Abstract
Several snake venom secreted phospholipases A2 (sPLA2s) including OS2 exert a variety of pharmacological effects ranging from central neurotoxicity to anti-HIV activity by mechanisms that are not yet fully understood. To conclusively address the role of enzymatic activity and map the key structural elements of OS2 responsible for its pharmacological properties, we have prepared single point OS2 mutants at the catalytic site and large chimeras between OS2 and OS1, an homologous but non toxic sPLA2. Most importantly, we found that the enzymatic activity of the active site mutant H48Q is 500-fold lower than that of the wild-type protein, while central neurotoxicity is only 16-fold lower, providing convincing evidence that catalytic activity is at most a minor factor that determines central neurotoxicity. The chimera approach has identified the N-terminal region (residues 1–22) of OS2, but not the central one (residues 58–89), as crucial for both enzymatic activity and pharmacological effects. The C-terminal region of OS2 (residues 102–119) was found to be critical for enzymatic activity, but not for central neurotoxicity and anti-HIV activity, allowing us to further dissociate enzymatic activity and pharmacological effects. Finally, direct binding studies with the C-terminal chimera which poorly binds to phospholipids while it is still neurotoxic, led to the identification of a subset of brain N-type receptors which may be directly involved in central neurotoxicity.
Secreted phospholipases A2 (sPLA2s) comprise a large family of structurally conserved enzymes that catalyze the hydrolysis of glycerophospholipids at the sn-2 position to release free fatty acids and lysophospholipids. These enzymes were first discovered in insect and snake venoms, and subsequently in mammalian tissues, plants, bacteria, fungi and viruses (1–6). Most sPLA2s share a common set of structural features including a relatively small molecular mass (14–19 kDa), a compact structure with several disulfides, and a conserved Ca2+-dependent catalytic mechanism.
To date, up to 12 different sPLA2s that belong to three main structural collections have been identified in mammals (1, 7–9). Despite an important knowledge accumulated at the molecular level (1, 9–11), the exact biological functions of most mammalian sPLA2s remain to be elucidated. Known functions include lipid digestion, host defence and lipid mediator production during normal and inflammatory processes. Some of these biological roles have been attributed to the first identified sPLA2s, namely the group IB, IIA, V and X enzymes. Almost nothing is known about the biological functions of the more recently identified sPLA2s IID, IIE, IIF, III, XIIA and XIIB (8, 12).
Much more is known about the pharmacological effects of snake venom sPLA2s. More than 280 venom enzymes have been reported over several decades (http://sdmc.lit.org.sg/Templar/DB/snaketoxin_PLA2/index.html.), and many of them display a wide spectrum of toxic effects including neurotoxic, myotoxic, cardiotoxic, cytotoxic, anticoagulant, convulsant, hypotensive, and pro-inflammatory effects (2, 13–16). In vitro, they can also modulate cell migration and cell proliferation (17, 18), have antibacterial activity (19), and display potent inhibitory effects against HIV-1 (20) and Plasmodium falciparum, the most deadly malaria parasite (21). Because of this tremendous array of pharmacological effects, it is possible that mammalian sPLA2s may exert much more diverse functions than those currently known (22).
Although many venom sPLA2s share similar structural features and catalytic activity, not all venom enzymes exhibit all of the above pharmacological effects (2). Furthermore, some venom sPLA2s have no catalytic activity while they exert various toxic and pharmacological effects (14, 16, 18–20). The absence of direct correlation between catalytic activity and pharmacological effects has led to the hypothesis that the specific actions of sPLA2 is due to the presence of pharmacological sites on the sPLA2 surface which are separated from or overlap with the catalytic site. These pharmacological sites would allow the sPLA2 to bind specifically to soluble proteins or membrane-bound proteins that participate to the sPLA2 mechanism of action (23, 24). Since this hypothesis was proposed, a collection of binding proteins has been identified using several toxic snake venom sPLA2s (25–28). Besides β-bungarotoxin (29), early studies with the neurotoxic sPLA2 OS2 from the Australian Taipan snake Oxyuranus scutellatus scutellatus have led to the identification of two families of binding proteins called N- and M-type receptors (25, 30). The N-type receptors bind OS2 with picomolar affinities, are present in mammalian brain and other tissues, and are made up of multiple proteins with molecular masses of 18–24 kDa, 36–51 kDa and 85 kDa. Other neurotoxic sPLA2s bind to the N-type receptors with high affinities while non toxic sPLA2s including OS1 bind with much lower affinities, suggesting that these receptors are involved in sPLA2 central neurotoxicity. Conversely, the M-type receptor, which binds with high affinity both toxic and non toxic sPLA2s including OS2 and OS1, consists of a single protein of 180 kDa, and belongs to the C-type lectin superfamily (25). Importantly, the M-type receptor binds several mammalian sPLA2s (31, 32), suggesting that these proteins are the endogenous ligands for this receptor, and possibly for the collection of binding proteins initially identified with venom sPLA2s. Binding studies with ammodytoxins from the long-nosed viper Vipera ammodytes ammodytes have also led to the identification and characterization of several proteins in the brain that appear related to N- and M-type receptors ((27) and references therein).
Despite the identification of the above binding targets and many structure-function studies based on amino acid modification, site-directed mutagenesis and structural comparison (33–36), the molecular mechanisms underlying many of the pharmacological actions of venom sPLA2s are not yet fully understood (2, 14, 37, 38). In particular, the neurotoxicity of venom sPLA2s, which has been one of the most studied pharmacological effects, is currently thought to involve both catalytic activity and binding to target proteins on presynaptic membranes (39, 40). The assumption that enzymatic activity is required for neurotoxicity is however essentially based on old studies showing that alkylation of the active site histidine of several neurotoxic sPLA2s by p-bromo-phenacyl-bromide substantially decreases their neurotoxicity (41–43). However, there was a greater loss of enzymatic activity than neurotoxicity, suggesting that other factors must also contribute to neurotoxicity. The fact that alkylation of different sPLA2s by p-bromo-phenacyl-bromide leads to conformational changes at the surface of the enzyme (44–46) raises the possibility that the loss of neurotoxicity may not be due to a decrease of catalytic activity per se, but rather to an altered ability of the enzyme to interact with high affinity with its specific binding protein target on neuronal membranes. On the other hand, significant efforts have been made to identify the sPLA2 pharmacological site involved in neurotoxicity, but its exact location still remains ill-defined (2, 33, 35, 47). In the case of ammodytoxins, a series of structure-function studies have shown that residues located at both the N-terminal and C-terminal regions of these sPLA2s are important for central neurotoxicity ((47) and references therein).
As indicated above, OS2 has initially served as a key tool to identify the N- and M-type sPLA2 receptors (25, 30). It is highly neurotoxic in mice and rats (30, 48), blocks acetylcholine release in Aplysia neurons (49), triggers cell migration (17), potentiates proinflammatory cellular signaling (50), and we report here that it can also affect chick neuromuscular transmission, be myotoxic, and exert anti-HIV and antimalarial activities in vitro. The main purpose of this work was thus to determine more conclusively the role of catalytic activity, to identify the location of the OS2 pharmacological sites responsible for central neurotoxicity and other pharmacological effects, and to analyze the relationship with M-type and N-type receptors. Based on the accumulated knowledge on sPLA2 catalytic activity and the complexicity of the reaction (51), we reasoned that the best way to probe the role of enzymatic activity was to produce OS2 derivatives with single point mutations in the active site, rather than on the interfacial binding surface of the sPLA2. To determine the location of the pharmacological sites on OS2 and explain the large difference in neurotoxicity between OS2 and the structurally-related OS1, we prepared large chimeras between the two proteins by grafting the divergent N-terminal, central and C-terminal regions of OS1 onto the OS2 structure.
EXPERIMENTAL PROCEDURES
Construction of the OS2 synthetic gene and mutants
A number of considerations have been taken into account for the production of OS2. First, as we could not obtain mRNA from the venom gland of the Australian Taipan snake, we constructed a synthetic gene for OS2 based on the sequence of the venom protein (52). Second, since our experience indicates that some sPLA2s cannot be easily refolded when produced in E. coli while they can be obtained in a native form from insect cell systems and vice-versa (11, 31), we tried expression of OS2 in both S2 Drosophila cells and E. coli. Finally, since we found that the use of E. coli preferred codons is not critical when the sPLA2 is fused to the N-terminal sequence of proteins such as glutathione S-transferase or thioredoxin (unpublished data and (53)), we designed the OS2 synthetic gene using Drosophila preferential codons (54) that should allow expression in both insect cells and E. coli. The synthetic cDNA sequence (available on request) was designed using the backtranslate program from the Genetic Computer Group and assembled from three sets of six partially overlapping oligonucleotides (40-mer) in a three-step PCR process using Dynazyme polymerase (Finnzyme laboratories). In the first round of PCR (final volume 20 µl), each set of oligonucleotides (5 µg each, 3 forward and 3 reverse, 20-mer complementary sequences) was annealed (60 °C/20 s), elongated (72 °C/20 s) and denaturated (94 °C/20 s) for 25 cycles. The DNA assemblies were further amplified in a second round of PCR (25 cycles, each 94 °C/20 s, 60 °C/20 s, 72 °C/20 s) using the two external oligonucleotides. The three PCR products were purified on agarose gel and used for a last round of PCR using the two oligonucleotides flanking the full OS2 synthetic gene. The final PCR product was tailed with Taq polymerase, subcloned into the pGEM-T easy vector (Promega Corp.) and sequenced. The OS2 cDNA was subcloned in frame into the S2 expression vector harboring the human group IIA sPLA2 signal peptide or into the pAB3 vector (11). Point mutants were prepared in a two-step PCR standard procedure (55) using complementary primers containing the mutation and primers flanking the OS2 synthetic gene inserted into the pAB3-OS2 vector. Chimeras were constructed by inserting by PCR synthetic DNA fragments coding for the N-terminal, central and C-terminal regions of OS1 (Fig. 1) into the pAB3-OS2 construction. All the final PCR products were entirely sequenced.
Fig. 1.
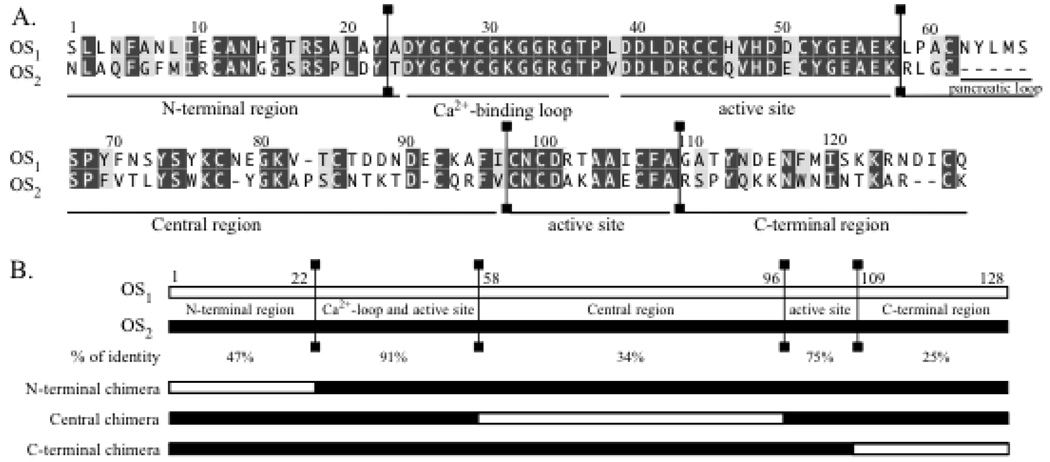
Alignment of the amino acid sequences of OS1 and OS2 and schematic representation of the chimeras between OS1 and OS2. (A) Alignment of the amino acid sequences of OS1 and OS2 (52). (B) Schematic representation of the chimeras between OS1 and OS2. The conserved and divergent regions between OS1 and OS2 are separated by vertical lines in panels A and B. The percentage of identity between the different regions are indicated. The N-terminal (1–22), central (58–96) and C-terminal (109–128) regions of OS1 have been introduced into the OS2 sequence to construct N-terminal, central and C-terminal chimeras, respectively.
Production of recombinant OS2 mutants and chimeras in Drosophila S2 cells and E. coli
Initial attempts to express OS2 in Drosophila S2 cells were unsuccessful, although we used a protocol similar to the one used for several mouse and human sPLA2s (11). Conversely, wild-type (WT) OS2, single point mutants and chimeras could be produced in E. coli using the pAB3 vector (11). The sPLA2s were produced as insoluble C-terminal fusion proteins with the truncated glutathione S-transferase (8.7 kDa) and the factor Xa/trypsin proteolytic site (Ile-Glu-Gly-Arg) located before the N-terminal residue of the mature sPLA2. Expression of fusion proteins as inclusion bodies in E. coli BL21 cells and sulfonation from 100 mg of protein were performed as described (56). For refolding, the sulfonated protein was dissolved in 500 ml of 6 M guanidine-HCl, 50 mM Tris-HCl pH 8.0 and dialyzed (8 kDa membranes, Spectrum laboratories) against 8 liters of 0.7 M guanidine-HCl, 50 mM Tris-HCl pH 8.0, 5 mM EDTA and 5 mM L-cysteine (5 mM L-methionine was added for the central and C-terminal chimeras to prevent methionine oxidation) for 48 h at 4 °C. For the N-terminal chimera the sulfonated protein was resuspended in 500 ml of 8 M urea, 0.1 M NH4Cl, 50 mM Tris-HCl pH 8.0 and refolded by dialysis against 8 liters of 1.6 M urea, 0.1 M NH4Cl, 50 mM Tris-HCl pH 8.0, 5 mM CaCl2 and 5 mM L-cysteine for 48 h at 4 °C. The refolded protein was then dialysed against 8 liters of 50 mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM CaCl2 and clarified by centrifugation (10,000 g, 30 min). The protein solution was subjected to trypsin digestion (1:30 trypsin:protein) which was monitored online by E. coli enzymatic assays (11) and mass spectrometry, and the reaction was stopped by adding 1% acetic acid and 1 mM phenylmethylsulfonyl fluoride. The digested protein was concentrated to 20 ml by ultrafiltration using an Amicon stirred cell concentrator with a YM10 membrane, and the buffer was exchanged to 1% acetic acid/10% acetonitrile (ACN). The refolded protein was purified sequentially by cation exchange HPLC on a Spherogel TSK SP-5PW column (3.3 ml, 10 µm, 7.5 × 75 mm, Tosoh Biosep, 1 ml/min, linear gradient of ammonium acetate (0 to 1 M, pH 6.8 over 50 min) in 10% ACN) followed by C18 reverse phase HPLC (19.3 ml, 10 × 250 mm, 100 Å, 5 µm, Beckman, 4 ml/min, linear gradient of ACN in 0.1% TFA (10–28% ACN over 18 min, 28–35% ACN over 42 min). The lyophilized proteins were quantified by OD280nm using their calculated molar absorption coefficients, and analyzed by MALDI-TOF mass spectrometry, SDS-polyacrylamide gel electrophoresis, and circular dichroism (CD). MALDI-TOF analysis of sPLA2s was carried out on an Applied Biosystems voyager DE-PRO mass spectrometer in linear mode with sinapinic acid as a matrix, using external calibration. The secondary structure of all proteins was evaluated by CD using a Jasco PTC-423S (J-810) apparatus and analyzed with the program CDNN (CD Spectra deconvolution, version 2.1). Proteins were diluted to 0.1 or 0.2 mg/ml in 10 mM Na+-borate pH 8.0, 0.1 mM EDTA and spectra were recorded at 20 °C with a scanning speed of 20 nm/min between 190 and 260 nm. The final CD spectrum was obtained from the average of 15 runs.
Enzymatic activity and interfacial binding to phospholipid vesicles
E. coli enzymatic assays were performed using [3H]oleate-radiolabeled membranes (11). The headgroup substrate specificity was measured on large unilamellar vesicles of pure POPG, POPS, or POPC with the fatty acid-binding protein assay as described (11). The initial velocities were measured at 37 °C in 1.3 ml of Hanks’ balanced salt solution containing 1.3 mM Ca2+, 0.9 mM Mg2+, 30 µM phospholipid extruded vesicles, 1 µM 11-dansyl-undecanoic acid, and 10 µg of rat liver fatty acid binding protein. Excitation was at 350 nm and emission at 500 nm. Assays were calibrated by adding known amounts of oleic acid and measuring the decrease in fluorescence. Reactions were started by adding sPLA2. Binding studies of sPLA2s to sucrose-loaded, 0.1 µm unilamellar vesicles of diether phospholipids (20 mole% DOetPS/80 mole% DOetPC) were performed as described previously (11). sPLA2 binding studies were carried out in 5 mM MOPS, pH 7.4, 0.1 M KCl, 2 mM CaCl2 at room temperature using the centrifugation method in which the amount of enzyme remaining in the supernatant above pelleted vesicles is quantified by enzymatic assays (for high specific activity enzymes) or silver-stained 16% SDS-PAGE gels (for both low and high specific activity enzymes). Assays were made in the presence of 0.5 µg of sPLA2 and different concentrations of phospholipid vesicles in a total volume of 100 µl to measure the affinity (Kd) of the sPLA2 for the phospholipid vesicle.
Receptor binding assays and cross-linking studies
Competition binding assays between iodinated OS2 and unlabeled OS2 mutants on N-type and M-type receptors were performed under equilibrium binding conditions as described (20, 30, 31), except that OS2 was labeled to a specific activity of 3000–3500 cpm/fmol using lactoperoxidase as follows. The iodination was performed in a total volume of 60 µl of 0.1 M NaH2PO4 pH 6.5 containing 0.5 nmole OS2, 0.5 nmole 125I-Na (Perkin Elmer), 0.5 µg lactoperoxidase (Sigma L8257), and 20 µl of hydrogen peroxide (Sigma H1009) diluted 1/40,000 in H2O. The reaction was initiated by adding 10 µl hydrogen peroxide at 0 and 10 min. After incubation at room temperature for 20 min, the mixture was diluted to 1 ml with H2O/ ACN/TFA (90/10/0.1) and applied to a C18 end-capped reverse phase HPLC column (Merck purospher STAR, 55 × 4 mm, 3 µm, 100 Å, bed volume 0.7 ml). Elution was performed at 1 ml/min using a linear gradient of ACN in 0.1% TFA (10–25% ACN over 10 min, 25–50% ACN over 50 min). Fractions containing active iodinated OS2 were identified by binding assays on M- and N-type receptors. Binding on M-type receptors was carried out on membranes from HEK 293 cells (ATCC CRL-1573) transfected with either rabbit or mouse cloned M-type receptors (31, 57). Binding on N-type receptors was carried out on mouse brain membranes (30) or HeLa P4 cell membranes (20). Briefly, membranes, 125I-OS2 and unlabeled sPLA2s were incubated at room temperature in 0.5 ml of binding buffer (140 mM NaCl, 0.1 mM CaCl2, 20 mM Tris-HCl pH 7.4 and 0.1% bovine serum albumin). Incubations were started by addition of membranes and filtered after 1 h through GF/C glass fiber filters pre-soaked in 0.5% polyethyleneimine, 10 mM Tris-HCl pH 7.4. Iodination and binding experiments with the C-terminal chimera were performed as for WT OS2. Cross-linking experiments on mouse brain membranes were performed as described (30) using 200 µM of suberic acid bis-N-hydroxysuccinimide ester (DSS, Sigma).
Central neurotoxicity
Central neurotoxicity was evaluated by measuring the minimal lethal doses (LD100) after intracerebroventricular injection of sPLA2s (5 µl, dissolved in 0.9% NaCl) into BALB/c male mice (42–45 days old, 18–20 g) (58). LD100 is defined as the lowest amount of sPLA2 that kills all mice (n≥2), half of this dose being not lethal 4 h after injection (n≥2). All procedures were performed in accordance with the European Commission Council Directives.
Chick isolated biventer cervicis muscle assays
Biventer cervicis muscles were removed from male chicks (4–10 days-old), mounted in 5 ml organ baths and maintained at 34 °C under 1 g resting tension in Krebs solution of the following composition (mM): NaCl 118.4; KCl 4.7; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.0; glucose 11.1; CaCl2 2.5, bubbled continuously with 95% O2 and 5% CO2. Isometric contractions were measured via a Grass transducer (FT03) connected to a Maclab/8 system. Twitches were evoked by stimulating the motor nerve (supramaximal voltage, 0.2 ms, 0.1 Hz) via silver electrodes connected to a Grass S88 stimulator. Nerve mediated (indirectly evoked) twitches were confirmed by abolition of twitches after addition of 10 µM (+)-tubocurarine (Sigma). In the absence of electrical stimulation, responses (directly evoked) to exogenous acetylcholine (ACh, Sigma, 1 mM, 30s), carbachol (CCh, 20 mM, 60 s) and potassium chloride (KCl, 40 mM, 30 s) were obtained prior to the addition of sPLA2 and at the end of the experiment. This allows the classification of the sPLA2 effects observed in the chick preparation into presynaptic or postsynaptic actions. Preparations were allowed to equilibrate for at least 30 min with continuous stimulation before addition of sPLA2.
Myotoxic effects on mice
sPLA2s were dissolved in phosphate-buffered saline (PBS : 0.12 M NaCl, 0.04 M sodium phosphate, pH 7.2). Aliquots of 100 µl, containing 5 µg of sPLA2, were injected intramuscularly in the right thigh of CD-1 mice (18–20 g). Control mice received 100 µl of PBS under otherwise identical conditions. Three hours after injection, a blood sample obtained by cutting the tip of the tail was collected into heparinized capillary tubes and centrifuged. Plasma creatine kinase activity was determined using the Sigma kit No. 47-UV (Sigma-Aldrich). Creatine kinase activity is expressed in units U/l, where one unit is defined as the activity of the enzyme that produces 1 µmol of NADH/min under the conditions of the assay. Mice were observed for 8 h for symptoms of systemic toxicity. Statistical analysis was performed by determining the significance of the differences of the means of all experimental groups by ANOVA. Comparisons between pairs of means were performed by the Tukey Kramer method.
Anti-HIV-1 activity
Single rounds of HIV-1BRU virus infection in HeLa P4 cells were performed as described previously (20). Briefly, HeLa P4 cells were seeded into 24-well plates (80,000 cells per well) and infected the next day with 100 µl of HIV-1BRU viral supernatant (100 ng of Gag p24). Virus and cells were left in contact for 8 h at 37 °C in the absence or presence of the different sPLA2s, and the culture medium was then replaced with fresh medium containing 50 µM 3’-azido-3’-deoxythymidine (AZT, Sigma) to prevent for another round of infection. Two days after infection, HeLa P4 cells were lysed and β-galactosidase activity was measured as an index of virus infection.
Antimalarial activity
Inhibitory effect toward P. falciparum intraerythrocytic development was assayed as previously described (21) in complete medium containing either 8% heat-inactivated human serum or 0.5% AlbuMAX II, a culture medium supplement based on calf serum lipid-enriched albumin (InVitroGen). Various concentrations of each sPLA2 (100 µl/well) were distributed in a 96-well plate, then 100 µl of a P. falciparum culture (1.5% parasitemia and 4% hematocrit) was added. After 24 h of incubation, 0.5 µCi 3H-hypoxanthine (ICN Biomedicals) was added per well, and parasites were grown for an additional 24 h. Plates were freeze-thawed and harvested on filters. Dried filters were moistened in scintillation liquid mixture (OptiScint, Hisafe) and counted in a 1450 Microbeta counter (Wallac, Perkin Elmer).
RESULTS
Rationale for the design and recombinant production of wild-type OS2, single point mutants and chimeras
Because the production of recombinant sPLA2s can be problematic (11), we initially tried to express WT OS2 from a synthetic gene in Drosophila S2 cells and E. coli using the pAB3 expression vector system (see methods). Only the expression in E. coli was successful. Two different site-directed mutagenesis approaches were then developed to investigate the structure-function relationships of OS2. First, we prepared single point mutants of OS2 (glycine-30 to serine, histidine-48 to glutamine and aspartate-49 to lysine) with the aim to abolish its enzymatic activity, but not its biological effects and/or binding properties to receptors (Fig. 1 and Fig. 2). These three residues are absolutely conserved in all active sPLA2s, are located in the active site or Ca2+-binding loop, and are known to be essential to catalysis (51). Moreover, venom sPLA2s that harbor similar mutations occur naturally, are catalytically inactive, yet they can exert diverse pharmacological effects (59–61). Mutation of Histidine-48 by glutamine has been shown to dramatically reduce enzymatic activity (62, 63). Mutations of glycine-30 and aspartate-49 are known to affect the binding of substrate and Ca2+, an essential cofactor for sPLA2 activity and substrate binding (60, 64, 65). All three mutations were thus expected to produce catalytically inactive mutants of OS2 for distinct reasons. We also constructed the OS2 double mutant K31L-R34S to analyze the role of amino acids at positions 31 and 34, which are hypervariable within the highly conserved Ca2+-binding loop of sPLA2s (66). Lysine-31 and arginine-34 of OS2 were respectively replaced by leucine and serine which are found in the non toxic porcine pancreatic sPLA2 (52).
Fig. 2.
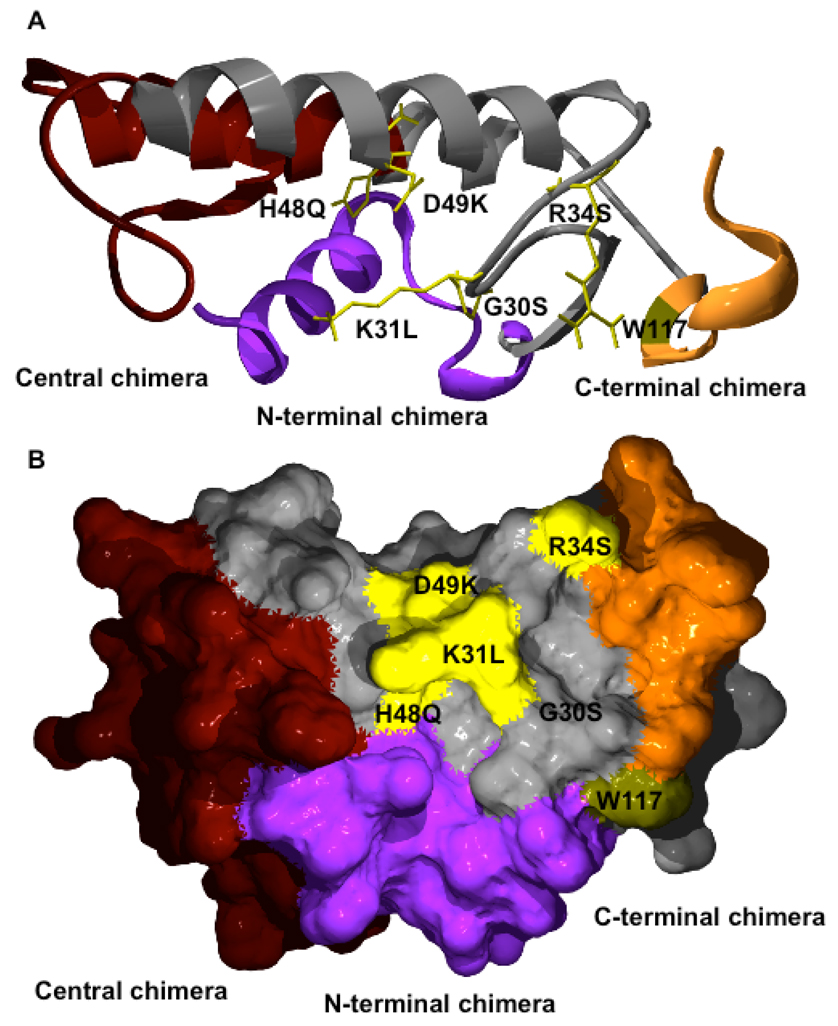
Structural model of WT OS2 showing the location of the different mutations. An OS2 model was build using the SwissPDB protein viewer software and the crystal structure of notexin (PDB 1AE7) as template. The location of the single point mutations and W117 are shown. The N-terminal, central, and C-terminal regions exchanged in the chimeras are shown in blue, brown, and dark yellow, respectively. The conserved backbone between OS1 and OS2 is shown in grey. A, Ribbon view of OS2 with the putative interfacial binding surface facing down. B, Molecular surface view after rotating the molecule by 90°.
In the second approach, we designed chimeras between OS2 and OS1 in an effort to identify the main regions of OS2 which are responsible for its molecular properties and biological effects. OS1 was also purified from Taipan snake venom and is structurally homologous to OS2, but has quite distinct biological properties (30, 52, 58). Pharmacologically, OS1 binds only very weakly to N-type receptors, has a lower specific activity on phospholipid substrates (see below), and is neither neurotoxic, nor myotoxic, nor able to potently inhibit HIV-1 replication ((20, 30) and below). However, both OS1 and OS2 are high affinity ligands for the M-type receptor (58), and both also have antimalarial activity (see below). Structurally, OS1 is 54% identical to OS2 (Fig. 1B). The level of identity is very high in the active site and Ca2+-binding loop, while it is much lower in the N-terminal, central, and C-terminal regions (Fig. 1B). A three-dimensional model of OS2 was produced by homology modeling using the x-ray structure of notexin as a template (Fig. 2). A model for OS1 (not shown) was also produced using as template the three-dimensional structure of the acidic sPLA2 from Ophiophagus hannah venom (pdb structure 1gp7). Based on the major structural differences between the two proteins, we constructed three different chimeras (Fig. 1 and Fig. 2). Importantly, all the residues in the protein segments that were exchanged in the chimera constructs and that point toward the core of the protein are identical between OS1 and OS2 except for the conservative change of Met-8 in OS2 being replaced with Leu in OS1. Thus, all but one of the residue changes occur at the molecular surface, and segment exchanges should not cause an adverse structural alteration of the chimeric proteins.
Recombinant expression and structural characterization of OS2, single point mutants and chimeras in E. coli
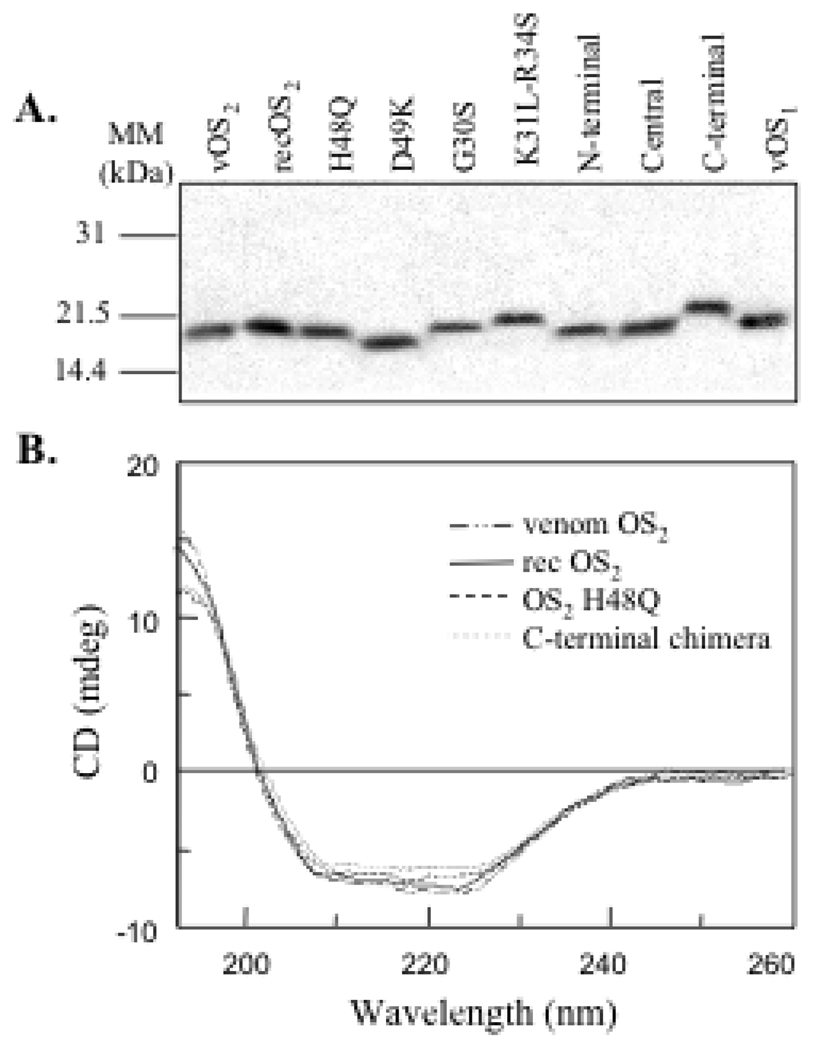
All OS2 proteins could be produced from E. coli with high yields (Table 1). The HPLC-purified proteins migrated as single bands of the expected size on a Laemmli SDS gel (Fig. 3A). For all sPLA2s, the molecular masses measured by MALDI-TOF mass spectrometry matchedthe calculated masses within less than 1 Da (Table 1), clearly establishing that all the disulfide bonds were formed in the proteins, and that residue side chains had not been chemically oxidized or further modified during the refolding and purification processes. We also compared the overall conformation and secondary structure of venom, WT recombinant OS2, and its mutants by CD spectroscopy. The CD spectra were very similar for all proteins, including the three chimeras (Fig. 3B and not shown). Taken together, these results indicate that the recombinant proteins are pure and properly folded with all their disulfide bonds appropriately formed.
TABLE 1. Recombinant production of OS2 and its mutants.
All proteins were produced using the pAB3 E.coli expression system. The yield of production is the amount of pure protein obtained per liter of E. coli culture. The proteins were analyzed by MALDI-TOF mass spectrometry to confirm that all the disulfide bonds are formed and that proteins have not been covalently modified during purification.
| sPLA2 | Yield of production | Calculated mass | Measured mass | Δmass |
|---|---|---|---|---|
| mg/liter | Dalton | Dalton | ||
| OS2 WT | 5 | 13317.08 | 13317.86 | 0.78 |
| OS2 H48Q | 2.6 | 13308.07 | 13307.27 | 0.80 |
| OS2 D49K | 6.4 | 13330.17 | 13329.84 | 0.33 |
| OS2 G30S | 1.3 | 13347.11 | 13347.89 | 0.78 |
| OS2 K31L-R34S | 1.5 | 13232.96 | 13233.79 | 0.83 |
| N-terminal chimera | 0.3 | 13278.02 | 13277.30 | 0.72 |
| Central chimera | 1 | 13992.66 | 13993.29 | 0.63 |
| C-terminal chimera | 2.6 | 13429.10 | 13430.12 | 1.02 |
Fig. 3.
Analysis of the OS2 mutants and chimeras by SDS-PAGE electrophoresis and CD. (A) One µg of sPLA2 was loaded on a 14% SDS-polyacrylamide gel under reducing conditions and stained with Coomassie brilliant blue. The apparent mobility appeared to be very sensitive to the pI of the protein (B) CD spectra of recombinant WT OS2, H48Q mutant and C-terminal chimera compared to that of venom OS2. The CD spectra of all the other mutants are identical to those shown here.
Lethal potency of OS2 and its mutants in mice
Both venom and recombinant WT OS2 are highly neurotoxic to mice following intracerebroventricular injection with an identical LD100 of 2 µg/kg (Table 2). Interestingly, the active site mutant H48Q is still neurotoxic (33 µg/kg), although the LD100 is 16-fold lower than that of WT OS2. Conversely, the two other active site mutants G30S and D49K have very low or no toxicity with LD100 values of 3,330 µg/kg or higher (Table 2). The double mutant K31L-R34S appears to be nearly as toxic as WT OS2, suggesting that positions 31 and 34 are not critical, at least when mutated into leucine and serine. In marked contrast with WT OS2, OS1 displays a very low toxicity with an LD100 of 3,500 µg/kg. The N-terminal chimera is not toxic at a concentration as high as 1,500 µg/kg, and thus behaves similarly to OS1. However, the central and C-terminal chimeras retain high toxicities, with LD100 values of 70 and 33 µg/kg, respectively.
TABLE 2. Biological effects of OS2 and its mutants.
Minimal lethal dose (LD100) was measured by intracisternal injection of sPLA2 into adult Balb/C male mice. LD100 is defined as the lowest amount of sPLA2 that is lethal to all mice (n≥2), half of this dose being not lethal to mice 4 hours after injection (n≥2). HIV-1 inhibition was determined by infection of HeLa P4 cells with HIV-1BRU in the absence or presence of various concentrations of sPLA2 for 8 hours. The level of viral infection was evaluated 2 days after infection. Antimalarial activity was measured by incubating various concentrations of sPLA2 with an asynchronous culture of P. falciparum (1.5% parasitemia and 2% hematocrit) grown in the presence of 8% human serum or 0.5% AlbuMAX II. The inhibition of growth was determined by 3H-hypoxanthine incorporation. The IC50 value is defined as the concentration of sPLA2 that inhibits 50% of HIV-1BRU infection or parasite growth. The values are representative of at least 2 independent experiments with SEM values lower than 50%.
| sPLA2s | LD100 | IC50 value | ||
|---|---|---|---|---|
| Lethality | HIV inhibition | Antimalarial activity | ||
| human serum | AlbuMAX II | |||
| µg/kg | nM | |||
| venom OS2 | 2 | 35 | 3.1 | >2,500 |
| rec OS2 WT | 2 | 33 | 2.1 | >1,250 |
| OS2 H48Q | 33 | >1,000 | >250 | >1,250 |
| OS2 D49K | >3,330 | >1,000 | >250 | >1,250 |
| OS2 G30S | 3,330 | >1,000 | >250 | >1,250 |
| OS2 K31L-R34S | 7 | 23 | 0.1 | 378 |
| N-terminal | >1,500 | >1,000 | >250 | >1,250 |
| Central | 70 | 110 | 0.1 | 680 |
| C-terminal | 33 | 60 | 17.9 | >1,250 |
| venom OS1 | 3,500 | 300 | 0.07 | 1,000 |
Enzymatic properties of OS2 and its mutants
The enzymatic properties of WT recombinant OS2 were found to be identical to those of OS2 purified from venom (Table 3). WT OS2 shows a high specific activity on E. coli membranes and is more efficient on the anionic phospholipid POPG than on the anionic phospholipid POPS or the zwitterionic phospholipid POPC (Table 3). As expected, mutations of G30, H48 and D49 in the Ca2+-loop and active site of OS2 dramatically affected the catalytic activity, but did not alter the headgroup substrate specificity. Indeed, the specific activities of H48Q and G30S mutants were both reduced by about 500-fold. The activity of OS2 D49K was reduced to undetectable levels. The mutations K31L and R34S in the Ca2+-loop did not affect the specific activity and only slightly modified the headgroup substrate specificity of OS2.
TABLE 3. Enzymatic properties of OS2 and its mutants.
The specific activities were measured on [3H]oleate-radiolabeled E.coli membranes and on vesicles composed of the single phospholipid POPG, POPS or POPC using the real-time fluorometric assay employing the fatty acid binding protein. Interfacial binding of sPLA2 was determined by the centrifugation method on sucrose-loaded vesicles of 20 mole% DOetPS/ 80 mole% DOetPC. The results are representative of at least duplicate experiments with SEM values lower than 40%.
| sPLA2 | Specific activity | Interfacial binding | |||
|---|---|---|---|---|---|
| E.coli | POPG | POPS | POPC | DOetPS/PC | |
| dpm/(pmol × min) | µmol/(min×mg) | Kd (mM) c | |||
| venom OS2 | 1090 | 433 | 26 | 53.7 | 0.023 |
| rec OS2 WT | 1000 | 471 | 23 | 59.1 | 0.074 |
| OS2 H48Q | 2 | 1.2 | 0.05 | 0.05 | 0.040 |
| OS2 D49K | 0a | nd b | nd b | nd b | no binding at 1 mM |
| OS2 G30S | 2.3 | 0.60 | 0.03 | 0.03 | no binding at 1 mM |
| OS2 K31L-R34S | 950 | 169 | 3.10 | 61.8 | 0.007 |
| N-term. chimera | 1 | 2.60 | 0.003 | 0.02 | no binding at 1 mM |
| Central chimera | 5060 | 131 | 5.8 | 86 | 0.015 |
| C-term. chimera | 1.9 | 0.01 | 0.17 | 0.18 | no binding at 1 mM |
| venom OS1 | 10 | 2.40 | 2.2 | 9.7 | no binding at 1 mM |
No sPLA2 activity is detected at 1 µM after 2 h of incubation.
Not determined.
The indicated Kd values are averaged values of Kd values measured by SDS-PAGE analysis (for sPLA2s with low specific activity), or by both SDS-PAGE and sPLA2 assays (for sPLA2s with high specific activity).
In the case of the OS2/OS1 chimeras, a strong decrease in specific activity was observed for the N-terminal (1,000-fold) and C-terminal (500-fold) chimeras (Table 3). Conversely, the central chimera retains a high specific activity, which can be up to 5-fold higher than that of WT OS2. Some changes in the headgroup substrate specificities were observed for the central chimera and the C-terminal chimera, which appeared to have a substrate specificity more similar to that of OS1.
We also measured the binding properties of the different sPLA2s to diether phospholipid vesicles made up of 20 mole% DOetPS/ 80 mole% DOetPC. WT OS2 binds much more tightly to these phospholipid vesicles than OS1 (Table 3). The double mutant K31L/R34S and the active site mutant H48Q also bind tightly, while G30S and D49K do not bind at all under our binding assay conditions. Among the three chimeras, the central chimera binds as tightly as WT OS2, while the two other chimeras do not bind. When the binding of WT OS2 was measured in the absence of free Ca2+ (2 mM EDTA instead of 2 mM CaCl2 in binding buffer, see methods), the sPLA2 was completely unable to bind to vesicles (not shown).
Binding of OS2 and its mutants to M-type and N-type receptors measured by competition binding assays with iodinated OS2
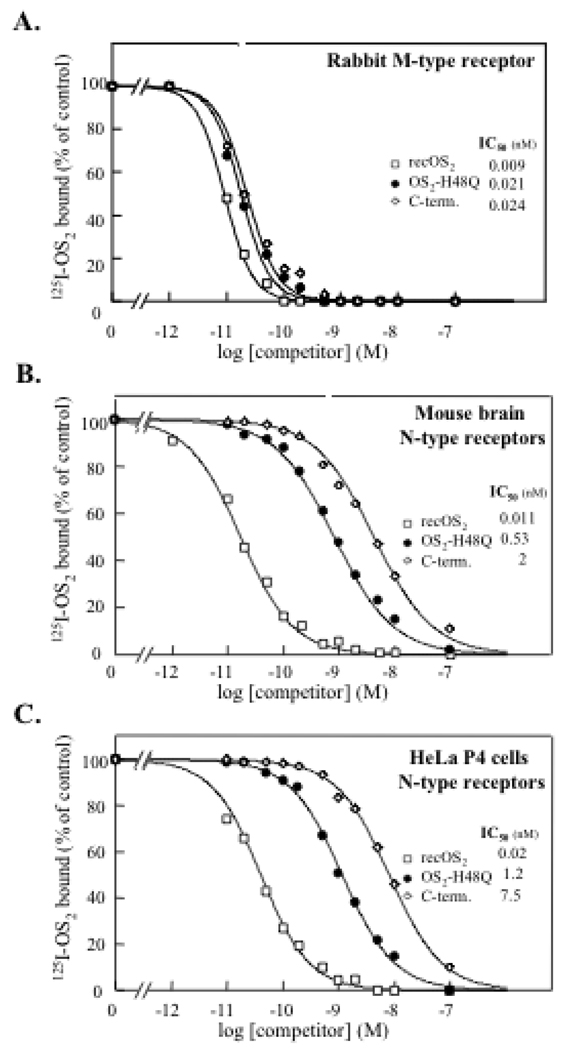
Binding studies on mouse and rabbit M-type receptor show that most mutants bind to this receptor with K0.5 values similar to that of WT OS2 (Fig. 4A and Table 4). The only exceptions were the N-terminal and central chimeras which show 4- to 30-fold lower affinities. The fact that all mutants including chimeras bind to the M-type receptor with high affinities further indicates that all of them are properly folded. Finally, the double mutant K31L/R34S was constructed to evaluate the contribution of residues 31 and 34 to M-type receptor binding. Indeed, we previously found that mutation of Leu-31 in the pancreatic sPLA2 decreased its affinity for the receptor, suggesting that this position is important (52). We also noticed that the positions 31 and 34 are the only two hypervariable positions within the conserved Ca2+-loop, suggesting that the nature of the residues at these positions may determine, at least in part, the different binding properties of sPLA2s to the receptor (52). In the OS2 double mutant K31L/R34S, the OS2 residues have been replaced by those of the pancreatic sPLA2 with the goal to decrease the binding affinity. The fact that no decrease was observed suggest that the positions 31 and 34 are not crucial for the OS2 interaction, or that these mutations can be accomodated in the OS2 structure to maintain a high affinity. Further work including co-crystallization of the sPLA2-receptor complex is required to address the contribution of residues at positions 31 and 34 in the sPLA2-M-type receptor interaction.
Fig. 4.
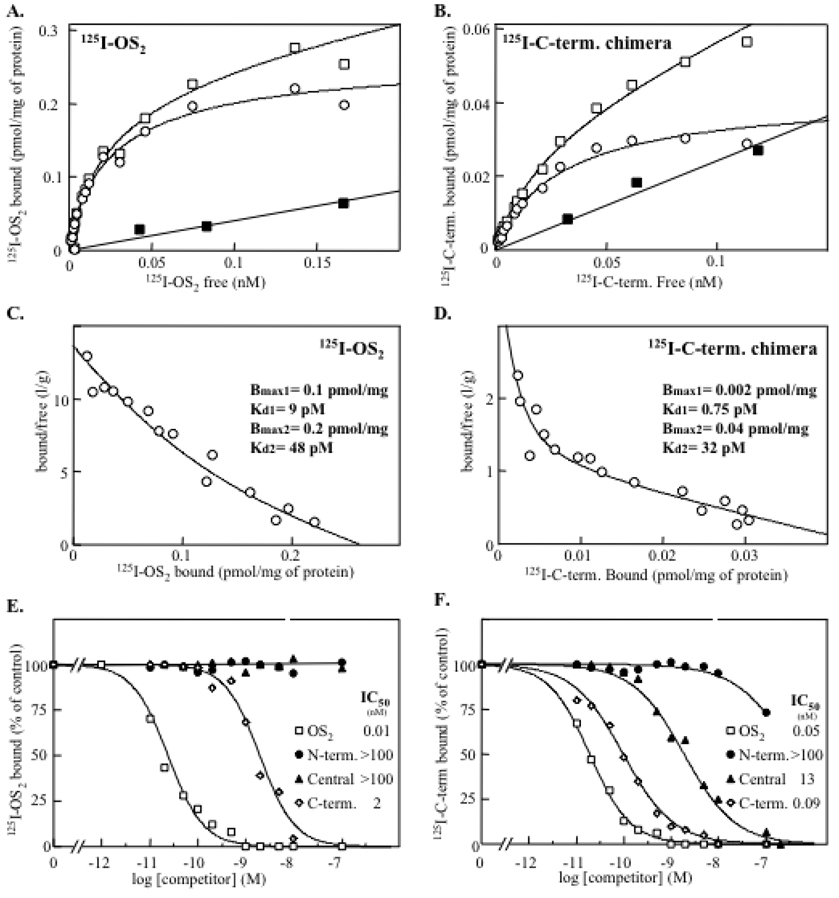
Binding properties of OS2 and its mutants to M- and N-type receptors measured by competition assays with iodinated WT OS2. Competition experiments between 125I-OS2 and unlabeled WT recombinant OS2 (*), the mutant H48Q (▯) and the C-terminal chimera (↓) for binding to (A) the cloned rabbit M-type receptor expressed in HEK 293 cells, (B) N-type receptors from mouse brain membranes, and (C) N-type like receptors from HeLa P4 cell membranes. Membranes were incubated with 125I-OS2 and various concentrations of unlabeled competitors. Results are expressed as percentage of the 125I-OS2 specific binding measured in the absence of competitor. The membrane concentration was adjusted to obtain a specific binding of 10–20% of the total radiolabeled ligand added (55 pM). The non specific binding was measured in the presence of 100 nM unlabeled OS2 and was below 10% of total binding.
TABLE 4. Binding properties of OS2 and its mutants to M-type and N-type receptors.
Competition binding assays between iodinated OS2 and unlabeled mutants or chimeras were performed on recombinant M-type receptor or N-type receptors from mouse brain membranes and Hela P4 cell lysates. The K0.5 value is defined as the concentration of sPLA2 competitor which inhibits 50% of the specific binding. K0.5 values are representative of at least three independent experiments with SEM values lower than 30%.
| sPLA2s | K0.5 value | |||
|---|---|---|---|---|
| Rabbit M-type | Mouse M-type | Mouse N-type | HelaP4 N-type | |
| nM | ||||
| Venom OS2 | 0.009 | 0.07 | 0.011 | 0.02 |
| rec OS2 WT | 0.009 | 0.07 | 0.011 | 0.02 |
| OS2 H48Q | 0.021 | 0.19 | 0.53 | 1.2 |
| OS2 D49K | 0.026 | 0.49 | 13.5 | 0.96 |
| OS2 G30S | 0.035 | 0.2 | 200 | 400 |
| OS2 K31L-R34S | 0.008 | 0.047 | 0.045 | 0.028 |
| N-terminal chimera | 0.19 | 1.4 | >500 | >500 |
| Central chimera | 0.037 | 2.2 | >300 | >300 |
| C-terminal chimera | 0.024 | 0.03 | 2 | 7.5 |
| venom OS1 | 0.1 | 0.15 | >1000 | >1000 |
We next investigated the binding properties of the different mutants to N-type receptors present in mouse brain membranes and human HeLa P4 cells. These two sources of N-type receptors were chosen because of their potential implication in two of the pharmacological models used in this study, i.e. neurotoxicity in mouse brain and inhibition of HIV replication. Binding to N-type receptors was found to be sensitive to the different mutations analyzed, but to a different extent (Figs. 4B and 4C, and Table 4). The mutant K31L-R34S retained an affinity close to that of WT OS2 for both mouse brain and HeLa P4 N-type receptors. The mutant H48Q was also able to efficiently bind to the N-type receptors expressed in mouse brain and HeLa P4 cells, yet its affinity was 50-fold lower than that of WT OS2. D49K and the C-terminal chimera also bind to the N-type receptors with nanomolar affinities, even though their affinities are 180-to 1,230-fold lower than for WT OS2. Mutation of glycine-30 produces a very dramatic effect, decreasing the affinity by a factor of about 20,000. Finally, the N-terminal and central chimeras were unable to compete with iodinated OS2 for binding to mouse brain and Hela P4 cell membranes.
Binding of OS2 and its mutants as revealed by cross-linking of labeled OS2 to mouse brain membranes
Because some of the above binding results did not correlate well with the central neurotoxic properties of OS2 mutants (Table 2), while a relationship between sPLA2 neurotoxicity and N-type receptor binding was previously proposed (30, 67), we analyzed the binding properties of the OS2 mutants by cross-linking experiments of radiolabeled OS2 to mouse brain membranes (Fig. 5). These experiments constituted another method to visualize the ability of the OS2 mutants to bind to N-type receptor proteins. When 125I-OS2 was allowed to bind to mouse brain membranes and subsequently cross-linked with DSS, several proteins of 14, 18, 39 and 50 kDa were labeled (Fig. 5). The cross-linking of these proteins is specific because it is fully prevented by an excess of unlabeled OS2 (300 nM), but not OS1, which is unable to bind to N-type receptors (Table 4). In agreement with the competition binding assays (Table 4), the mutant K31L-R34S completely prevented the cross-linking of 125I-OS2 to the N-type receptors. Similarly, the mutants H48Q and D49K decreased the labeling, whereas the mutant G30S and the N-terminal chimera had no effect. However, the central and C-terminal chimeras appeared more potent than expected from the competition assays (Fig. 4), especially for the C-terminal chimera which efficiently prevented the cross-linking of iodinated OS2.
Fig. 5.
Binding properties of WT OS2 and its mutants as revealed by cross-linking experiments of WT 125I-OS2 to mouse brain membranes. Membranes (300 µg of protein/ml) were incubated with 200 pM 125I-OS2 in the absence (Ø) or presence of unlabeled sPLA2s (300 nM), centrifuged, cross-linked with 200 µM DSS and loaded on a 10% SDS-polyacrylamide gel under reducing conditions. The gel was stained with Coomassie brilliant blue, dried and autoradiographed at −70 °C for 7 days. After correction for the molecular mass of labeled OS2 assuming a 1:1 stoechiometry, OS2 binding proteins with molecular masses of 14, 18, 39 and 50 kDa are detected. The most representative experiment out of three experiments performed on two different mouse brain membranes is shown.
Binding properties of iodinated C-terminal chimera
To reconcile the above results, we postulated that WT OS2 binds with high affinities to at least two different populations of binding sites in mouse brain, while the central and C-terminal chimeras bind with high affinity to only a subset of these receptors. This receptor subset would correspond to those visualized by cross-linking experiments (Fig. 5). According to this hypothesis, the WT OS2-specific population(s) of binding sites (which could be proteins and/or phospholipids) should be abundant in mouse brain membranes and responsible for most of the signal measured with iodinated WT OS2 in competition binding assays (Fig. 4). Additionally, the two chimeras should have weak affinity for these binding sites, and thus they should not compete efficiently (Fig. 4).
To investigate this hypothesis, we iodinated the central and C-terminal chimeras to perform direct binding studies and identify a subset of OS2 binding sites. Binding assays with the central chimera could not be done as it became highly oxidized during the iodination procedure with lactoperoxidase (data not shown), even when iodination was performed under very mild conditions (68). Conversely, the C-terminal chimera could be iodinated without oxidation and used in direct binding experiments. Fig. 6 shows a comparison of the binding properties of iodinated WT OS2 and C-terminal chimera obtained in parallel experiments on the same mouse brain membrane preparations. Fig. 6A and B show that the binding of both proteins to mouse brain membranes is saturable and specific. In both cases, the Scatchard plots of the specific binding are best fitted by curvilinear curves, suggesting that the two ligands bind to at least two distinct families of binding sites with very high affinities in the pM range (Fig. 6C and D). However, the total number of binding sites for the chimera (0.042 pmol/mg of total protein) appears to be about 7-fold lower than that for WT OS2 (0.3 pmol/mg of total protein). This result plus the fact that WT OS2 competes with the labeled chimera for binding (Fig. 6F) indicates that the chimera indeed binds to a minor subset of WT OS2 binding sites. Additionally, when the competition binding assays for the different chimeras were performed using the iodinated C-terminal chimera as labeled ligand, the affinities were much higher than those obtained using labeled WT OS2 (Fig. 6E and F). Together, the binding properties of the labeled C-terminal chimera support the hypothesis of at least two major populations of OS2 binding sites, among which one binds with high affinity the central and C-terminal chimeras and has molecular masses corresponding to those revealed by the cross-linking experiments (Fig. 5). The curvilinear curve obtained with the iodinated C-terminal chimera suggests that the binding sites recognized by WT OS2 and the C-terminal chimera are still complex in nature and may be represented by several independent targets.
Fig. 6.
Comparison of the binding properties of iodinated WT OS2 and C-terminal chimera to mouse brain membranes. All of the binding experiments were performed in parallel and on the same membrane preparations (n=3). (A and B), Direct equilibrium binding assays with the two iodinated ligands. Membranes were incubated with various concentrations of labeled ligands in the absence (*) or presence (—) of 100 nM unlabeled homologous ligand. The specific binding (▯) represents the difference between total (*) and non specific binding (—). (C and D), Scatchard plot analysis of the specific binding. (E and F), Competition experiments between iodinated ligands and unlabeled WT recombinant OS2 and the different chimeras. Membranes were incubated with 50 pM of WT 125I-OS2 or 125I-C-terminal chimera and various concentrations of unlabeled competitors. Results are expressed as the percentage of 125I-OS2 specific binding measured in the absence of competitor. The non specific binding was measured in the presence of 100 nM unlabeled homologous ligand and was below 15% of total binding.
Effects of OS2 and its mutants on neuromuscular transmission
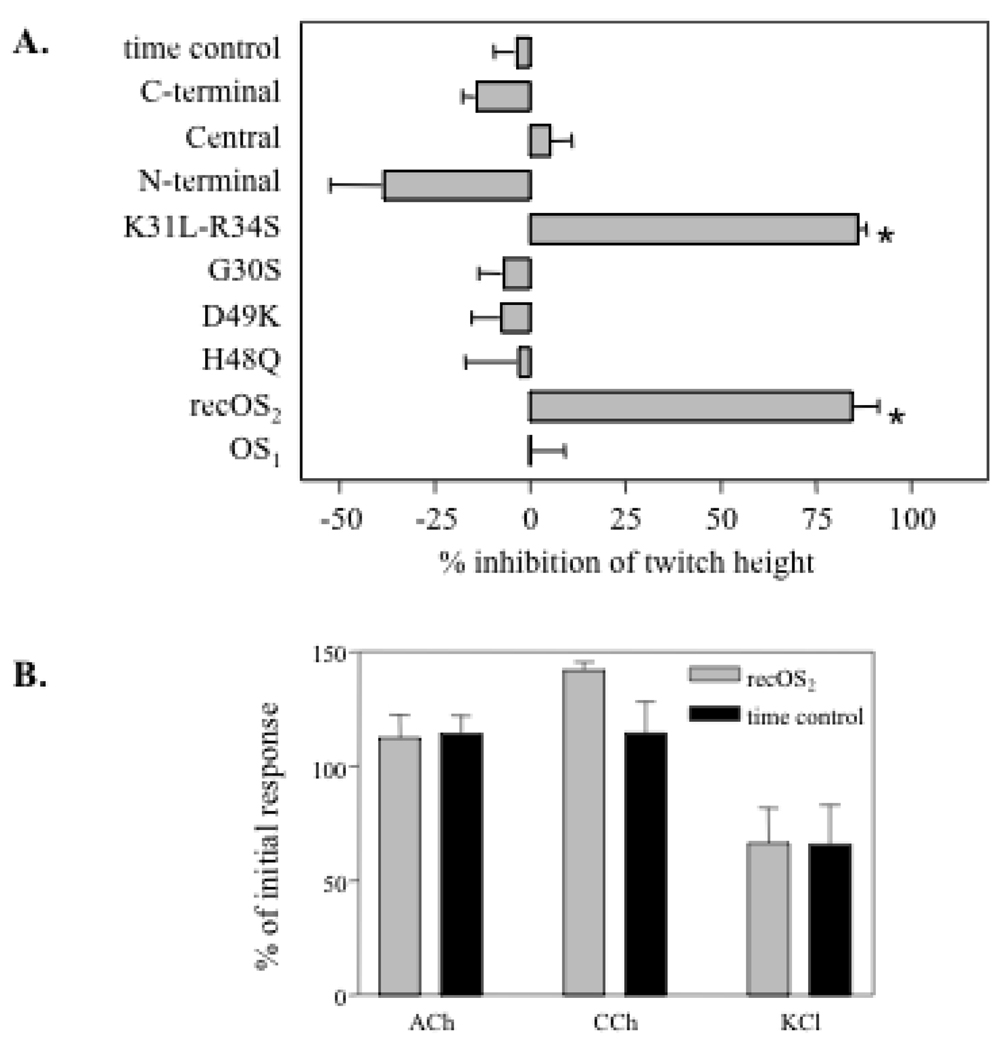
Neurotoxic snake venom sPLA2s are called β-neurotoxins because they affect neuronal or neuromuscular transmission by acting presynaptically, even though some of them can also exert postsynaptic and myotoxic effects (2, 14, 69). OS2 is lethal when injected intra-cerebroventricularly into rat or mouse brain (30, 48, 70), and has been shown to block acetylcholine release in a neuro-neuronal synapse in Aplysia (49), suggesting that OS2 is also a β-neurotoxin. To confirm that OS2 acts presynaptically at a vertebrate neuromuscular junction, we analyzed its effects and those of its mutants on the chick biventer cervicis neuromuscular preparation (Fig. 7). Only WT OS2 and the K31L-R34S mutant, when tested at 200 nM, were able to reduce the height of indirect twitches evoked by electrical nerve stimulation (Fig. 7A). At lower concentrations of 20 and 50 nM, WT OS2 had no twitch reduction effect, indicating that OS2 is not very potent on this neuromuscular preparation. In line with this rather low potency, all the other mutants and chimeras were found to be inactive when assayed at 200 nM. We next analyzed the effect of WT OS2 on contractions of the chick biventer cervicis preparation directly evoked by various agonists including acetylcholine, carbachol and potassium chloride (Fig. 7B). No significant inhibition of the contractions was observed in the presence of WT OS2 (Fig. 7B) or mutants (not shown) at 200 nM.
Fig. 7.
Effects of OS2 and its mutants on neuromuscular transmission in the chick biventer cervicis muscle preparation. A, Effects of recombinant OS2, OS1, mutants and chimeras (200 nM) on indirect twitches evoked by electrical nerve stimulation. The percentage of inhibition of twitch height was measured 3 h after the addition of sPLA2 to the bath. B, effects of WT recombinant OS2 on contractures of the chick biventer cervicis preparation in response to acetylcholine (ACh, 1 mM), carbachol (CCh, 20mM), and potassium chloride (KCl, 40 mM) compared to time control (—). All experiments were performed in triplicate. * p< 0.001 significantly different from time control (unpaired t-test assuming unequal variance).
Myotoxic effects of OS2 and its mutants
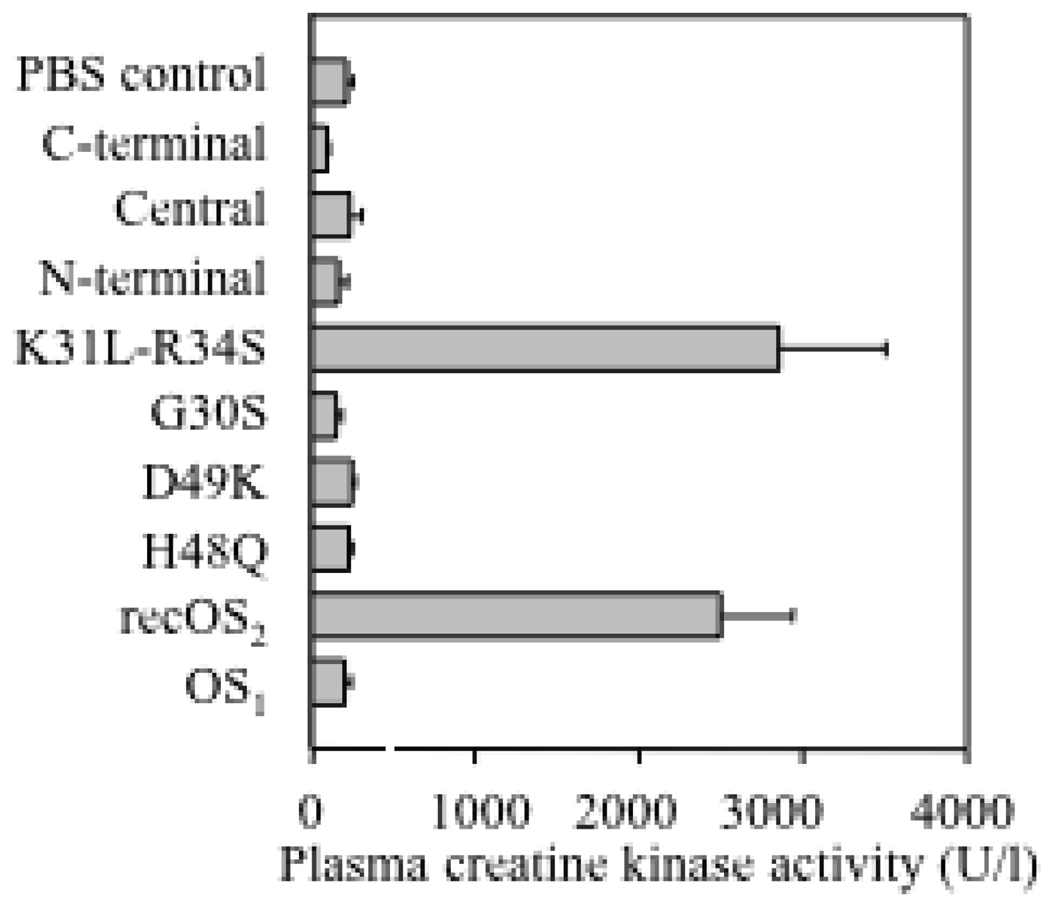
We also analyzed whether OS2 can exert myotoxic effects when injected intramuscularly into mice (Fig. 8). Only the groups of mice injected with recombinant WT OS2 and the mutant K31L-R34S showed signs of myotoxic intoxication, as measured by plasma creatine kinase activity. In both cases, mice also developed signs of neurotoxicity, with paralysis of the limbs and respiratory difficulties. Three out of the five mice injected with recombinant WT OS2 and the mutant K31L-R34S died within 8 hr. In addition, mice in these two experimental groups showed macroscopic swelling and immobilization of the injected limb consistent with myotoxic effects, as previously observed for other myotoxins (71). In contrast, mice injected with the other mutants, chimeras, and venom OS1 did not show any evidence of intoxication, nor local myotoxic effects at the site of injection.
Fig. 8.
Myotoxic effects of OS2 and its mutants. Three hours after intramuscular injection of sPLA2 (5 µg) into mice, the plasma was collected and creatine kinase activity was measured as an index of myotoxicity. One unit of creatine kinase activity is defined as the amount of enzyme that produces 1 µmol of NADH/min.
Anti-Plasmodium activity of OS2 and its mutants
WT OS2 and its mutants were tested for their ability to inhibit the intra-erythrocytic growth of P. falciparum, the causative agent of the most severe form of human malaria (Table 2). The effect of sPLA2s was assayed in the presence of human serum (8%) or in a semi-defined medium (0.5% AlbuMAX II, a serum substitute devoid of lipoproteins) to evaluate whether sPLA2s act directly or indirectly by modification of serum components including hydrolysis of lipoprotein phospholipids (72). In the presence of serum, both OS1 and WT OS2 inhibited P. falciparum growth. Remarkably, OS1 was even more potent than OS2 (Table 2). The mutants H48Q, D49K, G30S and the N-terminal chimera, that display extremely low enzymatic activities on all types of phospholipids (Table 3), were totally inactive against P. falciparum. Conversely, the mutant K31L/R34S, the central chimera, and to a lesser extent the C-terminal chimera, were still able to inhibit the growth of the parasite. The results were dramatically different in the presence of AlbuMAX, where most of the sPLA2s were inactive. Under those conditions, only the sPLA2s which are extremely potent in the presence of human serum were still capable of preventing the growth of the parasite, yet at much higher concentrations (Table 2).
Inhibition of HIV replication by OS2 and its mutants
We previously found that several but not all venom sPLA2s can block the entry of HIV-1 into host cells (20). Venom and recombinant WT OS2 as well as the mutant K31L-R34S were found to be inhibitors of HIV-1 infectivity with IC50 values in the nM range (Table 2). Conversely, at concentrations up to 1 µM, the mutants H48Q, D49K, G30S and the N-terminal chimera did not inhibit HIV-1 infection. However, the central and C-terminal chimeras still retained a fairly potent antiviral activity, with IC50 values between those of WT OS2 and OS1 (Table 2).
DISCUSSION
A large number of studies using in silico protein sequence comparisons, chemical modification of amino acids, and more recently site-directed mutagenesis on different toxic sPLA2s have been carried out over the past 30 years (23, 35, 43). However, the results from these studies have remained difficult to interpret for several reasons: i) sPLA2s are quite compact molecules and some mutations may cause structural changes at sites remote from the mutation site; ii) they probably exert most of their biological properties through relatively large molecular surfaces encompassing several discrete domains (i.e. the N-terminal helix, Ca2+-loop, etc..); iii) their mechanism of action may include several steps (39, 40); iv) several studies have been based on chemical modification or mutagenesis of single amino acids, which in most cases have led to only relatively modest effects; v) the results obtained for a particular venom sPLA2 may not apply to other sPLA2s; vi) some amino acids that are important for catalytic activity may also be involved in receptor binding or biological activities.
The main goal of our study was to obtain new insights into the structure-function relationships of venom sPLA2s using OS2 from the Australian Taipan snake venom as a template, and with a particular focus on its central neurotoxic effects. Our first approach employed site-directed mutagenesis at the active site which, to our knowledge, has never been used to establish the relationship between enzymatic catalysis and central neurotoxicity. The second approach used large chimeras between OS1 and OS2, and is based on the observation that the two proteins from the same Taipan venom display very distinct catalytic, toxic, and N-type receptor binding properties. The aim of this second approach was to produce very dramatic changes in the molecular and pharmacological properties of OS2, and thus map unambiguously which of the three regions plays the major role. A similar chimeric approach was used for ammodytoxins, but in that case, the predicted C-terminal neurotoxic region of ammodytoxin A was introduced into the non toxic ammodytoxin I2, and only mild effects were observed (34). As discussed below in more details, our results clearly indicate that : i) based on the clear dissociation observed for the H48Q mutant and the C-terminal chimera, the catalytic activity of OS2 is responsible for little, if any, of the central neurotoxicity; ii) the N-terminal region of OS2 and some well conserved residues of the Ca2+-loop, but not the central and C-terminal regions, are key elements for central neurotoxicity and several other pharmacological effects; iii) The same N-terminal region allows OS2 to bind to a subset of N-type receptors which is likely to be associated with central neurotoxicity; iv) The antimalarial effect of OS2 and OS1 appears to be dependent on the catalytic activity of these enzymes on serum lipoproteins. Together, these findings support the view that a single sPLA2 exerts its different pharmacological effects through distinct mechanisms that depend or not on its enzymatic activity (2).
Enzymatic properties of the OS2 mutants and chimeras
Our two approaches have generated OS2 mutants which have dramatically lower enzymatic activity for different reasons. The single point mutants G30S, H48Q and D49K have sPLA2 activity reduced by 500-fold or more, essentially because they cannot efficiently bind or hydrolyze phospholipids in their active site (51). Conversely, the N-terminal and C-terminal chimeras have reduced activity because they cannot efficiently bind to the phospholipid membrane interface. Indeed, the sPLA2 catalysis consists of two distinct steps, in which the enzyme first binds to a surface of about 40–50 phospholipids at the phospholipid-water interface (interfacial binding) and then forms a Michaelis-Menten complex by binding a single phospholipid molecule into the active site (51). These two steps involve two topologically distinct sPLA2 domains, ie the interfacial binding surface and the active site slot. The active site contains six residues which are conserved in all active sPLA2s among which G30, H48 and D49 play major catalytic roles. Conversely, the interfacial binding surface comprises at least 10–15 residues which are not conserved between sPLA2s and which can be located in the N-terminal, central and C-terminal regions of sPLA2s. The nature of these residues will determine the affinity of the enzyme for different lipid interfaces. Over the years, it has become clear that the interfacial binding of sPLA2s is the key step that governs and explains most of the differences in specific activities on different phospholipid substrates. From a large number of studies, first performed on venom sPLA2s and then on the various mammalian enzymes, it is now clear that while basic, neutral, and aliphatic residues found in the interfacial binding domain contribute to phospholipid binding, a few aromatic residues, especially tryptophans, play major roles in sPLA2 binding, particularly to zwitterionic phospholipids (73–81).
The 500- to 1,000-fold drop in catalytic activity of the N-terminal and C-terminal chimeras is largely explained by their decreased binding affinity for phospholipid vesicles (Table 3). This indicates that the N-terminal and C-terminal regions of OS2, but not the central one, contain key residues which are involved in high affinity interfacial binding and are not present in OS1. Among these residues, OS2 has a tryptophan located in the C-terminal region (Fig. 2 and position 117 in Fig. 1) that would explain at least in part why the C-terminal chimera is less active than WT OS2 (Table 3). Accordingly, mutation of asparagine to a tryptophan at this position (N117W) in the pancreatic group IB sPLA2 results in a 18-fold increase in interfacial binding (78). OS2 has a second tryptophan at position 75 (Fig. 1), but this residue is located on the β-wing structure in the central region, far away from the interfacial binding domain, and thus most probably does not participate in interfacial binding. This is consistent with the fact that for many sPLA2s, the critical interfacial binding residues are present in the N-terminal and C-terminal regions (79, 80, 82–86). The slightly altered enzymatic activity and substrate specificity of the central chimera (Table 3) may be related at least in part to the deletion of the OS1 pancreatic loop, whose deletion in the pancreatic group IB sPLA2 produced similar effects (87).
The H48Q mutant does not show altered interfacial binding under our experimental conditions, indicating that the drop in activity is essentially due to its inability to hydrolyze phospholipids in the active site. Conversely, the very low enzymatic activity of the OS2 active site mutants G30S and D49K is due not only to their inability to bind the substrate at the active site, but also to altered interfacial binding (Table 3). Previous studies on the pancreatic group IB and human group IIA sPLA2s have shown that the mutations G30S, H48Q and D49K largely alter phospholipid catalysis, but have little effects on interfacial binding (62–65, 88). Similar conclusions were drawn from enzymatic studies and crystal structures of venom sPLA2s harboring the natural mutations G30S, H48Q and D49K (59–61, 89). However, detailed studies on the role of the Ca2+ cofactor in catalysis by pancreatic and bee venom sPLA2s indicated that the interfacial binding and the binding at the active site are interconnected, where the Ca2+-dependent binding of the substrate at the active site partially drives the interfacial binding of sPLA2 by mass action (81, 90, 91). Under our phospholipid vesicle assay conditions (20 mole% DOetPS/ 80 mole% DOetPC), the mutants G30S and D49K do not bind efficiently to the vesicles, which can be explained at least in part by the fact that they cannot bind Ca2+ and thus their substrate at the active site. This result also agrees with the absence of vesicle binding for WT OS2 when Ca2+ is omitted (not shown). Additionally, the structural data accumulated so far also suggest that the mutations G30S, H48Q and D49K locally alter to different extents the conformations of the Ca2+-loop and N-terminal helix, with conformational changes induced by the mutation H48Q being smaller than those induced by mutation of Asp-49 (61, 63, 92, 93). On the other hand, deletion of the C-terminal region of the pancreatic sPLA2 affects substrate binding at the active site (90). In the case of His-48 alkylation, an allosteric coupling between the catalytic site and the membrane binding surface of the sPLA2 has been proposed (43–46, 94). Furthermore, the hydrogen bonding network connecting the active site to the interfacial binding domain and which involves His-48 is likely to be important for the conformational stability of sPLA2s (46, 92). Finally, a recent study showed that binding of an hydrophobic ligand in the active site of a Lys-49 venom sPLA2 induces conformational changes in the C-terminal domain (95). In summary, it is likely that the drop in activity observed in the case of the OS2 active site mutants (Table 3) is due in large part to the point mutation affecting a key catalytic functionality, ie phospholipid hydrolysis for the H48Q and Ca2+-dependent phospholipid binding at the active site for G30S and D49K. However, we propose that these mutations may also induce small (H48Q) or significant (G30S and D49K) conformational changes at the surface of the protein, especially on the N-terminal alpha-helix. These conformational changes would represent the molecular basis for the loss of central neurotoxicity and binding properties to N-type receptors of these mutants (see below).
Probing the role of sPLA2 activity in central neurotoxicity of OS2
Our first aim was to analyze the role of sPLA2 activity by preparing catalytically inactive mutants of OS2 and determining whether these mutants are still neurotoxic when injected intracerebroventricularly into mice. OS2 is one of the most neurotoxic sPLA2s by this route (30). The results obtained with the mutant H48Q indicate a clear dissociation, since this mutant is only 16-fold less neurotoxic, but 500-fold less catalytically active than WT OS2. This result is in accordance with the partial dissociation obtained in the past by alkylation of His-48 on several neurotoxic sPLA2s (41–43, 96). The results obtained with the mutants G30S and D49K support the view that sPLA2 activity may be required for neurotoxicity since these two mutants have lost both properties (Table 2 and Table 3). However, as explained above, their loss of neurotoxicity may be due to a conformational change at the surface of the protein induced by the point mutation. It may be also possible that surface residues from the Ca2+-loop including G30 more directly participate to the interaction with the neurotoxic cellular targets. Our data obtained with the central and C-terminal chimera further indicate that there is no direct correlation between enzymatic activity and neurotoxicity. Indeed, the central chimera is at least as active as WT OS2, but has a 35-fold lower neurotoxicity, and the C-terminal chimera has 500-fold lower activity, but only 16-fold less central neurotoxicity. The fact that both the H48Q mutant and the C-terminal chimera have dramatically reduced enzymatic activity on both anionic and zwitterionic phospholipids likely indicates that their catalytic activity is also very low on cellular phospholipids in vivo, although this hypothesis should deserve attention in future studies.
The fact that the H48Q mutant and the C-terminal chimera retain most of the central neurotoxicity of WT OS2, yet show a much more drastic loss in enzymatic activity on all types of anionic and zwitterionic phospholipid substrates (Table 3) indicates that the enzymatic activity is at most a minor factor involved in central neurotoxicity. If we assume that the 16-fold decrease in central neurotoxicity is due to mutations that cause a small structural change in the neurotoxic site of OS2 which in turn affects the energetics of its binding to brain components required for central neurotoxicity (a 16-fold factor would correspond to a loss of binding energy to brain component(s) of about 1.5 kcal/mol), the catalytic activity of OS2 would in fact not be required for central neurotoxicity. According to this hypothesis, the neurotoxic action of OS2 would simply be due to the specific binding of the sPLA2 to its cellular neuronal targets via the neurotoxic site. OS2, and possibly other neurotoxic sPLA2s, would then behave like many other distinct neurotoxins which are devoid of catalytic activity and bind to specific membrane targets (97–99). This view is reminiscent of the situation observed for the K-49 myotoxic sPLA2s, which exert their myotoxic effect independently of enzymatic activity, but via a myotoxic site located on the C-terminal region of the sPLA2 (36). It is also reminiscent of the fact that the major anticoagulant properties of sPLA2s do not require enzymatic activity, but rather a specific binding to factor Xa (15, 100). The production of OS2 mutants fully devoid of catalytic activity yet still highly neurotoxic is however required to validate this hypothesis.
Mapping the region of OS2 essential for its central neurotoxicity
The results obtained with the chimeras indicate that the N-terminal region of OS2 contains key residues which are not present in OS1 and constitute the major structural determinants of the neurotoxic site. The slight decrease in central neurotoxicity observed for the central and C-terminal chimeras suggests that a few residues from these regions contribute to neurotoxicity, or that their mutations mildly affect the structure of the N-terminal region. Similarly, the results obtained with the mutants G30S and D49K indicate that strictly conserved residues from the Ca2+-loop are either key structural elements of the neurotoxic site or that these residues contribute to the conformational stability of the neurotoxic site. The neurotoxic site of OS2 is thus overlapping with the interfacial binding domain, and this explains why the sPLA2 activity seems to be involved, although no obvious correlation can be found. The proposed location of the OS2 neurotoxic site agrees with several other studies based on protein sequence alignments, crystal structure analyses, amino acid modifications and site-directed mutagenesis (35, 43, 61, 67, 84, 96, 101). However, it is in apparent contradiction with some studies indicating that the β-wing or the C-terminal domains are involved ((47, 102, 103) and references therein). Importantly, it should be noted that the modifications or mutations performed in these latter studies led in most cases to a small decrease in neurotoxicity, lower than 10-fold, which is comparable to the relatively modest 16- and 35-fold decreases observed here for the central and C-terminal chimeras. Our results using the single point mutations D49K, G30S and the chimeras led to more consistent decrease or even complete loss of neurotoxicity, unambiguously indicating that the Ca2+-loop and the N-terminal region are crucially involved. Finally, it is remarkable that the single point mutation G30S completely inactivates OS2, and virtually converts this latter into OS1 in terms of molecular and functional properties (except for the antimalarial activity, see below). The same is also observed for the N-terminal chimera, and a similar result was previously observed for the bee venom sPLA2 when mutated at the functionally homologous glycine-12 (67).
Binding properties of the OS2 mutants and chimeras to M-type and N-type receptors and relationship with central neurotoxicity
The pancreatic sPLA2 has previously been shown to bind to the M-type receptor through residues located in or close to the Ca2+-loop, and this binding leads to an inhibition of sPLA2 activity (25, 32). However, the binding does not appear to be related to enzymatic activity since both catalytically active and inactive enzymes can bind to the M-type receptor (25, 32, 50). In the case of OS2, we also found that the active site mutants can bind to rabbit or mouse M-type receptors with high affinity (Table 4). Since the active site residues, in particular His-48 and Asp-49, probably do not directly interact with residues from the receptor, it is likely that the small shifts in affinity observed for the H48Q and D49K mutants (Table 4) are due to mutation-induced conformational changes occurring on the molecular surface of OS2 (see above). For chimeras, we found that they all bind to the M-type receptor. This result was expected since both OS1 and OS2 bind to this receptor (58). Since OS1 and both neurotoxic and non neurotoxic mutants of OS2 can bind with similar affinities to this receptor while several other neurotoxic sPLA2s do not bind to this receptor (58), it is unlikely that the M-type receptor plays a critical role in central neurotoxicity. This contrasts with the proposed role of the M-type receptor in the neurotoxicity of ammodytoxins (39).
The N-type receptors for OS2 and other neurotoxic sPLA2s were initially identified in rat brain membranes (30), and then in several peripheral tissues and cells (25), including the HeLa P4 cells used for HIV studies (see below). Although not yet cloned, these receptors are likely to form a family of binding proteins with molecular masses distinct from that of the M-type receptor (25). The physiological roles of these receptors are still unclear, but they have been proposed to participate in the neurotoxic and anti-HIV activities of venom sPLA2s (20, 30). The binding properties of the different mutants to the N-type receptors expressed in mouse brain microsomal membranes and HeLa P4 cells follow the same trend. As measured by competition studies using iodinated WT OS2, mutations in the active site decrease the affinity of OS2 for the N-type receptors, but there is no obvious relationship between receptor affinity and enzymatic activity or interfacial binding to purified lipid vesicles. As discussed above, the shift in affinity may be explained by the fact that mutations in the active site lead to conformational changes in the Ca2+-loop and N-terminal surface of OS2 that directly affect the binding properties. This explanation appears more likely than the one assuming that the receptor has a molecular arm penetrating directly into the active site. A similar shift in affinity was observed when OS2 was alkylated by p-bromo-phenacyl-bromide (unpublished data). The absence of relationship between binding and enzymatic activity was also observed for the chimeras, where all three possibilities were found, ie loss of both receptor binding and enzymatic activity (N-terminal chimera), loss of binding but not sPLA2 activity (central chimera), or loss of more enzymatic activity than binding (C-terminal chimera).
The apparent discrepancies observed between cross-linking experiments and competition assays with the central and C-terminal chimeras against labeled WT OS2 prompted us to investigate their direct binding properties. The radiolabeled C-terminal chimera appeared to bind to a minor subset of OS2 binding sites which displays high affinity for WT OS2, as well as for the central and C-terminal chimeras, but not for the N-terminal chimera (Fig. 6). These results restore at least partially the correlation between the neurotoxicity and the affinity of OS2 mutants for this subset of N-type binding sites, and thus suggest that the high affinity binding sites for the C-terminal chimera are more directly involved in central neurotoxicity. The molecular nature and complexity of these binding sites are unknown, but they most likely consist of several proteins, as revealed by the cross-linking experiments and the curvilinear Scatchard plot analysis (Fig. 5 and Fig. 6). The nature of the other subset of WT OS2 binding sites on which the C-terminal chimera binds with low affinity is also unknown, but the fact that the C-terminal chimera cannot bind efficiently to phospholipid vesicles raises the possibility that they are lipidic in nature. These latter binding sites would not be important for central neurotoxicity. The N-terminal region of OS2, but not the central region, appears to be critical for the interaction with the two subsets of N-type OS2 binding sites. On the other hand, the C-terminal region of OS2 appears important only for binding to the subset which may be lipidic in nature. The critical role of the N-terminal region in central neurotoxicity of OS2 and interaction with these subsets of N-type receptors is in agreement with the results previously obtained for bee venom sPLA2 (67) and other neurotoxic sPLA2s including taipoxin, Pa-11, crotoxin (104) and ammodytoxins ((105) and references therein). The relationships between the ammodytoxin binding proteins which have been identified (calmodulin, R25 and 14-3-3 protein isoforms) and these subsets of N-type receptors are still unclear. However, our results are in agreement with some of the structure-function studies on ammodytoxins indicating that residues in the N-terminal and C-terminal regions, but not the β-wing (which is within the OS1/OS2 region swapped in the central chimera), are involved in binding to calmodulin and R25 (34, 47, 84).
Inhibition of neuromuscular transmission and myotoxic effects induced by OS2
Similarly to many neurotoxic snake venom sPLA2s which are called β-neurotoxins and include β-Bungarotoxin, crotoxin, textilotoxin, notexin, and taipoxin (69, 106), we found that OS2, but not OS1, acts pre-synaptically on a chick biventer cervicis neuromuscular preparation, indicating that OS2 is also a β-neurotoxin. At 200 nM, we did not observe any evidence for a post-synaptic effect, which fits with the fact that β-neurotoxins exert post-synaptic effects only at concentrations higher than those required for their neurotoxic effects (107). Except for K31L/R34S, none of the OS2 mutants had any inhibitory effect at this concentration on the chick biventer cervicis preparation, hampering the analysis of the structure-function relationships of OS2 in this system. The relatively low activity of OS2 in this model is probably linked to the resistance of the chick preparation to the different β-neurotoxins from Australian elapids (106), although we observed that OS2 is highly neurotoxic when directly injected in chick brain (unpublished data).
Several group IA and IIA venom sPLA2s which are catalytically active (D49) or inactive (K49) act as potent myotoxins that induce muscle necrosis by disrupting the integrity of the plasma membrane (14). The myotoxicity of group I and II sPLA2s may depend on different structural determinants. The primary mechanism of action of both active and inactive group II myotoxic sPLA2s is independent of sPLA2 activity, and is likely to be due to basic and aromatic residues in the C-terminal region that allow the toxin to bind directly to phospholipids or yet ill-defined protein targets on the surface of skeletal muscle cells (14, 35, 36, 95, 108). On the other hand, all potent myotoxic group I sPLA2s were found to lack the pancreatic loop and to have a cluster of surface residues including R15, V88, A100, N108, and cationic and hydrophobic residues at positions 83 and 109 (109). Here, we found that OS2, but not OS1, exerts myotoxic effects with histological and biochemical features similar to those of group I and II sPLA2 myotoxins. OS2 has most of the residues associated with myotoxicity in group I sPLA2s, and lacks the pancreatic loop, whereas OS1 lacks V88 and has the pancreatic loop (Fig 1). Like myotoxic group II sPLA2s, OS2, but not OS1, also has basic and aromatic residues in its C-terminal region (Fig. 1), including W117 (Fig. 1 and Fig. 2), which probably plays an important role (110, 111). The role of these residues and sPLA2 activity could not be analyzed in detail since all the OS2 mutants, except K31L-R34S, appeared to lack myotoxic activity. Since most of the sPLA2 mutants that have lost myotoxicity have also lost enzymatic activity, one might conclude at first sight that sPLA2 activity is critical. However, the relationship is still unclear since the central chimera is not myotoxic while it has a high sPLA2 activity. Since most OS2 mutants bind to the M-type receptor while they are no longer myotoxic, it is unlikely that this receptor is involved in myotoxicity, at least in the mouse. It is also unlikely because the receptor is expressed at very low levels in adult mouse skeletal muscle, and because the very potent K49 myotoxins Ba-II and Ba-IV purified from Bothrops asper do not bind to the endogenous or cloned mouse M-type receptor (MR, BL, JMG, and GL, unpublished data).
Overall, the same trend was observed for the different OS2 mutants at the chick neuromuscular junction and for myotoxicity. The fact that all enzymatically inactive OS2 mutants are inefficient suggests that catalytic activity is essential for the two types of toxicity. However, the fact that the central chimera is also inactive in both cases, while it has a high level of enzymatic activity, suggests that both catalytic activity and other cellular factor(s) are critical for peripheral neurotoxicity and myotoxicity, with the central region of OS2 containing some key structural determinants. Importantly, these observations contrast with those observed for central neurotoxicity, where the H48Q mutant and the central and C-terminal chimeras were nearly as active as WT OS2 (see above). This suggests that the neurotoxic mechanisms operating at the peripheral neuromuscular junction and those occuring in the brain may be distinct. Very recently, Montecucco et al. proposed that the enzymatic activity of different venom sPLA2s is a key factor for peripheral neurotoxicity (40). This proposal was based on the fact that equimolar mixtures of lysophospholipids and fatty acids closely mimic the biological effects of venom sPLA2s at the mouse neuromuscular junction. Unfortunately, no mutated neurotoxic sPLA2s were used in this study. This proposal fits our results at the neuromuscular junction, but contrasts with our findings regarding central neurotoxicity. Since Montecucco et al. do not exclude the contribution of sPLA2 receptors in their proposed mechanism of action (40), it may be possible that different receptors are targeted at the peripheral and central nervous systems. The subtype of brain receptors identified with the C-terminal chimera would be one of these receptors.
Anti-malarial and Anti-HIV activity of OS2 mutants and chimeras
Several venom sPLA2s have been shown to kill the parasite P. falciparum during its intra-erythrocytic development (21) by an indirect mechanism mediated by hydrolysis of serum lipoproteins and production of lipid products which are toxic for the parasite (72). Our results with OS2 and its mutants are in accordance with this mechanism. Indeed, the inhibitory potency of the mutants correlates with their relative decrease in enzymatic activity and is dependent on the presence of serum. In this case, OS1 and the central chimera are as active as, or even more active than WT OS2. Whether their effects are related to particular enzymatic properties (Table 3) toward serum lipoproteins requires further investigation.
Our previous studies have shown that several venom sPLA2s can also inhibit HIV replication by a mechanism that is still ill-defined, but that may not be associated with catalytic activity, since catalytically inactive K-49 sPLA2s can inhibit HIV while sPLA2 catalytic products and inhibitors do not block infection (20, 37). Among all the sPLA2s tested, bee venom sPLA2 appears to be the most potent with an IC50 of 0.6 nM. WT OS2 is about 60-fold less potent with an IC50 of 35 nM, yet it is more potent than OS1 (Table 2). This relatively low inhibitory activity has prevented a detailed analysis of the OS2 antiviral structure-function relationships. Nevertheless, our results show that, as for central neurotoxicity, the N-terminal, but not the central and C-terminal regions of OS2 is critical. At first sight, our results support the view that catalytic activity is important. However, the result with the C-terminal chimera which is still potent against HIV while it has a low enzymatic activity supports the view that this mutant binds to a subtype of N-receptors which may be similar to the one identified in mouse brain.
Concluding remarks
Our results demonstrate that some biological effects of OS2 are due to its enzymatic activity (for example the antimalarial effect), while others including central neurotoxicity are more related to specific binding to target cells than enzymatic activity. The most obvious dissociation between enzymatic activity and central neurotoxicity was observed with the H48Q mutant and the C-terminal chimera. Based on the chimera approach, both the N- and C-terminal regions are important for catalytic activity, but only the N-terminal is critical for central neurotoxicity and anti-HIV activity. Based on G30S and D49K mutants, the Ca2+-loop appears important for all activities, although it is highly conserved among sPLA2s. Based on the central chimera, the β-wing structure did not appear to play a key role in the molecular properties or biological activities of OS2. Amazingly, no clear role has been so far attributed to this particular structure or to the surface residues located in the two large alpha-helices on the back of the sPLA2 (grey scaffold in Fig. 2). This suggests that these two regions play only a structural role, serving as a rigid scaffold on which the functionally important and flexible domains containing the key residues involved in the specific sPLA2 functions are anchored (92). Finally, our findings may help to develop novel strategies to neutralize the toxicity of venom sPLA2s (28) or to engineer sPLA2s with more specific functions. It will be interesting to analyze in particular i) the effect of sPLA2 competitive inhibitors or soluble M-type receptors on the biological effects of OS2 and other sPLA2s, and ii) the effects of peptides from the N-terminal alpha helix or the Ca2+-loop for their neurotoxic or anti-HIV properties, or for their inhibition of the sPLA2 action, as these peptides may be agonists or antagonists. Short peptides derived from the C-terminal region of myotoxic K49 sPLA2s where are (36, 112).
ACKNOWLEDGEMENTS
We thank Catherine Le Calvez, Sabine Scarzello, and Romain Gauthier for expert assistance in the structural characterization and computer modeling of the OS2 protein. We are grateful to Carine Mounier, Fanny Surrel, and Cassian Bon for a critical reading of the manuscript.
This work was supported in part by CNRS (to G.L.), the Association pour la Recherche sur le Cancer (to G.L.), and National Institutes of Health Grant HL36236 (to M.H.G.). M.R. L.D.R. and E.B. are respectively supported by fellowships from the Fondation de la Recherche Médicale, the INSERM/NH&MRC (ID 194470) grant and the Canadian Institute of Health Research in partnership with the Arthritis Society.
The abbreviations used are
- ACN
acetonitrile
- CD
circular dichroism
- DSS
Suberic acid bis-N-hydroxysuccinimide ester
- HIV-1
human immunodeficiency virus-1
- LD100
lethal dose for 100% of the population tested
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- OS1
Oxyuranus scutellatus scutellatus toxin 1
- OS2
Oxyuranus scutellatus scutellatus toxin 2
- recOS2
recombinant OS2
- vOS2
venom OS2
- POPG/S/C
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol /serine /choline
- sPLA2
secreted phospholipase A2
- TFA
trifluoracetic acid
- WT
Wild-Type
REFERENCES
- 1.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim. Biophys. Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 2.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 4.Zadori Z, Szelei J, Lacoste MC, Li Y, Gariepy S, Raymond P, Allaire M, Nabi IR, Tijssen P. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell. 2001;1:291–302. doi: 10.1016/s1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 5.Soragni E, Bolchi A, Balestrini R, Gambaretto C, Percudani R, Bonfante P, Ottonello S. A nutrient-regulated, dual localization phospholipase A(2) in the symbiotic fungus Tuber borchii. Embo J. 2001;20:5079–5090. doi: 10.1093/emboj/20.18.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagiec MJ, Lei B, Parker SK, Vasil ML, Matsumoto M, Ireland RM, Beres SB, Hoe NP, Musser JM. Analysis of a novel prophage-encoded group A Streptococcus extracellular phospholipase A(2) J. Biol. Chem. 2004;279:45909–45918. doi: 10.1074/jbc.M405434200. [DOI] [PubMed] [Google Scholar]
- 7.Hanasaki K, Arita H. Biological functions of group X secretory PLA2. Adv. Exp. Med. Biol. 2003;525:93–96. doi: 10.1007/978-1-4419-9194-2_18. [DOI] [PubMed] [Google Scholar]
- 8.Rouault M, Bollinger JG, Lazdunski M, Gelb MH, Lambeau G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry. 2003;42:11494–11503. doi: 10.1021/bi0349930. [DOI] [PubMed] [Google Scholar]
- 9.Diaz BL, Arm JP. Phospholipase A2. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:87–97. doi: 10.1016/s0952-3278(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 10.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 11.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 12.Murakami M, Masuda S, Shimbara S, Ishikawa Y, Ishii T, Kudo I. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A(2) J. Biol. Chem. 2005;280:24987–24998. doi: 10.1074/jbc.M502088200. [DOI] [PubMed] [Google Scholar]
- 13.Chwetzoff S, Tsunasawa S, Sakiyama F, Menez A. Nigexine, a phospholipase A2 from cobra venom with cytotoxic properties not related to esterase activity. Purification, amino acid sequence, and biological properties. J. Biol. Chem. 1989;264:13289–13297. [PubMed] [Google Scholar]
- 14.Gutierrez JM, Ownby CL. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Kini RM. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon. 2005;45:1147–1161. doi: 10.1016/j.toxicon.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Zuliani JP, Fernandes CM, Zamuner SR, Gutierrez JM, Teixeira CF. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45:335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo MT, Nguyen E, Aldo-Benson M, Lambeau G. Secreted phospholipase A2 induces vascular endothelial cell migration. Blood. 2000;96:3809–3815. [PubMed] [Google Scholar]
- 18.Rufini S, Cesaroni MP, Balestro N, Luly P. Proliferative effect of ammodytin L from the venom of Vipera ammodytes on 208F rat fibroblasts in culture. Biochem. J. 1996;320:467–472. doi: 10.1042/bj3200467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santamaria C, Larios S, Angulo Y, Pizarro-Cerda J, Gorvel JP, Moreno E, Lomonte B. Antimicrobial activity of myotoxic phospholipases A2 from crotalid snake venoms and synthetic peptide variants derived from their C-terminal region. Toxicon. 2005;45:807–815. doi: 10.1016/j.toxicon.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Fenard D, Lambeau G, Valentin E, Lefebvre JC, Lazdunski M, Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Invest. 1999;104:611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deregnaucourt C, Schrevel J. Bee venom phospholipase A2 induces stage-specific growth arrest of the intraerythrocytic Plasmodium falciparum via modifications of human serum components. J. Biol. Chem. 2000;275:39973–39980. doi: 10.1074/jbc.M006712200. [DOI] [PubMed] [Google Scholar]
- 22.Valentin E, Lambeau G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie. 2000;82:815–831. doi: 10.1016/s0300-9084(00)01168-8. [DOI] [PubMed] [Google Scholar]
- 23.Jeng TW, Hendon RA, Fraenkel-Conrat H. Search for relationships among the hemolytic, phospholipolytic, and neurotoxic activities of snake venoms. Proc. Natl. Acad. Sci. U S A. 1978;75:600–604. doi: 10.1073/pnas.75.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kini RM, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27:613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 25.Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999;20:162–170. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 26.Stefansson S, Kini RM, Evans HJ. The basic phospholipase A2 from Naja nigricollis venom inhibits the prothrombinase complex by a novel nonenzymatic mechanism. Biochemistry. 1990;29:7742–7746. doi: 10.1021/bi00485a024. [DOI] [PubMed] [Google Scholar]
- 27.Sribar J, Sherman NE, Prijatelj P, Faure G, Gubensek F, Fox JW, Aitken A, Pungercar J, Krizaj I. The neurotoxic phospholipase A2 associates, through a non-phosphorylated binding motif, with 14-3-3 protein gamma and epsilon isoforms. Biochem. Biophys. Res. Commun. 2003;302:691–696. doi: 10.1016/s0006-291x(03)00228-6. [DOI] [PubMed] [Google Scholar]
- 28.Lizano S, Domont G, Perales J. Natural phospholipase A(2) myotoxin inhibitor proteins from snakes, mammals and plants. Toxicon. 2003;42:963–977. doi: 10.1016/j.toxicon.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Rehm H. Molecular aspects of neuronal voltage-dependent K+ channels. Eur. J. Biochem. 1991;202:701–713. doi: 10.1111/j.1432-1033.1991.tb16425.x. [DOI] [PubMed] [Google Scholar]
- 30.Lambeau G, Barhanin J, Schweitz H, Qar J, Lazdunski M. Identification and properties of very high affinity brain membrane-binding sites for a neurotoxic phospholipase from the Taipan venom. J. Biol. Chem. 1989;264:11503–11510. [PubMed] [Google Scholar]
- 31.Cupillard L, Mulherkar R, Gomez N, Kadam S, Valentin E, Lazdunski M, Lambeau G. Both group IB and group IIA secreted phospholipases A2 are natural ligands of the mouse M-type receptor. J. Biol. Chem. 1999;274:7043–7051. doi: 10.1074/jbc.274.11.7043. [DOI] [PubMed] [Google Scholar]
- 32.Hanasaki K, Arita H. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 2002;68–69:71–82. doi: 10.1016/s0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 33.Soares AM, Giglio JR. Chemical modifications of phospholipases A2 from snake venoms: effects on catalytic and pharmacological properties. Toxicon. 2003;42:855–868. doi: 10.1016/j.toxicon.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Prijatelj P, Krizaj I, Kralj B, Gubensek F, Pungercar J. The C-terminal region of ammodytoxins is important but not sufficient for neurotoxicity. Eur. J. Biochem. 2002;269:5759–5764. doi: 10.1046/j.1432-1033.2002.03301.x. [DOI] [PubMed] [Google Scholar]
- 35.Chioato L, Ward RJ. Mapping structural determinants of biological activities in snake venom phospholipases A2 by sequence analysis and site directed mutagenesis. Toxicon. 2003;42:869–883. doi: 10.1016/j.toxicon.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Lomonte B, Angulo Y, Calderon L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon. 2003;42:885–901. doi: 10.1016/j.toxicon.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Fenard D, Lambeau G, Maurin T, Lefebvre JC, Doglio A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of t-cell tropic hiv-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001;60:341–347. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- 38.Petan T, Krizaj I, Gelb MH, Pungercar J. Ammodytoxins, potent presynaptic neurotoxins, are also highly efficient phospholipase A2 enzymes. Biochemistry. 2005;44:12535–12545. doi: 10.1021/bi051024r. [DOI] [PubMed] [Google Scholar]
- 39.Krizaj I, Gubensek F. Neuronal receptors for phospholipases A(2)and beta-neurotoxicity. Biochimie. 2000;82:807–814. doi: 10.1016/s0300-9084(00)01172-x. [DOI] [PubMed] [Google Scholar]
- 40.Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 41.Fohlman J, Eaker D, Dowdall MJ, Lullmann-Rauch R, Sjodin T, Leander S. Chemical modification of taipoxin and the consequences for phospholipase activity, pathophysiology, and inhibition of high-affinity choline uptake. Eur. J. Biochem. 1979;94:531–540. doi: 10.1111/j.1432-1033.1979.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang CC, Su MJ. Presynaptic toxicity of the histidine-modified, phospholipase A2-inactive, beta-bungarotoxin, crotoxin and notexin. Toxicon. 1982;20:895–905. doi: 10.1016/0041-0101(82)90077-0. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg P, Ghassemi A, Condrea E, Dhillon D, Yang CC. Do chemical modifications dissociate between the enzymatic and pharmacological activities of beta bungarotoxin and notexin? Toxicon. 1989;27:137–159. doi: 10.1016/0041-0101(89)90128-1. [DOI] [PubMed] [Google Scholar]
- 44.Renetseder R, Dijkstra BW, Huizinga K, Kalk KH, Drenth J. Crystal structure of bovine pancreatic phospholipase A2 covalently inhibited by p-bromo-phenacyl-bromide. J. Mol. Biol. 1988;200:181–188. doi: 10.1016/0022-2836(88)90342-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Tang L, Wang X, Zhou Y, Lin Z. Structure of a snake venom phospholipase A2 modified by p-bromo- phenacyl-bromide [In Process Citation] Toxicon. 1998;36:875–886. doi: 10.1016/s0041-0101(97)00169-4. [DOI] [PubMed] [Google Scholar]
- 46.Tatulian SA. Structural effects of covalent inhibition of phospholipase A2 suggest allosteric coupling between membrane binding and catalytic sites. Biophys. J. 2003;84:1773–1783. doi: 10.1016/S0006-3495(03)74985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanovski G, Petan T, Krizaj I, Gelb MH, Gubensek F, Pungercar J. Basic amino acid residues in the beta-structure region contribute, but not critically, to presynaptic neurotoxicity of ammodytoxin A. Biochim. Biophys. Acta. 2004;1702:217–225. doi: 10.1016/j.bbapap.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Kolko M, Bruhn T, Christensen T, Lazdunski M, Lambeau G, Bazan NG, Diemer NH. Secretory phospholipase A2 potentiates glutamate-induced rat striatal neuronal cell death in vivo. Neurosci. Lett. 1999;274:167–170. doi: 10.1016/s0304-3940(99)00709-0. [DOI] [PubMed] [Google Scholar]
- 49.Fossier P, Lambeau G, Lazdunski M, Baux G. Inhibition of ACh release at an Aplysia synapse by neurotoxic phospholipases A2: specific receptors and mechanisms of action. J. Physiol. 1995;489:29–40. doi: 10.1113/jphysiol.1995.sp021027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck S, Lambeau G, Scholz-Pedretti K, Gelb MH, Janssen MJ, Edwards SH, Wilton DC, Pfeilschifter J, Kaszkin M. Potentiation of TNFalpha -induced sPLA2-IIA expression in mesangial cells by an autocrine loop involving secreted phospholipase A2 and PPARalpha activation. J. Biol. Chem. 2003;278:29799–29812. doi: 10.1074/jbc.M211763200. [DOI] [PubMed] [Google Scholar]
- 51.Berg OG, Gelb MH, Tsai MD, Jain MK. Interfacial enzymology: the secreted phospholipase A2 paradigm. Chem. Rev. 2001;101:2613–2654. doi: 10.1021/cr990139w. [DOI] [PubMed] [Google Scholar]
- 52.Lambeau G, Ancian P, Nicolas JP, Beiboer S, Moinier D, Verheij H, Lazdunski M. Structural elements of secretory phospholipases A2 involved in the binding to M-type receptors. J. Biol. Chem. 1995;270:5534–5540. doi: 10.1074/jbc.270.10.5534. [DOI] [PubMed] [Google Scholar]
- 53.Chen GT, Inouye M. Role of the AGA/AGG codons, the rarest codons in global gene expression in Escherichia coli. Genes Dev. 1994;8:2641–2652. doi: 10.1101/gad.8.21.2641. [DOI] [PubMed] [Google Scholar]
- 54.Moriyama EN, Powell JR. Codon usage bias and tRNA abundance in Drosophila. J. Mol. Evol. 1997;45:514–523. doi: 10.1007/pl00006256. [DOI] [PubMed] [Google Scholar]
- 55.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 56.Valentin E, Koduri RS, Scimeca J-C, Carle G, Gelb MH, Lazdunski M, Lambeau G. Cloning and recombinant expression of a novel mouse secreted phospholipase A2. J. Biol. Chem. 1999;274:19152–19160. doi: 10.1074/jbc.274.27.19152. [DOI] [PubMed] [Google Scholar]
- 57.Lambeau G, Ancian P, Barhanin J, Lazdunski M. Cloning and expression of a membrane receptor for secretory phospholipases A2. J. Biol. Chem. 1994;269:1575–1578. [PubMed] [Google Scholar]
- 58.Lambeau G, Schmid-Alliana A, Lazdunski M, Barhanin J. Identification and purification of a very high affinity binding protein for toxic phospholipases A2 in skeletal muscle. J. Biol. Chem. 1990;265:9526–9532. [PubMed] [Google Scholar]
- 59.Lind P, Eaker D. complete amino-acid sequence of a non-neurotoxic, non-enzymatic phospholipase A2 homolog from the venom of the Australian tiger snake Notechis scutatus scutatus. Eur. J. Biochem. 1980;111:403–409. doi: 10.1111/j.1432-1033.1980.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 60.Scott DL, Achari A, Vidal JC, Sigler PB. Crystallographic and biochemical studies of the (inactive) Lys-49 phospholipase A2 from the venom of Agkistridon piscivorus piscivorus. J. Biol. Chem. 1992;267:22645–22657. [PubMed] [Google Scholar]
- 61.Georgieva DN, Perbandt M, Rypniewski W, Hristov K, Genov N, Betzel C. The X-ray structure of a snake venom Gln48 phospholipase A2 at 1.9A resolution reveals anion-binding sites. Biochem. Biophys. Res. Commun. 2004;316:33–38. doi: 10.1016/j.bbrc.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 62.Janssen MJ, van de Wiel WA, Beiboer SH, van Kampen MD, Verheij HM, Slotboom AJ, Egmond MR. Catalytic role of the active site histidine of porcine pancreatic phospholipase A2 probed by the variants H48Q, H48N and H48K. Protein Eng. 1999;12:497–503. doi: 10.1093/protein/12.6.497. [DOI] [PubMed] [Google Scholar]
- 63.Edwards SH, Thompson D, Baker SF, Wood SP, Wilton DC. The crystal structure of the H48Q active site mutant of human group IIA secreted phospholipase A2 at 1.5 A resolution provides an insight into the catalytic mechanism. Biochemistry. 2002;41:15468–15476. doi: 10.1021/bi020485z. [DOI] [PubMed] [Google Scholar]
- 64.Van Den Bergh CJ, Slotboom AJ, Verheij HM, De Haas GH. The role of aspartic acid-49 in the active site of phospholipase A2. A site-specific mutagenesis study of porcine pancreatic phospholipase A2 and the rationale of the enzymatic activity of [lysine49]phospholipase A2 from Agkistrodon piscivorus piscivorus venom. Eur. J. Biochem. 1988;176:353–357. doi: 10.1111/j.1432-1033.1988.tb14288.x. [DOI] [PubMed] [Google Scholar]
- 65.Bekkers AC, Franken PA, Toxopeus E, Verheij HM, De Haas G. The importance of glycine-30 for enzymatic activity of phospholipase A2. Biochim. Biophys. Acta. 1991;1076:374–378. doi: 10.1016/0167-4838(91)90479-j. [DOI] [PubMed] [Google Scholar]
- 66.Van Den Bergh CJ, Slotboom AJ, Verheij HM, De Haas GH. The role of Asp-49 and other conserved amino acids in phospholipases A2 and their importance for enzymatic activity. J. Cell. Biochem. 1989;39:379–390. doi: 10.1002/jcb.240390404. [DOI] [PubMed] [Google Scholar]
- 67.Nicolas JP, Lin Y, Lambeau G, Ghomashchi F, Lazdunski M, Gelb MH. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 1997;272:7173–7180. doi: 10.1074/jbc.272.11.7173. [DOI] [PubMed] [Google Scholar]
- 68.Chizzonite R, Truitt T, Podlaski FJ, Wolitzky AG, Quinn PM, Nunes P, Stern AS, Gately MK. IL-12: monoclonal antibodies specific for the 40-kDa subunit block receptor binding and biologic activity on activated human lymphoblasts. J. Immunol. 1991;147:1548–1556. [PubMed] [Google Scholar]
- 69.Hawgood B, Bon C. Snake Venom Presynaptic Toxins. In: Tu AT, editor. Handbook of Natural Toxins. New York: Marcel Dekker; 1991. pp. 3–52. [Google Scholar]
- 70.Gandolfo G, Lambeau G, Lazdunski M, Gottesmann C. Effects on behavior and EEG of single chain phospholipases A2 from snake and bee venoms injected into rat brain. Research for a functional antagonism. Pharmacol. and Toxicol. 1996;78:341–347. doi: 10.1111/j.1600-0773.1996.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 71.Gutierrez JM, Lomonte B. phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33:1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 72.Guillaume C, Deregnaucourt C, Clavey V, Schrevel J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004;43:311–318. doi: 10.1016/j.toxicon.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Viljoen CC, Visser L, Botes DP. An essential tryptophan in the active site of phospholipase A2 from the venom of Bitis gabonica. Biochim. Biophys. Acta. 1976;438:424–436. doi: 10.1016/0005-2744(76)90259-x. [DOI] [PubMed] [Google Scholar]
- 74.Nishida S, Tamiya N. Tryptophan residues of phospholipase A2 from the venom of an Australian elapid snake (Pseudechis australis) Toxicon. 1991;29:429–439. doi: 10.1016/0041-0101(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 75.Chang LS, Kuo KW, Chang CC. Identification of functional involvement of tryptophan residues in phospholipase A2 from Naja naja atra (Taiwan cobra) snake venom. Biochim. Biophys. Acta. 1993;1202:216–220. doi: 10.1016/0167-4838(93)90007-e. [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Zhu H, Huang B, Rogers J, Yu BZ, Kumar A, Jain MK, Sundaralingam M, Tsai MD. Phospholipase A2 engineering. Probing the structural and functional roles of N-terminal residues with site-directed mutagenesis, X-ray, and NMR. Biochemistry. 1995;34:7322–7334. doi: 10.1021/bi00022a005. [DOI] [PubMed] [Google Scholar]
- 77.Han SK, Kim KP, Koduri R, Bittova L, Munoz NM, Leff AR, Wilton DC, Gelb MH, Cho W. Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J. Biol. Chem. 1999;274:11881–11888. doi: 10.1074/jbc.274.17.11881. [DOI] [PubMed] [Google Scholar]
- 78.Janssen MJ, Burghout PJ, Verheij HM, Slotboom AJ, Egmond MR. Introduction of a C-terminal aromatic sequence from snake venom phospholipases A2 into the porcine pancreatic isozyme dramatically changes the interfacial kinetics. Eur. J. Biochem. 1999;263:782–788. doi: 10.1046/j.1432-1327.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- 79.Bezzine S, Bollinger JG, Singer AG, Veatch SL, Keller SL, Gelb MH. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J. Biol. Chem. 2002;277:48523–48534. doi: 10.1074/jbc.M203137200. [DOI] [PubMed] [Google Scholar]
- 80.Beers SA, Buckland AG, Giles N, Gelb MH, Wilton DC. Effect of tryptophan insertions on the properties of the human group IIA phospholipase A2: mutagenesis produces an enzyme with characteristics similar to those of the human group V phospholipase A2. Biochemistry. 2003;42:7326–7338. doi: 10.1021/bi0343222. [DOI] [PubMed] [Google Scholar]
- 81.Bollinger JG, Diraviyam K, Ghomashchi F, Murray D, Gelb MH. Interfacial binding of bee venom secreted phospholipase A2 to membranes occurs predominantly by a nonelectrostatic mechanism. Biochemistry. 2004;43:13293–13304. doi: 10.1021/bi049390i. [DOI] [PubMed] [Google Scholar]
- 82.Snitko Y, Han SK, Lee BI, Cho W. Differential interfacial and substrate binding modes of mammalian pancreatic phospholipases A2: a comparison among human, bovine, and porcine enzymes. Biochemistry. 1999;38:7803–7810. doi: 10.1021/bi990600e. [DOI] [PubMed] [Google Scholar]
- 83.Canaan S, Nielsen R, Ghomashchi F, Robinson BH, Gelb MH. Unusual mode of binding of human group IIA secreted phospholipase A2 to anionic interfaces as studied by continuous wave and time domain electron paramagnetic resonance spectroscopy. J. Biol. Chem. 2002;277:30984–30990. doi: 10.1074/jbc.M203649200. [DOI] [PubMed] [Google Scholar]
- 84.Petan T, Krizaj I, Gubensek F, Pungercar J. Phenylalanine-24 in the N-terminal region of ammodytoxins is important for both enzymic activity and presynaptic toxicity. Biochem. J. 2002;363:353–358. doi: 10.1042/0264-6021:3630353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sa JM, Chioato L, Ferreira TL, De Oliveira AH, Ruller R, Rosa JC, Greene LJ, Ward RJ. Topology of the substrate-binding site of a Lys49-phospholipase A2 influences Ca2+-independent membrane-damaging activity. Biochem. J. 2004;382:191–198. doi: 10.1042/BJ20031946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin S, Pande AH, Nemec KN, He X, Tatulian SA. Evidence for the regulatory role of the N-terminal helix of secretory phospholipase A2 from studies on native and chimeric proteins. J. Biol. Chem. 2005;280:36773–36783. doi: 10.1074/jbc.M506789200. [DOI] [PubMed] [Google Scholar]
- 87.Kuipers OP, Thunnissen MM, de Geus P, Dijkstra BW, Drenth J, Verheij HM, de Haas GH. Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop. Science. 1989;244:82–85. doi: 10.1126/science.2704992. [DOI] [PubMed] [Google Scholar]
- 88.Li Y, Yu BZ, Zhu H, Jain MK, Tsai MD. Phospholipase A2 engineering. Structural and functional roles of the highly conserved active site residue aspartate-49. Biochemistry. 1994;33:14714–14722. doi: 10.1021/bi00253a009. [DOI] [PubMed] [Google Scholar]
- 89.Ward RJ, Chioato L, de Oliveira AH, Ruller R, Sa JM. Active-site mutagenesis of a Lys49-phospholipase A2: biological and membrane-disrupting activities in the absence of catalysis. Biochem. J. 2002;362:89–96. doi: 10.1042/0264-6021:3620089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang B, Yu BZ, Rogers J, Byeon IJ, Sekar K, Chen X, Sundaralingam M, Tsai MD, Jain MK. Phospholipase A2 engineering. Deletion of the C-terminus segment changes substrate specificity and uncouples calcium and substrate binding at the zwitterionic interface. Biochemistry. 1996;35:12164–12174. doi: 10.1021/bi960234o. [DOI] [PubMed] [Google Scholar]
- 91.Yu BZ, Rogers J, Nicol GR, Theopold KH, Seshadri K, Vishweshwara S, Jain MK. Catalytic significance of the specificity of divalent cations as KS* and kcat* cofactors for secreted phospholipase A2. Biochemistry. 1998;37:12576–12587. doi: 10.1021/bi9728607. [DOI] [PubMed] [Google Scholar]
- 92.Yuan C, Byeon IJ, Poi MJ, Tsai MD. Structural analysis of phospholipase A2 from functional perspective. 2. Characterization of a molten globule-like state induced by site-specific mutagenesis. Biochemistry. 1999;38:2919–2929. doi: 10.1021/bi9822123. [DOI] [PubMed] [Google Scholar]
- 93.Sekar K, Biswas R, Li Y, Tsai M, Sundaralingam M. Structures of the catalytic site mutants D99A and H48Q and the calcium- loop mutant D49E of phospholipase A2. Acta Crystallogr. D Biol. Crystallogr. 1999;55:443–447. doi: 10.1107/s0907444998013699. [DOI] [PubMed] [Google Scholar]
- 94.Chwetzoff S, Couderc J, Frachon P, Menez A. Evidence that the anti-coagulant and lethal properties of a basic phospholipase A2 from snake venom are unrelated. FEBS Lett. 1989;248:1–4. doi: 10.1016/0014-5793(89)80419-3. [DOI] [PubMed] [Google Scholar]
- 95.Ambrosio AL, Nonato MC, de Araujo HS, Arni R, Ward RJ, Ownby CL, de Souza DH, Garratt RC. A molecular mechanism for Lys49-phospholipase A2 activity based on ligand-induced conformational change. J. Biol. Chem. 2005;280:7326–7335. doi: 10.1074/jbc.M410588200. [DOI] [PubMed] [Google Scholar]
- 96.Soares AM, Mancin AC, Cecchini AL, Arantes EC, Franca SC, Gutierrez JM, Giglio JR. Effects of chemical modifications of crotoxin B, the phospholipase A(2) subunit of crotoxin from Crotalus durissus terrificus snake venom, on its enzymatic and pharmacological activities. Int. J. Biochem. Cell. Biol. 2001;33:877–888. doi: 10.1016/s1357-2725(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 97.Massensini AR, Romano-Silva MA, Gomez MV. Sodium channel toxins and neurotransmitter release. Neurochem. Res. 2003;28:1607–1611. doi: 10.1023/a:1025643030044. [DOI] [PubMed] [Google Scholar]
- 98.Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 99.Rajendra W, Armugam A, Jeyaseelan K. Neuroprotection and peptide toxins. Brain Res. Brain Res. Rev. 2004;45:125–141. doi: 10.1016/j.brainresrev.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Mounier CM, Bon C, Kini RM. Anticoagulant venom and mammalian secreted phospholipases A(2): protein- versus phospholipid-dependent mechanism of action. Haemostasis. 2001;31:279–287. doi: 10.1159/000048074. [DOI] [PubMed] [Google Scholar]
- 101.Yang CC, Chang LS. The N-terminal amino group essential for the biological activity of notexin from Notechis scutatus scutatus venom. Biochim. Biophys. Acta. 1990;1040:35–42. doi: 10.1016/0167-4838(90)90143-4. [DOI] [PubMed] [Google Scholar]
- 102.Mollier P, Chwetzoff S, Bouet F, Harvey AL, Menez A. Tryptophan 110, a residue involved in the toxic activity but not in the enzymatic activity of notexin. Eur. J. Biochem. 1989;185:263–270. doi: 10.1111/j.1432-1033.1989.tb15111.x. [DOI] [PubMed] [Google Scholar]
- 103.Yang CC, Chang LS. Dissociation of lethal toxicity and enzymic activity of notexin from Notechis scutatus scutatus (Australian tiger snake) venom by modification of tyrosine residues. Biochem. J. 1991;280:739–744. doi: 10.1042/bj2800739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tzeng MC, Yen CH, Tsai MD. Binding proteins on synaptic membranes for certain phospholipases A2 with presynaptic toxicity. Adv. Exp. Med. Biol. 1996;391:271–278. doi: 10.1007/978-1-4613-0361-9_21. [DOI] [PubMed] [Google Scholar]
- 105.Sribar J, Copic A, Poljsak-Prijatelj M, Kuret J, Logonder U, Gubensek F, Krizaj I. R25 is an intracellular membrane receptor for a snake venom secretory phospholipase A(2) FEBS Lett. 2003;553:309–314. doi: 10.1016/s0014-5793(03)01035-4. [DOI] [PubMed] [Google Scholar]
- 106.Hodgson WC, Wickramaratna JC. In vitro neuromuscular activity of snake venoms. Clin. Exp. Pharmacol. Physiol. 2002;29:807–814. doi: 10.1046/j.1440-1681.2002.03740.x. [DOI] [PubMed] [Google Scholar]
- 107.Rowan EG, Harvey AL, Menez A. Neuromuscular effects of nigexine, a basic phospholipase A2 from Naja nigricollis venom. Toxicon. 1991;29:371–374. doi: 10.1016/0041-0101(91)90290-8. [DOI] [PubMed] [Google Scholar]
- 108.Yamazaki Y, Matsunaga Y, Nakano Y, Morita T. Identification of vascular endothelial growth factor receptor-binding protein in the venom of eastern cottonmouth. A new role of snake venom myotoxic Lys49-phospholipase A2. J. Biol. Chem. 2005;280:29989–29992. doi: 10.1074/jbc.C500236200. [DOI] [PubMed] [Google Scholar]
- 109.Alape-Giron A, Persson B, Cederlund E, Flores-Diaz M, Gutierrez JM, Thelestam M, Bergman T, Jornvall H. Elapid venom toxins: multiple recruitments of ancient scaffolds. Eur. J. Biochem. 1999;259:225–234. doi: 10.1046/j.1432-1327.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 110.Lomonte B, Pizarro-Cerda J, Angulo Y, Gorvel JP, Moreno E. Tyr-->Trp-substituted peptide 115–129 of a Lys49 phospholipase A(2) expresses enhanced membrane-damaging activities and reproduces its in vivo myotoxic effect. Biochim. Biophys. Acta. 1999;1461:19–26. doi: 10.1016/s0005-2736(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 111.Chioato L, De Oliveira AH, Ruller R, Sa JM, Ward RJ. Distinct sites for myotoxic and membrane-damaging activities in the C-terminal region of a Lys49-phospholipase A2. Biochem. J. 2002;366:971–976. doi: 10.1042/BJ20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nunez CE, Angulo Y, Lomonte B. Identification of the myotoxic site of the Lys49 phospholipase A(2) from Agkistrodon piscivorus piscivorus snake venom: synthetic C-terminal peptides from Lys49, but not from Asp49 myotoxins, exert membrane-damaging activities. Toxicon. 2001;39:1587–1594. doi: 10.1016/s0041-0101(01)00141-6. [DOI] [PubMed] [Google Scholar]