Abstract
Developing autoreactive B cells may edit (change) their specificity by secondary H or L chain gene rearrangement. Recently, using mice hemizygous for a site-directed VDJH and VJκ transgene (tg) encoding an auotreactive antibody, we reported ongoing L chain editing not only in bone marrow cells with a pre-B/immature B cell phenotype but also in immature/transitional splenic B cells. Using the same transgenic model, we report here that editing at the H chain locus appears to occur exclusively in bone marrow cells with a pro-B phenotype. H chain editing is shown to involve VH replacement at the tg allele or VH rearrangement at the wild-type (wt) allele when the tg is inactivated by nonproductive VH replacement. VH replacement/rearrangement at the tg/wt alleles was found to entail diverse usage of VH genes. Importantly, whereas the development of edited B cells expressing the wt allele was dependent on the λ5 component of the surrogate L chain, the development of B cells expressing the tg allele, including those with VH replacement, appeared λ5 independent. We suggest the unique CDR3 region of the tg-encoded μH chain is responsible for the λ5 independence of tg-expressing B cells.
Keywords: B cell differentiation, autoreactive B cells, V(D)J rearrangement, VH replacement
INTRODUCTION
In response to encounter with self-antigen, autoreactive B cells may undergo secondary L chain gene rearrangement and edit their antigen receptor specificity by expression of a new L chain (1-5). B cells may also edit their antigen receptor specificity by a process known as VH replacement, in which case the targeting of a cryptic recombination signal sequence (cRSS)2 within a rearranged VH gene results in its replacement by an upstream VH gene (6-12).
VH replacement may occur during early B cell differentiation at H chain alleles with a nonproductive VDJH rearrangement (6, 7, 13). In humans, VH replacement has been estimated to occur in 5% or more of developing B cells based on the recovery of potential VH replacement products (“footprints”) in analyzed H chain sequences (13). In mice, the relative frequency at which rearranged VH genes may be targeted for replacement has been estimated to be 1-7% based on the recovery of dsDNA breaks at cRSS sites in VH1 and VH5 genes relative to the recovery of breaks at the JH2 RSS (14). Relatively few dsDNA breaks have been reported at the highly conserved cRSS in the 3′ region of murine VH genes (12, 14). Nonetheless, VH replacement at this 3′ cRSS site has been shown to rescue B cell differentiation in transgenic mice with non-productively rearranged VH genes at both alleles (VDJH−/VDJH−) (15, 16).
There is evidence that VH replacement may also occur relatively late in B cell differentiation. For example, VH replacement in the transgene (tg) mouse model reported by Cascalho et al. (17) was found to occur not only in bone marrow (BM) but also in spleen (SPL) (18), consistent with the observed high frequency of mutation in the replaced VH genes of these mice (19). VH replaced and somatically mutated genes also have been recovered in clonally expanded subpopulations of human tonsillar and synovial B cells (20, 21), suggesting that VH replacement may have occurred before or during the mutation and expansion of these cell populations. The basis for VH replacement during late B cell differentiation is not clear; it could possibly reflect loss of a functional H chain or expression of a self-reactive H chain. Consistent with the latter possibility is the finding of VH replacement in murine B cells with a site-directed (sd) tg coding for a self-reactive H chain (10).
VH replacements that are nonproductive may result in VH rearrangement at the second allele. Taki et al. (11) first reported an example of this in mice hemizygous for a sd-tg (VHT15) inserted into the DHQ52-JH region. More than half of the B cells in these mice were found to express the H chain allotype of the wt allele instead of that of the tg allele. Sekiguchi et al. (22) reported similar findings in transgenic mice hemizygous for a sd-tg (56R) that codes for a H chain with anti-self (DNA) specificity. The induction of chronic graft-versus host disease in 56R mice resulted in a relatively high frequency of B cells with VH replacements at the 56R allele or VH rearrangements at the wt allele in cases where 56R was inactivated. What was left unclear, as discussed by the authors (22), is whether VH replacement/rearrangement at the tg/wt alleles occurred during early or late B cell differentiation and whether it reflected incomplete allelic exclusion, loss of a functional H chain or autoimmune receptor editing.
To address the above issues regarding VH replacement/rearrangement at the tg/wt alleles in mice hemizygous for 56R and in mice hemizygous for both 56R and the sd-tg, Vκ8 (23), we asked the following: when during B cell differentiation does H chain editing occur? As reported here, we found VH replacement/rearrangement at the tg/wt alleles to occur in BM cells with a pro-B phenotype. The differentiation of pro-B cells is well-known to be dependent on the surrogate L (SL) chain (rev in (24-26)), and consistent with expectation, the development of H chain edited B cells expressing the allotype of the wt allele (IgHb) in 56R and 56RVκ8 mice was clearly dependent on the presence of the λ5 component of the SL chain. In contrast, the development of B cells expressing the tg allotype (IgHa), including those with a replaced 56R VH gene segment, appeared λ5 independent. We suggest the apparent λ5 independence of edited and unedited 56R-expressing B cells may reflect a property of the CDR3 region of the 56R μH chain, a region that is retained in 56R μH chains with a replaced VH region. These and other aspects of H chain editing are discussed and contrasted with previous findings on L chain editing in mice bearing 56R.
MATERIALS AND METHODS
Mice
H/L chain transgenic mice hemizygous for the sd-tgs, 3H9 and Vκ8 (3H9Vκ8 mice) or 3H9(56R) and Vκ8 (56RVκ8 mice), were produced by crossing C.B-17 scid/scid mice doubly homozygous for these tgs (e.g. 3H9/3H9, Vκ8/Vκ8, scid/scid mice) with C.B-17 mice (27). To produce mice hemizygous for the above H chain sd-tgs alone, here simply designated as 3H9 and 56R mice, we crossed C.B-17 scid/scid mice homozygous for 3H9 or 3H9(56R) with C.B-17 mice. Genotyping of Ig transgenic mice was done by PCR as described previously (10, 23, 28). 56R and 56RVk8 mice lacking the λ5 component of the surrogate L chain (56Rλ5−/− and 56RVκ8λ5−/− mice) were obtained by selective backcrossing of transgenic mice with C.B-17λ5−/− mice provided by R. R. Hardy (Fox Chase Cancer Center). Genotyping of mice for λ5 was done by PCR using forward (5′ CACTCATTCTAGCCTCTAGTCCGTG) and reverse (5′ TCCGCCCGGGCATGAAGCTCAGAGTAGGACAGACTC) primers under the following conditions: 5 min at 95°C, followed by 34 cycles (30s at 94°C, 30 s at 72°C, 1 min at 72°C) and a final 5 min extension at 72°C. For non-transgenic controls we used C.B-17 or C.B-17 scid/+ mice; the scid mutation is recessive and C.B-17 scid/+ mice have a wt phenotype (27, 29). BALB/c mice lacking RAG1 (RAG1−/− mice) (30) or TdT (TdT−/− mice) (31) were provided by R. R. Hardy. 56RVκ8 RAG1−/− mice were selectively bred as described earlier (28). Investigators interested in obtaining mice homozygous for the H and L chain tgs reported here should contact the Mutant Mouse Regional Resource Center at the University of North Carolina, Chapel Hill (www.mmrrc.org). All of the mice used in this study, including BALB/c and C57BL/6 × BALB/c F1 mice, were produced in the Laboratory Animal Facility of the Fox Chase Cancer Center. Mice were used between 8-15 weeks of age according to the protocols approved by the Animal Care and Use Committee of this institution.
Flow Cytometry
For most analyses, BM and SPL cells were stained for two or more of the following cell surface markers: CD23, CD43, CD93, CD45 (B220), IgM, IgMa, IgMb, IgDa, IgDb and Vλx using Cy7PE-anti-CD23 (B3B4), Fluorescein (FL)-anti-CD43 (S7), PE-anti-CD93 (AA4.1), APC-anti-CD45/B220 (RA3-6B2), FL- or PE-anti-IgM (331.1), FL-anti-IgMa (RS3.1), biotin-anti-IgMb (AF6-78), FL-anti-IgDa (AMS9.1), PE-anti-IgDb (217-270) and biotin-anti-Vλx (10C5). All of the above reagents were made in this laboratory except Cy7PE-anti-CD23, PE-anti-CD93, FL-anti-IgDa and PE-anti-IgDb from BD Biosciences. Biotin conjugated reagents were visualized by a second-step incubation with QDot605-streptavidin (Invitrogen). Intracellular staining with Alexa 488 conjugated anti-TdT (19.3) (a gift from J. Kearney, University of Alabama) was performed with fixed permeabilized cells (BD Cytofix/Cytoperm Kit). Cells were stained for 30 min and washed twice with cytoperm buffer at 4° C. For intracellular staining with Cy5-SL-156 (a gift from R. R. Hardy), cells were fixed and permeablilized using a CALTAG Fix and Perm Cell Permeabilization Kit (Invitrogen) according to manufacturer’s instructions. Analyses were performed with LSRII and FACS VantageSE/DiVa flow cytometers (BD Biosciences) using FlowJo software (TreeStar). Forward and side scatter were set to exclude nonlymphoid cells. For cell surface staining propidium iodide was used to exclude dead cells.
Cell sorting of BM pro-B (B220+CD43+sIgM−), pre-B (B220+CD43−sIgM−) and B (B220+sIgM+) cells and, immature/transitional splenic B cells with a T3 phenotype (B220+CD93+CD23highIgM−/lowIgD+) (32, 33), was performed with a FACS VantageSE/Diva flow cytometer. Cells were stained as described previously (32). The pro-B subsets, B (B220+CD24−CD43+BP-1−sIgM−), C (B220+CD24−CD43+BP-1+sIgM−) and C’ (B220+CD24+ CD43+BP-1+sIgM−) (34) were distinguished using FL-anti-CD43 (S7), Alexa 594-anti-CD24/HSA (30F1), APC-anti-CD45/B220 (RA3-6B2), biotin-anti-IgM (331.12) and PE-anti-BP-1. Before sorting the pro-B subsets, monocytes, macrophages, granulocytes, dendritic cells, erythroid cells and T lymphocytes were excluded using reagents specific for the cell surface markers, CD11b [Cy7APC-anti-CD11b/Mac-1 (M1/70)], GR1 (Cy7APC-GR1), Ly6C (Cy5PE-anti-Ly6C) Ter 119 (Cy5PE-Ter 11) and CD3 (Cy5PE-500A.A2). Biotin-anti-IgM (331.12), FL-anti-CD43 (S7), and APC-anti-CD45/B220 (RA3-6B2) were made in this laboratory. All other reagents were gifts from R. R. Hardy.
Amplification of Genomic DNA
Genomic DNA was isolated from SPL or BM cells using a DNeasy Tissue Kit according to the manufacturer’s protocol (Qiagen, Valencia CA). PCR assays of genomic DNA were carried out in a 25 μl volume containing 100 ng of genomic DNA and 0.5 units of Platinum Taq DNA polymerase (Invitrogen). To determine if 56R was intact, PCR was performed with primers specific for genomic sequence 5′ of the 56R targeting vector (MB 726, 5′ GATAACTCAGACACAAGTGAATGACAG) and for the vector’s neomycin-resistance (neoR) gene (MB 725, 5′ GCATTGTCTGAGTAGGTGTCATTC) (10) with the following thermocycler conditions: 35 cycles of 94°C for 30 s, 65°C for 30 s, 72° for 1 min. To amplify VH replacement products, PCR primers specific for the VH gene families, VH7183 (MB 650) (35), VHQ52 (MB 647) (35), VH3609 (MB 749) (36) and VHJ558 (MB 756, 5′ GAGATTTATCCTGGAAGTGGTAATAC), were used together with a primer specific for the CDR3 of 56R VH region (37). The MB 756 primer does not recognize the 56R VH region (E.L. Prak and L. Yunk, University of PA, unpublished observation). The conditions were as follows: 35 cycles of 94°C for 30 s, 59°C (53°C for MB 756) 30 s and 72°C for 30 s. To amplify VH rearrangements at the wt heavy-chain allele we used MB 647, 749 and 649 (a VHJ558 specific primer (35)) together with a 3′ JH2-specific primer (MB 711, 5′ GGATCCCAATGACCCTTTCTGA). The conditions were 30 s at 94°C, 30 s at 59°C and 1 min at 72°C for 35 cycles. For amplifying VH rearrangements in DNA of non-transgenic mice, we used 26 cycles. In some assays, VH replacements/rearrangements in serially diluted pro-B cell DNA were PCR amplified in the presence (or absence) of a constant amount of inert carrier (liver) DNA from RAG1−/− mice. VJκ rearrangements were amplified using MB 759, a Vκ-specific degenerate primer (38), and MB 735, a 3′ Jκ2-specific primer (39). The conditions were 30 s at 94°C, 30 s at 61°C and 1 min at 72° C for 26 cycles. To control for the amount of genomic DNA in each PCR reaction, the beta 2 microglobulin (β2M) gene was amplified for 25 cycles as previously described (32).
Southern Analysis
PCR products were subjected to agarose gel electrophoresis, transferred to nylon membranes by a downward alkaline transfer method and hybridized to random-primed 32P-labeled DNA probes. In each case, the probe was a gel-purified PCR product: the β2M PCR probe was made as described previously (32); the 56R probe was a 1,500 bp fragment amplified from plasmid DNA containing the 56R region using primers MB 724 (5′ ATCTACATAGCTAGAGAGCTAGAGC) and MB 725; the V gene-specific probes for VHQ52, VH3609 and VHJ558 gene families were 700 bp DNA fragments amplified with primers MB 701 (5′ CTAATACGACTCACTATAGGGC) and MB 671 (5′ CATGGCCACCAGATTCTTATCAG) from previously sequenced cDNA clones obtained from 56RVk8 hybridomas or spleen cells using the technique, SMART (switching mechanism at the 5′ end of the RNA transcript), as described in Ref 32; and the Jκ probe for VJκ rearrangements was a 1.7 kb fragment from pJκ and spans the Jκ region (40).
SMART cDNA preparation and 5′ RACE anchored PCR amplification
Total RNA from 1 × 106 hybridoma cells or 5 × 105 sorted IgMb-expressing SPL cells was isolated using an RNeasy mini kit (Qiagen). To synthesize full-length cDNA, we used a modified version of the SMART (switching mechanism at 5′ end of RNA transcript) technique (41) with one-tenth of the RNA, as described previously (32). The SMART cDNA was then subjected to 5′ Rapid Amplification of cDNA Ends (RACE) RT-PCR in order to amplify μH chain cDNA products using a reverse primer (MB 671) complementary to the constant region of the μH chain and a 5′ Universal primer mix, as described previously (32). The size of the μH chain 5′ RACE amplified PCR product was approximately 700 bp.
Cloning and sequencing
PCR-amplified VH replacement products from the 56R allele and μH chain SMART PCR products corresponding to the wt allele, as well as PCR-amplified VJκ rearrangement products, were electrophoresed through 2% NuSieve agarose, excised and the DNA recovered using QIAEX II Gel Extraction Kit as recommended by the manufacturer (Qiagen). Gel-purified VJκ products were re-amplified for 25 cycles using semi-nested primers, MB 759 and a 3′ Jk1-specific primer (MB 735, 5′ TCTCCAGAGAACATGTCTAGC), followed by removal of unused primers using a QIAquick Purification kit as directed by manufacturer (Qiagen). Purified PCR products were cloned using the TOPO TA cloning kit (Invitrogen) and plasmid DNA was isolated using Perfect Prep Plasmid Mini kit (Eppendorf). Cycle sequencing was performed with the ABI Prism dye terminator reaction kit and a model 3100 genetic analyzer (Applied Biosystems) using an oligonucleotide primer complementary to the constant region of the μH chain (MB 671) for SMART cDNA clones and a universal-sequencing primer for clones corresponding to VH replacements and VJκ rearrangements. DNA sequences were analyzed using the Ig Basic Local Alignment Research Tool (Ig BLAST) (www.ncbi.nlm.nih.gov/BLAST). This program provides the germline VH, DH and JH genes that show the closest match to the query sequence, with a minimum of 5 consecutive nucleotides required for DH and JH gene assignments. Individual VH families and members were designated according to Johnston et al. (42).
RESULTS
B cells expressing the IgH allotype of the wt allele (IgHb) in 56RVκ8 mice
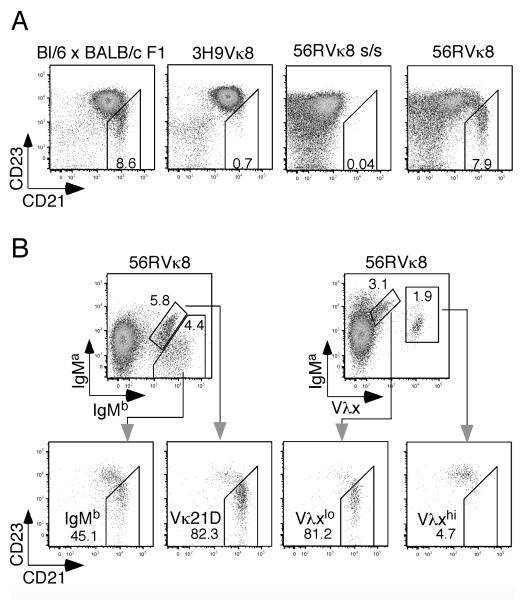
As we noted in a recent paper (32), H/L chain transgenic mice hemizygous for the sd-tgs, 56R and Vκ8 (56RVκ8 mice), were found to contain splenic B cells expressing the μH chain allotype of the endogenous wt allele (μb) instead of the tg allele (μa). Approximately ~7% of B220+ gated splenic B cells expressed surface IgMb (sIgMb+) rather than surface IgMa (sIgMa+) as shown in the FACS profiles of Figure 1 (top row). Very few H chain edited sIgMb expressing cells (≤0.5%) were seen in the SPL of 3H9Vκ8 mice. Note that splenic B cells in 56RVκ8 mice showed much less sIgMa on a per cell basis than the 3H9Vκ8 controls. Also, as shown previously (28), 56RVκ8 mice contain approximately 5-fold fewer splenic B cells (~5 × 106) than non-transgenic controls (~30 × 106).
Figure 1.
Detection of B cells expressing the wt H chain allotype (IgHb) in the SPL of 56R and 56RVκ8 mice. Flow cytometric dot plots of IgMa versus IgMb and IgDa versus IgDb for B220 gated lymphocytes are shown for individual mice of the indicated genotypes (BL/6 × BALB/c F1, 56RVκ8, 3H9Vκ8, 56R, 3H9 and RAG1−/−). B220+ cells staining positive for both sIgMa and sIgMb in 56RVκ8 mice (top row) do not actually express IgMb but instead correspond to L chain edited B cells expressing sIgMa molecules with Vκ21D and the unedited 56R μH chain, a H/L chain pair that is cross reactive with the AF6-78 anti-IgMb reagent (32). L chain edited sIgMa+ cells represent ≥40% of the splenic B cells in 56RVκ8 mice; the remaining sIgMa+ cells express the Vκ8 tg (32). The numbers indicate the percentage of cells in each quadrant. The mean percentage (±SEM) of sIgMb+ and sIgDb+ cells for seven analyzed 56RVκ8 mice was 6.7 ± 1.5 and 4.9 ± 0.8, respectively. The profiles shown here and in Figures 5, 6 and 7 are representative of two or more independent experiments in which two mice of each genotype were analyzed.
The cell preparations used for analysis in the top row of Figure 1 were also analyzed with anti-IgD allotype reagents (see middle row). As indicated, ~5% of B220+ gated splenic B cells in 56RVκ8 mice were positive for surface IgDb (sIgDb+). Similar results were obtained with transgenic mice containing 56R only (Figure 1, bottom row). The H chains encoded by 3H9 and 56R differ only at position 56 in the VH region: at this position there is an aspartate in 3H9 and an arginine in 56R (4). We conclude that this single amino acid difference must be responsible for the greater frequency of sIgMb+/sIgDb+ cells in 56R and 56RVκ8 than 3H9 and 3H9Vκ8 mice.
sIgMb+ B cells show disruption and inactivation of the 56R allele
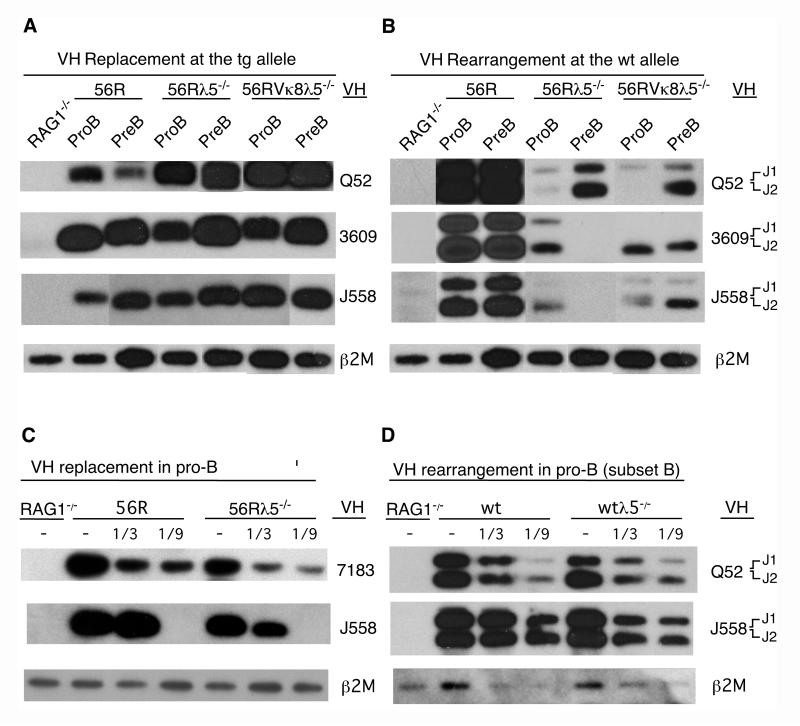
Silencing of 56R in sIgMb+ B cells could involve one or two rearrangement events (VH to VDJH or VH to DH to VDJH) as illustrated in Figure 2C. Although DH elements are normally deleted during VH rearrangement, the VDJH coding segment of 56R is flanked by upstream DH elements (4, 10). DH invasion into the 3′ cRSS region of the 56R VH gene segment would disrupt and inactivate the tg. The tg would also be inactivated by a non-productive VH replacement of the 56R VH gene segment. DH invasion and/or VH replacement would remove the neoR marker upstream of 56R (56R neoR). To test for loss of the 56R neoR marker, we used PCR and the appropriate primers (illustrated in Figure 2A) to amplify a portion of the 56R neoR gene in sorted B220+sIgMb+ splenic B cells pooled from five donor 56RVκ8 mice. As a positive control for full retention of the 56R neoR marker we used DNA from BM of 56RVκ8 RAG1−/− mice, in which no DH rearrangement or VH replacement is possible. For the negative control we used DNA from splenic B cells of C.B-17 mice. PCR products were hybridized with a 56R neoR-specific probe. As shown in Figure 2B, we observed a dramatic loss of the 56R neoR marker in sIgMb+ B cells. Little or no loss of this marker was seen in the sIgMa+ splenic B cell population of 56RVκ8 mice, consistent with our earlier findings that ≥80% of IgMa-expressing splenic B cell hybridomas from 56RVκ8 mice contain an unaltered 56R tg (32).
Figure 2.
VH replacement in sIgMb+ and sIgMa+ splenic B cells of 56RVκ8 mice and in sIgMa+ splenic B cells of 56RVκ8λ5−/− mice. A. Cartoon depicting the positions of VH3609 and VHQ52 genes relative to 56R, the inserted neomycin-resistant (neoR) gene and the primers used for PCR amplification of neo DNA and VHQ52 orVH3609 replacements of the 56R VH gene segment. B. Southern blot analysis of PCR amplified products showing loss of the neo marker in sIgMb+ cells of 56RVκ8 mice; VH replacement of the 56R VH gene segment by VHQ52 and VH3609 in sIgMb+ cells of 56RVκ8 mice; and replacement of the 56R VH gene segment by VHQ52 and VH3609 in sIgMa+ splenic cells of 56RVκ8 and 56RVκ8λ5−/− mice. 56RVκ8 RAG1−/− and C.B-17 scid/+ (wt) SPL cells served as negative controls. β2M served as a control for DNA loading (see Materials and Methods for details). C. Cartoon depicting VH replacement of the 56R VH region. The arrows in the top schematic illustrate rearrangement of a DH element followed by that of a VH element (1 + 2) and rearrangement of a VH element alone (3). The resulting products are illustrated in the middle and bottom schematic.
We next asked whether loss of the 56R neoR marker in sIgMb+ cells might be attributed in part to replacement of the 56R VH gene segment with an upstream VH gene. To test for VH replacement, DNA from sorted B220+sIgMb+ splenic B cells of 56RVκ8 mice was PCR amplified using primers (illustrated in Figure 2A) specific for the DH-proximal (3′) VHQ52 or DH-distal (5′) VH3609 gene families and the CDR3 region of 56R. PCR products were hybridized with specific VH gene family probes. Clear evidence for replacement of the 56R VH region by members of the 3′ VHQ52 and 5′ VH3609 families in sIgMb+ splenic B cells is shown in Figure 2B. VH replacement was also readily evident in the sIgMa+ splenic B cell population of 56RVκ8 mice, even though most members in this population retain an unaltered 56R allele (32). No VH replacement products were recovered from splenic B cells of 56RVκ8 RAG1−/− and non-transgenic C.B-17 mice, the negative controls. Note that the level of VH replacement in sIgMa+ splenic B cells from 56RVκ8 mice was comparable to that in sIgMa+ splenic B cells from 56RVκ8λ5−/− mice; this finding is relevant to results presented later.
VH3609 replacement of the 56R VH gene
To examine the structure of VH replacements in sIgMb-expressing B cells of 56RVκ8 mice, PCR amplified VH3609 replacements of the 56R VH gene illustrated in Figure 2B were cloned from sorted IgMb+ splenic B cells and sequenced. As shown in Figure 3A, the sequences for the CDR3 region of all twenty-one clones examined showed evidence of two rearrangement events, the first being a DH invasion (rearrangement) into the 3′ cRSS region of the 56R VH gene followed by a second event in which an upstream VH gene was joined to the rearranged DH element. All but two of the nineteen rearrangements shown in Figure 3A contained a stop codon in the V region (noted in bold). Two of the stop codons were located in the μH constant region (noted by the asterisk). Two replacement products were in-frame (noted by the † caret); the one utilizing VH3609.12.174 contained an unusually long CDR3 that might have excluded SL or L chain pairing and subsequent membrane expression. All of the rearrangements were unique and therefore represented independent events. Most of them contained N additions at the VH-DH and DH-56R junctions; some junctions showed palindromic (P) additions (underlined nucleotides). The observed loss of an intact 56R in sIgMb+ cells (Figure 2B), together with our finding that all but two VH3609 replacements recovered from these cells were nonproductive, strongly suggests that disruption and silencing of 56R precedes and allows VH rearrangement to occur at the wt allele.
Figure 3.
VH replacement of the 56R VH region in 56RVκ8 mice. A. Examples of VH3609-DH-56R joints isolated from sorted IgMb-expressing splenic B cells. The top sequence is the original 56R CDR3 region beginning with the first nucleotide after the 3′ cRSS; the sequences below are the junctional regions originating from VH3609-DH secondary rearrangements at the 56R VH cryptic heptamer. The bolded numbers above the VH3609 sequences correspond to the gene numbers within the family and locus according to Johnston et al (42). Designation of DH sequences is based on the identity of at least 4 nucleotides with a known DH segment. Nucleotide sequences of nontemplated (N) and palindromic (P) nucleotides are indicated at the junctions with P nucleotides underlined. The bolded sequence indicates a stop codon. Symbols: single asterisk indicates a TGA stop codon is in the μH chain constant region, double asterisk denotes sequences obtained twice and the caret (†) indicates an in-frame rearrangement. B. Examples of the VH3609-56R and VH3609-DH-56R joints isolated from sorted IgMa-expressing splenic B cells. The first two sequence joints represent replacement of the 56R VH region by direct secondary rearrangement of VH3609 genes; below are the junctional regions originating from VH3609-DH secondary rearrangements at the 56R VH cryptic heptamer. The sequence format is the same as in A. VH replacement products in A and B were amplified by genomic PCR using a forward primer specific for the VH3609 gene family and a reverse primer complementary to the 56R DJH junction.
Consistent with expectation, all VH3609 replacement products recovered from sorted sIgMa+ splenic B cells were in-frame (Figure 3B). Fifteen of seventeen replacements showed clear evidence of two events; i.e., DH rearrangement followed by joining of an upstream VH gene to the rearranged DH element. Direct joining of a VH gene into the 3′ cRSS region of the 56R VH gene was seen in two cases (top two sequences in Figure 3B). Most of the seventeen clones contained N additions and all but two were unique. Our recovery of productive VH3609 replacements from sorted sIgMa+ splenic cells of 56RVκ8 mice indicate the presence of an additional subset of H chain edited B cells distinct from that of sIgMb+ B cells. This subset can be inferred to represent a minor cell population as we previously found most IgMa-expressing splenic hybridomas in 56RVκ8 mice to contain an unaltered 56R (32).
sIgMb+ B cells show VH gene diversity and include cells with a marginal zone phenotype
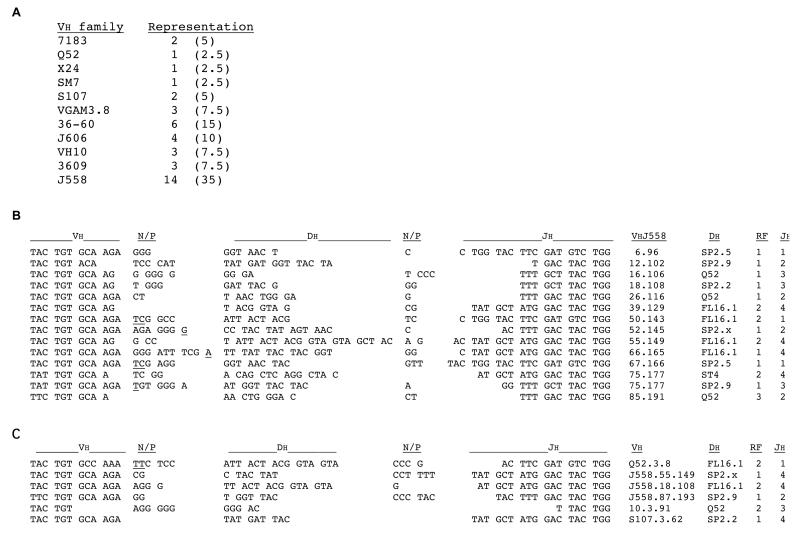
To test whether rearrangement at the wt H chain allele in 56RVκ8 mice involved widespread usage of VH genes, we synthesized full-length cDNA from sorted sIgMb+ splenic B cells of 56RVκ8 mice using a modified version of the SMART technique (41) and then amplified the cDNA using 5′ anchored PCR (see Materials and Methods). This approach was used to ensure recovery of VDJH rearrangements irrespective of the particular VH gene used. PCR products were digested with Ssp1, which cuts at a restriction site unique to the CDR3 region of 56R. The majority of products were not cut by this enzyme (results not shown) and therefore corresponded to VDJH rearrangements originating from the wt allele. Cloning and sequencing of these wt allele products showed diverse usage of both 3′ and 5′ VH genes (Figure 4A). Eleven of the fifteen known VH families were represented among the 40 clones sequenced, all of which were unique. VH usage appeared random with VHJ558, the most distal and complex 5′ VH family (42, 43), accounting for 35% of the recovered VH rearrangements. All but 4 of the 40 cloned sequences showed N additions at their VH-DH junction (illustrated for VHJ558 rearrangements in Figure 4B). Of particular interest is that 35% (5/14) of the VHJ558 rearrangements showed the DH element to be in reading frame (RF) 2 or 3. The relatively high usage of RF2, which for all 40 cloned sequences was 32% (13/40), stands in sharp contrast to the 5-10% usage of RF2 in normal B cells (44). RF2 was also used in three of six IgMb-producing hybridomas (Figure 4C) that we recovered earlier from a single 56RVκ8 mouse (32).
Figure 4.
Analysis of productive VDJH rearrangements at the wt allele in IgMb-expressing splenic B cells from 56RVκ8 mice. A. Representation of VH gene families. VDJH-Cμ sequences from sorted IgMb-expressing splenic B cells were amplified by 5′ RACE PCR from a SMART cDNA library, then cloned and sequenced. Nucleotide sequence analysis of 40 unique cDNA clones identified usage of 11 VH gene families, as listed. The number (and percentage) of clones obtained for each VH family is indicated in the right column. B. Junctional region sequences for productive VDJH rearrangements involving the VHJ558 gene family in sorted IgMb-expressing splenic B cells. C. Junctional region sequences for productive VDJH rearrangements from IgMb-expressing B cell hybridomas. Results in B and C were obtained using SMART cDNA clones. The sequences and VH gene nomenclature are represented as in Figure 3. The DH and JH gene segments utilized and DH reading frame (RF) are indicated on the far right side of the Figure.
B cells that produce polyreactive anti-self antibodies often show VDJH rearrangements with the DH element in RF2 or RF3 (45-49). This association, coupled with the known fact that self-reactive B cells tend to localize in the marginal zone (MZ) (50-53), led us to ask the following: Do 56RVκ8 mice contain splenic B cells with a MZ phenotype (CD21high CD23low) and, if so, does this cell population include sIgMb+ B cells? As shown in Figure 5A, about eight percent of B220+ splenic lymphocytes in 56RVκ8 mice displayed a MZ phenotype. The non-transgenic controls (BL/6 × BALB/c F1 mice) showed a comparable percentage of MZ splenic B cells. Few, if any, MZ splenic B cells were detectable in 3H9Vκ8 and 56RVκ8 SCID control mice. 56RVκ8 SCID mice cannot receptor edit and develop unedited B cells only (28). Thus, our results with the two transgenic controls clearly demonstrate that the development of the MZ splenic B cell population in 56RVκ8 mice depends on the presence of the 56R tg and the ability of these mice to undergo receptor editing. Figure 5B shows that splenic B cells with a MZ phenotype included sIgMb+ B cells (bottom row, left panel). The analysis of five individual 56RVκ8 mice revealed that approximately 40% (39.6% ± 4.3) of the B220+IgMb+ gated splenic B cells were CD21high CD23low (results not shown). This result, along with our finding that one-third of the VDJH rearrangements recovered from sIgMb+ cells contained the DH element in RF2, leaves open the possibility that a significant proportion of these H chain edited cells may produce IgM antibodies with low self-reactivity. Whether in fact the IgMb+ cell population in 56RVκ8 mice includes autoreactive B cells will require screening a large number of IgMb-expressing B cell hybridomas for reactivity to potential self-antigens.
Figure 5.
Marginal zone (MZ) splenic cells in 56RVκ8 mice include H and L chain edited B cells. A. Flow cytometric dot plots of CD21 versus CD23 for B220 gated lymphocytes showing presence of MZ splenic B cells (CD21+CD23−) in 56RVκ8 mice and the non-transgenic (BL/6 × BALB/c) F1 control, but not in 3H9Vκ8 or 56RVκ8 scid/scid (s/s) mice. B. Flow cytometric dot plots of IgMa versus IgMb and IgMa versus Vλx for B220 gated lymphocytes of 56RVκ8 mice. Lower dot plots show CD21/CD23 staining profiles for cells expressing sIgMb, Vκ21D (corresponding to sIgMa/sIgMb doubly stained cells (32)) and for cells expressing low (lo) and high (hi) surface Vλx (Vλxlo and Vλxhi). Numbers within and outside the boxed areas in A and B indicate the percentage of cells with the indicated phenotype.
Figure 5B also shows that the MZ B cell population in 56RVκ8 mice included L chain edited sIgMa+ B cells expressing the Vλx editor (54, 55). Two distinct Vλx expressing sIgMa+ B cell populations were identified in our earlier work (32): one expressed low surface Vλx (Vλxlow), the other high surface Vλx (Vλxhigh). Interestingly, most Vλxlow splenic B cells displayed a MZ phenotype but not Vλxhigh splenic B cells. The basis for this difference is not clear. The majority of splenic B cells doubly stained for sIgMa and sIgMb also displayed a MZ phenotype; these B cells correspond to Vκ21D edited B cells as they were shown earlier to express 56R/Vκ21D containing IgMa molecules that cross-react with the AF6-78 anti-IgMb reagent (32). The MZ phenotype of Vλxlow- and Vκ21D-expressing B cells in 56RVκ8 mice is consistent with our earlier evidence that these L chain edited cells retain low self-reactivity (32). Our findings are also consistent with those of Li et al. (51), who found that partially self-reactive B cells in 56R mice, due to the expression of both an editor (κ) and non-editor (λ) L chain, accumulate in the MZ.
VH replacement/rearrangement at the tg/wt alleles is initiated during pro-B cell differentiation
The presence of N additions at the VH-DH junctions in VH replaced tg and rearranged wt alleles of 56RVκ8 mice suggested that such secondary rearrangement is occurring during pro-B cell differentiation. N additions are catalyzed by the enzyme, TdT (31, 56, 57), the expression of which is normally restricted to the pro-B stage (58). To test whether this restriction also applied to 56RVκ8 mice, sIgM− gated lymphocytes in BM were stained intracellularly with Alexa 488 conjugated anti-TdT. Pro-B (B220+ CD43+sIgM−) and pre-B (B220+CD43−sIgM−) cell fractions, depicted in Figure 6A, were distinguished according to Hardy et al. (34). As shown in Figure 6B, TdT protein was detectable in pro-B cells of wt and 56RVκ8 mice. TdT was not detectable in 56RVκ8 pre-B and B (B220+sIgM+) BM cells and immature/transitional (T3) splenic B cells (B220+CD93+CD23high IgM−/lowIgD+) (Figure 6B). Thymocytes from BALB/c and BALB/cTdT−/− mice served as positive and negative controls, respectively. From these results we conclude H chain editing is occurring during pro-B cell differentiation. In support of this conclusion, VHQ52, VH3609 and VHJ558 replacements/rearrangements were readily detectable at the tg/wt alleles in pro-B cells of 56RVκ8 mice; they were also readily detectable in pro-B cells of 3H9Vκ8 mice (Figure 6C). The occurrence of VH replacement in pro-B cells of 3H9Vκ8 mice is not surprising as VH replacement in B cell hybridomas derived from 3H9 transgenic mice was reported earlier by Chen et al. (10). However, despite VH rearrangement on the wt allele at levels similar to that in 56RVκ8 mice, we found 3H9Vκ8 mice to contain at least 10-fold fewer IgMb+ splenic B cells than 56RVκ8 mice (Figure 1). We infer there must be little or no “space” or selection for H chain edited B cells expressing IgMb in 3H9Vκ8 mice. Indeed, 3H9Vκ8 mice have been found to contain normal numbers of splenic B cells and no detectable edited B cells (32, 59), suggesting that there is no strong selection for either H or L chain edited B cells in these mice. In contrast, both H and L chain edited splenic B cells are readily detectable in 56RVκ8 mice (this paper and (32)). Edited 56RVκ8 B cells would be expected to have a selective advantage over unedited 56RVκ8 B cells as the latter would be subject to deletion due to the strong anti-self (DNA) affinity of the 56R μH chain (54, 60).
Figure 6.
VH replacement/rearrangement at the tg/wt alleles in 56RVκ8 mice is initiated in the TdT+ pro-B cell fraction. A. Flow cytometric dot plots of B220 versus CD43 for sIgM− gated BM cells from RAG1−/−, C.B17 scid/+ (wt) and 56RVκ8 mice. Numbers within boxed areas indicate percentage of pro-B (B220+CD43+sIgM−) and pre-B (B220+CD43−sIgM−) cells. B. Histograms of TdT staining (all in bold) for pro-B and pre-B cells from BM of wt and 56RVκ8 mice and for splenic B (B220+IgM+) and immature/transitional T3 splenic B (B220+sIgM−/lowsIgD+CD23+CD93+) cells from 56RVκ8 mice._Thymocytes from BALB/c and BALB/cTdT−/− mice served as a positive and negative controls, respectively (TdT+ contrl and TdT− contrl). Max, maximum. C. VH replacement at the tg allele in 56RVκ8 and 3H9Vκ8 B lineage cells. PCR was performed with genomic DNA from BM pro- and pre-B cells of 56RVκ8 and 3H9Vκ8 mice using primers specific for VHQ52 or VH3609 and a primer specific for the CDR3 region of 56R. D. VH rearrangement at the wt allele in 56RVκ8 and 3H9Vκ8 B lineage cells. PCR performed as in C, but with primers specific for VHQ52, VH3609 or VHJ558 and a 3′ JH2-specific primer. Genomic DNA from BM of 56RVκ8 RAG1−/− mice (RAG1−/−) served as a negative control. E. β2 microglobulin (β2M) was amplified by PCR as a control for genomic DNA input.
Development of IgHb- but not IgHa-expressing B cells appears SL chain dependent
Critical to normal B cell differentiation is the expression of the SL chain. The SL chain is known to serve both as a chaperone for intracellular μH chain transport in pro-B cells and as a component of the pre-BCR complex responsible for signaling the clonal expansion of late pro-B cells (rev in (24-26)). As H chain editing at the wt (IgHb) allele in 56RVκ8 mice was shown to occur at the pro-B cell stage (Figure 6) and involve diverse usage of VH genes (Figure 4), we expected the development of IgHb+ pro-B cells would show SL chain dependency. Consistent with expectation, we found transgenic mice lacking the λ5 component of the SL chain (56Rλ5−/− and 56RVκ8λ5−/− mice) to be deficient in sIgMb+/sIgDb+ splenic B cells compared to 56R and 56RVκ8 mice. This is illustrated in Figure 7A using anti-IgD allotype reagents; note that the percentage of sIgDb+ splenic cells was 5-10 fold lower in the SPL of λ5− than λ5+ transgenic mice.
Figure 7.
Development of IgHa- (but not IgHb-) expressing B cells is independent of the λ5 component of the SL chain. A, Flow cytometric dot plots of IgDa versus IgDb and IgDa versus Vλx for B220-gated splenic lymphocytes from 56R, 56Rλ5−/−, 56RVκ8 and 56RVκ8λ5−/− mice. Spleen cells from (C57BL/6 (Bl/6) × BALB/c)F1 and BALB/c RAG1−/− (RAG1−/−) mice served as positive and negative controls, respectively, for Ig allotype staining. B, Flow cytometric dot plots of B220 versus CD43 to distinguish pro-B (B220+CD43+IgM−) and pre-B (B220+CD43−IgM−) cell fractions in B220+IgM−-gated BM cells of non-transgenic C.B-17 mice with and without λ5 (wt and wtλ5−/−), 56R and 56λ5−/− mice and RAG1−/− mice. C, Flow cytometric dot plots of BP-1 versus CD24 to distinguish pro-B cell subsets (B, B’, C and C’) in B220+CD43+IgM− -gated BM cells of wt, wtλ5−/−, 56R, 56Rλ5−/− and RAG1−/− mice. D, Vκ rearrangement to Jκ1 and Jκ2 in pro-B cells (subset B’) (B220+CD24+CD43+BP-1−IgM−) of 56R and 56Rλ5−/− mice and pro-B cells (subset B) (B220+CD24−CD43+BP-1−IgM−) of C.B-17 (+/+) mice. Pre-B (B220+CD43−IgM−) cells from wt mice and BM cells from RAG1−/− mice served as positive and negative controls. PCR amplification of β2M served as a control for genomic DNA input.
The development of sIgDa+ splenic B cells, including those expressing the Vλx editor, was unaffected by the absence of λ5 (Figure 7A). The comparable percentages of sIgDa+ (and not shown, sIgMa+) splenic B cells in 56R and 56Rλ5−/− mice suggested equally efficient 56R promotion of B cell differentiation in the presence or absence of λ5. To test whether this was true, we compared 56R and 56Rλ5−/− BM for representation of pro-B (B220+ CD43+ IgM−), pre-B (B220+ CD43−IgM−) and immature B (B220+IgM+) cells. As indicated in Figure 7B and Table I, the percentages of pro-B, pre-B and B cells in 56Rλ5−/− mice were comparable to those in 56R mice. In contrast, and consistent with previous findings (61, 62), non-transgenic wtλ5−/− mice were deficient in pre-B and B cells compared to the wt controls (Figure 7B). We conclude that the development of 56R-expressing B cells does not require the λ5 component of the SL chain.
Table I.
Representation of B cell subsets in bone marrow of 56R and 56Rλ5−/− mice
| Mice |
No. |
pro-B1 |
pre-B1 |
B2 |
|---|---|---|---|---|
| 56R | 7 | 6.7 (±1.4) | 93.3 (±2.2) | 6.0 (±1.6) |
| 56Rλ5−/− | 7 | 11.9 (±2.9) | 88.0 (±2.9) | 6.3 (±2.4) |
Mean percentage (±SEM) of pro-B (B220+CD43+slgM−), pre-B (B220+CD43−slgM−) in B220+slgM− gated lymphocytes.
Mean percentage (±SEM) of immature B (B220+slgM+) lymphocytes.
We also tested 56R and 56Rλ5−/− mice for their content of the pro/pre-B cells in subset B (pro-B/pre-BI), C (small pre-BII), and C’ (large pre-BII) (34, 63). These subsets were distinguished according to Hardy et al. (34) using the heat stable antigen, HSA/CD24 (64), and the early B lineage marker, BP-1 (65). Cells staining BP-1− CD24−/low and BP-1−CD24high were designated as subsets B and B’, respectively. Note that impairment in the differentiation of wt pro-B cells lacking λ5 was first evident at the subset B to C transition as wtλ5−/− mice showed a much higher percentage of cells in subset B than wt mice (Figure 7C). In both 56Rλ5−/− and 56R mice, subsets B and C were missing. Thus, in 56Rλ5−/− and 56R mice, cells in the B’ subset appeared to transit directly to the C’ stage. This anomaly in representation of pro/pre-B subsets was also observed earlier in 3H9 transgenic SCID mice, which we interpreted to reflect tg-induced accelerated B cell differentiation (66). Additional evidence for accelerated B cell differentiation in 56R and 56Rλ5−/− mice is the increased level of premature VJκ rearrangement in the pro-B cell fraction of these mice relative to that in the non-transgenic controls (Figure 7D). Premature VJκ rearrangement in pro-B cells of wt mice has been reported previously (58, 67, 68) and has been shown to be elevated in developing fetal B cells of μH chain transgenic mice relative to non-transgenic controls (69). As expected, most (70%) of the forty-two VJκ1 rearrangements recovered from the B’ subset of 56R and 56Rλ5−/− mice were out-of-frame (see supplemental Figure), consistent with little or no cellular selection for in-frame rearrangements at the pro-B stage. A high frequency (71%) of out-of-frame rearrangements has also been reported for VJκ sequences recovered from wt pro-B cells (70).
To test whether the 56R μH chain is able to associate with the SL chain, pro-B cells of SCID mice homozygous for 56R (56R/56R SCID mice) were stained intracellularly with an antibody (SL-156) specific for μH-SL chain complexes (71). Pro-B cells of wt and 56R/56R mice lacking λ5 (56R/56Rλ5−/−) served as positive and negative controls, respectively. As shown in Figure 8, the percentage of SL-156+ pro-B cells in 56R/56R SCID (and 3H9/3H9 SCID) mice was comparable to that in pro-B cells of wt mice. We conclude that the 56R μH chain can associate with the SL chain even though such an association appears not to be required for early B cell differentiation in 56R mice. The ability of the 56R μH chain to promote B cell differentiation independently of λ5 is not without precedent. Cell surface expression of functional μH chains in the absence of SL chain has been reported by others (49, 72-75). As a group, these previously reported “autonomous” μH chains included a variety of different VH regions, such that most would be expected to have the capacity to associate with the SL chain; however, such association appears not to be required for transport of these μH chains to the cell surface.
Figure 8.
Evidence for association of 56R μH chain with SL chain. Flow cytometric dot plots showing side scatter (SSC) versus intracellular staining with SL-156 antibody of pro-B (B220+CD43+IgM−) cells from wt mice, SCID mice homozygous for 56R (56R/56Rs/s), SCID mice homozygous for 3H9 (3H9/3H9s/s) and 56R/56Rλ5−/− mice. Numbers within boxed areas indicate percentage of cells with the indicated phenotype.
Undiminished VH replacement in pro-B cells of 56R mice lacking λ5
Although the development of B cells expressing the 56R allele (IgHa) appeared λ5 independent, we wondered whether this included the development of H chain edited B cells with a productive VH replacement. If the early development of such cells were λ5 independent, we would expect comparable levels of VH replacement in the pro/pre-B cell populations of 56Rλ5−/− and 56R mice. Conversely, if the development of 56R-expressing B cells with VH replacement were λ5 dependent we would expect a reduced level of VH replacement in pro/pre-B cell populations of 56Rλ5−/− mice compared to that in control 56R pro/pre-B cells. To test which of these possibilities might apply, we compared the relative level of VH replacement in sorted pro-B and pre-B cells from 56R and 56Rλ5−/− mice. As shown in Figure 9A and 9C, we found replacement of the 56R VH gene segment by members of the 3′ VHQ52/VH7183 and 5′ VH3609/VHJ558 gene families to be comparable in pro-B and pre-B cells of 56Rλ5−/− (and 56RVκ8λ5−/−) mice to that in 56R mice. We also observed comparable levels of VH replacement in sIgMa+ peripheral (splenic) B cells of 56RVκ8 and 56RVκ8λ5−/− mice (see Figure 2B), indicating that H chain edited sIgMa+ B cells exit the BM and reach the SPL with similar efficiency in these mice. We conclude λ5 is not critical for development of H chain edited and unedited sIgMa+ B cells. As discussed later, the unique CDR3 region of the 56R gene segment, which is retained in VH replaced alleles (see Figure 3), may be responsible for the apparent λ5 independence of all developing IgHa expressing B cells in 56R mice.
Figure 9.
A and B. Relative levels of VH replacement/rearrangement at the tg/wt alleles in pro-B and pre-B cells of 56Rλ5−/− and 56RVκ8λ5−/− mice compared to that in pro-B and pre-B cells of 56R mice. A. VH replacement at the 56R: Genomic DNA was subjected to PCR amplification for VH replacement products using a primer specific for members of the Q52, 3609 or J558 VH gene families and a 56R CDR3-specific primer. The VHJ558 primer (MB 756) used to detect VH replacement does not recognize the 56R VH region. B. VDJH rearrangement at the wt allele: Genomic DNA was subjected to PCR amplification using the same VH gene family specific primers as in A and a 3′ JH2-specific primer. C. Semi-quantitative analysis of VH replacement in pro-B cells of 56R and 56Rλ5−/− mice. VH replacements in serially diluted pro-B cell DNA were PCR amplified for 35 cycles in the presence of a constant amount of carrier (liver) DNA from RAG1−/− mice using a primer specific for the VH7183 or VHJ558 (MB 756) gene family together with a 56R CDR3 specific primer. D. Semi-quantitative analysis of VH rearrangement in pro-B cells (subset B) of wt and wt λ5−/− mice. Serial dilutions of genomic DNA from sorted pro-B cells were subjected to 26-27 cycles of PCR amplification using a primer specific for the VHQ52 or VHJ558 (MB 649) gene family and a 3′ JH2-specific primer. Southern blots of PCR products shown panels A, B and C were hybridized with probes corresponding to the VH gene family being tested for replacement/rearrangement; in D, blots were hybridized with a JH1-3 probe. Genomic DNA from BM of 56RVκ8 RAG1−/− (RAG1−/−) mice served as a negative control for all blots. To control for input genomic DNA, PCR amplification of part of the β2M gene was performed for 25 cycles and the Southern blot hybridized with a β2M probe.
In contrast to the above, the level of VH rearrangement at the wt allele was less in pro/pre-B cells of 56Rλ5−/− (and 56RVκ8λ5−/−) mice than in pro/pre-B cells of 56R mice (Figure 9B). We interpret the reduced VH rearrangement in the 56Rλ5−/− pro-B cell population to reflect impaired survival and expansion of H chain edited pro-B cells with productive VH rearrangements at their wt allele, consistent with the approximately 10-fold fewer sIgDb+ (and sIgMb+) splenic B cells in 56Rλ5−/− than 56R mice (Figure 7A and results not shown). One might suggest that the results shown in Figure 9B could also reflect less initiation of VH rearrangement at the wt allele in pro-B cells of 56Rλ5−/− than 56R mice. We cannot exclude this possibility. However, given that VH replacement occurs with similar frequency in pro-B cells of 56Rλ5−/− and 56R mice, we would expect nonproductive VH replacement at the tg allele and initiation of VH rearrangement at the wt allele also to occur with similar frequency in these mice. Moreover, if initiation of VH rearrangement in general were λ5 dependent, we would expect early pro-B cells of wtλ5−/− mice to show less VH rearrangement than early pro-B cells of wt mice. However, when we tested wt and wtλ5−/− pro-B cells at the pro/pre-BI stage (corresponding to subset B), the stage at which VH rearrangement is known to be initiated (58, 67, 76-78), we observed similar levels of 3′ and 5′ VH rearrangement in both cases (Figure 9D) in agreement with the earlier findings of Ehlich et al. (67). These investigators also found rearrangement of VH genes to be similar in early pro-B cells with and without λ5, although 3′ and 5′ VH rearrangements were not separately distinguished as in the present study.
DISCUSSION
VH replacement/rearrangement at the tg/wt alleles occurs during pro-B cell differentiation
We have provided evidence that H chain editing, as scored by VH replacement/rearrangement at the tg/wt alleles in mice hemizygous for 56R, occurs during pro-B cell differentiation. The evidence is twofold: First, secondary VH rearrangements at the tg and wt alleles were recovered from sorted 56RVκ8 BM cells with a pro-B phenotype (Figures 6C, 9B and 9C)). Second, virtually all such VH rearrangements recovered from H chain edited B cells contained N additions. TdT is responsible for N additions (31, 56, 57) and was shown to be restricted to the pro-B stage of differentiation in 56RVκ8 mice (Figure 6B). These results suggest that H chain editing in 56RVκ8 (and 56R) mice occurs before detectable expression of the autoreactive μH chain on the cell surface and therefore does not appear to be induced by cell interaction with self-antigen. The occurrence of H chain editing may simply reflect a low frequency of spontaneous DH invasion and VH replacement at the active 56R allele in developing pro-B cells in the BM. When this results in 56R inactivation, VH rearrangement is allowed at the wt allele. Given this scenario, H chain edited B cells would be relatively rare in the BM. Indeed, as shown in our earlier study (32), sIgMb+ edited B cells were not detectable by flow cytometry in the BM of 56RVκ8 mice. Selective expansion and accumulation of sIgMb+ edited B cells in peripheral lymphoid tissues presumably accounts for our ability to detect these cells in the SPL.
We cannot formally exclude the possibility that expression of the 56R μH chain in developing B cells causes some of these cells to down regulate their sIgM, revert to the pro-B cell stage and undergo VH replacement/rearrangement at their tg/wt alleles. The idea that developing autoreactive B cells, upon encounter with self-antigen, down-regulate their cell surface expression of antigen receptor and revert to an earlier stage of differentiation has been suggested by Tze et al.(79). In this scenario, loss of cell surface antigen receptor is postulated to trigger secondary L chain rearrangement (L chain editing). H chain editing, however, does not appear to fit the scenario suggested for L chain editing. If the pro-B cell fraction in 56R and 56RVκ8 mice were contaminated with many reverted B cells lacking sIgM, we would expect the signature of these cells to be clearly evident; i.e., we would expect to find many functional (in-frame) L chain rearrangements in the pro-B cell fraction. However, the majority of VJκ1 rearrangements (70%) recovered from pro-B cells (subset B’) of 56R and 56Rλ5−/− mice were out-of-frame (see supplemental Figure). A comparable frequency of out-of-frame VJκ rearrangements (71%) has been reported for wt pro-B cells (70).
Consistent with our evidence for H chain editing at the pro-B cell stage are reports from other groups. Davila et al. (14) reported that pro-B cells from μMT mice (80) with a block in pro-B cell differentiation show a frequency of dsDNA breaks at cRSS sites in VH1 and VH5 genes similar to that observed in wt pro-B cells. As the breaks in μMT pro-B cells could not derive from pre-B or immature B cells, there is no evidence to suggest that VH replacement in wt pro-B cells is dependent on prior encounter of pre-B or immature B cells with self-antigen and reversion to the pro-B cell stage. Indeed, in transgenic mice with nonproductive VH rearrangements at both alleles, antigen independent VH replacement does occur in developing pro-B cells and results in a B cell population expressing a diverse repertoire of VH regions (15, 16).
H and L chain editing differences in 56RVκ8 mice
Based on this and earlier reports (4, 32, 54), two key differences between H and L chain editing in 56RVκ8 mice with a BALB/c genetic background should be noted. First, L chain editing occurs in immature B cells with down regulated sIgM (sIgM−/lo). These cells are found not only in the BM but also in the SPL where they display an immature/transitional phenotype. H chain editing, on the other hand, appears to occur exclusively in the BM during pro-B cell differentiation before expression of detectable cell surface antigen receptor. Although H chain editing appears not to be induced by self-antigen, we presume that antigen dependent selection plays a major role in the expansion and maintenance of H (and L) chain edited B cells. Second, L chain edited B cells show very restricted usage of VL genes, as only a few L chains are able to veto or reduce the anti-DNA binding affinity of the 56R H chain. In contrast, H chain edited B cells show diverse usage of VH genes, including 3′ VH genes (e.g. VHQ52) and 5′ VH genes (e.g., VH 3609 and VHJ558).
IgHa- and IgHb-expressing B cells show differential SL chain dependence
Compelling evidence for the differential SL chain dependence of IgHa- and IgHb- expressing B cells was provided by the following observations: Splenic B cells expressing the allotype of the 56R allele (IgHa) were equally represented in λ5− and λ5+ transgenic mice (Figure 7A). Moreover, the representation of pro-B (and pro-B subsets), pre-B and B cells in the BM of 56Rλ5−/− mice was found to be comparable to that in 56R controls (Figure 7B and 7C). Thus, the development of 56R-expressing B cells appeared λ5 independent. What was not clear, however, is whether this included pro-B cells expressing a 56R encoded μH chain with a replaced VH region. To address this issue, we compared pro-B cells from 56R and 56Rλ5−/− mice for their relative level of VH replacement. If the survival and expansion of H chain edited pro-B cells with productive VH replacement were dependent on λ5, such cells would be under represented in the pro-B cell population of 56Rλ5−/− mice and we would thus expect the pro-B cell population of 56Rλ5−/− mice to show less VH replacement than that of 56R mice. But no apparent reduction in level of VH replacement was observed in 56Rλ5−/− or 56RVκ8λ5−/− mice (Figure 9, A and C). Moreover, the level of VH replacement in sIgMa+ splenic B cells of 56RVκ8λ5−/− mice was found to be comparable to that in sIgMa+ splenic B cells of 56RVκ8 mice (Figure 2B), indicating that H chain edited peripheral B cells expressing the tg allotype were similarly represented in transgenic mice with and without λ5. We therefore conclude that SL chain is not essential for the development of edited and unedited B cells expressing the 56R μH chain. On the other hand, the SL chain did appear essential for the development of H chain edited B cells expressing the wt allele (IgHb). 56Rλ5−/− and 56RVκ8λ5−/− pro-B cells showed less VH rearrangement at their wt allele than 56R pro-B cells (Figure 9B). In addition, 56Rλ5−/− and 56RVκ8λ5−/− mice contained 5 to10-fold less sIgMb+/sIgDb+ splenic B cells than 56R and 56RVκ8 mice, respectively (Figure 7 and results not shown). Our results with 56RVκ8 and 56RVκ8λ5−/− mice clearly indicate that Vκ8 is not able to substitute for the role of λ5 in the development of edited pro-B cells expressing the wt H chain allele.
Precedent for the promotion of B cell differentiation by μH chains in the absence of the SL chain comes from a number of earlier studies. Schuh et al. (74) reported that Sp6 transgenic mice lacking RAG2 and λ5 were able to give rise to pre-B cells; furthermore, induced expression of Sp6 μH chain in CD19+ BM cell cultures from these mice resulted in cell proliferation. Galler et al. (72) showed that a variety of μH chains could be expressed on the cell surface of cultured CD19+ BM cells in the absence of each or all components of the SL chain (λ5, VpreB1 and VpreB2) and could mediate down regulation of TdT and RAG1/2. Transport of μH chains to the cell surface in the absence of the SL chain was shown to be dependent on Igα. Su et al. (75) also reported Igα-dependent expression of polyclonal SL− pre-BCRs in BM cultures from mice lacking the SLP-65 adapter protein and λ5. Incubation of these cells with anti-μH antibody resulted in tyrosine phosphorylation and Ca2+ release, indicating that SL− pre-BCRs are able to transduce signals to the interior of the cell. In humans, close to 10% of productive VDJH rearrangements have been found to code for μH chains that, in the absence of SL chain, can be expressed on the cell surface and be secreted (73). Transcripts for such autonomous μH chains were detectable in pro-B but not pre-B or B cells suggesting that cells expressing these μH chains are negatively selected (73). Evidence for SL chain-dependent negative selection of pro-B cells expressing autonomous μH chains was also recently reported in mice (49). Finally, there are examples of μH chains that support early B cell development despite their inability to efficiently pair with the SL chain; these include μH chains with VH regions encoded by VH81X or VH11 (81-85).
What allows some μH chains to be transported to the cell surface in the absence of the SL chain is not clear. μH chains are generally retained in the endoplasmic reticulum in a partially folded state by the chaperone, BiP, prior to their displacement by the SL chain (86-88). Some μH chains may fail to be displaced from BiP because the structure of their CDR3 region inhibits or prevents pairing with the SL chain. For example, among four murine μH chains containing the same VH gene segment (VH81X) but different CDR3 regions, two were found not to bind SL chain (85, 89). For other μH chains, a particular CDR3 structure may confer SL chain independence, such that these (autonomous) μH chains do not require the SL chain for displacement from BiP and transport to the cell surface (26). In humans and mice, the CDR3 regions of autonomous μH chains show a high proportion of positively charged amino acids (arginine and lysine) in their CDR3 region (49, 73). For example, autonomous μH chains in pro-B cells of mice lacking both SL and L chains were found to show two or more basic amino acids (arginine and lysine) in their CDR3 region. These μH chains also showed a strong preference for DH gene usage in RF2 or RF3 and autoreactivity to nuclear antigens (chromatin, ssDNA and dsDNA) and cardiolipin (49).
The disproportionate representation of positively charged amino acids in H chains with autoreactivity to nuclear antigens (e.g., DNA) has long been recognized and extensively studied ((90-92) and references therein). In the case of the 56R μH chain, the arginine within the CDR3 region at position 96, and two arginines within the CDR2 region at positions 53 and 56 appear critical for the strong autoreactivity and negative selection of immature B cells expressing this μH chain (4). Negative selection (deletion) of 56R-expressisng B cells is apparent at the pre-B to B cell transition (60) although cells may escape deletion by expressing a L chain editor to veto (reduce) the anti-self reactivity of the 56R μH chain (4, 32, 55). However, as indicated in this report, we find no evidence for SL chain dependent deletion of 56R-expressing cells at the pro-B to pre-B transition, including those with VH replacement. If 56R-expressing cells were to be deleted or negatively selected at the pro-B to pre-B transition, we would have expected to see, for example, fewer pre-B cells in 56R than 56Rλ5−/− mice. But no differences were evident in the representation of any of the analyzed early B cell subsets in 56R versus 56Rλ5−/− mice. Given that 56R μH chains with VH replacement retain the same CDR3 region as unedited 56R μH chains (see Figure 3), leads us to conclude that this region is responsible for the apparent SL chain independence of developing 56R-expressing B cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Kappes, R. R. Hardy, K. Campbell, D. Wiest for review of this manuscript; and R. Brooks and K. Trush for help in formatting the text and figures. We especially want to thank E. L. Prak and L. Yunk for sharing their unpublished results and for the sequence of primer MB 756, R. R. Hardy for mice, reagents and help with the sorting of B cell subsets, J. F. Kearney for Alexa 488 conjugated anti-TdT, P-A. Cazenave for the anti-Vλx (10C5) hybridoma and R. E. Spallone for administrative support. Assistance from personnel in the following CORE facilities of the Fox Chase Cancer Center is gratefully acknowledged: the Flow Cytometry and Cell Sorting Facility, Hybridoma Facility, Laboratory Animal Resources, DNA Sequencing Facility and the Biochemistry and Biotechnology Facility.
Abbreviations used in this paper
- cRSS
cryptic recombination signal sequence
- β2M
beta 2 microglobulin
- BM
bone marrow
- SPL
spleen
- tg
transgene
- sd
site-directed
- SL
surrogate light
- wt
wild-type
- sIgM
surface IgM
- sIgD
surface IgD;
- RF
reading frame
- FL
fluorescein
- SMART
switching mechanism at 5′ end f the RNA transcript’
- MZ
marginal zone
Footnotes
This work was supported by National Institutes of Health Grants, CA06927 and CA04946, and an appropriation from the Commonwealth of Pennsylvania.
DISCLOSURES The authors have no financial conflict of interest.
REFERENCES
- 1.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radic MZ, Erickson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their receptors. J. Exp. Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: An approach by autoreactive B cells to escape tolerance. J. Exp. Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Jiang Y, Prak EL, Radic MZ, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 5.Casellas R, Zhang Q, Zheng NY, Mathias MD, Smith K, Wilson PC. Igk allelic inclusion is a consequence of receptor editing. J. Exp. Med. 2007;204:153–160. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinfield R, Hardy RR, Tarlinton D, Dangl J, Herzenberg LA, Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. Nature. 1986;322:843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- 7.Reth M, Gehrmann P, Petrac E, Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. Nature. 1986;322:840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- 8.Covey LR, Ferrier P, Alt FW. VH to VHDJH rearrangement is mediated by the internal VH heptamer. Int. Immunol. 1990;2:579–583. doi: 10.1093/intimm/2.6.579. [DOI] [PubMed] [Google Scholar]
- 9.Usuda S, Takemori T, Matsuoka M, Shirasawa T, Yoshida K, Mori A, Ishizaka K, Sakano H. Immunoglobulin V gene replacement is caused by the intramolecular DNA deletion mechanism. EMBO. J. 1992;11:611–618. doi: 10.1002/j.1460-2075.1992.tb05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: A mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 11.Taki S, Schwenk F, Rajewsky K. Rearrangement of upstream DH and VH genes to a rearranged immunoglobulin variable region gene inserted into the DQ52-JH region of the immunoglobulin heavy chain locus. Eur. J. Immunol. 1995;25:1888–1896. doi: 10.1002/eji.1830250715. [DOI] [PubMed] [Google Scholar]
- 12.Watson LC, Moffatt-Blue CS, McDonald RZ, Kompfner E, Ait-Azzouzene D, Nemazee D, Theofilopoulos AN, Kono DH, Feeney AJ. Paucity of V-D-D-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT−/− and TdT+/+ mice. J. Immunol. 2006;177:1120–1128. doi: 10.4049/jimmunol.177.2.1120. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zemlin M, Wang YH, Munfus D, Huye LE, Findley HW, Bridges SL, Roth DB, Burrows PD, Cooper MD. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 14.Davila M, Liu F, Cowell LG, Lieberman AE, Heikamp E, Patel A, Kelsoe G. Multiple, conserved cryptic recombination signals in VH gene segments: detection of cleavage products only in pro B cells. J. Exp. Med. 2007;204:3195–3208. doi: 10.1084/jem.20071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koralov SB, Novobrantseva TI, Konigsmann J, Ehlich A, Rajewsky K. Antibody repertoires generated by VH replacement and direct VH to JH joining. Immunity. 2006;25:43–53. doi: 10.1016/j.immuni.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Lutz J, Muller W, Jack HM. VH replacement rescues progenitor B cells with two nonproductive VDJ alleles. J. Immunol. 2006;177:7007–7014. doi: 10.4049/jimmunol.177.10.7007. [DOI] [PubMed] [Google Scholar]
- 17.Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 18.Bertrand FE, Golub R, Wu GE. V(H) gene replacement occurs in the spleen and bone marrow of non-autoimmune quasi-monoclonal mice. Eur. J. Immunol. 1998;28:3362–3370. doi: 10.1002/(SICI)1521-4141(199810)28:10<3362::AID-IMMU3362>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Cascalho M, Wong J, Wabl M. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. J. Immunol. 1997;159:5795–5801. [PubMed] [Google Scholar]
- 20.Wilson PC, Wilson K, Liu YJ, Banchereau J, Pascual V, Capra JD. Receptor Revision of Immunoglobulin Heavy Chain Variable Region Genes in Normal Human B Lymphocytes. J. Exp. Med. 2000;191:1881–1894. doi: 10.1084/jem.191.11.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh K, Meffre E, Albesiano E, Farber A, Dines D, Stein P, Asnis SE, Furie RA, Jain RI, Chiorazzi N. Immunoglobulin heavy chain variable region gene replacement As a mechanism for receptor revision in rheumatoid arthritis synovial tissue B lymphocytes. J. Exp. Med. 2000;192:1151–1164. doi: 10.1084/jem.192.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekiguchi DR, Eisenberg RA, Weigert M. Secondary heavy chain rearrangement: a mechanism for generating anti-double-stranded DNA B cells. J. Exp. Med. 2003;197:27–39. doi: 10.1084/jem.20020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prak EL, Weigert M. Light chain replacement: A new model for antibody gene rearrangement. J. Exp. Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat. Rev. Immunol. 2005;5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- 25.Melchers F, Haasner D, Grawunder U, Kalberer C, Karasuyama H, Winkler T, Rolink AG. Roles of IgH and L chains and of surrogate H and L chains in the development of cells of the B lymphocyte lineage. Annu. Rev. Immunol. 1994;12:209–225. doi: 10.1146/annurev.iy.12.040194.001233. [DOI] [PubMed] [Google Scholar]
- 26.Vettermann C, Herrmann K, Jack HM. Powered by pairing: The surrogate light chain amplifies immunoglobulin heavy chain signaling and preselects the antibody repertoire. Seminars in Immunol. 2006;18:44–55. doi: 10.1016/j.smim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Kiefer K, Oshinsky J, Kim J, Nakajima PB, Bosma GC, Bosma MJ. The catalytic subunit of DNA-protein kinase (DNA-PKcs) is not required for Ig class-switch recombination. Proc. Natl. Acad. Sci. U S A. 2007;104:2843–2848. doi: 10.1073/pnas.0611359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosma GC, Oshinsky J, Kiefer K, Nakajima PB, Charan D, Congelton C, Radic M, Bosma MJ. Development of functional B cells in a line of SCID mice with transgenes coding for anti-double-stranded DNA antibody. J. Immunol. 2006;176:889–898. doi: 10.4049/jimmunol.176.2.889. [DOI] [PubMed] [Google Scholar]
- 29.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 30.Spanopoulou E, Roman CA, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzweig MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B -cell differentiation in Rag-1- deficient mice. Genes & Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 31.Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [published erratum appears in Science 1993 Dec 24;262(5142):1957] [DOI] [PubMed] [Google Scholar]
- 32.Kiefer K, Nakajima PB, Oshinsky J, Seeholzer SH, Radic M, Bosma GC, Bosma MJ. Antigen receptor editing in anti-DNA transitional B cells deficient for surface IgM. J. Immunol. 2008;180:6094–6106. doi: 10.4049/jimmunol.180.9.6094. [DOI] [PubMed] [Google Scholar]
- 33.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seijen AM, Seijen HG, Bos NA. Systematic design of mouse Vh gene family-specific oligonucleotides. J. Immunol. Methods. 2001;254:161–168. doi: 10.1016/s0022-1759(01)00396-9. [DOI] [PubMed] [Google Scholar]
- 36.Angelin-Duclos C, Calame K. Evidence that immunoglobulin VH-DJ recombination does not require germ line transcription of the recombining variable gene segment. Mol. Cell Biol. 1998;18:6253–6264. doi: 10.1128/mcb.18.11.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in nonautoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 38.Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 39.Ruetsch NR, Bosma GC, Bosma MJ. Unexpected rearrangement and expression of the immunoglobulin lambda1 locus in scid mice. J. Exp. Med. 2000;191:1933–1943. doi: 10.1084/jem.191.11.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleclough C, Perry RP, Karjalainen K, Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981;290:372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- 41.Chenchik A, Zhu Y, Diatchenko L, Li R, Hill J, Siebert PD. Generation and use of high quality cDNA from small amounts of total RNA by SMART PCR. In: Siebert P, larrick J, editors. RT-PCR methods for gene cloning and analysis. BioTechniques Books; Natick, MA: 1998. p. 305. [Google Scholar]
- 42.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J. Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 43.Mainville CA, Sheehan KM, Klaman LD, Giorgetti CA, Press JL, Brodeur PH. Deletional mapping of fifteen mouse VH gene families reveals a common organization for three Igh haplotypes. J. Immunol. 1996;156:1038–1046. [PubMed] [Google Scholar]
- 44.Gu H, Kitamura D, Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991;65:47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- 45.Eilat D, Webster DM, Rees AR. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J. Immunol. 1988;141:1745–1753. [PubMed] [Google Scholar]
- 46.Marion TN, Tillman DM, Jou NT. Interclonal and intraclonal diversity among anti-DNA antibodies from an (NZB × NZW)F1 mouse. J. Immunol. 1990;145:2322–2332. [PubMed] [Google Scholar]
- 47.Novick KE, Fasy TM, Losman MJ, Monestier M. Polyreactive IgM antibodies generated from autoimmune mice and selected for histone-binding activity. Int. Immunol. 1992;4:1103–1111. doi: 10.1093/intimm/4.10.1103. [DOI] [PubMed] [Google Scholar]
- 48.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 1990;171:265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keenan RA, De Riva A, Corleis B, Hepburn L, Licence S, Winkler TH, Martensson IL. Censoring of Autoreactive B Cell Development by the Pre-B Cell Receptor. Science. 2008 doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 50.Kanayama N, Cascalho M, Ohmori H. Analysis of marginal zone B cell development in the mouse with limited B cell diversity: role of the antigen receptor signals in the recruitment of B cells to the marginal zone. J. Immunol. 2005;174:1438–1445. doi: 10.4049/jimmunol.174.3.1438. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandik-Nayak L, Racz J, Sleckman BP, Allen PM. Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J. Exp. Med. 2006;203:1985–1998. doi: 10.1084/jem.20060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen L, Brill-Dashoff J, Shinton SA, Asano M, Hardy RR, Hayakawa K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 2005;23:297–308. doi: 10.1016/j.immuni.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Chen C, Radic MZ, Erikson J, Camper SA, Litwin S, Hardy RR, Weigert M. Deletion and Editing of B cells that express antibodies to DNA. J. Immunol. 1994;152:1970–1982. [PubMed] [Google Scholar]
- 55.Li Y, Louzoun Y, Weigert M. Editing anti-DNA B cells by Vlambdax. J. Exp. Med. 2004;199:337–346. doi: 10.1084/jem.20031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komori T, Okada A, Stewart V, Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 57.Landau NR, Schatz DG, Rosa M, Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol. Cell Biol. 1987;7:3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J. Exp. Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, Weigert M. The site and stage of anti-DNA B-cell deletion. Nature. 1995a;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 61.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of l5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 62.Mundt C, Licence S, Shimizu T, Melchers F, Martensson IL. Loss of precursor B cell expansion but not allelic exclusion in VpreB1/VpreB2 double-deficient mice. J. Exp. Med. 2001;193:435–445. doi: 10.1084/jem.193.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rolink A, Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991;66:1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- 64.Bruce J, Symington FW, McKearn TJ, Sprent J. A Monoclonal Antibody Discriminating Between Subsets of T and B Cells. J. Immunol. 1981;127:2496–2501. [PubMed] [Google Scholar]
- 65.Cooper MD, Mulvaney D, Coutinho A, Cazenave PA. A novel cell surface molecule on early B-lineage cells. Nature. 1986;321:616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- 66.Chang Y, Bosma MJ, Bosma GC. Extended duration of DH-JH rearrangement in immunoglobulin heavy chain transgenic mice: Implications for regulation of allelic exclusion. J. Exp. Med. 1999;189:1295–1305. doi: 10.1084/jem.189.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehlich A, Schaal S, Gu H, Kitamura D, Muller W, Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 68.Constantinescu A, Schlissel MS. Changes in locus-specific V(D)J recombinase activity induced by immunoglobulin gene products during B cell development. J. Exp. Med. 1997;185:609–620. doi: 10.1084/jem.185.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlissel MS, Morrow T. Ig heavy chain protein controls B cell development by regulating gem-line transcription and retargeting V(D)J recombination. J. Immunol. 1994;153:1645–1657. [PubMed] [Google Scholar]
- 70.Victor KD, Vu K, Feeney AJ. Limited junctional diversity in kappa light chains. Junctional sequences from CD43+B220+ early B cell progenitors resemble those from peripheral B cells. J. Immunol. 1994;152:3467–3475. [PubMed] [Google Scholar]
- 71.Winkler TH, Rolink A, Melchers F, Karasuyama H. Precursor B cells of mouse bone marrow express two different complexes with the surrogate light chain on the surface. Eur. J. Immunol. 1995;25:446–450. doi: 10.1002/eji.1830250221. [DOI] [PubMed] [Google Scholar]
- 72.Galler GR, Mundt C, Parker M, Pelanda R, Martensson IL, Winkler TH. Surface mu heavy chain signals down-regulation of the V(D)J-recombinase machinery in the absence of surrogate light chain components. J. Exp. Med. 2004;199:1523–1532. doi: 10.1084/jem.20031523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minegishi Y, Conley ME. Negative selection at the pre-BCR checkpoint elicited by human mu heavy chains with unusual CDR3 regions. Immunity. 2001;14:631–641. doi: 10.1016/s1074-7613(01)00131-5. [DOI] [PubMed] [Google Scholar]
- 74.Schuh W, Meister S, Roth E, Jack HM. Cutting edge: signaling and cell surface expression of a mu H chain in the absence of lambda 5: a paradigm revisited. J. Immunol. 2003;171:3343–3347. doi: 10.4049/jimmunol.171.7.3343. [DOI] [PubMed] [Google Scholar]
- 75.Su YW, Flemming A, Wossning T, Hobeika E, Reth M, Jumaa H. Identification of a pre-BCR lacking surrogate light chain. J. Exp. Med. 2003;198:1699–1706. doi: 10.1084/jem.20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr. Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 77.ten Boekel E, Melchers F, Rolink A. The status of Ig loci rearrangements in single cells from different stages of B cell development. Int. Immunol. 1995;7:1013–1019. doi: 10.1093/intimm/7.6.1013. [DOI] [PubMed] [Google Scholar]
- 78.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 79.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, Kraus M, Hardy RR, Schlissel MS, Rajewsky K, Behrens TW. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell deficient mouse by targeted disruption of the membrane exon of the immunoglobulin m chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 81.Yancopoulos GD, Desiderio SV, Paskind M, Kearney JF, Baltimore D, Alt FW. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- 82.Malynn BA, Yancopoulos GD, Barth JE, Bona CA, Alt FW. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J. Exp. Med. 1990;171:843–859. doi: 10.1084/jem.171.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marshall AJ, Wu GE, Paige GJ. Frequency of VH81X usage during B cell development: initial decline in usage is independent of Ig heavy chain cell surface expression. J. Immunol. 1996;156:2077–2084. [PubMed] [Google Scholar]
- 84.Wasserman R, Li Y-S, Shinton SA, Carmack CE, Manser T, Wiest DL, Hayakawa K, Hardy RR. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J. Exp. Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keyna U, Beck-Engeser GB, Jongstra J, Applequist SE, Jack H-M. Surrogate light chain-dependent selection of Ig heavy chain V regions. J. Immunol. 1995;155:5536–5542. [PubMed] [Google Scholar]
- 86.Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 87.Hendershot LM. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J. Cell Biol. 1990;111:829–837. doi: 10.1083/jcb.111.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanhove M, Usherwood YK, Hendershot LM. Unassembled Ig heavy chains do not cycle from BiP in vivo but require light chains to trigger their release. Immunity. 2001;15:105–114. doi: 10.1016/s1074-7613(01)00163-7. [DOI] [PubMed] [Google Scholar]
- 89.Kline GH, Hartwell L, Beck-Engeser GB, Keyna U, Zaharevitz S, Klinman NR, Jack HM. Pre-B cell receptor-mediated selection of pre-B cells synthesizing functional mu heavy chains. J. Immunol. 1998;161:1608–1618. [PubMed] [Google Scholar]
- 90.Krishnan MR, Marion TN. Structural similarity of antibody variable regions from immune and autoimmune anti-DNA antibodies. J. Immunol. 1993;150:4948–4957. [PubMed] [Google Scholar]
- 91.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 92.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert M. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc. Natl. Acad. Sci. (USA) 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.