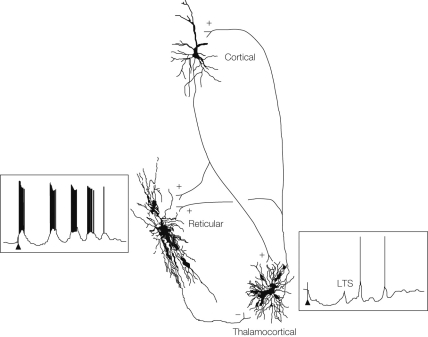
Thalamocortical (TC) neurons lead double lives: In a depolarized state near threshold excitatory synaptic input evokes regular spiking that faithfully “relays” information to the cortex, but when the cell is hyperpolarized, the same excitatory input can lead to a low-threshold spike (LTS), triggered by low the threshold calcium T-currents (Figure 1). LTSs in TC cells occur during sleep, when the membrane potential is hyperpolarized as a consequence of reduced levels of neuromodulatory inputs and the opening of potassium currents. The LTSs are prominent features of sleep spindles, but can also appear in states of alertness. We have known that TC cell have this dual function since it was first reported by Jahnsen and Llinás (1984), but only recently has the distribution of T-currents in the TC cells been explored.
Figure 1.
Sleep, epilepsy and thalamic reticular inhibitory neurons. Steriade M. Cortical, reticular and thalamocortical neurons showing connectivity (+ excitation, − inhibition), intracellular recordings and anatomical reconstructions from cats. Cortical stimulation (arrows) leads to a sequence of spindle waves in the reticular cell and to a series of low-threshold spikes (LTS) in the thalamocortical cell. From Steriade (2005).
T-current recorded from the somas of dissociated TC cells lacking dendrites have much smaller amplitudes than in cells recorded from thalamic slices, which suggests that the T-channels are mainly dendritic (Destexhe et al., 1998). Are the T-currents distributed uniformly in the dendrites, or are they concentrated more proximally or more distally? In this issue, Zomorrodi et al. (2008) have modeled these possibilities and concluded that the lowest threshold LTS occurs when the T-current are located proximally. Most of their simulations were based on a 3 compartment model, consisting of a soma, a proximal dendritic segment and a distal dendritic segment. They also reconstructed a complete TC cell and used simulations of the more accurate morphology to show that the results were even more robust than for the 3 compartment model. Confirmation of the prediction that T-current is concentrated in the proximal dendrites of TC cells awaits high-resolution electron microscopic imaging of the Cav3.1 and Cav3.3 T-channels. In neurons of the reticular nucleus of the thalamus, which also exhibit an LTS, the T-current is concentrated in the distal dendrites (Kovács et al., 2009), where they generate a prolonged LTS (Destexhe et al. 1996).
Although the proximal localization of T-currents in the dendrites of TC cells may require the least current to trigger an LTS when current is injected in the soma, it is not clear whether this is also true for synaptic conductance changes in the dendrites. Another open issue is whether single dendrites behave like functional units, as occurs in hippocampal cells (Gasparini et al., 2004)? If so, input from a cluster of synapses on a single dendrite could be sufficient to trigger an LTS. Or is the TC cell electrically compact? In a previous compartmental model of the TC cell (Destexhe et al., 1998), reconstructed from a 200 μm slice, the dendrites were much shorter and more electrically compact than the reconstructed TC cell in the current study, which had dendrites extending 400 μm from the soma (Zomorrodi et al., 2008).
The H-current, a non-specific cation current that is activated by membrane hyperpolarization, also participates in the LTS in TC cells, particularly during sleep spindles. The effects of its distribution within the TC cell could also be studied with compartmental models, in conjunction with the distribution of the T-currents. The optimal distributions of these currents may not be independent of each other, so they need to be jointly varied.
The calcium that enters the neuron during an LTS must be internally bound or extruded to maintain the equilibrium of free calcium in the cell in the long term. This is accomplished by Ca2+/Na+ metabolic exchangers in the plasma membrane. Reducing the number of T-channels needed to trigger an LTS would reduce the number of calcium ions that need to be extruded later, and hence would reduce the energy that the TC cell must expend to function. In the hippocampus, nonmyelinated axons have a fast sodium current and delayed potassium current, which reduces the overlap of the currents and minimizes the cost of an action potential (Alle et al., 2009). Similarly, pyramidal neurons and fast-spiking interneurons in the cerebral cortex also minimize energy expenditure for the patterns of action potentials they generate in vivo (Hasenstaub et al. 2009). This may be a general principle for neural information processing systems (Laughlin et al., 1998; Laughlin and Sejnowski, 2003).
References
- Alle H., Roth A., Geiger J. R. P. (2009). Energy-efficient action potentials in hippocampal mossy fibers. Science 325, 1405–1408 10.1126/science.1174331 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Contreras D., Steriade M., Sejnowski T. J., Huguenard J. R. (1996). In vivo, ln vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons, J. Neurosci. 16, 169–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A., Neubig M., Ulrich D., Huguenard J. (1998). Dendritic low threshold calcium currents in thalamic relay cells. J. Neurosci. 18, 3574–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S., Migliore M., Magee J. C. (2004). On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J. Neurosci. 24, 11046–11056 10.1523/JNEUROSCI.2520-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A., Otte S., Callaway E., Sejnowski T. J. (2009). Metabolic constraints on the biophysics of action potential generation, Neuron. [Google Scholar]
- Jahnsen H., Llinás R. (1984). Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J. Physiol. 349, 205–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács K., Sik A., Ricketts C., Timofeev I. (2009). Subcellular distribution of low-voltage activated T-type Ca(2+) channel subunits (Ca(v)3.1 and Ca(v)3.3) in reticular thalamic neurons of the cat. J. Neurosci. Res. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Laughlin S. B., Sejnowski T. J. (2003). Communication in Neuronal Networks, Science 301, 1870–1874 10.1126/science.1089662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin S. B., de Ruyter van Steveninck R. R., Anderson J. C. (1998). The metabolic cost of neural information. Nat. Neurosci. 1, 36–41 10.1038/236 [DOI] [PubMed] [Google Scholar]
- Steriade M. (2005). Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 28, 317–324 10.1016/j.tins.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Zomorrodi R., Kröger H., Timofeev I. (2008). Modeling thalamocortical cell: impact of Ca2+ channel distribution and cell geometry on firing pattern. Front. Comput. Neurosci. 2, 1–11 10.3389/neuro.10.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]