Abstract
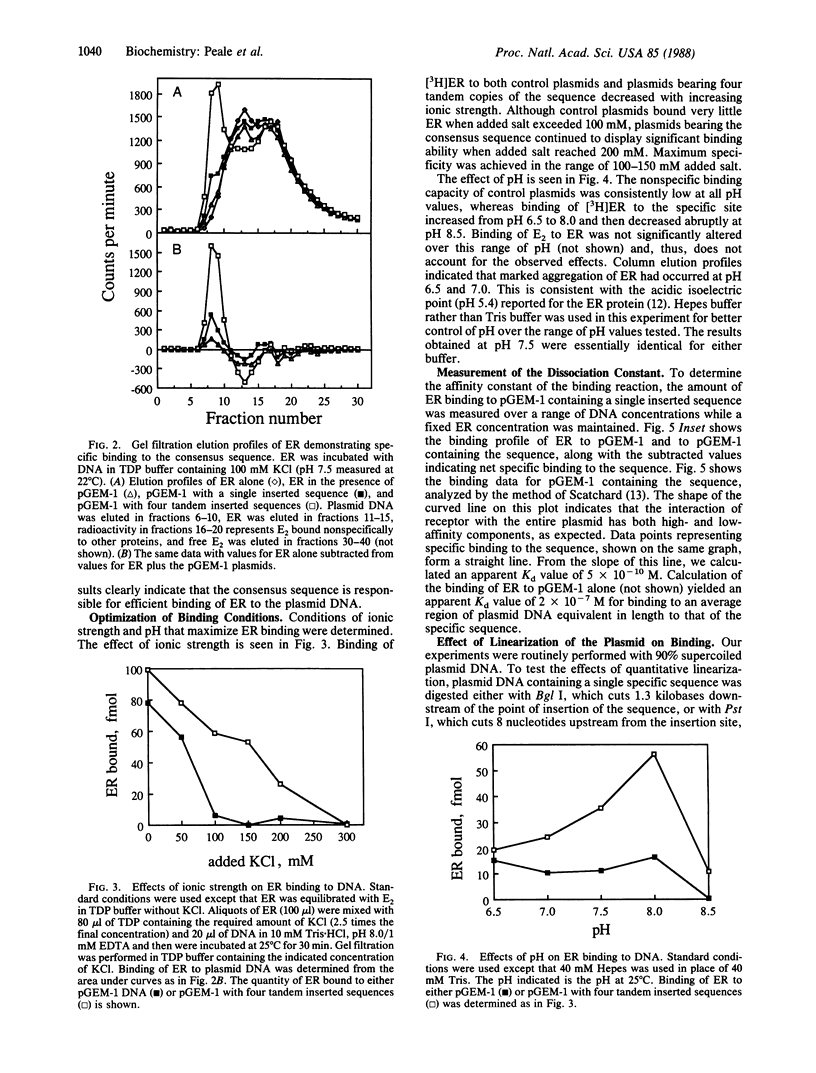
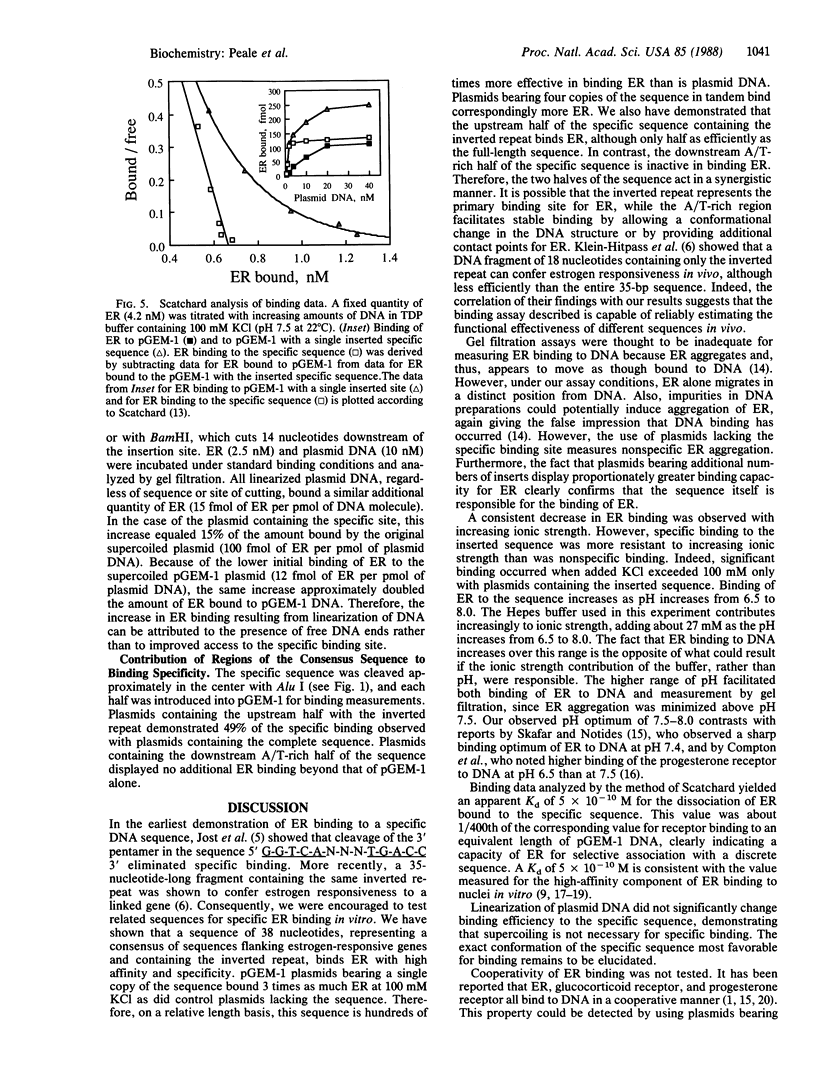
It has been shown previously that a short sequence from the 5' regulatory region of the Xenopus laevis vitellogenin gene A2, when appropriately placed, can confer estrogen responsiveness to another gene. Using the Xenopus sequence and similar sequences from the 5' regulatory regions of other estrogen-responsive genes, we derived a consensus sequence 38 nucleotides long. The sequence contains an inverted repeat (5' C-A-G-G-T-C-A-G-A-G-T-G-A-C-C-T-G 3') and an A/T-rich region. Plasmids carrying a single copy of the sequence bound 3-fold-more partially purified estrogen receptor (ER) than did control plasmids when assayed by gel filtration. Maximum specificity for ER binding occurs at 100-150 mM ionic strength and pH 7.5-8.0. Plasmids carrying multiple copies of the sequence bound correspondingly more ER. The dissociation constant for ER bound to the sequence is 0.5 nM. This value is lower by a factor of about 400 than the dissociation constant for ER bound to an equivalent length of plasmid DNA. Portions of the consensus sequence were evaluated for binding efficiency. Plasmids containing the inverted repeat alone bound ER, though less efficiently than did plasmids containing the entire sequence. The A/T-rich region alone was ineffective in binding ER. Linearization of the plasmid DNA did not enhance specific binding efficiency for ER. This model system represents an effective tool for characterization of ER binding to DNA sequences involved in the regulation of gene expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch J. B. Identification and sequence analysis of the 5' end of the major chicken vitellogenin gene. Nucleic Acids Res. 1984 Jan 25;12(2):1117–1135. doi: 10.1093/nar/12.2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comptom J. G., Schrader W. T., O'Malley B. W. Progesterone receptor interaction in the 5'-flanking regulatory region of the ovalbumin gene. Adv Exp Med Biol. 1986;196:291–304. doi: 10.1007/978-1-4684-5101-6_19. [DOI] [PubMed] [Google Scholar]
- Compton J. G., Schrader W. T., O'Malley B. W. Progesterone receptor binding to DNA: studies by sedimentation velocity methods. J Steroid Biochem. 1984 Jan;20(1):89–94. doi: 10.1016/0022-4731(84)90193-6. [DOI] [PubMed] [Google Scholar]
- Cushing C. L., Bambara R. A., Hilf R. Interactions of estrogen-receptor and antiestrogen-receptor complexes with nuclei in vitro. Endocrinology. 1985 Jun;116(6):2419–2429. doi: 10.1210/endo-116-6-2419. [DOI] [PubMed] [Google Scholar]
- Druege P. M., Klein-Hitpass L., Green S., Stack G., Chambon P., Ryffel G. U. Introduction of estrogen-responsiveness into mammalian cell lines. Nucleic Acids Res. 1986 Dec 9;14(23):9329–9337. doi: 10.1093/nar/14.23.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haché R. J., Wiskocil R., Vasa M., Roy R. N., Lau P. C., Deeley R. G. The 5' noncoding and flanking regions of the avian very low density apolipoprotein II and serum albumin genes. Homologies with the egg white protein genes. J Biol Chem. 1983 Apr 10;258(7):4556–4564. [PubMed] [Google Scholar]
- Jost J. P., Seldran M., Geiser M. Preferential binding of estrogen-receptor complex to a region containing the estrogen-dependent hypomethylation site preceding the chicken vitellogenin II gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):429–433. doi: 10.1073/pnas.81.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klinge C. M., Bambara R. A., Zain S., Hilf R. Estrogen receptor binding to nuclei from normal and neoplastic rat mammary tissues in vitro. Cancer Res. 1987 Jun 1;47(11):2852–2859. [PubMed] [Google Scholar]
- Kon O. L., Spelsberg T. C. Nuclear binding of estrogen-receptor complex: receptor-specific nuclear acceptor sites. Endocrinology. 1982 Dec;111(6):1925–1935. doi: 10.1210/endo-111-6-1925. [DOI] [PubMed] [Google Scholar]
- Pavlik E. J., Coulson P. B. Hydroxylapatite "batch" assay for estrogen receptors: increased sensitivity over present receptor assays. J Steroid Biochem. 1976 May;7(5):357–368. doi: 10.1016/0022-4731(76)90095-9. [DOI] [PubMed] [Google Scholar]
- Skafar D. F., Notides A. C. Modulation of the estrogen receptor's affinity for DNA by estradiol. J Biol Chem. 1985 Oct 5;260(22):12208–12213. [PubMed] [Google Scholar]
- Thibodeau S. N., Sullivan W. P., Jiang N. S. Analysis of the estrogen receptor from human uterus and breast tumor tissue by isoelectric focusing. J Clin Endocrinol Metab. 1983 Oct;57(4):741–748. doi: 10.1210/jcem-57-4-741. [DOI] [PubMed] [Google Scholar]
- Walker P., Germond J. E., Brown-Luedi M., Givel F., Wahli W. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDLII genes. Nucleic Acids Res. 1984 Nov 26;12(22):8611–8626. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichman B. M., Notides A. C. Estradiol-binding kinetics of the activated and nonactivated estrogen receptor. J Biol Chem. 1977 Dec 25;252(24):8856–8862. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem. 1974 Nov 25;249(22):7076–7086. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- de Boer W., Snippe L., Ab G., Gruber M. Interaction of calf uterine estrogen receptors with chicken target cell nuclei. J Steroid Biochem. 1984 Jan;20(1):387–390. doi: 10.1016/0022-4731(84)90239-5. [DOI] [PubMed] [Google Scholar]


