Abstract
Rationale
The transition from infrequent and controlled cocaine use to dependence may involve enduring changes in neurobiology as a consequence of persistent drug use.
Objective
The present study utilized an intravenous drug self-administration protocol of increasing cocaine access to evaluate potential changes in dopamine function in vivo, including changes in sensitivity to psychostimulants.
Materials and methods
Drug-naïve rhesus monkeys were provided limited access (1 h) to cocaine self-administration for 60 days followed by 60 days under an extended access condition (4 h). Basal levels of striatal extracellular dopamine and its metabolites, as well as the effectiveness of cocaine and amphetamine to elevate dopamine, were determined with in vivo microdialysis before the initiation of cocaine self-administration and during limited and extended access. The effect of cocaine and amphetamine on the acoustic startle response was also examined to assess complementary behavioral changes as a function of drug history.
Results
Extended access to cocaine self-administration lead to increased daily intake compared to limited access conditions but did not result in escalated intake over time. However, cocaine- and amphetamine-induced increases in striatal dopamine were diminished as a function of cocaine self-administration history. Surprisingly, there was no effect of drug-taking history on sensitivity to psychostimulant-induced enhancement of startle amplitude.
Conclusions
The present experiments provide evidence of a hypofunctional dopamine system that is not associated with an escalation in drug intake or reflected in measures of acoustic startle.
Keywords: Cocaine, Startle, Microdialysis, Self-administration, Access
Introduction
The transition from infrequent and controlled cocaine use to dependence may involve lasting changes in neurobiological function as a consequence of persistent drug use. These changes manifest in the increased consumption of drug over time and the inability to modulate that intake despite the aversive consequences (Siegel 1984; Gawin and Ellinwood 1989; Leshner 1997). Animal models of drug self-administration have been used to demonstrate many of the major hallmarks of drug abuse. In rodents, short periods of cocaine access (1 h) produce a consistent pattern of self-administration behavior with a moderate level of drug intake, whereas prolonged cocaine availability (6 h or more) can result in a progressive escalation in drug intake similar to human patterns of cocaine abuse (Ahmed and Koob 1998, 1999). Employing this model of chronic, elevated cocaine intake may be useful in characterizing changes in abuse-related behavior and neurobiological adaptations as a function of drug history.
Psychostimulants, such as cocaine and amphetamine, increase extracellular levels of monoamines. However, it is the mesocorticolimbic dopamine projections to the striatum and cortex that play the primary role in the reinforcing effects of cocaine (Wise and Bozarth 1985). Studies comparing short versus long access to cocaine self-administration in rodents have found noticeable differences in dopamine system function with prolonged cocaine exposure. Rodents provided long access are more sensitive to the behavioral-suppressant effects of a dopamine receptor antagonist suggesting a change in receptor number or function as a result of increased cocaine intake (Ahmed and Koob 2004). Rodents provided long access also show deficits in cognitive function following extended drug experience (Briand et al. 2008) and significant decreases in dopamine D2 receptor mRNA and protein levels (Mantsch et al. 2004; Briand et al. 2008). Synaptic reorganization is also evident with increased density of dendritic spines on neurons in the prefrontal cortex and the nucleus accumbens (Ferrario et al. 2005). Hence, long-term, repeated administration of cocaine can induce a variety of neuroadaptations within the dopamine system.
Acoustic startle provides a simple and reliable behavioral measure to characterize the consequence of cocaine self-administration history on the status of the dopamine system and sensitivity to the acute effects of psychostimulants. Acoustic startle is a sensorimotor reflex in response to an intense, abrupt auditory stimulus. The reflex is an innate defense mechanism and is mediated by a well-characterized three-synapse simple circuit (Davis et al. 1982; Lee et al. 1996; Lang et al. 2000). This response is likewise sensitive to dopaminergic modulation by both direct and indirect dopamine agonists (Davis et al. 1975, 1986b; Davis 1985; Meloni and Davis 1999). Moreover, chronic cocaine users in various phases of drug withdrawal have been shown to have diminished responses to startle stimuli (Efferen et al. 2000) as do rats given daily cocaine for 8 weeks (Adams et al. 2001). This may be the result of persistent changes to the dopamine system as a consequence of an extensive drug-taking history.
The present study investigated the effect of extended intravenous (i.v.) cocaine self-administration history on subsequent dopamine system function in nonhuman primates. Rhesus monkeys were chosen as subjects because of their close physiological and behavioral relationship to humans, as well as for their longevity which allows for a more rigorous, within-subjects design that covers months of cocaine access as documented in humans. Naïve monkeys were trained on a fixed ratio (FR) 20 schedule of cocaine reinforcement. Following acquisition of self-administration, cocaine access was limited to 1 h/day for 60 days (limited access) and then increased to 4 h/day of extended access for 60 days (extended access). Basal levels of striatal extracellular dopamine and its primary metabolites, as well as the effectiveness of cocaine and amphetamine to elevate dopamine, were determined prior to cocaine self-administration and during limited and extended access conditions. In vivo microdialysis was complemented with behavioral measures of acoustic startle. Baseline measures of startle amplitude prior to cocaine self-administration and during limited and extended access conditions were used to determine any progressive changes in basal startle response. In addition, measures of startle amplitude in response to cocaine and amphetamine challenges also assessed progressive changes in sensitivity to psychostimulants. The overall objective was to determine, in vivo, whether the tone and responsiveness of the dopamine system changes as a result of cocaine self-administration history.
Materials and methods
Subjects
Four male and two female adult (6–8 years old) rhesus monkeys (Macaca mulatta) weighing between 9 and 15 kg served as subjects. All monkeys were experimentally naïve at the start of the present experiments. Between daily experimental sessions, monkeys were individually housed and provided ad libitum access to food (Purina Monkey Chow, fresh fruit and vegetables) and water daily. Animal use procedures were in strict accordance with the National Institutes of Health’s “Guideline for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Emory University.
Self-administration
Monkeys were surgically implanted with chronic indwelling i.v. catheters. Implantation was done under a combination of Telazol and isoflurane (1.0–2.0%) anesthesia using aseptic techniques. One end of a silicone catheter was inserted into either the femoral or jugular vein and advanced into the vena cava. The distal end of the catheter was routed subcutaneously and attached to a vascular access port (Access Technologies, Skokie, IL, USA) in the intrascapular region. Preoperative antibiotic (Rocephin) and postoperative analgesic (Banamine) were administered according to veterinary staff direction. Catheters were flushed daily with 1.0 mL of heparinized (100 U/mL) saline to maintain patency.
During self-administration sessions, each monkey was seated in a commercially available primate chair (Primate Products, Miami, FL, USA). A response panel equipped with a lever and stimulus lights was attached to the front of the chair. The skin over the vascular access port was cleaned with 95% ethanol and betadine, and then a special right-angle Huber needle (Access Technologies, Skokie, IL, USA) was inserted into the port. The chair was then enclosed in a ventilated, sound-attenuating chamber (Med Associates, St. Albans, VT, USA). Polyvinyl chloride tubing connected the Huber needle to a motor-driven syringe pump (Model PhD 2000; Harvard Apparatus, Holliston, MA, USA) located outside the test chamber containing the drug solution. When activated, the pump delivered a unit dose of 0.1 mg/kg/infusion (−) cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) in a volume of 2.0 mL during a 7.0-s infusion. Med-PC (Med Associates, St. Albans, VT, USA) software systems controlled all experimental events and data collection.
Each monkey was trained on an FR schedule of drug self-administration. FR self-administration training began with an FR 1 and increased sequentially when at least three infusions were earned in three consecutive testing days at each FR until FR 20 was reached. Only five infusions were available for each session during FR self-administration training. It took on average about 30 sessions to complete FR self-administration training. Each session began with the presentation of a red stimulus light. The completion of a FR response requirement changed the stimulus light from red to white for 15 s and initiated a 0.1-mg/kg infusion of cocaine. The infusion was followed by a 60-s timeout during which stimulus lights were extinguished and responding had no scheduled consequences. At the end of the timeout, the red light was presented again to initiate the next FR component. Once the terminal schedule was reached (FR 20), the sessions were limited to 1 h each day, 6 days/week, but no limit was placed on the number of available infusions. Subjects were maintained on this limited access schedule for 60 days (10 weeks). The limited access condition was then followed by 60 days (10 weeks) on an extended access schedule during which the session length was increased to 4 h each day with a limit of 60 available infusions to prevent adverse effects. Finally, the limited access condition was reinstated to determine if the history of extended access resulted in a persistent escalation of cocaine self-administration.
In vivo microdialysis
For in vivo microdialysis experiments, subjects were implanted bilaterally with guide cannulae (CMA 12; CMA Microdialysis, Stockholm, Sweden) targeted to the caudate nucleus, a target region of dense dopamine terminals. Before surgery, monkeys were sedated with Telazol then supplemented with inhaled isoflurane (1.0–2.0%) to maintain depth of anesthesia during the procedure. A stereotaxic apparatus was used for proper positioning of the animal’s head and to ensure insertion of the guide cannulae to the appropriate depth based on coordinates derived from a standard macaque atlas. A small burr hole for the guide cannulae was made above the caudate nucleus using a trephine drill. Once implanted, Teflon screws and cranioplastic cement held the guides securely in place, and a protective chamber with removable cap (IPI-J1 and ICO-J0; Crist Instrument Company, Hagerstown, MD, USA) prevented access to the site by the monkeys. Analgesics and antibiotics were prescribed as necessary to alleviate any discomfort associated with surgery, and a 2-week recovery period preceded any planned microdialysis experiments. Three subjects lost their dialysis chambers prior to the completion of all baseline experiments in the drug-naïve state and were, therefore, not included in the data presented.
Three sets of microdialysis experiments were conducted throughout this study. Changes in dopamine levels in the caudate in response to an acute drug challenge were determined before the initiation of self-administration in the drug-naïve state, during limited access, and again during extended access. During each set of experiments, the monkeys were seated in a primate chair and placed inside a ventilated, sound-attenuating chamber as previously described. The cap of the chamber was removed and 24 mm microdialysis probes (CMA 12, custom made) were inserted into both guide cannulae. A microinjection pump (Harvard Apparatus, Holliston, MA) located outside the chamber continuously delivered artificial cerebrospinal fluid (aCSF) (Na2HPO4, 1.0 mM; NaCl, 150 mM; KCl, 3 mM; CaCl, 1.3 mM; MgCl, 1.0 mM; and ascorbic acid, 0.15 mM) through the probes via FEP Teflon tubing (CMA, 1.2 μL internal volume/ 100 mm length) at a perfusion flow rate of 2.0 μL/min. Following a 1-h equilibrium period, six samples of baseline dialysate (basal samples) were collected before an acute i.v. injection of either cocaine (0.3 or 1.0 mg/kg) or amphetamine (0.1 or 0.3 mg/kg) was administered through the vascular access port. Samples of dialysate were then collected every 10 min for 2 h following the injection. Finally, a high potassium (K+) aCSF solution containing 54 mM KCl and 103 mM NaCl was substituted for the standard aCSF at the end of the session to induce voltage-dependent dopamine release. This release of dopamine served as an indication of site viability across several experimental determinations. Additionally, the probes were tested in vitro before and after each in vivo experiment to determine the probe efficiency and performance.
Small-bore high-pressure liquid chromatography (HPLC) and electrochemical detection quantified the levels of dopamine and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), according to well-established analytical procedures (Czoty et al. 2000). The HPLC system consisted of a small-bore (3 mm internal diameter×100 mm) column (5 μm C18 stationary phase; Thermo Hypersil; Keystone Scientific Operations, Bellefonte, PA, USA) with commercially available mobile phase (ESA Biosciences, Chelmsford, MA, USA) delivered by an ESA model 582 solvent delivery pump at a flow of 0.6 μL/min. After loading the dialysis samples into the refrigerated sample tray (ESA model 542), they were automatically mixed with 3 μL of ascorbate oxidase and 5 μL of this mixture was injected into the HPLC system. Electrochemical analyses were performed with an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential= 350 mV) and an ESA Coulchem II detector. The potential of channel 1 was set to −150 mV for oxidation and channel 2 was set to 275 mV for reduction.
A full range of dopamine standards (0.5–25 nM) was analyzed before and after each set of experimental samples to evaluate the possible degradation of dopamine. Levels of dopamine below 0.5 nM were considered below the limits of detection. Chromatograms were generated using EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA, USA). This software was also used to analyze the chromatograms by comparing the estimated concentration of dopamine and its metabolites to the standards. The change in dopamine and metabolite concentrations was then presented as a percentage of their basal levels as a function of drug dose over time. The percent change in dopamine and metabolite levels for the left and right caudate were averaged for each monkey at each dose of drug.
Acoustic startle
A detailed account of the apparatus used to quantify acoustic startle responses in rhesus monkeys has been described previously (Winslow et al. 2002). Briefly, startle testing was conducted in a sound-attenuating chamber equipped with a small ventilation fan and two wall-mounted, high-frequency audio speakers. Background noise (64 dB) was produced by a white noise generator (Lafayette, Model 15800; Lafayette, IN, USA) and delivered through the speakers. The speakers also delivered the startle stimulus, a 50-ms burst of white noise of varying intensities. Whole-body startle responses were measured with an Endevco 2217E accelerometer connected to a Model 104 Endevco amplifier (Endevco, San Juan Capistrano, CA, USA) inside the testing chamber. The accelerometer was center-mounted underneath the upper panel of a two-panel platform. The panels were bolted together and separated by heavy compression springs at each corner. On startle testing days, each monkey was placed inside a custom-built Lexan primate restraint box (25×25×56 cm) designed to support the monkey in an upright position while still allowing free movement within the apparatus. Subjects were positioned on the platform inside the testing chamber. Movement of the restraint box, resulting from whole-body startle responses, displaced the accelerometer and produced a voltage signal that was integrated by the Endevco amplifier to produce an output voltage signal proportional to the chamber’s displacement velocity. Startle amplitude was defined by the maximum accelerometer voltage generated within the first 200 ms of the acoustic stimulus presentation.
Three sets of acoustic startle experiments were conducted throughout this study. The stimulus–response relationship between startle stimulus intensity and startle amplitude was determined before the initiation of self-administration in the drug-naïve state, during limited access, and again during extended access. Within each set of experiments, a startle test session was conducted 3 days/week. A 24-min test session began with a 5-min acclimation period then consisted of six consecutive blocks of three stimulus intensities (95, 105, and 115 dB) for a total of 18 presentations. The order of the stimulus presentations was systematically varied for each block with an interstimulus interval of 60 s. Background movement was also sampled by measuring accelerometer voltage generated in the absence of any startle stimulus 30 s before each startle presentation. This measure was defined as basal activity to provide a measure of general activity. Two baseline test sessions were conducted prior to any drug sessions. Immediately prior to the start of a drug test session, intramuscular injections were given of either saline or drug (0.3, 1.0, and 1.7 mg/kg cocaine and 0.1, 0.3, and 1.0 mg/kg amphetamine). All saline and drug doses were randomized for each monkey and tested twice for a total of 16 test sessions for each set of startle experiments.
Study timeline
Subjects underwent the first set of microdialysis and acoustic startle experiments in the drug-naïve state (Fig. 1). Microdialysis experiments were conducted once every 2 weeks and acoustic startle test sessions were conducted three times per week (Monday, Thursday, and Friday). Immediately following these initial determinations, subjects were trained under the FR schedule of cocaine self-administration. Once the terminal FR 20 schedule was reached, sessions were limited to 1 h each day, 6 days/week. Subjects were maintained under this limited access condition for 60 test sessions. The second set of microdialysis and acoustic startle experiments began after 36 test sessions were completed. On days when subjects were not involved in either microdialysis or acoustic startle experiments, cocaine self-administration sessions were conducted as normal. Subsequently, the extended access condition (FR 20, 4 h each day) and the last set of microdialysis and acoustic startle experiments followed the same schedule described above for the limited access condition. Finally, the limited access condition was reinstated for 12 sessions to evaluate changes in the level of cocaine intake due to a history of extended access to cocaine.
Fig. 1.
Study timeline for each drug access condition including each set of microdialysis and acoustic startle experiments (see the “Materials and methods” section for detailed explanation)
Data analysis
A paired t test compared the average number of infusions earned during the first and tenth week of the extended access condition to determine if cocaine intake escalated during the 10 weeks of cocaine self-administration. In addition, daily intake during hour 1 for the first 12 days (week 1) of extended access was also compared by one-way repeated-measures analysis of variance (ANOVA).
For microdialysis, dopamine levels are expressed as nanomolar concentration in dialysate, unadjusted for probe recovery. Mean baseline dopamine concentrations for an individual subject were defined as the mean of the dopamine concentration of the six samples preceding drug treatment. The dopamine concentration for each 10-min sample was expressed as a percentage of the mean baseline value. Individual subject means were well-reflected in the group data. The peak dopamine responses for each set of microdialysis experiments were analyzed by one-way repeated-measures ANOVA to determine the effect of cocaine self-administration history on the ability of acute administration of cocaine and amphetamine to increase dopamine concentrations above baseline. Mean baseline dopamine and metabolite data were also analyzed by one-way repeated-measures ANOVA to identify any significant effect of drug history on basal levels.
The startle amplitude for each stimulus intensity is the average of two test sessions conducted for each drug dose per animal. Each test session presented the stimulus intensities six times in random order. Within each set of startle experiments, a two-way repeated-measures ANOVA was conducted for cocaine and amphetamine with startle amplitude response as the dependent measure. Differences between drug-naïve, limited access, and extended access conditions at the midrange dose of cocaine and amphetamine were analyzed by an additional two-way repeated-measures ANOVA. The Tukey’s post hoc test for multiple comparisons was used for all follow-up analyses.
Results
Self-administration behavior
Table 1 lists the total levels of drug intake (in milligrams per kilogram) in individual animals for each phase of cocaine access. Self-administration training sessions allowed for a maximum of five cocaine infusions per session (0.1 mg/kg/infusion). Training generally took about 30 sessions and total cocaine intake for all monkeys averaged 9.3±1.3 mg/kg. Following acquisition of self-administration, cocaine became available under limited access conditions. Self-administration sessions were performed 6 days/week, and over the 10 weeks of limited access, cocaine intake averaged 82.4±13.3 mg/kg. When cocaine access was increased to 4 h/day during the 10 weeks of extended access, total intake for each monkey increased considerably above their limited access levels averaging 245.0±27.8 mg/kg for the entire group.
Table 1.
Total cocaine intake (in milligrams per kilogram) during FR 20 self-administration training and limited and extended access conditions for individual monkeys
| Monkey | Training (~30days) | Limited access (10weeks) | Extended access (10weeks) |
|---|---|---|---|
| RBl6 (F) | 13.80 | 113.70 | 345.80 |
| RDn8 (F) | 4.47 | 131.30 | 315.10 |
| RJl8 (M) | 8.90 | 74.40 | 225.60 |
| RKn8 (M) | 8.30 | 50.80 | 186.90 |
| ROf8 (M) | 10.90 | 65.70 | 202.20 |
| RYt8 (M) | 9.40 | 58.50 | 194.10 |
| Mean±SEM | 9.3±1.3 | 82.4±13.3 | 245.0±27.8 |
The letter in parentheses (F or M) indicates gender
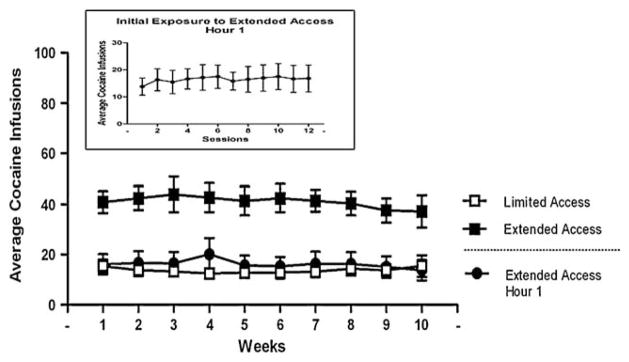
Figure 2 illustrates, on the other hand, that, as a group, drug intake did not show any escalation over the course of the 10-week extended access. A paired t test comparing the average intake during the first week to the tenth week revealed no significant difference in the level of intake throughout the extended access condition. Similarly, during the first hour of extended access sessions, cocaine intake remained unchanged from that seen during the 1 h of limited access each week, further indicating the stability of self-administration behavior. As shown in the inset of Fig. 2, there was also no increase in daily cocaine intake during hour 1 over the first 12 days of extended access. Finally, a 2-week return to limited access conditions was accompanied by a level of cocaine intake indistinguishable from that seen during the first 10 weeks (data not shown).
Fig. 2.
Average number of cocaine infusions (mean±SEM) earned each week of limited (open squares) and extended access (filled squares) conditions. Also shown is the average number of infusions earned during hour 1 of extended access (filled circles). Inset average number of cocaine infusions (mean±SEM) earned during hour 1 over the first 12 sessions of extended access
In vivo microdialysis
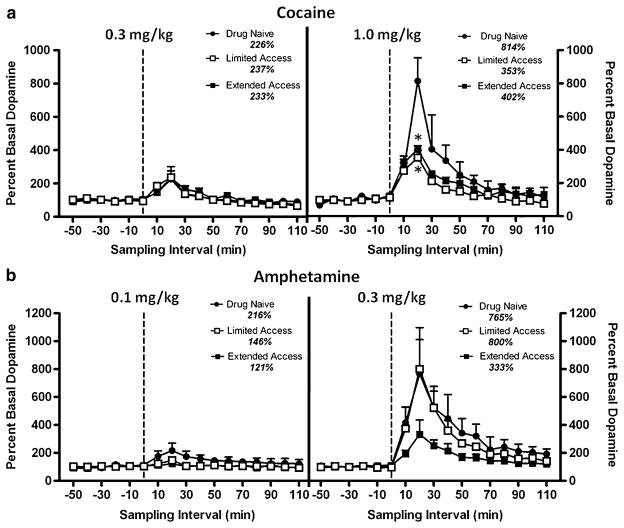
Cocaine (0.3 and 1.0 mg/kg) and amphetamine (0.1 and 0.3 mg/kg) both elicited dose-dependent increases in extracellular dopamine (Fig. 3a, b). The peak dopamine response (percent of basal levels) occurred within 20 min of the drug administration and gradually returned toward baseline levels over the remaining 2-h session. As cocaine self-administration history progressed, there was a significant decline in cocaine-induced increases in dopamine during limited and extended access (F(2,4)=8.125, p=0.039) at the highest dose. There was also a non-significant downward trend in amphetamine-induced increases in dopamine during extended access at the highest dose. There was no significant difference in the peak dopamine effect in response to the lower doses of cocaine (0.3 mg/kg) or amphetamine (0.1 mg/kg) at any time point. Note that the highest doses of cocaine (1.0 mg/kg) and amphetamine (0.3 mg/kg) also produced a comparable increase in dopamine at their peak in the drug-naive state, 814% and 765%, respectively. This potency comparison was used to determine dose selection in acoustic startle experiments.
Fig. 3.
Effects of cocaine (a) and amphetamine (b) on extracellular levels of dopamine before drug self-administration (drug-naïve) and following 10 weeks of limited and extended access (n=3). Dopamine levels are expressed as the mean (±SEM) percentage of baseline levels during 10-min sampling intervals beginning 1 h after probe implantation. Dashed vertical line at time point 0-min represents administration of drug after collection of six baseline samples. The peak percent increase in dopamine in response to both doses of cocaine and amphetamine is indicated for each drug access condition. The asterisk indicates significant difference from the drug-naïve state at time point 20-min
Table 2 shows that measures of basal dopamine (average of the six baseline samples taken before administration of drug) remained consistent across multiple experiments within individual subjects and that similar levels of dopamine were observed in all three monkeys. There was no significant change in the nanomolar concentration of dopamine as a function of cocaine self-administration history, although levels were less than 70% of those observed in the drug-naïve state during limited and extended access conditions. Also included in Table 2 are the basal concentrations of the major dopamine metabolites, DOPAC and HVA. There was a significant decrease in both metabolites as a function of cocaine self-administration history (DOPAC: F(2,4)=13.452, p=0.017; HVA: F(2,4)= 10.318, p=0.026). Note that the ratio of dopamine/DOPAC and dopamine/HVA was consistent across all conditions. There was no change in in vitro probe recovery of dopamine or its metabolites across multiple determinations (data not shown).
Table 2.
Baseline dopamine and metabolites
| DA | DOPAC | HVA | DA/DOPAC | DA/HVA | |
|---|---|---|---|---|---|
| Drug-naïve | |||||
| RYt8 | 8.93±2.3 | 331.0±99.2 | 4,210±1,024 | 0.027 | 0.0021 |
| RKn8 | 11.25±4.5 | 451.7±31.5 | 5,381±635.9 | 0.025 | 0.0021 |
| RDn8 | 14.00±4.0 | 232.4±51.5 | 4,209±1,367 | 0.060 | 0.0033 |
| Mean | 10.88±0.4 | 355.0±11.0 | 4,625±114.8 | 0.031 | 0.0024 |
| Limited access | |||||
| RYt8 | 7.44±2.6 | 221.4±42.2 | 3,610±651.8 | 0.034 | 0.0021 |
| RKn8 | 9.16±3.3 | 250.0±28.2 | 4,612±1,399 | 0.037 | 0.0020 |
| RDn8 | 4.93±3.1 | 175.1±33.0 | 2,712±583.7 | 0.028 | 0.0018 |
| Mean | 7.28±0.3 | 213.6±3.9a | 3,678±104.7a | 0.034 | 0.0020 |
| Extended access | |||||
| RYt8 | 8.39±4.4 | 194.8±128.7 | 3,156±282.1 | 0.043 | 0.0027 |
| RKn8 | 11.47±4.0 | 274.9±50.3 | 4,814±754.1 | 0.042 | 0.0024 |
| RDn8 | 2.53±1.0 | 105.1±61.6 | 3,083±761.9 | 0.024 | 0.0008 |
| Mean | 7.45±0.4 | 192.1±9.4a | 3,711±121.4a | 0.038 | 0.0020 |
Data represent nanomolar concentrations of dopamine and its metabolites, DOPAC and HVA, uncorrected for probe recovery, with SD for individual animals and SEM for the group mean
Significantly different from the drug-naïve state
Acoustic startle
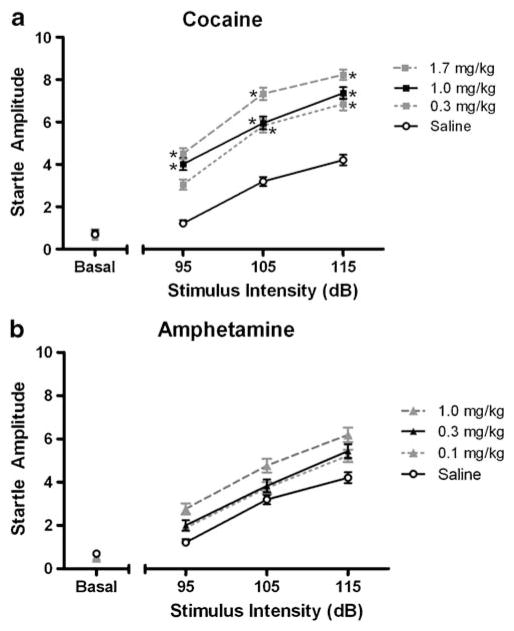
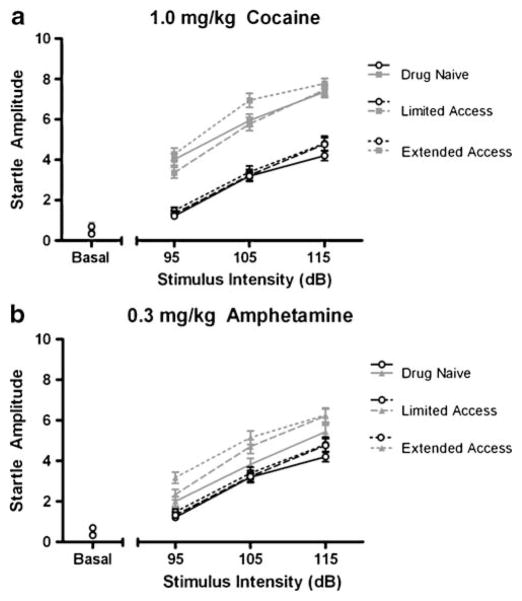
As expected, acoustic startle was robust and its amplitude increased with stimulus intensity. There was a significant main effect of acute cocaine challenge on acoustic startle (drug-naïve [F(3, 15)=35.713, p<0.001]; limited access [F(3, 15)=13.677, p<0.001]; extended access [F(3,15)= 31.718, p<0.001]) that was not seen with amphetamine. No dose of amphetamine produced a startle response greater than the saline control even with repeated, long-term cocaine self-administration history. The first set of startle experiments during the drug-naïve state is depicted in Fig. 4a, b and is typical of the stimulus–response relationship seen during limited and extended access. For each set of startle determinations, cocaine consistently induced a greater enhancement of startle than any dose of amphetamine. Although cocaine treatment enhanced the startle response above that of saline, the effect was not dose-dependent nor did the effectiveness of cocaine change as cocaine self-administration history increased from limited to extended access conditions. Startle amplitude at the midrange dose tested for both cocaine and amphetamine is graphed in Fig. 5a, b for each set of startle experiments. Cocaine self-administration history had no effect on baseline startle or startle amplitude in response to acute stimulant challenges with either cocaine or amphetamine.
Fig. 4.
Effects of cocaine (a) and amphetamine (b) on average startle amplitude for drug-naïve animals (n=6). Drug-naïve subjects were given either saline or drug immediately prior to the start of an experimental session. Graphs depict mean startle amplitude for increasing drug doses as a function of acoustic stimulus intensities (in decibels). Data are collapsed across repeated blocks of stimulus presentation. The asterisk indicates significant difference from saline at each stimulus intensity
Fig. 5.
Average startle amplitude as a function of cocaine self-administration history (n=6). For each set of acoustic startle experiments (drug-naïve, limited access, and extended access), subjects were given either saline or drug immediately prior to the start of an experimental session. Graphs depict mean startle amplitude as a function of acoustic stimulus intensities (in decibels) at the midrange dose tested for cocaine (1.0 mg/kg) and amphetamine (0.3 mg/kg) for each set of startle experiments. Data are collapsed across repeated blocks of stimulus presentation. Open circles represent saline in both graphs
Discussion
The objective of the present study was to establish a well-documented history of cocaine self-administration in nonhuman primates and to evaluate potential changes in dopamine system function. Cocaine- and amphetamine-induced increases in striatal dopamine diminished following a history of cocaine self-administration, indicating tolerance to the neurochemical effects of these psychomotor stimulants. This diminished dopamine response was not associated with a significant decrease in basal dopamine, although there was a significant decrease in DOPAC and HVA levels during the limited access condition. Interestingly, these changes in dopamine neurochemistry were not dependent upon the emergence of an escalating pattern of drug intake. During the extended access condition, there was no escalation in the number of cocaine infusions earned over the 60-day period. This was surprising considering rodent studies employing a similar dosing regimen have repeatedly shown an escalating pattern of cocaine intake in subjects that have experienced up to 6 h of daily cocaine access (Ahmed and Koob 1999; Ferrario et al. 2005; Knackstedt and Kalivas 2007). In acoustic startle experiments, acute administration of cocaine enhanced the startle response, but there was no effect of self-administration history on baseline startle amplitude or sensitivity to cocaine-induced enhancement of startle. Overall, the present experiments indicate that nonhuman primates with a history of cocaine self-administration show evidence of tolerance to cocaine-and amphetamine-induced increases in striatal dopamine. This effect on dopamine neurochemistry, however, was not associated with an escalation in drug intake and was not reflected in behavioral measures of acoustic startle.
Neuroimaging studies in cocaine-dependent human subjects have associated long-term cocaine use with a decrease in dopamine neurotransmission. Functional changes in the dopamine system in dependent subjects can be characterized by marked decreases in drug-induced dopamine release, increases in dopamine transporter density, and decreases in dopamine receptor binding, consistent with a hypofunctional dopamine system (Volkow et al. 1990, 1997; Malison et al. 1998; Martinez et al. 2007). These human studies are consistent with findings reported in rodents showing a diminished dopamine response to cocaine following prolonged access to cocaine. An acute cocaine challenge in rodents with a history of continuous cocaine self-administration for 24 h failed to significantly increase dopamine levels above that seen in drug-naïve animals (Mateo et al. 2005). Similar to the current study, there was also no difference in the cocaine-induced dopamine response between rodents provided extended access (6 h) to cocaine self-administration and those with only limited access (1 h) (Ahmed et al. 2003). The strength of the current study was the use of a within-subject, longitudinal design that allowed for the evaluation of striatal dopamine from a drug-naive state through two different conditions of cocaine self-administration in nonhuman primates.
Acoustic startle is an innate reflex elicited by a sudden and intense auditory stimulus. The reflex is mediated by a simple three-synapse circuit (Davis et al. 1982; Lang et al. 2000; Lee et al. 1996) and is sensitive to dopaminergic modulation by both direct and indirect dopamine agonists (Davis et al. 1986b; Meloni and Davis 1999). Furthermore, chronic cocaine users have been shown to have diminished responses to startle stimuli presumably as a consequence of an extensive drug-taking history (Efferen et al. 2000). It was, therefore, hypothesized that enduring neuroadaptive changes in the dopamine system due to chronic cocaine exposure would alter the responsiveness to acoustic startle. Moreover, it was hypothesized that the enhanced startle amplitude following acute stimulant challenge would also be diminished, further indicating a tolerance to psychostimulants. The present study is the first to characterize the effect of cocaine self-administration history on subsequent acoustic startle responding in nonhuman primates. However, there was no evidence that cocaine self-administration history affected basal or stimulant-induced enhancement of startle amplitude. The consistency of startle-inducing acoustic and tactile stimuli (air puffs) following several days or weeks of continuous cocaine exposure is well-documented by the literature derived from rodent studies. Compared to saline-treated groups, maximum tactile startle responses were largely unchanged in rodents with a history of cocaine self-administration or chronic cocaine infusions (Mansbach et al. 1994; Barros and Miczek 1996; Mutschler and Miczek 1998a, b). The magnitude of the acoustic startle response was similarly unaffected by long-term cocaine exposure whether self-administered or by chronic infusion (Mansbach et al. 1994). Similarly, there was no change in baseline (saline) responding to acoustic stimuli at any time point in this study.
Acute administration of cocaine enhanced the acoustic startle response, although the effect was not dose-dependent. A ceiling effect may explain the lack of a significant dose-related effect with cocaine. Interesting, sensitivity to cocaine-induced enhancement of startle was not influenced by cocaine self-administration history even though there was evidence of a decreased dopamine response determined with in vivo microdialysis. While the dopamine response to acute cocaine challenge was blunted with continued cocaine self-administration history, the increase observed may have been sufficient to modulate startle. Additionally, cocaine has equipotent affinity for the dopamine and serotonin transporters (Rothman and Baumann 2003) and selective serotonin receptor agonists alone have been shown to increase the magnitude of the acoustic startle response in rodents (Svensson and Ahlenius 1983; Davis et al. 1986a; Nanry and Tilson 1989). A potential role for serotonin is supported by the ineffectiveness of amphetamine to enhance acoustic startle in the present study. Unlike cocaine, amphetamine has very low affinity for the serotonin transporter (Rothman and Baumann 2003). Amphetamine is a potent dopamine transporter blocker and a releaser of dopamine (Sulzer et al. 1995), but acute administration of amphetamine did not have a significant effect on startle amplitude.
Literature derived from rodent studies also suggests that the behavioral-stimulant effects of cocaine may be insensitive to changes induced by chronic cocaine self-administration. Rodents trained to self-administer cocaine under a long access schedule (6 h/session) escalate their drug intake over time; however, they show the same level of cocaine-induced locomotor activity as their short access counterparts (1 h/session) (Ben-Shahar et al. 2004, 2005; Ferrario et al. 2005; Ahmed and Cador 2006; Knackstedt and Kalivas 2007). Moreover, consistent behavioral-stimulant effects were observed even though long access to cocaine was associated with increased motivational effects of cocaine, increased spine density in the nucleus accumbens core, enhanced synaptic activity, and synaptic reorganization (Ben-Shahar et al. 2004; Ferrario et al. 2005; Knackstedt and Kalivas 2007). Clearly, changes in neurochemistry are not necessarily reflected across all behavioral measures.
Interestingly, there was no escalation in drug intake during the extended access condition. Compared to the limited access condition, drug intake increased markedly when subjects were given three extra hours of drug access. However, drug intake was remarkably stable over the 60 days of extended access. Moreover, drug intake during the first hour of extended access was virtually identical to drug intake during the 1-h limited access condition. This contrasts with a number of rodent studies reporting an escalation in cocaine intake during extended access conditions (Ahmed and Koob 1998, 1999; Ben-Shahar et al. 2004; Mantsch et al. 2004; Ferrario et al. 2005). Rodents provided long access to cocaine increase their drug intake significantly within five self-administration sessions and continue to increase thereafter. Furthermore, increases in cocaine intake are seen in rodents within the first hour of long access conditions, whereas intake remains unchanged with repeated testing under limited access conditions. In the current study, a similar reinforcement schedule was used in the nonhuman primate model including prolonged self-administration sessions for 60 days providing ample opportunity for an escalated pattern of cocaine intake to emerge. In fact, a consistent level of cocaine intake was also noted previously in rhesus monkeys provided supplemental drug intake under a FR schedule several days a week. Even with months of supplemental drug intake, there was no indication of an escalated pattern of cocaine intake over time (Henry and Howell 2009). Taken together, these nonhuman primate studies demonstrate that supplemental drug intake or extended access to cocaine self-administration do not result in escalation of cocaine intake. Nonetheless, the present findings did show a significant effect of cocaine self-administration history on subsequent neurochemical measures which lends support for the utility of the nonhuman primate as a model in which to study the impact of extended cocaine access on neurochemistry.
In summary, rhesus monkeys with a progressive history of cocaine self-administration developed evidence of tolerance to stimulant-induced increases in striatal dopamine as determined with in vivo microdialysis. This neuroadaptive response was not associated with an escalated pattern of cocaine intake. In contrast, behavioral measures of stimulant-enhanced acoustic startle responding were unaffected as cocaine exposure increased despite the blunted dopamine response to cocaine. The results of this study demonstrate that an escalated pattern of drug use is not necessary for drug history to alter the responsiveness of the dopamine system. Duration of cocaine access may be a more important variable in determining the neurobiological effects of cocaine self-administration history in nonhuman primates.
Acknowledgments
This study was supported by USPHS grants DA016589 and DA00517 to LH, DA018294 to MD, and RR00165.
Contributor Information
Porche’ Kirkland Henry, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA.
Michael Davis, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA. Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA 30322, USA.
Leonard L. Howell, Email: lhowell@emory.edu, Yerkes National Primate Research Center, Emory University, Atlanta, GA 30322, USA. Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA 30322, USA
References
- Adams JU, Efferen TR, Duncan EJ, Rotrosen J. Prepulse inhibition of the acoustic startle response in cocaine-withdrawn rats. Pharmacol Biochem Behav. 2001;68:753–759. doi: 10.1016/s0091-3057(01)00478-6. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology. 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent Alterations in Cognitive Function and Prefrontal Dopamine D2 Receptors Following Extended, but Not Limited, Access to Self-Administered Cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Davis M. Cocaine: excitatory effects on sensorimotor reactivity measured with acoustic startle. Psychopharmacology. 1985;86:31–36. doi: 10.1007/BF00431680. [DOI] [PubMed] [Google Scholar]
- Davis M, Svensson TH, Aghajanian GK. Effects of D- and L-amphetamine on habituation and sensitization of the acoustic startle response in rats. Psychopharmacologia. 1975;43:1–11. doi: 10.1007/BF00437607. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Cassella JV, Wrean WH, Kehne JH. Serotonin receptor subtype agonists: differential effects on sensorimotor reactivity measured with acoustic startle. Psychopharmacol Bull. 1986a;22:837–843. [PubMed] [Google Scholar]
- Davis M, Commissaris RL, Cassella JV, Yang S, Dember L, Harty TP. Differential effects of dopamine agonists on acoustically and electrically elicited startle responses: comparison to effects of strychnine. Brain Res. 1986b;371:58–69. doi: 10.1016/0006-8993(86)90810-3. [DOI] [PubMed] [Google Scholar]
- Efferen TR, Duncan EJ, Szilagyi S, Chakravorty S, Adams JU, Gonzenbach S, Angrist B, Butler PD, Rotrosen J. Diminished acoustic startle in chronic cocaine users. Neuropsychopharmacology. 2000;22:89–96. doi: 10.1016/S0893-133X(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Ellinwood EH., Jr Cocaine dependence. Annu Rev Med. 1989;40:149–161. doi: 10.1146/annurev.me.40.020189.001053. [DOI] [PubMed] [Google Scholar]
- Henry PK, Howell LL. Cocaine-induced reinstatement during limited and extended drug access conditions in rhesus monkeys. Psychopharmacology. 2009 doi: 10.1007/s00213-009-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Markou A, Patrick GA. Lack of altered startle responding in rats following termination of self-administered or noncontingently infused cocaine. Pharmacol Biochem Behav. 1994;48:453–458. doi: 10.1016/0091-3057(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Enhancement of the acoustic startle response in rats by the dopamine D1 receptor agonist SKF 82958. Psychopharmacology. 1999;144:373–380. doi: 10.1007/s002130051020. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from a self-administered or non-contingent cocaine binge: differences in ultrasonic distress vocalizations in rats. Psychopharmacology. 1998a;136:402–408. doi: 10.1007/s002130050584. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA. Withdrawal from i.v. cocaine “binges” in rats: ultrasonic distress calls and startle. Psychopharmacology. 1998b;135:161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- Nanry KP, Tilson HA. The role of 5HT1A receptors in the modulation of the acoustic startle reflex in rats. Psychopharmacology. 1989;97:507–513. doi: 10.1007/BF00439556. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Siegel RK. Changing patterns of cocaine use: longitudinal observations, consequences, and treatment. NIDA Res Monogr. 1984;50:92–110. [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L, Ahlenius S. Enhancement by the putative 5-HT receptor agonist 8-OH-2-(di-n-propylamino) tetralin of the acoustic startle response in the rat. Psychopharmacology. 1983;79:104–107. doi: 10.1007/BF00427793. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Parr LA, Davis M. Acoustic startle, prepulse inhibition, and fear-potentiated startle measured in rhesus monkeys. Biol Psychiatry. 2002;51:859–866. doi: 10.1016/s0006-3223(02)01345-8. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain mechanisms of drug reward and euphoria. Psychiatr Med. 1985;3(4):445–460. [PubMed] [Google Scholar]