Abstract
Rationale
The progression of addiction from controlled to compulsive drug use leads to serious adverse consequences, including a greater propensity to relapse to drug use after sustained periods of abstinence.
Objective
The present study assessed the potential effects of cocaine self-administration history on the magnitude and persistence of cocaine-induced reinstatement in rhesus monkeys (n=6).
Methods
During a 3-month period of limited access to cocaine self-administration under a second-order schedule, subjects could take a maximum of 0.5 mg/kg per session 5 days per week. During a subsequent 3-month period of extended access to cocaine self-administration, subjects could take an additional 3.0 mg/kg under a fixed-ratio 20 schedule 3 days per week. Reinstatement effects were evaluated on six separate occasions that included limited and extended access conditions. Saline was substituted for cocaine, and once extinction criteria were met (response rates <20% of cocaine-maintained rates), response-independent cocaine (0.1 mg/kg) was administered i.v. prior to extinction sessions. Reinstatement was defined as a restoration of drug-appropriate responding above extinction criteria. Reinstatement experiments were conducted repeatedly on a daily basis until response rates returned to extinction levels. Peak response rates provided a measure of reinstatement magnitude whereas number of sessions required to meet extinction levels provided a measure of reinstatement persistence.
Results
Both the magnitude and persistence of reinstatement were consistent across all determinations regardless of drug history.
Conclusions
The results indicate that reinstatement under the second-order schedule is remarkably stable even when supplemental drug intake is provided over several months.
Keywords: Cocaine, Self-administration, Reinstatement, Second-order schedule, Operant behavior, Rhesus monkeys
Introduction
Cocaine addiction is a chronic, relapsing disorder resulting in a condition where the individual is unable to remain abstinent for extended periods of time. Preventing relapse to drug use is one of the most difficult obstacles in treating cocaine addiction (O’Brien 2005). There is substantial evidence that environmental stimuli associated with cocaine use and acute re-exposure to cocaine are critical determinants of relapse (Jaffe et al. 1989; Rohsenow et al. 1990). Drug self-administration in animals is a validated technique to model drug use in humans and the environmental cues associated with this behavior (Everitt and Robbins 2000). Moreover, the reinstatement paradigm provides a well-characterized preclinical model with relevance toward understanding drug relapse in humans (Stewart and de Wit 1987). In the reinstatement procedure, extinction of drug-maintained responding is followed by exposure to a stimulus (response-independent drug injections, drug-conditioned contextual cues, or stressful stimuli) which restores drug-appropriate responding (for review, see Shalev et al. 2002). This procedure has been used to model the human condition of relapse since conditions that are reported to provoke relapse in humans will also reinstate extinguished drug taking behavior in laboratory animals (Shaham et al. 2003).
Extended access to cocaine self-administration in rodents has been proposed to model the escalating patterns of drug intake seen in cocaine dependence (Ahmed and Koob 1998, 1999; Deroche-Gamonet et al. 2004; Koob et al. 2004). Rodents provided extended access to cocaine have been evaluated for changes in reinstatement modeling drug-seeking behavior under a variety of conditions. Groups extinguished from long-access cocaine self-administration (6 h or more daily) reinstated to lower doses of cocaine than groups extinguished from short-access (less than 3 h daily) exposure (Mantsch et al. 2004; Ahmed and Cador 2006; Knackstedt and Kalivas 2007). Additionally, Ferrario et al. (2005) demonstrated enhanced drug-seeking in rodents even after 47 days of abstinence indicating that the self-administration experience is associated with persistent increases in drug-seeking behavior. Altogether, researchers find in rodents that the length of cocaine access can profoundly influence the sensitivity to drug-induced reinstatement with long-access groups consistently displaying higher response rates relative to their short-access counterparts (Mantsch et al. 2004; Ferrario et al. 2005; Ahmed and Cador 2006; Kippin et al. 2006; Knackstedt and Kalivas 2007).
The aim of the present study was to extend these findings and develop a model of extended cocaine access in nonhuman primates in order to evaluate the potential effect of cocaine self-administration history on cocaine-induced reinstatement. Using a long-term, within-subject design, rhesus monkeys were initially trained to self-administer cocaine under a second-order schedule. During limited access conditions, the number of cocaine infusions earned during 1 h test sessions was limited to five infusions. Following the limited access condition, daily sessions included an additional 2 h of cocaine access under a fixed-ratio schedule of reinforcement 3 days per week. Reinstatement probes were performed during the limited and extended access conditions to evaluate the potential consequences of drug intake on subsequent reinstatement effects. It was hypothesized that cocaine-induced reinstatement would be enhanced persistently in monkeys given extended cocaine access over the course of several months.
Materials and methods
Subjects
Six adult (6–8 years old) male rhesus monkeys (Macaca mulatta) weighing between 13 and 15 kg served as subjects. All monkeys were experimentally naïve at the start of the present experiments. Between daily experimental sessions, monkeys were individually housed, provided access to food daily (Purina Monkey Chow, fresh fruit and vegetables), and unlimited access to water. Animal use procedures were in strict accordance with the NIH “Guideline for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Emory University.
Catheter implantation
Monkeys were surgically implanted with chronic indwelling intravenous catheters. Implantation was done under a combination of Telazol and isoflurane (1.0–2.0%) anesthesia using aseptic techniques. One end of a silicone catheter was inserted into either the femoral or jugular vein and advanced into the vena cava. The distal end of the catheter was routed subcutaneously and attached to a vascular access port (Access Technologies, Skokie, IL, USA) in the intrascapular region. Preoperative antibiotic (Rochephin) and postoperative analgesic (Banamine) were administered according to veterinary staff direction. Catheters were flushed daily with 1.0 ml of heparinized (100 U/ml) saline to maintain patency.
Apparatus
During experimental sessions, each monkey was seated in a commercially available primate chair (Primate Products, Miami, FL, USA). A response panel equipped with a lever and stimulus lights was attached to the front of the chair. The skin over the vascular access port was cleaned with 95% ethanol and betadine, and then a special right-angle Huber needle (Access Technologies) was inserted into the port. The chair was then enclosed in a ventilated, sound-attenuating chamber (Med Associates, St. Albans, VT, USA). Polyvinylchloride (PVC) tubing connected the Huber needle to a motor-driven syringe pump (Model PhD 2000, Harvard Apparatus, Holliston, MA) located outside the test chamber containing the drug solution. When activated, the pump delivered a unit dose of 0.1 mg/kg/infusion (−) cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) in a volume of 2.0 ml during a 7.0-s infusion. Med-PC (Med Associates) software systems controlled all experimental events and data collection.
Self-administration
Each monkey was trained on a second-order, fixed-interval (FI) 600-s schedule with fixed-ratio (FR) 20 response components (FI 600; FR 20:S). Responding was initiated using an FR 1 schedule such that each response in the presence of a red light produced an i.v. infusion of 0.1 mg/kg cocaine and a 15-s illumination of a white light. There was a 2-h limited hold in which the subject could complete the ratio requirement. Daily sessions were limited to five injections. The ratio value was increased gradually as responding increased, from the initial FR 1 to FR 2, FR 5, FR 10, and ultimately FR 20. When the schedule value reached FR 20, drug injections no longer followed the completion of each FR but instead followed an increasing number of FR components completed during an FI 30-s schedule. A 2-s white light was presented upon completion of each FR 20 component during the interval. Once the criteria of at least four infusions for three consecutive days was met at this duration, the FI was increased to 60 s, and subsequently increased in 60-s intervals until the FI duration reached 600 s. Under the terminal schedule, each test session began with a 600-s presentation of a red stimulus light. During this 600-s interval, completion of an FR 20 response requirement changed the stimulus light from red to white for 2 s. At the end of the 600-s interval, the subject had a 60-s limited hold to complete one additional FR 20. Completion of this terminal FR 20 before the limited hold elapsed changed the stimulus light from red to white for 15 s and initiated a 0.1 mg/kg infusion of cocaine. The infusion was followed by a 60-s timeout during which stimulus lights were extinguished and responding had no scheduled consequences. Each daily session consisted of five consecutive 600-s components allowing for a total drug intake of 0.5 mg/kg of cocaine. The initial training phase required approximately 100 sessions. Once responding stabilized under the terminal schedule, subjects were maintained on this limited access condition (five injections/day during 1-h sessions) for an additional 3 months. Note the 0.1 mg/kg/infusion maintenance dose of cocaine is typically on the ascending limb or the peak of the dose–response curve in rhesus monkeys maintained under the second-order schedule utilized (Lindsey et al. 2004; Howell et al. 2007).
The limited access condition was then followed by 3 months of extended access conditions during which monkeys had an opportunity 3 days a week to increase the amount of cocaine self-administered. On extended access days, sessions began with the second-order schedule, as described. This was followed by two additional hours on a simple FR 20 schedule for a total session length of 3 h. During the additional 2 h of extended access, a green stimulus light illuminated the chamber and each FR 20 completed resulted in the presentation of the 15-s white light and a 0.1 mg/kg infusion of cocaine. Each infusion received was followed by a 60-s timeout. Monkeys had the opportunity to take an additional 30 infusions (3.0 mg/kg total) during the extended access condition in addition to the 0.5 mg/kg available during the 1 h second-order schedule.
Reinstatement
Every 4 weeks, reinstatement experiments were conducted during the limited and extended access conditions using the second-order schedule. Cocaine self-administration behavior was extinguished by substituting saline for cocaine in the infusion syringe and omitting the presentation of the drug-paired stimulus lights. Extinction sessions were otherwise identical to the cocaine self-administration protocol. Extinction sessions continued until response rates dropped below 20% of the rate formerly maintained by cocaine self-administration. To reinstate extinguished self-administration behavior, animals were given a response-independent injection of the maintenance dose of cocaine (0.1 mg/kg) at the start of the session and the drug-paired stimulus lights were restored, but saline continued to be available in the infusion syringe. Response-independent injections of cocaine were administered each day until responding under reinstatement conditions declined to that of extinction levels (<20%). At the conclusion of each reinstatement protocol, monkeys returned to their limited or extended access self-administration condition to reestablish baseline levels of responding until the next scheduled reinstatement determination. During the limited access condition, responding was maintained by the second-order schedule only. During the extended access conditions, responding was maintained by the second-order and FR schedules as described previously.
Data analysis
Mean session response rates were determined for individual subjects by dividing the total number of responses emitted in the presence of the discriminative stimuli (red or green lights) by the total session duration. The magnitude of reinstatement was the peak response rate observed on the first day of each series of reinstatement determinations. The persistence of reinstatement was the number of days necessary to reduce response rate to extinction levels (<20% of cocaine baseline). Response rate data were derived for individual subjects and compared to their own baseline performance (percent of baseline) so that each subject served as its own control. Response rates were then averaged for each drug access condition for group statistical analyses. Within each access condition, a repeated measures ANOVA was performed across each reinstatement determination with peak reinstatement effect and days to reach extinction criteria as the factors. Differences between limited and extended access conditions were analyzed by direct comparisons using Student’s t test. All statistical tests were performed as indicated with commercially available statistical analysis software (SigmaStat; Systat Software, Inc., Point Richmond. CA).
Results
Self-administration
During the first 3 months of stable self-administration under the second-order schedule, monkeys earned an average of 4.6±0.1 infusions of cocaine daily and maintained consistent rates of responding (averaging 0.90±0.15 responses/second). One-way repeated measures ANOVA for the number of infusions earned and daily response rates did not show any significant difference in either variable during the first 3 months of the limited access condition. As expected, monkeys consistently earned nearly all available cocaine infusions and maintained stable response rates over the 3 months of limited access. The average daily number of infusions during the last 5 days of months 1–3 was 4.3± 0.2, 4.8±0.1, and 4.6±0.2, respectively. Mean response rate during the last 5 days of months 1–3 was 0.95±0.30, 0.93± 0.23, and 0.82±0.28, respectively.
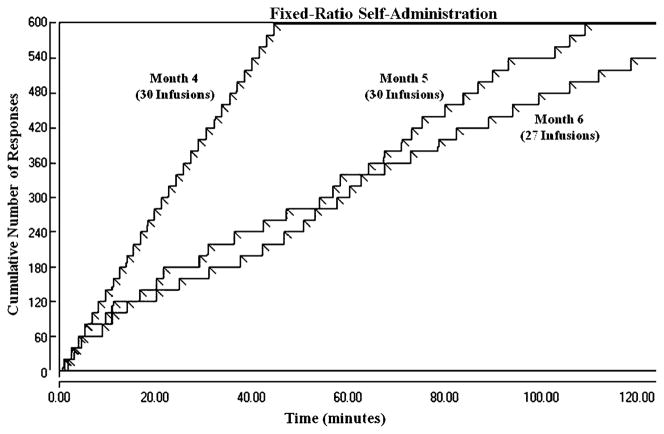
During extended access conditions, behavior during the second-order schedule remained consistent with that observed during limited access conditions. The average daily number of cocaine infusions earned was 4.3±0.2, and the mean response rate was 0.83±0.18. One-way repeated measures ANOVA for the number of infusions earned and response rates during the second-order schedule did not show any significant difference in either variable over three consecutive months of the extended access condition. Similarly, the number of infusions earned during the FR 20 schedule did not show any significant difference over three consecutive months of the extended access condition. The average daily number of infusions during the last 5 days of months 4–6 was 23.3±3.0, 20.5±3.3, and 19.7±3.2, respectively. However, one-way repeated measures ANOVA revealed a decrease in the response rate during the FR 20 schedule after the first month of extended access [F (2,8)=10.214, p=0.006]. Mean response rate during the last 5 days of months 4–6 was 0.19±0.08, 0.10±0.03, and 0.08 ±0.02, respectively. Although monkeys were taking the same number of infusions during the FR 20 component, the pattern in which they took the infusions changed over time. Instead of taking all the infusions within the first hour of the component, they were now taking several infusions earlier in the 2-h component and spacing the remaining infusions over the course of the session. Figure 1 shows the progressive change in response pattern during the FR 20 schedule over the 3 months of extended access in a representative monkey.
Fig. 1.
Cumulative records of the 2-h FR 20 schedule during extended access conditions for subject RLq7. The records compare the distribution of responses and cocaine injections over 2 h. Each diagonal line represents one infusion of 0.1 mg/kg cocaine and is followed by a 1-min timeout
Reinstatement
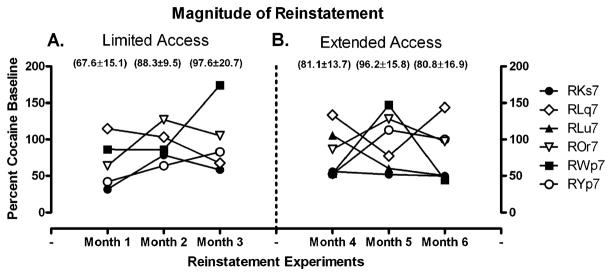
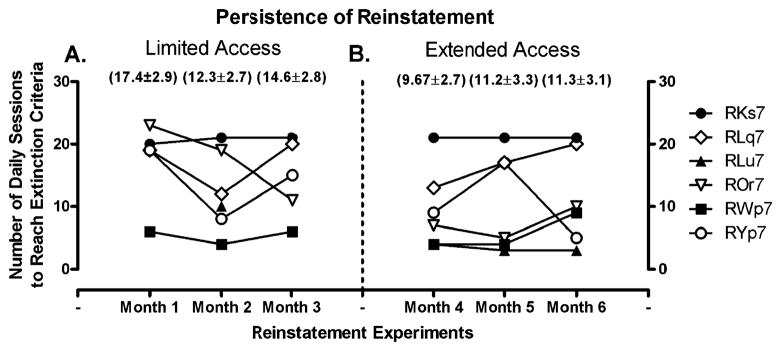
After each block of extinction, responding was reinstated with a response-independent injection of the maintenance dose of cocaine (0.1 mg/kg) immediately prior to a saline self-administration session. The drug was administered on consecutive days until response rates declined to extinction levels. Peak response rates occurred on the first day of reinstatement and gradually declined over consecutive sessions. Figure 2 shows response rates on the first day of reinstatement as a percentage of each monkey’s self-administration baseline. One-way repeated measures ANOVA did not reveal any significant difference in mean response rates over consecutive months of limited or extended access conditions. Lastly, Fig. 3 shows the persistence of cocaine-induced reinstatement. There was no significant difference in the number of sessions required to meet extinction criteria during the 3 months of limited or extended access conditions. Regardless of self-administration history, the persistence of reinstatement was similar across multiple determinations, averaging 12.6±1.2 days over all blocks of reinstatement.
Fig. 2.
Magnitude of cocaine-induced reinstatement of operant behavior in monkeys following each month of limited (a) or extended (b) access conditions. Percent of baseline (last 5 days of cocaine self-administration each month) on the first day of reinstatement during each block of reinstatement is shown for individual monkeys. The numbers in parentheses are the group averages for each condition. Note that subject RLu7 did not reinstate during months 1 and 3
Fig. 3.
Persistence of cocaine-induced reinstatement following each month of limited (a) or extended (b) access conditions. The number of daily sessions required to return to extinction criteria (<20% of cocaine-maintained responding) during each block of reinstatement is shown for individual monkeys. The numbers in parentheses are the group averages for each experiment
Discussion
The present study evaluated the potential influence of cocaine self-administration history on cocaine-induced reinstatement in nonhuman primates. Rhesus monkeys trained on a second-order schedule of cocaine self-administration had limited drug access for 3 months followed by a period of increased drug intake under an FR schedule during 3 months of extended drug access. As drug intake increased from limited to extended access conditions, self-administration behavior under the second-order schedule was stable over the course of the study. Cocaine-induced reinstatement was limited to a single dose but evaluated monthly during each access condition to characterize any progressive changes as a result of chronic drug exposure. The results indicate that the magnitude and persistence of reinstatement under the second-order schedule were remarkably stable even when supplemental drug intake was provided over several months.
Numerous studies indicate that prolonged cocaine access can potentiate cocaine-induced reinstatement in rodents (Mantsch et al. 2004; Ferrario et al. 2005; Ahmed and Cador 2006; Kippin et al. 2006; Knackstedt and Kalivas 2007), suggesting that increases in drug intake induce a sensitized response to drug-induced reinstatement. However, there has been recent evidence in rodents highlighting the importance of behavioral history on cocaine-induced reinstatement. In one study (Kippin et al. 2006), rats that self-administered cocaine for 6 h/day for 14 days showed more robust reinstatement than rats that received response-independent infusions of an equivalent dose of cocaine. As cocaine intake was matched across subjects, it was the operant conditioning history that apparently enhanced responding during drug-induced reinstatement. Keiflin et al. (2008) also found that operant conditioning history influenced the levels of responding during cocaine-induced reinstatement. In the latter study, two groups of rats maintained similar cocaine intake while their responding was varied by either increasing the unit dose or the FR requirement. The subjects were then evaluated for dose-dependent changes in cocaine-induced reinstatement. For both groups, the conditions that produced the greatest rates of responding during self-administration were associated with enhanced responding during drug-induced reinstatement. Taken together, these studies suggest that operant conditioning history is a major determinant of drug-induced reinstatement.
In the current study, cocaine intake was limited under a second-order schedule of reinforcement. An important property of second-order schedules is that response-dependent, drug-paired stimuli can maintain high rates of operant behavior. Moreover, performance maintained by second-order schedules is remarkably stable over extended periods, and frequency of reinforcement is relatively insensitive to changes in response rate (Spear et al. 1991; Schindler et al. 2002). This consistency was seen during the second-order schedule of both the limited and extended access conditions. All animals received extensive self-administration training on the second-order schedule over 5–6 months in order to establish stable behavioral baselines prior to the initiation of extinction and reinstatement determinations under limited access conditions. Once responding was stabilized, each month of limited access resulted in a similar number of drug infusions and consistent rates of responding. Even after increased drug intake during the FR schedule of the extended access sessions, the number of infusions and response rates during the preceding second-order schedule remained constant each month and comparable to that seen during the 3-month limited access condition. Hence, the consistency in drug-induced reinstatement may be attributed to the stability of the performance maintained by the second-order schedule employed and the extensive self-administration training prior to reinstatement determinations. Also, it is important to note that the paired brief stimulus was not presented during the extinction sessions. Accordingly, the conditioned reinforcing effects of the drug-paired stimulus during reinstatement sessions may have contributed to the stability of behavior observed.
Interestingly, there was no escalation in drug intake during the extended access condition. The FR schedule allowed subjects to increase their cocaine intake without the limitations imposed by the second-order component, and compared to the limited access condition, drug intake increased markedly. There was also a progressive change in the pattern of responding during the FR schedule during each month of extended access, resulting in significant reductions in mean session response rates. However, this change in pattern of responding was not reflected in daily cocaine intake. Drug intake during the FR schedule was remarkably stable over the 3 months of extended access. This contrasts with a number of rodent studies reporting an escalation in cocaine intake during extended access conditions (Ahmed and Koob 1999; Ferrario et al. 2005; Knackstedt and Kalivas 2007). The stability of drug intake in the present study may reflect species differences or important procedural differences. Rodent studies reporting an escalation in cocaine intake typically have used the same FR schedule of reinforcement during limited and extended access conditions. In the present study, the monkeys had an extensive history of cocaine self-administration under a second-order schedule before supplemental drug intake was provided intermittently under a separate FR schedule.
In summary, the results obtained indicate that rhesus monkeys with an extensive cocaine self-administration history under a second-order schedule of reinforcement show robust but consistent cocaine-induced reinstatement effects. Extended access to cocaine was not sufficient to enhance reinstatement of extinguished self-administration. Both the magnitude and persistence of reinstatement were remarkably stable. Similarly, others have reported that supplemental cocaine intake under an FR schedule did not alter the reinforcing effectiveness of cocaine in rhesus monkeys (Czoty et al. 2006). The short-term stability of cocaine-induced reinstatement over repeated determinations has been documented previously in nonhuman primates (Khroyan et al. 2000). The present study extends these findings by documenting the long-term stability of reinstatement effects even following an intervening period of supplemental drug intake under a different schedule of reinforcement. Collectively, the results further demonstrate that operant conditioning history and the schedule of reinforcement employed can have a major influence on subsequent drug-induced reinstatement.
Acknowledgments
Supported by USPHS grants DA016589, DA00517, and RR00165
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend. 2006;85:213–220. doi: 10.1016/j.drugalcdep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Vouillac C, Cador M. Level of operant training rather than cocaine intake predicts level of reinstatement. Psychopharmacology. 2008;197:247–261. doi: 10.1007/s00213-007-1026-2. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology. 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Spear DJ, Muntaner C, Goldberg SR, Katz JL. Methohexital and cocaine self-administration under fixed-ratio and second-order schedules. Pharmacol Biochem Behav. 1991;38:411–416. doi: 10.1016/0091-3057(91)90300-q. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer; New York: 1987. pp. 211–227. [Google Scholar]