Abstract
Vertebrate gastrulation entails massive cell movements that establish and shape the germ layers. During gastrulation, the individual cell behaviors are strictly coordinated in time and space by various signaling pathways. These pathways instruct the cells about proliferation, shape, fate and migration into proper location. Convergence and extension (C&E) movements during vertebrate gastrulation play a major role in the shaping of the embryonic body. In vertebrates, the Wnt/Planar Cell Polarity (Wnt/PCP) pathway is a key regulator of C&E movements, essential for several polarized cell behaviors, including directed cell migration, and mediolateral and radial cell intercalation. However, the molecular mechanisms underlying the acquisition of planar cell polarity by highly dynamic mesenchymal cells engaged in C&E is still not well understood. Each cell in the embryo needs spatial and temporal information, which are required for its appropriate behavior, and the Wnt/PCP pathway provides this information by a yet unclear mechanism. Here we review new evidence implicating the Wnt/PCP pathway in specific cell behaviors required for C&E during zebrafish gastrulation, in comparison to other vertebrates. We also discuss findings on the molecular regulation and the interaction of the Wnt/PCP pathway with other signaling pathways during gastrulation movements.
Introduction
The non-canonical Wnt/PCP pathway, initially discovered in Drosophila melanogaster and discussed in other chapters of this volume, has now been implicated in many processes during vertebrate development. C&E is the first such process in which the key role of the Wnt/PCP pathway has been recognized. Zebrafish embryos carrying mutations in the Wnt/PCP pathway components show defective gastrulation C&E movements. In particular, they include: trilobite (tri) coding for a zebrafish homologue of Strabismus/Van Gogh like 2 (Vangl2) [1], knypek (kny) encoding a membrane glypican 4 [2], as well as silberblick (slb) and pipetail (ppt) coding for the secreted ligands Wnt11 and Wnt5a, respectively [3,4]. The requirement for Wnt/PCP signaling during gastrulation appears to be important selectively for C&E gastrulation movements. Indeed, the Wnt/PCP mutants undergo normal epiboly and internalization without affecting cell fates. These defects result in embryos with shortened anterior-posterior body axis and wider dorsal structures like the notochord or somites.
In Drosophila, the establishment of planar polarity manifests by the coordinated polarization of cells within the plane of the epithelium, perpendicular to the typical apico-basal polarization of an epithelial cell sheet [5,6]. In these epithelia, neighbor relationships remain constant. However, during dynamic vertebrate gastrulation movements of mesenchymal-type cells, the Wnt/PCP components control much more complex cell behaviors.
1. Morphogenetic processes during zebrafish gastrulation
The first gastrulation movement during zebrafish embryogenesis is epiboly (Fig. 1A–B). At the beginning of epiboly (4.0 hours post fertilization, hpf) the blastula consists of blastomeres piled upon the yolk cell. Epiboly starts as the yolk cell domes into the blastoderm. As epiboly proceeds, the blastoderm thins and spreads from the animal to the vegetal pole to enclose the entire yolk cell at 9.5 hpf. During this process, massive radial intercalations occur, whereby the deep blastomeres intercalate into more superficial layers, leading to the thinning and spreading of the blastoderm [7,8].
Fig. 1. Morphogenetic movements involved in zebrafish gastrulation.
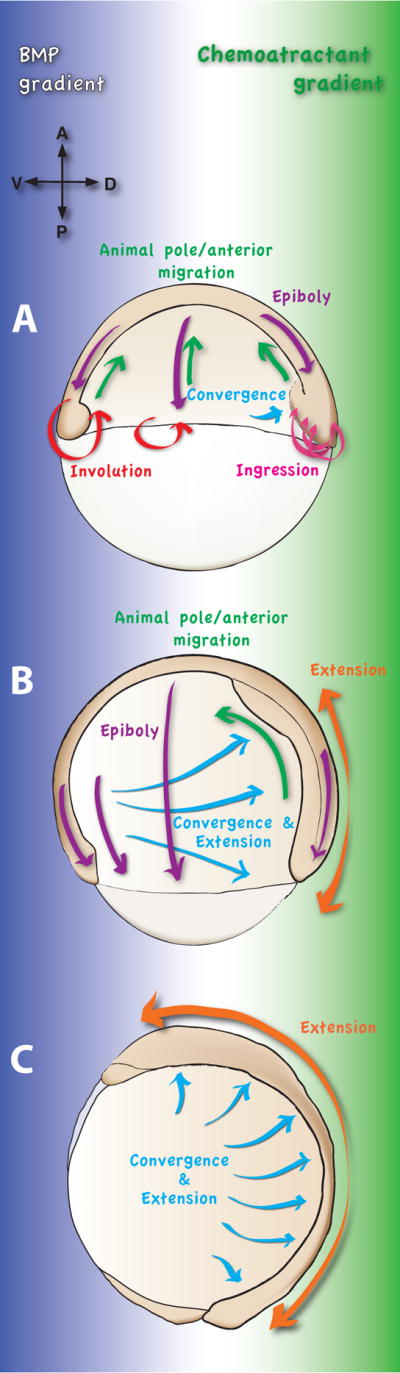
(A), at the beginning of gastrulation (shield stage), the blastoderm envelops half of the syncytial yolk cell. The dorsal side is noticeable at this stage by a thickening of the blastoderm, called the embryonic shield. At the blastoderm margin, the prospective mesendodermal cells undergo internalization. Internalization occurs by involution of cell sheaths in the ventral and lateral domains and by ingression of individual cells in the dorsal domain. The internalized cells migrate towards the animal pole/anterior (green arrow), while the epiboly movement (purple arrow) towards the vegetal pole continues. In the dorsal shield region, some convergence movements are observed (blue arrow).
(B), at midgastrulation (75% epiboly), the major changes in mesendodermal cell movements include the termination of the internalization and the shift from the animal pole/anterior migration to convergence towards dorsal (blue arrow). However, the animal pole/anterior migration continues in the dorsal region (green arrow). These movements contribute a major part to extension of the anterior-posterior axis (orange arrow). In the ventral domain, the cells do not undergo convergence towards dorsal, but migrate towards the vegetal pole (yellow arrow). Epiboly continues at this stage until the blastoderm encloses the entire yolk cell at 9.5 hpf (purple arrows).
(C), at tail bud stage, the entire yolk cell is covered by the blastoderm and epiboly is completed. Convergence of the lateral and dorsal cell populations towards the dorsal midline and their simultaneous extension along the anterior-posterior axis is the major cell movement (blue arrows). Dorsal cell populations undergo fast extension (orange arrow) and modest convergence.
A hypothetical gradient of a chemoattractant emanating from the dorsal midline (green) and a ventral to dorsal BMP gradient (dark blue) present in the gastrula tissues and proposed to influence gastrulation cell movements are shown in the background.
When the blastoderm covers half of the yolk (50% epiboly), the second gastrulation movement, internalization or emboly, takes place (Fig. 1A). At this stage, the blastoderm margin (equivalent of the blastopore in Xenopus and primitive streak in mouse and chick) thickens by accumulation of mesendodermal progenitors, giving rise to the germ ring. The internalization occurs around the entire circumference of the blastoderm margin. In the dorsal part of the gastrula, the germ ring becomes thicker, forming the embryonic shield, which is the zebrafish equivalent to the Spemann-Mangold gastrula organizer and Hensen’s node in Xenopus and amniotes, respectively. Recently, high-resolution microscopy, following almost all cells in the embryo throughout gastrulation revealed, with an unprecedented precision, that in the dorsal region, the internalization occurs by the ingression of individual cells and in the ventral and lateral regions, it occurs largely by involution of sheets of cells [9]. The internalization of mesodermal cells and their migration away from the blastopore and towards the animal pole contributes to the anterior-posterior extension. Upon internalization, the endodermal cells behave differently adopting a random walk behavior, which allows their spreading over the yolk surface [10].
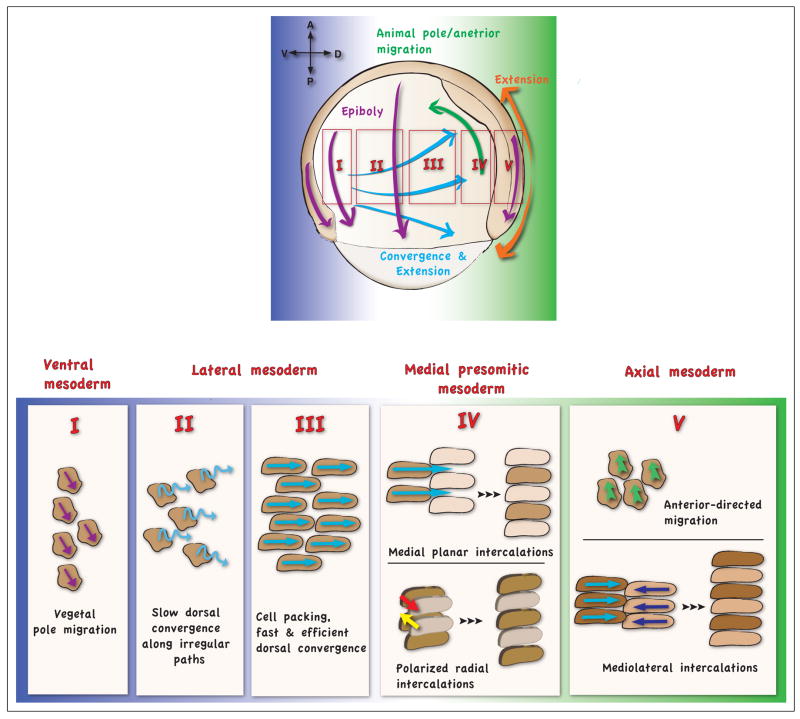
The main cell movements following internalization are C&E movements (Fig. 1 and 2). At midgastrulation (7.5hpf), lateral mesodermal and endodermal cells shift their trajectories from the anterior and random migration, respectively, to dorsalward migration [10,11]. C&E is the major morphogenetic process, which shapes the body axis, narrowing all the germ layers in the mediolateral direction and extending them along the anterior-posterior axis at the dorsal midline [8,11]. The C&E cell behaviors are regulated in time and space in a dynamic manner. Indeed, C&E movements entail multiple cell behaviors depending on the cell’s position along the dorsal-ventral and anterior-posterior embryonic axes. Several distinct domains based on differences in cell behaviors have been identified (Fig. 2) [12,13]. In the ventral domain, known as the “no convergence, no extension zone”, cells do not undergo C&E, instead migrating towards the vegetal pole [12]. Whereas in the lateral domain, the directed cell migration towards the dorsal axis is the key C&E movement. Although, in the Xenopus embryo, C&E movements take place in the context of cell sheets [14] and mediolateral intercalation behavior (MIB) is the predominant cell behavior, in zebrafish at mid-gastrulation the mesendodermal cells migrate as individuals. Only the cell behaviors in the dorsal domain of the zebrafish gastrula are similar to Xenopus convergent extension. In the anterior dorsal domain, the prechordal mesoderm cells in both species engage in directed anterior migration (Fig. 2). The posterior dorsal domain is characterized by limited convergence and strong extension of the embryonic axis where MIB is the predominant cell behavior. In this domain, polarized radial intercalation is also known to contribute to C&E movements [15].
Fig 2. Different cell behaviors along the dorso-ventral axis of the zebrafish gastrula.
During zebrafish gastrulation the cell movements change over time, depending on the gastrulation stage, but also in space, depending on the localization of the cells in particular domains. The midgastrula stage is represented. Several domains can be distinguished along the dorso-ventral gastrula axis according to the type of mesodermal cell movements (showed by magnified I, II, III, IV, V regions). In the most ventral domain (I) the cells undergo migration towards the vegetal pole. In the ventro-lateral domain (II) the cells undergo slow convergence movements along irregular migration paths, as cells located closer to the animal pole bias their trajectories animally and those closer to the vegetal pole bias their trajectories vegetally, the entire population converges and also extends along the anterior-posterior axis. In the lateral domain (III) the cells undergo fast convergence movements along straight migration paths; the cell packing is increased. In the latero-dorsal domain (IV), migration and the cell packing are increased and the cells undergo polarized radial and medio-lateral intercalation movements that drive modest convergence and extension. In the dorsal domain, the most anterior cells undergo anterior directed migration, while more posterior cells undergo massive cell intercalation movements contributing to fast extension and modest convergence.
2. Specific gastrulation cell behaviors regulated by the Wnt/PCP pathway in zebrafish
The cell motility analyses in Wnt/PCP mutant embryos identified the roles of Wnt/PCP signaling in the gastrulation cell movements. Various types of cell behaviors driving C&E movements are under the control of this pathway.
2.1. Anterior migration of prechordal mesoderm cells
After internalization, axial and paraxial mesoderm cells migrate from the marginal region toward the animal pole (Fig. 1A–B). Only cells at the anterior edge of prechordal mesoderm actively migrate and MIB is not observed in this group of cells [16,17]. Recent analysis revealed that this cell movement is regulated by the Wnt/PCP pathway. In slb/wnt11 mutants, both the average velocity and persistence of the anterior migration of the prechordal mesoderm cells are significantly reduced relative to wild type [18,19]. Pseudopod-like processes are observed in the anterior edge of prechordal mesoderm cells in both wild type and slb/wnt11 embryos. Interestingly, the direction of the process is randomized in slb/wnt11 mutants, suggesting that the cell polarity is affected [18]. Ubiquitous expression of wnt11 rescues the defect of cell migration in slb/wnt11 mutant, suggesting that Wnt/PCP functions as a permissive cue [18].
2.2. Mediolateral and polarized radial intercalation in the dorsal domain
Within the dorsal domain, the chordamesoderm undergoes three-fold higher extension than the adjacent presomitic mesoderm (PSM) [20,21]. MIB drives C&E in the axial domain, while a cooperation of MIB and polarized radial intercalations accounts for C&E in the paraxial domain [15,20]. MIB is best understood in the Xenopus gastrula. Time-lapse analysis in tissue explants revealed that MIB occurs via a stereotypical sequence of cell behaviors. In the early gastrula explants, mesodemal cells exhibit a rounder shape and extend randomly-directed lamellipodia. By midgastrulation, the orientation of the lamellipodia becomes more stable and cells show a bipolar shape and are mediolaterally aligned. These cells elongate with their long axes oriented perpendicular to the dorsal midline and intercalate between their medial and lateral neighbors (Fig. 2) [22,23].
In zebrafish, the dorsal mesodermal and ectodermal cells also become highly elongated with their long axes oriented perpendicular to the dorsal midline by late gastrulation [1,2,12,24]. In the chordamesoderm, MIB is thought to be the major cell behavior for C&E movements (Fig. 2) [20]. Surprisingly, in no tail mutants (the zebrafish homolog of the mouse T-box gene Brachyury), in which the MIB is defective, the notochord extends without convergence, suggesting that MIB is not the only process contributing to axis extension [20]. In kny or tri mutants, the cells are defective in mediolateral (ML) elongation and the notochord is wider than in wild-type embryos indicating that these cells have strongly reduced ML polarity and that ML intercalations are impaired [1,2,25].
Recently, Yin et al. reported that radial intercalation contributes to C&E movements in the medial PSM [15]. Radial intercalation is the movement of cells from a deeper layer to a more superficial one, and/or vice versa, which leads to the spreading and thinning of the tissue (Fig. 2) [26]. Cell-tracing analysis revealed that the number of cells entering and leaving the middle layer of PSM is balanced. However, radial intercalations of cells entering the layer preferentially separate the anterior and posterior rather than medial and lateral neighboring cells, thus driving the extension of the PSM. In contrast, in tri;kny double mutant embryos, the rate of the AP directed radial intercalations in PSM is decreased and ML intercalations are increased. These results indicate that Wnt/PCP signaling regulates the AP bias of radial intercalation by providing cell polarity to different PSM layers [15].
2.3. Slow and fast dorsal-directed migration in the lateral domain
The cells in the lateral domain show dynamic changes in their shape and movements during gastrulation (Fig. 2). At early gastrulation (6hpf), the cells located in the lateral domain migrate towards the animal pole and no dorsal-directed migration is observed. At mid-gastrulation (7.5hpf), these cells show amoeboid morphology and move dorsally along zigzagging trajectories. As these cells approach the dorsal midline, they converge with increasing speed and along straighter paths than those in more lateral regions [12,27]. At the end of gastrulation (9.5hpf), they become densely packed and exhibit mediolaterally elongated shape (Fig. 2). Thus, the cell-cell interactions are different between the slow lateral domain (loose cell-cell contacts) and the fast lateral domain (closely-packed cells).
Interestingly, in tri and kny mutants, fast dorsal migration is impaired and the cells undergo slow dorsal migration even at the late gastrula stage. Moreover, the cells are less polarized and stay rounder, indicating that the Wnt/PCP pathway is essential for the fast dorsal migration and a polarized cell shape, but is not required for the initial slow dorsal migration [1,2,13].
2.4. Oriented cell division
In Drosophila, the PCP pathway regulates spindle orientation during asymmetric cell division [28]. Oriented cell division is known to contribute to the elongation of Drosophila wing [29] and mammalian renal tubules [30]. In zebrafish gastrula, the cells in the dorsal ectoderm preferentially divide along the animal-vegetal axis, thus contributing to tissue extension [24,31]. This cell division orientation is misaligned in slb mutants, tri morphant embryos or gastrulae expressing dominant negative Dvl [31]. These results implicate the Wnt/PCP pathway in the orientation of cell division in zebrafish.
3. Wnt/PCP pathway regulates gastrulation in other vertebrates
In amniotes (human, mouse, chick), during early gastrulation, cells in the epiblast move towards the primitive streak (blastopore equivalent), where they ingress [32]. Recent data suggest the involvement of several Wnt/PCP components in avian gastrulation. In the chick embryo, perturbation of Wnt/PCP signalling by electroporation of dominant negative Dvl, leads to cell accumulation in the primitive streak [33]. wnt5a, wnt5b and wnt11b genes, homologues of the zebrafish and Xenopus non-canonical Wnt genes (wnt5a and wnt11) [3,4,18,34,35], are expressed in chick primitive streak and regulate mesodermal precursors’ internalization through the primitive streak [33,36]. Upon internalization, mesodermal and endodermal cells migrate away from the primitive streak, which is comparable to the movement of internalized cells in the zebrafish gastrula moving away from the margin towards the animal pole. Electroporation of mutant forms of Dvl and Prickle1 (Pk1) was shown to perturb paraxial and lateral plate mesoderm migration, respectively, after they emerge from the primitive streak [36]. However, in zebrafish, with the exception of the prechordal mesoderm cells, the Wnt/PCP pathway is not essential for the migration of mesendodermal precursors towards the animal pole upon internalization at the margin.
Furthermore, recent studies of avian gastrulation demonstrated that the Wnt/PCP signalling pathway is essential even before formation of the embryonic germ layers, in contrast to what is known in other vertebrates. Indeed, primitive streak formation is preceded by massive intercalation of epiblast cells, called the “polonaise movement” [37,38]. Inhibition of the Wnt/PCP pathway by electroporation of the Dvl mutant compromised these movements, showing their dependence on functional Wnt/PCP pathway. Further evidence showed that before primitive streak formation, Flamingo (Fmi), Vangl2 and Pk1 are all expressed in the epiblast region where the intercalation movements take place. In addition, simultaneous inhibition of Pk1, Fmi and Vangl2, by electroporation of antisense morpholino oligonucleotides (MOs) targeting these genes, compromised primitive streak but not mesoderm formation [39].
In mammals, similarly to zebrafish and Xenopus, mutations in the Wnt/PCP pathway genes impair C&E and neurulation. Indeed, mouse embryos harboring mutations in Vangl2 (loop-tail), Scribble1 (Circletail), Celsr1/Fmi (spin cycle and crash) and dvl-related genes all display shorter bodies and defects in neural tube closure [40–45]. In addition, mutations in Wnt signaling antagonists Sfrp1, Sfrp2, and Sfrp5, via misregulation of Wnt/PCP signaling, cause neural tube closure defects, as well as severe shortening of the anterior-posterior axis and widening of the notochord and somites that are similar to the defects observed in zebrafish and Xenopus Wnt/PCP-deficient embryos [46]. Additional evidence for the conserved involvement of the Wnt/PCP pathway in mammalian gastrulation came from a direct comparison of the function of PTK7 (Protein Kinase 7), an evolutionary conserved transmembrane protein with kinase homology domain, in mouse and Xenopus. PTK7 mouse mutants show typical C&E defects (short body axis, neural tube closure defects, misorientation of stereociliary bundles in the cochlea). Likewise, interference with Ptk7 function in Xenopus leads to typical C&E defects. In addition, a strong genetic interaction was described for PTK7 and Vangl2 (looptail) mutations, suggesting that these genes cooperate in the regulation of Wnt/PCP signaling [47].
Together, these results suggest that despite the morphological differences between zebrafish, Xenopus, mouse and avian gastrulae, the Wnt/PCP pathway’s essential role for morphogenetic movements that narrow and elongate tissues is conserved. However, the involvement of the Wnt/PCP pathways appears to be required earlier in avian embryos than in zebrafish or Xenopus.
4. Molecular regulation of the Wnt/PCP pathway
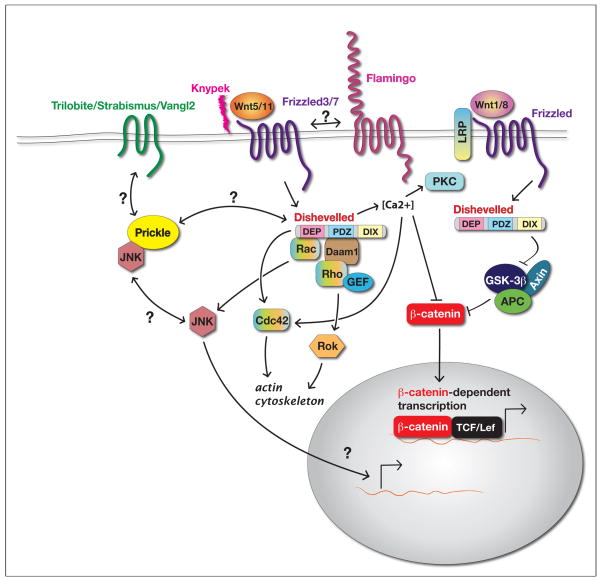
Wnt signaling activates multiple pathways, which are classified into either canonical or non-canonical. In the canonical, Wnt/β-catenin pathway, binding of a Wnt ligand to the Frizzled (Fz) receptor causes activation of the cytoplasmic protein Dvl (Fig. 3). Activated Dvl suppresses the degradation of β-catenin, which accumulates in the cytoplasm and subsequently in the nucleus, where it regulates the expression of downstream genes [48]. On the other hand, multiple β-catenin-independent, non-canonical, Wnt signaling pathways also have been identified in many invertebrate and vertebrate species. The Wnt/PCP pathway is one of these non-canonical Wnt pathways. The Wnt/PCP pathway has been most extensively studied in Drosophila where none of the known Wnt ligands are likely to mediate PCP signaling [49]. In contrast, from over 19 Wnt-encoding genes in the vertebrate genomes, four genes (in zebrafish, wnt4, wnt5, wnt5a and wnt11) encode ligands for the Wnt/PCP pathway [3,4,35,50,51], suggesting some divergence in the regulation of this pathway between Drosophila and vertebrates.
Fig 3. Schematic illustration of the canonical Wnt (Wnt/β-catenin) and the non-canonical Wnt/Planar Cell Polarity (Wnt/PCP) signaling pathways in vertebrates.
See text for details
4.1. The functions of the Wnt/PCP core components in vertebrates
Similarly to Drosophila, Fz and Dvl serve as core components of Wnt/PCP signaling in vertebrates (Fig. 3). Expression and epistasis analyses suggest that Fz2 and Fz7 act as receptors for Wnt5 and Wnt11 during C&E in zebrafish [52] and Xenopus [53]. In the zebrafish gastrula, overexpressed Fz7 accumulates locally at cell membranes juxtaposed to the cells overexpressing Wnt11, suggesting that Wnt11 regulates subcellular localization of Fz7 [54]. In Xenopus and zebrafish, Fz7 overexpression promotes membrane localization of Dvl, suggesting that there is a common signal transduction mechanism between Drosophila and vertebrates [55–57].
Tri/Vangl2 and Pk are also known to be involved in Wnt/PCP pathway activity in vertebrates (Fig. 3). The disruption of vangl2 function by mutations or antisense MOs-mediated gene knock down causes C&E defects in mouse (looptail), Xenopus and zebrafish (tri) [1,40,58–60]. Paralleling the situation in Drosophila, overexpression of Vangl2 in Xenopus and zebrafish embryos also causes cell polarity and consequently C&E defects [1,59,60]. In zebrafish, C&E phenotypes in tri single mutants, revealed by a shorter and wider body axis and cyclopia, are enhanced in tri;slb double mutants [61]. Notably, tri;kny double mutants display an almost additive C&E defect and highly penetrant cyclopia, a genetic interaction stronger than that expected from two genes working in a linear pathway [15,62]. Like Vangl2, both gain- and loss-of-function of Pk causes C&E defects [55,57,63]. Pk can bind to Dvl and inhibits membrane translocation of Dvl [57,63]. Moreover, Pk accelerates the degradation of Dvl in zebrafish [57]. These results suggest that Pk antagonizes Dvl and works to generate cell polarity by limiting the activity of the Wnt/PCP pathway. Whereas in Xenopus, Pk or Vangl2 overexpression causes activation of JNK pathway [55,60,63] which is known as a downstream signaling of the Wnt/PCP pathway. Together, Vangl2 and Pk are essential components of the Wnt/PCP pathway but they do not function simply as positive or negative component of this pathway.
Three Fmi homologs are known in zebrafish and mammals and they belong to an unusual class of adhesion GPCRs [64]. Fmi contains extracellular cadherin repeats and a seven-pass transmembrane domain, reminiscent of the G-protein coupled receptor (GPCR) family. Gene knockdown analyses by MOs revealed that Celsr1a and Celsr1b cooperatively regulate C&E movements in collaboration with the Wnt/PCP pathway during zebrafish gastrulation [65]. However, a more recent study in zebrafish shows that celsr2 gene plays an additional role during development. Via its extracellular domain, Celsr2 regulates cell adhesiveness during epiboly, independently of the Wnt/PCP pathway. Through its cytoplasmic domain, Celsr2 regulates C&E by indirectly modulating Dvl localization at the cell membrane [66]. Consistent with the analysis in Drosophila, the local accumulation of Fz7 induced by Wnt11 at cell-cell contacts is dependent on Celsr/Fmi proteins in zebrafish [54]. While in Drosophila, Fmi directly binds Fz [49], the intracellular domain of Celsr2 doesn’t bind to Fz7 in zebrafish [66]. Thus, in vertebrates, the molecular mechanism of how Fmi/Celsr proteins interact with Wnt/PCP pathway still remains elusive.
4.2. Other components of the Wnt/PCP pathway
Protocadherins Fat and Dachsous and the Golgi transmembrane/secreted protein Four-jointed (Fj) participate in tissue polarization in flies [67–69]. Dachsous, and Fat homologues have been described in the mouse [70]. Mice carrying a null mutation in the Fat4 gene exhibit mild C&E phenotypes and some defects seen in other Wnt/PCP pathway deficient mice. In addition, genetic interaction between Fat4 and Vangl2 has been observed [71]. These results suggest that the Fat/Fj pathway is conserved between Drosophila and vertebrates. In zebrafish, so far one gene encoding the protocadherin Fat has been identified [72], but its role in C&E and the Wnt/PCP pathway remains to be analyzed. Further analysis is needed to understand the role of the protocadherins during vertebrate C&E as well as their relationship to other components of the Wnt/PCP pathway. This will involve considerable efforts due to the redundancy of the Fat genes in vertebrates [70,71,73].
It is known that a family of heparan sulfate proteoglycans (HSPG) is also involved in the Wnt/PCP pathway in vertebrates. In zebrafish and Xenopus, Glypican4, a GPI-linked HSPG is thought to be a co-receptor along with Fz for Wnt ligands in the Wnt/PCP pathway [2,74]. Zebrafish kny/gpc4 mutants exhibit severe C&E defects due to impaired polarized cell behaviors. The cyclopia phenotype in slb/wnt11 mutants is enhanced by kny mutations suggesting that kny/gpc4 functionally interacts with slb/wnt11 [2]. In addition to kny/gpc4, embryos deficient for another glypican, gpc3 show C&E defects suggesting that the multiple glypicans are involved in Wnt/PCP signaling in zebrafish [75]. In Xenopus, a transmembrane HSPG, Syndecan4 (Syn4), has also been implicated in Wnt/PCP signaling and C&E gastrulation movements. Overexpression or gene knockdown by MOs targeting syn4 causes a C&E defect during gastrulation. Moreover, Syn4 directly interacts with Fz and Dvl and mediates translocation of Dvl to the plasma membrane. These results suggest that Syn4 works as a Wnt co-receptor with Fz and mediates activation of the Wnt/PCP pathway. Interestingly, Syn4 function is Fibronectin-dependent suggesting that Syn4 mediates the interaction of the Wnt/PCP pathway and extracellular matrix during gastrulation [76].
4.3. Downstream effectors of the Wnt/PCP pathway
The Wnt/PCP pathway regulates different cell behaviors, including cell elongation, cell body orientation, and localization of protrusive activities during C&E. Thus a key question is what molecular pathways downstream of the core Wnt/PCP components mediate these specific cell behaviors? In both Drosophila and vertebrates, members of the Rho family of GTPases have been shown to act downstream of the Wnt/PCP pathway (Fig. 3) [77–81]. Rho family GTPases, Rho, Rac and Cdc42 are implicated in the establishment of cell polarity and the regulation of cell motility through the reorganization of the actin cytoskeleton [82,83]. Rho and Rac are thought to be controlled by independent and parallel pathways during Wnt/PCP signaling and C&E movements [77,78,84]. Wnt/PCP signaling stimulates formation of a complex that includes Rho, Dvl and Daam1. The latter is a Formin-homology protein, first identified in vertebrates that binds both Rho and the PDZ domain of Dvl and mediates Rho activation [79]. Activated Rho stimulates activity of the Rho-associated kinase (Rok) and mediates cytoskeletal reorganization (Fig. 3) [85–88]. Expression of dominant negative forms of Rok2 (dnRok2) impairs both the velocity of dorsal cell migration and ML cell elongation in the zebrafish gastrula. Mosaic analysis revealed that transplanted wild-type cells within dnRok2 expressing gastrulae showed a normally elongated shape but a random orientation of their long axis [88]. This result suggests that ML-biased orientation and elongation of the cell body are independent properties and that Rok2 function is required cell-autonomously for cell elongation and both cell-autonomously and non-autonomously for cell orientation. Rok2 overexpression suppresses the slb/wnt11 mutant phenotype suggesting that zebrafish Rok2 regulates cell polarity acting downstream of Wnt11. In contrast to Rho, Rac functions independently of Daam1 (Fig. 3). Rac directly binds to the DEP domain of Dvl and activates JNK [78,89]. Rho and Rac have distinct functions during C&E movement in Xenopus. Dominant negative Rho expression significantly decreased the length-to-width ratio of the cells engaged in C&E. In contrast, activation of Rac greatly increased the number of filopodia per cell. Together these studies in zebrafish and Xenopus support a model whereby cell elongation is determined predominantly by the Rho/Rok2 pathway and filopodia activity by Rac [84]. Another Rho family GTPase, Cdc42, is thought to regulate C&E through the protein kinase C (PKC-α)-mediated Wnt/Ca2+ pathway, downstream of Fz [53,90]. In both Drosophila and Xenopus, it has been demonstrated that JNK is activated by Wnt/PCP signaling. Moreover, in Xenopus, JNK MO knockdown caused the C&E and cell adhesion defects suggesting that the activation of JNK is essential for the transduction of Wnt/PCP signaling [91]. In Drosophila, the JNK pathway has been suggested to regulate the transcription of target genes [77,92], but its function is largely unknown in vertebrates.
4.4. Mechanisms of cell polarization by the Wnt/PCP pathway
In Drosophila wing epithelium, each cell forms a proximal-distal orientated hair and this cell polarity corresponds to the subcellular localization of PCP proteins, which becomes elaborated during the pupal stage. The asymmetric subcellular localization of these proteins is dependent on the normal functions of all these genes [49,93]. More recently, it has been shown that the Fat/Fj pathway provides directional cues over entire tissues such as the wing, eye, and abdomen anlage. It is still controversial whether Fat/Fj pathway is upstream or functions in parallel to the PCP pathway [67,94–96].
Asymmetric localization of Wnt/PCP proteins has also been recently reported during vertebrate embryogenesis e.g. in the cochlea and interfollicular epidermis of mouse embryos. By mosaic analysis in the cochlea, Fz6 (and presumably Fz3) was shown to localize at the proximal hair cell membranes [97]. Immunostaining of the cochlea showed that Vangl2 localizes at the proximal edges of hair cells and/or adjacent distal edges of nonsensory supporting cells [98]. In contrast, Dvl1 and Dvl2 are localized at the distal edges of hair cells and/or the adjacent proximal edges of the nonsensory supporting cells [43,99]. In the interfollicular epidermis, all Vangl2, Celsr1 and Fz6 proteins are enriched at the anterior-posterior cell boundaries [100]. The asymmetric localization of these proteins is lost in Wnt/PCP mutant mice [43,97–100]. A different asymmetric localization is observed in zebrafish. In neuroectoderm, notochord and paraxial mesoderm, Pk-GFP fusion protein was observed to accumulate at the anterior cell edges [15,50], whereas Dvl-GFP fusion protein is localized at the posterior cell membrane during gastrulation [15]. This asymmetric localization of Pk and Dvl is lost in the Wnt/PCP mutants, suggesting that Wnt/PCP pathway provides the polarization of the dorsal mesoderm cells in an anterior to posterior direction [15]. The asymmetric localization of Dvl and Pk to the opposing cell membranes in the zebrafish resembles the localization of the Drosophila homologs to the proximal and distal cell edges in wing epithelium and argues for the functional conservation of the molecular mechanism of the Wnt/PCP signaling between Drosophila and vertebrates. In contrast, in Xenopus, Dvl was reported to accumulate around the medial and lateral tips of elongated cells in the dorsal marginal zone explants undergoing C&E [56]. This localization is lost in explants overexpressing Dvl that lacks the DEP domain. Consistently with the Drosophila PCP pathway, the subcellular localization of Dvl is dependent on Wnt/PCP signaling in vertebrates. However, the distinct localization of Dvl in Xenopus and zebrafish during mediolateral cell intercalation in dorsal mesoderm can be interpreted that in vertebrates, novel mechanisms may also operate.
5. Interactions of the Wnt/PCP pathway with other pathways implicated in C&E movements
Current evidence indicates that, in addition to the Wnt/PCP pathway, other pathways, like those involved in chemotaxis, cell-cell and cell-extracellular matrix adhesion, cell fate specification, also regulate C&E movements [101]. The major challenge is to understand the relationship between these pathways during the complex C&E movements.
5.1. Stat3
Stat3, the signal transducer and activator of transcription 3, has been proposed to act as an upstream input to the Wnt/PCP pathway during C&E [102,103]. Stat3 is activated by phosphorylation in the dorsal region of the zebrafish blastula, downstream of the maternal canonical Wnt signaling pathway [102]. The observations that the lateral mesodermal cells increase their migration speed and polarization as they move towards the dorsal midline during C&E, suggest the presence of a graded attractive signal emanating from the dorsal midline [1,11]. The activity of Stat3 is not required for cell fate specification in the zebrafish gastrula, but its activity was shown to be essential in the dorsal gastrula region, where it activates, cell autonomously, the anterior migration of dorsal mesodermal cells. Subsequent studies identified zinc transporter Liv1 as an essential mediator of the cell-autonomous activity of Stat3 in the dorsal mesoderm [104]. Stat3 also has a non cell-autonomous effect on the lateral mesodermal cells’ elongation and polarization as well as their dorsalward convergence. It was shown that expression of ΔN-Dvl, which specifically transduces Wnt/PCP signaling [4], can rescue the cell elongation but not the orientation defect of mesodermal cells in stat3-depleted embryos [103]. These data suggested that Stat3 activity is needed for cells to detect and align their long axes with the mediolateral embryonic axis. It is assumed that Stat3 acts, via an unknown, presumably diffusible signal that activates Dvl-Rho signaling in the Wnt/PCP pathway. However, the loss of Stat3 function does not interfere with the expression patterns of the Wnt/PCP genes. Moreover, Stat3 is required at midgastrulation for the initiation of the slow convergence of lateral mesoderm cells toward the dorsal midline, when the Wnt/PCP component, Tri/Vangl2, does not appear to be required [11]. It is then possible that the hypothesized diffusible signaling molecule downstream of Stat3, regulates the cell movements at the onset of C&E, and later its function is relayed (or completed) by Wnt/PCP signaling to achieve faster and more efficient convergence movements.
Identification of additional downstream effectors of Stat3 is needed in order to understand the molecular nature of the instructive diffusible signal that induces the dorsal convergence. An interesting area of investigation will be to determine which are the receptors for this unknown diffusible signal and which molecules in the Wnt/PCP pathway are involved directly in the ability of cells to sense the direction of cell elongation mediated by Stat3?
5.2. Chemotaxis
During zebrafish gastrulation, the directionality of cell movements is highly regulated. First, upon internalization, the mesodermal cells migrate towards the animal pole; then at midgastrulation they initiate directed migration towards the dorsal side of the embryo. The amoeboid morphology and trajectories of these cells are consistent with a chemotactic movement. For instance, mathematical models suggest that the dorsal convergence of lateral mesodermal cells could be regulated by two guidance cues present at different levels of the dorsal midline, or one guidance cue distributed along the midline, instructing the cells’ migration [11].
In Wnt/PCP mutants, the C&E movements are highly perturbed, however, not completely abolished, suggesting that different parallel pathways contribute to regulate these movements. Interestingly, Frizzled receptors have the structural characteristics of G-protein coupled receptors, which play crucial roles during chemotactic cell migration. Recent data in Drosophila suggest that the Fz receptors activate the canonical and Wnt/PCP pathway via the heterotrimeric G-protein subunit, Gα0 [105]. In zebrafish, it has been shown that Gα12/13 play an important role during C&E [25]. The overexpression or inhibition of these subunits phenocopies the defects associated with Wnt/PCP mutants: slb, tri and kny. These proteins are required for mediolateral cell elongation, efficient dorsalward cell migration during C&E and are cell-autonomously required for mediolateral cell intercalation. However, no direct link with the Wnt/PCP pathway has been established by these subunits. That the C&E defects are exacerbated in Gα12/13 depleted Wnt/PCP mutant embryos (slb, kny and tri) suggests a rather parallel function of these proteins with respect to the Wnt/PCP pathway [25]. It remains to be determined if the polarization and efficient directed migration of mesodermal cells during C&E movements of lateral mesoderm is downstream of any/which chemoattractant gradient.
5.3. BMP
During vertebrate gastrulation, BMPs (Bone Morphogenetic Protein), members of the TGFβ superfamily of ligands, are expressed in a ventral-to-dorsal gradient, which specifies cell fates in all germ layers [106,107]. Interestingly, the BMP gradient in the gastrulating zebrafish embryo was shown to regulate C&E movements by specifying different domains of cell behavior [12,108]. These data demonstrated that mesodermal cells require a moderate level of BMP signal in order to increase the convergence speed towards the dorsal midline. BMP acts by creating a gradient of cell adhesiveness increasing from ventral to dorsal by negatively regulating calcium-dependent cell-cell adhesion. This gradient is essential to determine the directionality of mesodermal cell movement in the lateral region of the zebrafish gastrula [108]. These results show the importance of cell adhesiveness in the control of directed migration during C&E movements. In this regard, the gradient of BMP signaling not only regulates cell fates but also the directionality of cell movement.
Interestingly, the inactivation of non-canonical wnts (wnt5 and wnt11) does not perturb the directionality of cell movement, and Wnt/PCP pathway is not required for the slow dorsal migration of mesodermal cells, suggesting that this cell guidance mediated by the BMP gradient is independent of Wnt/PCP signaling [108]. However, previous data showed that the low BMP levels in the more dorsal region of the zebrafish gastrula allow expression of wnt11 and wnt5 genes, and consequently Wnt/PCP-mediated cell polarization and MIB [12].
5.4. Cell-cell adhesion: Cadherins and Protocadherins
In vertebrates, the role of cell-cell communication by adhesion molecules in highly dynamic mesenchymal cells during gastrulation is still poorly understood. Several studies showed that classical Cadherins are required for proper gastrulation movements in zebrafish. E-cadherin/half baked/cdh1 mutants show several defects, including epiboly delay, defective anterior migration of prechordal mesoderm and C&E defects [7,109,110]. Recently, a study of the N-cadherin/cdh2 zebrafish mutant revealed that loss of N-cadherin destabilizes the cells protrusive activity, which is needed for proper cell traction during the intercalation movements [111]. Moreover, increasing evidence indicates that the Wnt/PCP pathway regulates cell behavior and movements by regulating the dynamic rearrangements of cell adhesion molecules. In particular, Wnt11 has been shown to regulate the cohesion of prechordal mesoderm cells in zebrafish by regulating the subcellular localization of E-cadherin [19]. A recent analysis of N-cadherin’s role during tail formation, which is thought to involve C&E movements, revealed genetic interaction with tri/vangl2. Additional data showed that in Wnt/PCP mutants, kny and tri/vangl2, N-cadherin cellular distribution and protein levels were not perturbed during tail morphogenesis. These observations suggest that N-cadherin and Tri/Vangl2 function in parallel pathways during tail morphogenesis [112].
Other adhesion molecules belonging to the cadherin family seem to collaborate with the Wnt/PCP pathway and play important roles during gastrulation. In Xenopus and zebrafish the paraxial protocadherin (PAPC), which is expressed in the mesoderm, is involved in the regulation of C&E movements and cell polarity during gastrulation [113,114]. Recent data in Xenopus show that XPAPC expression is positively regulated by XWnt-5A, but not XWnt-11 [115]. XPAPC regulates cell adhesive proprieties by down-regulating the adhesion activity of C-cadherin [116], but XPAPC can also interact with the Xenopus Fz7 receptor and control C&E movements through the small GTPases Rho and Rac1, and JNK [117]. In addition, XPAPC was shown to physically interact with Sprouty2, an inhibitor of the Wnt/PCP pathway, and to antagonize its function [118].
5.5. Cell adhesion to the Extracellular Matrix
Extracellular matrix (ECM) is assembled progressively during gastrulation as a fibrillar network in the extracellular space and at tissue boundaries by deposition of specific components (Fibronectin, Laminin, polysaccharides, proteoglycans). During Xenopus gastrulation, the interaction between Fibronectin and its cellular receptors, Integrins, is required for proper fibril assembly at the blastocoel roof and has been shown to regulate gastrulation movements and cell polarization [119,120]. Interestingly, in Xenopus gastrulae, disruption of the Wnt/PCP pathway, by inactivating Vangl2, Pk or Fz, impairs proper assembly of the Fibronectin network, which surrounds the mesodermal layer [59]. However, providing a properly organized ECM can rescue the mediolateral elongation of mesodermal cells in Pk-deficient explants, but not in Vangl2 or Fz deficient explants. Therefore, in contrast to Vangl2 and Fz, Pk is not essential for ECM-mediated cell polarization [121]. Recently, Wnt11 and Dvl were also shown to play important roles in Fibronectin network assembly in Xenopus via Integrins and the small GTPases Rho and Rac [122].
In zebrafish, recent results support the important role of the ECM during gastrulation. In particular, inhibition of matrix metalloproteinases MMP14a and MMP14b, which degrade and modify the ECM, impairs C&E. Moreover, MMP14 regulates mediolateral elongation of mesodermal cells during their migration towards the dorsal midline. More interestingly, genetic interaction between mmp14 (mmp14a, mmp14b) genes and the Wnt/PCP gene kny was described, suggesting that ECM remodeling via MMP14 and the Wnt/PCP pathway are both necessary for cell movements and cell polarity regulation during C&E [123].
Additional support for a direct link between the ECM molecules and cell behavior, came from the analysis of the extracellular polysaccharide, Hyaluronan (HA), an ECM component, which is synthesized by Has enzymes and then secreted into the intercellular space. Inhibition of Has2-mediated synthesis and the consequent loss of HA causes severe defects in dorsal convergence movements during zebrafish gastrulation that are correlated with defects in protrusive activity [124]. It is therefore important to consider the extracellular environment when analyzing the specific role of the Wnt/PCP pathway components in the polarizing signal transmission from cell to cell. This aspect could be an important difference between the mechanisms of PCP signaling in Drosophila epithelia and mesenchymal cell populations during vertebrate gastrulation.
5.6. Primary Cilia
Primary cilia are microtubule-based and evolutionarily conserved organelles at the surface of cells. They are assembled from a structure called the basal body, which is formed by a pair of centrioles and a complex of anchor proteins and built by intraflagellar transport proteins [125]. The first evidence implicating Wnt/PCP pathway in ciliogenesis, and vice versa came from studies of the inner ear in the mouse [98,99] (discussed in other chapters of this volume).
Studies in Xenopus implicated the Drosophila PCP effector proteins, Inturned and Fuzzy, in ciliogenesis. Interference with expression of these proteins has caused neural tube closure and C&E defects, which were enhanced by co-inhibition of PCP proteins (Xdd1, Fz8) [126]. Studies in ciliated ectoderm of Xenopus embryos revealed that Dvl and Inturned accumulate at the apical surface of ciliated cells where they govern the actin network assembly essential for proper ciliogenesis. In these cells, Dvl plays an essential role in the basal body docking at the apical cell surface. Interestingly, the basal body docking is mediated by vesicular trafficking, regulated by Dvl and the exocyst component Sec8. Sec8 and CLAMP, a protein associated with the basal body, interact in the ciliated cells and form highly polarized structures in the apical cell domain. This polarity is randomized by inhibition of Dvl, suggesting a key role of Dvl for the establishment of the basal body polarity [127].
However, little is known about the primary cilia in non-epithelial cells. During C&E in zebrafish, the sequence of cell behaviors regulated by Wnt/PCP pathway is complex, and the involvement of ciliary proteins needs to be investigated from this perspective. Interestingly, in Drosophila, primary cilia are absent and the only ciliated structures are sensory neurons and sperm. Therefore, the interaction of Wnt/PCP pathway with ciliogenesis appears to be an additional feature of the function of this pathway in vertebrates.
Concluding remarks
Our knowledge about Wnt/PCP pathway has greatly increased in both Drosophila and vertebrates in the last decade. Although numerous lines of evidence show that the requirement for Wnt/PCP function is conserved between species, much more is still to be discovered about the similarities and differences between the way that vertebrates and Drosophila use the Wnt/PCP pathway during development. In vertebrates, defective Wnt/PCP signaling perturbs cell polarity and consequently cell movements. Many of the molecular components of the Wnt/PCP pathway are conserved and are involved in the establishment of cell polarity in the plane of tissues. However, the subcellular localization of the Wnt/PCP components varies, not only between species but also between tissues. We are still far from a comprehensive understanding of the underlying molecular mechanism. The functional and physical interactions among the Wnt/PCP pathway components need to be analyzed in vertebrates. Furthermore, advances made over the past few years have brought evidence that besides the core Wnt/PCP components identified in Drosophila, in vertebrates several other pathways collaborate with the Wnt/PCP pathway during gastrulation, which make the understanding of this pathway even more challenging. We are convinced that development of genetic tools such as mutants in PCP pathway components in the mouse and zebrafish, as well as new methods of cell biological analyses in all vertebrate species will significantly accelerate progress in our understanding of this complex but also fascinating pathway in vertebrates.
Acknowledgments
We thank Diane Sepich, Simon Wu and Christina Speirs for discussions and constructive comments on the manuscript. The work on gastrulation in LSK lab is supported by Human Frontiers in Science Program and Award Number R01GM055101 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–5. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–64. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 3.Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, et al. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–34. [PubMed] [Google Scholar]
- 4.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 5.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 6.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–35. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 7.Kane DA, McFarland KN, Warga RM. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development. 2005;132:1105–16. doi: 10.1242/dev.01668. [DOI] [PubMed] [Google Scholar]
- 8.Warga RM, Kimmel CB. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569–80. doi: 10.1242/dev.108.4.569. [DOI] [PubMed] [Google Scholar]
- 9.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322:1065–9. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 10.Pezeron G, Mourrain P, Courty S, Ghislain J, Becker TS, Rosa FM, et al. Live analysis of endodermal layer formation identifies random walk as a novel gastrulation movement. Curr Biol. 2008;18:276–81. doi: 10.1016/j.cub.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Sepich DS, Calmelet C, Kiskowski M, Solnica-Krezel L. Initiation of convergence and extension movements of lateral mesoderm during zebrafish gastrulation. Dev Dyn. 2005;234:279–92. doi: 10.1002/dvdy.20507. [DOI] [PubMed] [Google Scholar]
- 12.Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- 13.Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis. 2000;27:159–73. doi: 10.1002/1526-968x(200008)27:4<159::aid-gene50>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, et al. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–32. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heisenberg CP, Tada M. Zebrafish gastrulation movements: bridging cell and developmental biology. Semin Cell Dev Biol. 2002;13:471–9. doi: 10.1016/s1084952102001003. [DOI] [PubMed] [Google Scholar]
- 17.Rohde LA, Heisenberg CP. Zebrafish gastrulation: cell movements, signals, and mechanisms. Int Rev Cytol. 2007;261:159–92. doi: 10.1016/S0074-7696(07)61004-3. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–84. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–64. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–87. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- 21.Wood A, Thorogood P. Patterns of cell behaviour underlying somitogenesis and notochord formation in intact vertebrate embryos. Dev Dyn. 1994;201:151–67. doi: 10.1002/aja.1002010206. [DOI] [PubMed] [Google Scholar]
- 22.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–14. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 23.Shih J, Keller R. Patterns of cell motility in the organizer and dorsal mesoderm of Xenopus laevis. Development. 1992;116:915–30. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- 24.Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–94. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- 25.Lin F, Sepich DS, Chen S, Topczewski J, Yin C, Solnica-Krezel L, et al. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169:777–87. doi: 10.1083/jcb.200501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson P, Keller R. Cell rearrangement during gastrulation of Xenopus: direct observation of cultured explants. Development. 1991;112:289–300. doi: 10.1242/dev.112.1.289. [DOI] [PubMed] [Google Scholar]
- 27.Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–55. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 28.Gho M, Schweisguth F. Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature. 1998;393:178–81. doi: 10.1038/30265. [DOI] [PubMed] [Google Scholar]
- 29.Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–4. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 30.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–3. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–93. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 32.Lawson A, Schoenwolf GC. Epiblast and primitive-streak origins of the endoderm in the gastrulating chick embryo. Development. 2003;130:3491–501. doi: 10.1242/dev.00579. [DOI] [PubMed] [Google Scholar]
- 33.Hardy KM, Garriock RJ, Yatskievych TA, D’Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–38. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 36.Sweetman D, Wagstaff L, Cooper O, Weijer C, Munsterberg A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev Biol. 2008;8:63. doi: 10.1186/1471-213X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui C, Yang X, Chuai M, Glazier JA, Weijer CJ. Analysis of tissue flow patterns during primitive streak formation in the chick embryo. Dev Biol. 2005;284:37–47. doi: 10.1016/j.ydbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 38.Chuai M, Zeng W, Yang X, Boychenko V, Glazier JA, Weijer CJ. Cell movement during chick primitive streak formation. Dev Biol. 2006;296:137–49. doi: 10.1016/j.ydbio.2006.04.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–52. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 40.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 41.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 42.Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 46.Satoh W, Matsuyama M, Takemura H, Aizawa S, Shimono A. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46:92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 48.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 49.Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–76. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 53.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- 54.Witzel S, Zimyanin V, Carreira-Barbosa F, Tada M, Heisenberg CP. Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J Cell Biol. 2006;175:791–802. doi: 10.1083/jcb.200606017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 56.Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–76. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–46. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 58.Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. Embo J. 2002;21:976–85. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–81. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 60.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–5. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 61.Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- 62.Marlow F, Zwartkruis F, Malicki J, Neuhauss SC, Abbas L, Weaver M, et al. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev Biol. 1998;203:382–99. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–9. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 64.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 65.Formstone CJ, Mason I. Combinatorial activity of Flamingo proteins directs convergence and extension within the early zebrafish embryo via the planar cell polarity pathway. Dev Biol. 2005;282:320–35. doi: 10.1016/j.ydbio.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, et al. Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development. 2009;136:383–92. doi: 10.1242/dev.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–88. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 68.Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–68. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- 69.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–96. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 70.Rock R, Schrauth S, Gessler M. Expression of mouse dchs1, fjx1, and fat-j suggests conservation of the planar cell polarity pathway identified in Drosophila. Dev Dyn. 2005;234:747–55. doi: 10.1002/dvdy.20515. [DOI] [PubMed] [Google Scholar]
- 71.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–5. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 72.Down M, Power M, Smith SI, Ralston K, Spanevello M, Burns GF, et al. Cloning and expression of the large zebrafish protocadherin gene, Fat. Gene Expr Patterns. 2005;5:483–90. doi: 10.1016/j.modgep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Ciani L, Patel A, Allen ND, ffrench-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–82. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–38. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 75.De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, et al. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–35. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- 77.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–88. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- 78.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 80.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–5. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 81.Zhu S, Liu L, Korzh V, Gong Z, Low BC. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal. 2006;18:359–72. doi: 10.1016/j.cellsig.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 82.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 83.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–35. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 85.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 86.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–24. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 88.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–84. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 89.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–18. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 90.Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244:342–57. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- 91.Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weber U, Paricio N, Mlodzik M. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 2000;127:3619–29. doi: 10.1242/dev.127.16.3619. [DOI] [PubMed] [Google Scholar]
- 93.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–76. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 94.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–7. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 95.Casal J, Struhl G, Lawrence PA. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–98. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 96.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–68. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heisenberg CP, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr Opin Genet Dev. 2008;18:311–6. doi: 10.1016/j.gde.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamashita S, Miyagi C, Carmany-Rampey A, Shimizu T, Fujii R, Schier AF, et al. Stat3 Controls Cell Movements during Zebrafish Gastrulation. Dev Cell. 2002;2:363–75. doi: 10.1016/s1534-5807(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 103.Miyagi C, Yamashita S, Ohba Y, Yoshizaki H, Matsuda M, Hirano T. STAT3 noncell-autonomously controls planar cell polarity during zebrafish convergence and extension. J Cell Biol. 2004;166:975–81. doi: 10.1083/jcb.200403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 105.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–22. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 106.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–19. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, et al. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–87. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 109.Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, et al. E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev. 2005;122:747–63. doi: 10.1016/j.mod.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 110.Montero JA, Carvalho L, Wilsch-Brauninger M, Kilian B, Mustafa C, Heisenberg CP. Shield formation at the onset of zebrafish gastrulation. Development. 2005;132:1187–98. doi: 10.1242/dev.01667. [DOI] [PubMed] [Google Scholar]
- 111.Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- 112.Harrington MJ, Hong E, Fasanmi O, Brewster R. Cadherin-mediated adhesion regulates posterior body formation. BMC Dev Biol. 2007;7:130. doi: 10.1186/1471-213X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–90. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–97. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–92. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 116.Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–13. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. Embo J. 2004;23:3259–69. doi: 10.1038/sj.emboj.7600332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y, Janicki P, Koster I, Berger CD, Wenzl C, Grosshans J, et al. Xenopus Paraxial Protocadherin regulates morphogenesis by antagonizing Sprouty. Genes Dev. 2008;22:878–83. doi: 10.1101/gad.452908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winklbauer R. Conditions for fibronectin fibril formation in the early Xenopus embryo. Dev Dyn. 1998;212:335–45. doi: 10.1002/(SICI)1097-0177(199807)212:3<335::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 120.Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol. 2003;13:1182–91. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- 121.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–93. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 122.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–32. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coyle RC, Latimer A, Jessen JR. Membrane-type 1 matrix metalloproteinase regulates cell migration during zebrafish gastrulation: evidence for an interaction with non-canonical Wnt signaling. Exp Cell Res. 2008;314:2150–62. doi: 10.1016/j.yexcr.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Bakkers J, Kramer C, Pothof J, Quaedvlieg NE, Spaink HP, Hammerschmidt M. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development. 2004;131:525–37. doi: 10.1242/dev.00954. [DOI] [PubMed] [Google Scholar]
- 125.Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11:9–19. doi: 10.1016/j.devcel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 126.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 127.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–9. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]