as six motion estimate covariates (49).
These smoothed contrast maps were then entered into a multiple linear regression analysis, in which contrasts were weighted to yield those brain areas that displayed a depressed group-specific decrease in activation across time. Specifically, our weights were as follows: first half-control (+1); second half-control (+1); first half-depressed (+1); second half-depressed (−3). The outcome of this analysis is essentially a weighted Group × Time interaction for the positive “enhance” vs. positive “suppress” as well as the “enhance” vs. “attend” condition contrast. To examine time as a continuous variable, we performed a second analysis in which we modulated the amplitude of each run in a linear fashion. Connectivity analyses were performed using the beta series correlation method described in (50). More details on this method can be found in the SI Text.
Univariate statistical maps were thresholded at P < 0.05, corrected for multiple comparisons using cluster-size thresholding based (k > 50 voxels) on Monte Carlo simulation (the AlphaSim program in AFNI) using a whole-brain mask. The connectivity statistical maps were thresholded at P < 0.05, small volume corrected for multiple comparisons using a PFC mask which included clusters found to be significant in a recent metaanalysis of fMRI studies of emotion regulation (51) and resulted in clusters exceeding a minimum of k = 15 voxels. For further details see the SI Text.
Supplementary Material
Acknowledgments.
We thank Michael Anderle, Ron Fisher, Carien van Reekum, Heather Urry, and Claire Wahmanholm for assistance and Lyn Abramson, Heleen Slagter, Melissa Rosenkranz, Brendon Nacewicz, and Andrew Fox for helpful comments. This work was supported by National Institutes of Health Grants: P50 MH069315, R01 MH043454-19 (to R.J.D.), Wyeth-Ayerst Pharmaceuticals (to N.H.K.), grants from the Fetzer Institute, the John W. Kluge Foundation and the Impact Foundation; and gifts from Bryant Wangard, Ralph Robinson, and Keith and Arlene Bronstein.
Footnotes
Conflict of interest statement: N.H.K. is a consultant related to the development of psychotropic agents (or serves on the Scientific Advisory Board) for the following companies: Astra Zeneca, Bristol-Myers-Squibb, CeNeRx Biopharma, Corcept, Cyberonics, Forest Laboratories, General Electric Corp., Jazz Pharmaceuticals, Eli Lilly, Neuronetics, Sanofi Syntholabs, and Wyeth Pharmaceuticals (which funded this study). N.H.K. has stock options in Corcept and CeNeRx and is principal owner of Promoter Neurosciences.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910651106/DCSupplemental.
Moreover, each of these effects remained significant after controlling for activity in the NAcc cluster on the negative trials.
References
- 1.American Psychiatric Association. Diagnostic Criteria from DSM-IV-TR. Arlington, VA: Am Psychiatric Assoc; 2000. p. xii. [Google Scholar]
- 2.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay LK, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 5.Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 8.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Myerson A. Anhedonia. Am J Psychiatry. 1922;2:87–103. [Google Scholar]
- 10.Meehl PE. Hedonic capacity: Some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 11.Sloan DM, Bradley MM, Dimoulas E, Lang PJ. Looking at facial expressions: Dysphoria and facial EMG. Biol Psychol. 2002;60:79–90. doi: 10.1016/s0301-0511(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 12.Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- 13.Fiorito ER, Simons RF. Emotional imagery and physical anhedonia. Psychophysiology. 1994;31:513–521. doi: 10.1111/j.1469-8986.1994.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaviani H, et al. Affective modulation of the startle response in depression: Influence of the severity of depression, anhedonia, and anxiety. J Affect Disord. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Germans MK, Kring AM. Hedonic deficit in anhedonia: Support for the role of approach motivation. Pers Individ Dif. 2000;28:14. [Google Scholar]
- 16.Berlin IG-S, L., Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison to healthy subjects. Eur Psychiatry. 1998;13:7. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 17.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Epstein J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 19.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry. 2006;60:974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Mitterschiffthaler MT, et al. Neural response to pleasant stimuli in anhedonia: An fMRI study. Neuroreport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- 22.Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 23.Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cogn Emot. 1998;12:34. [Google Scholar]
- 24.Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 27.Urry HL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- 29.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatric Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutson B, Wimmer GE. Splitting the difference: How does the brain code reward episodes? Ann NY Acad Sci. 2007;1104:54–69. doi: 10.1196/annals.1390.020. [DOI] [PubMed] [Google Scholar]
- 31.Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 32.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 33.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 35.Pizzagalli DA, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 37.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 38.Lewinsohn PM. In: The Psychology of Depression: Contemporary Theory and Research. Friedman RJ, Katz MM, editors. New York: Wiley; 1974. p. 30. [Google Scholar]
- 39.Hoberman HM, Lewinsohn P.M. In: Handbook of Depression: Treatment, Assessment, and Research. Beckham EE, Leber WR, editors. Homewood, IL: Dorsey; 1985. p. 43. [Google Scholar]
- 40.Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 41.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 42.Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- 45.Schaefer SM, et al. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cognit Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- 46.Ochsner KN, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Lang PJ, BM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Gainesville, FL: Univ of Florida; 2005. Technical Report A-6. [Google Scholar]
- 48.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 49.Johnstone T, et al. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 51.Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neurosci Biobehav Rev. 2009 doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 53.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 54.Petersson KM, Nichols TE, Poline JB, Holmes AP. Statistical limitations in functional neuroimaging. II. Signal detection and statistical inference. Philos Trans R Soc London Ser B. 1999;354:1261–1281. doi: 10.1098/rstb.1999.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.