Abstract
Previous epidemiologic studies suggest that the major histologic subtypes of epithelial ovarian cancer may have different risk factor profiles; however, no known prospective study has systematically examined differences in risk by subtype. The authors used Cox proportional hazards regression, stratified by histologic subtype and time period, to examine the association between ovarian cancer risk factors and incidence of serous invasive, endometrioid, and mucinous ovarian cancers in the US Nurses’ Health Study (1976–2006) and Nurses’ Health Study II (1989–2005). For each exposure, they calculated P-heterogeneity using a likelihood ratio test comparing models with separate estimates for the 3 subtypes versus a single estimate across subtypes. Analysis included 221,866 women and 721 cases with the histologies of interest (496 serous invasive, 139 endometrioid, 86 mucinous). In analyses of reproductive/hormonal exposures, the associations with age, duration of breastfeeding, age at natural menopause, and duration of estrogen use differed significantly by subtype (all P-heterogeneity ≤0.05). The associations with several nonreproductive exposures also appeared to vary by subtype, but only the association with smoking differed significantly (P-heterogeneity = 0.03). Results suggest that associations with several ovarian cancer risk factors vary by subtype, and these differences are consistent with known similarities between each major histologic subtype and its normal tissue counterpart.
Keywords: adenocarcinoma, mucinous; carcinoma, endometrioid; cystadenocarcinoma, serous; histology; ovarian neoplasms
Epithelial ovarian cancers often are analyzed as a single outcome in epidemiologic studies, despite evidence of differences in their natural history, morphology, and gene/protein expression (1–4). The most common histologic subtypes of epithelial ovarian cancer each resemble a different normal tissue in morphology and gene expression (4, 5), and previous studies suggest their etiology may differ as well. In a pooled analysis of 10 case-control studies, oral contraceptive use and parity were inversely associated with all subtypes, whereas associations with nonreproductive exposures, particularly body mass index and smoking, differed by subtype (6). Other studies have reported differences in associations with both reproductive and nonreproductive exposures for mucinous versus nonmucinous cancers (7–12).
Although these studies suggest that some associations differ by subtype, the data are inconsistent (6–10, 13, 14), and no known comprehensive, prospective analysis of differences in risk factors by histologic subtype has been published. In addition, most prior studies analyzed each subtype separately and did not report a statistical test comparing results across subtypes. We therefore used polytomous regression models to examine the association between known and suspected risk factors for ovarian cancer and incidence of the serous invasive, endometrioid, and mucinous subtypes in the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII).
MATERIALS AND METHODS
Study population
The NHS was established in 1976 and the NHSII in 1989 among 121,700 US female registered nurses aged 30–55 years and 116,430 US female registered nurses aged 25–42 years, respectively. Participants completed an initial questionnaire and biennial follow-up questionnaires, providing information on lifestyle factors and disease diagnoses. Follow-up is high in both cohorts; we obtained 95.2% of the total possible person-years through June 2006 in the NHS and 93.6% through June 2005 in the NHSII. The Committee on the Use of Human Subjects in Research at Brigham and Women's Hospital, Boston, Massachusetts, approved both studies.
Exposure data
We obtained information on exposures of interest from the biennial questionnaires. At baseline, participants reported their birth date, age at menarche, and height. We requested information on parity, oral contraceptive use, tubal ligation, hysterectomy/oophorectomy, menopausal status, age at menopause, postmenopausal hormone use, weight, physical activity, smoking status, and family history of breast/ovarian cancer on multiple questionnaires during follow-up. In our analysis, we updated values for these covariates when new data were available and otherwise carried forward values from the previous cycle. We requested data on total duration of breastfeeding across all pregnancies in 1986 (NHS) and 1993 (NHSII) and on duration of breastfeeding for each child in 1997 (NHSII only). Information on frequency of genital talc use was collected in 1982 (NHS only).
Identification of ovarian cancer cases
We collected information on new ovarian cancer diagnoses on each questionnaire. For all reported cases, as well as deaths due to ovarian cancer identified through family members, the National Death Index (15, 16), or the US Postal Service, we obtained medical records related to the diagnosis. A gynecologic pathologist (J. H.) blinded to exposure status reviewed the medical records to confirm the diagnosis, stage, histologic type/subtype, and invasiveness (17). Our analysis included cases of epithelial ovarian cancer (n = 885) and primary peritoneal cancer (n = 39) confirmed by pathology report review and diagnosed between baseline and June 2006 (NHS) or 2005 (NHSII).
Statistical analysis
Participants accrued person-time from the return date of the baseline questionnaire until the date of ovarian cancer diagnosis, diagnosis of any other cancer (excluding nonmelanoma skin cancer), bilateral oophorectomy, pelvic irradiation, death, or the end of follow-up. At baseline, we excluded women with bilateral oophorectomy (NHS: n = 7,669; NHSII: n = 2,229), menopause due to pelvic irradiation (NHS: n = 99; NHSII: n = 30), or cancer other than nonmelanoma skin cancer (NHS: n = 3,314; NHSII: n = 1,050). In addition, we excluded women with missing data on any exposure of interest except breastfeeding duration, talc use, and family history of ovarian cancer, which were not collected at baseline, and age at natural menopause, which was missing for women with a hysterectomy before menopause. We included missing indicators for these 4 exposures in our models to avoid excluding too many women from the analysis. Participants contributed person-time only for follow-up periods for which data were complete. Furthermore, we excluded person-time (≤0.3% of the total) when any continuous variable had an outlying value, using the generalized extreme studentized deviate many-outlier detection approach (18).
In analyses of reproductive/hormonal exposures, we modeled age, parity among parous women, duration of breastfeeding, duration of oral contraceptive use, age at natural menopause, and duration of postmenopausal use of unopposed estrogens as continuous variables to minimize the number of parameters in the model. We used binary variables to model menopausal status (postmenopause vs. premenopause/perimenopause), cohort (NHS vs. NHSII), and parity, tubal ligation, and hysterectomy without bilateral oophorectomy (yes/no). Because of evidence of a nonlinear association with age, we used a spline with a single knot at age 50 years to estimate linear associations with age separately for women younger than age 50 years versus 50 years of age or older.
In an alternative analysis, we modeled ovulatory years and duration of menopause instead of age, parity, duration of oral contraceptive use, and age at natural menopause. We calculated ovulatory years as current age (if premenopausal) or age at natural menopause minus age at menarche, years of oral contraceptive use, and parity (1 year per pregnancy), and we included a separate variable for total duration of breastfeeding. We calculated duration of menopause as current age minus age at natural menopause for postmenopausal women, and we coded premenopausal/perimenopausal women as 0. For women with an unknown age at natural menopause because of hysterectomy before menopause, we excluded person-time after hysterectomy.
For the nonreproductive exposures, we modeled body mass index (weight (kg)/height (m)2) and physical activity (cumulative average metabolic equivalent task-hours/week) continuously, regular genital talc use (≥once/week vs. <once/week) and family history of breast/ovarian cancer (yes/no) as binary variables, and smoking status as 2 indicator variables for past or current (vs. never) smoking. Metabolic equivalent task-hours captures both duration and intensity of activity (3 metabolic equivalent task-hours is equivalent to walking 2–2.9 mph for 1 hour (1 mile = 1.6 km)), and cumulative average levels better reflect long-term activity and minimize within-person variation. In the NHS, data on metabolic equivalent task-hours were not available until 1986; we therefore assigned all participants 0 activity from 1976 to 1986 and secondarily evaluated the association with physical activity with follow-up beginning in 1986.
We used Cox proportional hazards regression, stratified by time period, to model the incidence rate ratio and 95% confidence interval of epithelial ovarian cancer for each exposure in the NHS and NHSII combined. We then restricted the analysis to cases with serous invasive/poorly differentiated, endometrioid, or mucinous histology and used Cox proportional hazards regression, stratified by type of outcome and time period, to allow for different associations by histologic subtype (19). We used data augmentation, such that each participant had a separate observation for each subtype. We coded the event variable as 1 (failed) if the participant was diagnosed with the histologic subtype corresponding to that data row and as 0 otherwise; cases were censored for other subtypes at the time of diagnosis.
We compared a model that assumed different associations for all exposures by histologic subtype (full model) with a model with a single estimate across subtypes for one exposure at a time (reduced model). We calculated the P-heterogeneity using a likelihood ratio test, with the degrees of freedom equal to the difference between the numbers of parameters in the 2 models. Using a stepwise-down approach, we set exposures with a nonsignificant P-heterogeneity to have a single estimate across subtypes, so that the final model estimated 3 separate associations for exposures that differed significantly by subtype and a single estimate for all other exposures. All P values were 2-sided and were considered statistically significant if ≤0.05.
We evaluated goodness of fit by calculating the area under the receiver operating characteristic curve (AUC) for all cancers and stratified by subtype. For each observation, we determined a risk score using parameter estimates from the model, and we used the risk scores to calculate the Wilcoxon rank sum test statistic W by 5-year age group t. We calculated the Mann-Whitney 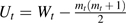 and
and 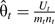 , where
, where 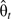 is the probability that a random case has a higher risk score than a random control within age group t. We calculated the variance of
is the probability that a random case has a higher risk score than a random control within age group t. We calculated the variance of 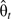 under the alternative hypothesis (20), and we calculated the overall AUC as the weighted average of
under the alternative hypothesis (20), and we calculated the overall AUC as the weighted average of 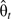 across t with weights = 1/var(
across t with weights = 1/var(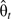 ).
).
We did not have adequate power to examine associations with clear-cell cancers separately because of the small number of cases (n = 48). However, we evaluated differences between serous versus nonserous (endometrioid/mucinous/clear-cell) and mucinous versus nonmucinous (serous/endometrioid/clear-cell) cancers. In secondary analyses, we examined differences between all 4 subtypes for the reproductive exposures only.
RESULTS
Our analysis included 221,866 women with 924 incident cases of confirmed epithelial ovarian cancer (NHS: 108,870 women and 797 cases; NHSII: 112,996 women and 127 cases). Of the cases of cancer, 496 were serous invasive (54%), 139 were endometrioid (15%), and 86 were mucinous (9%). The remaining 203 cases of cancer included 48 clear cell (5% of total), 71 noninvasive serous (8%), 21 carcinosarcoma (2%), 17 mixed (2%), and 46 other/unknown (5%).
In general, baseline characteristics of cases versus noncases were similar to those expected based on previous studies of known risk factors (Table 1). NHSII participants were younger than NHS participants and were more likely to have used oral contraceptives or have had a tubal ligation, were less likely to be parous or to smoke, were more physically active, and had lower mean parity but a longer mean duration of breastfeeding among parous women.
Table 1.
Baseline Characteristics of Epithelial Ovarian Cancer Cases and Noncases Among 108,870 Women in the NHS in 1976 and 112,996 Women in the NHSII in 1989
| NHS |
NHSII |
|||||||||
| Noncases (n = 108,073) | All Epithelial (n = 797) | Serous Invasive (n = 451) | Endometrioid (n = 115) | Mucinousa (n = 69) | Noncases (n = 112,869) | All Epithelial (n = 127) | Serous Invasive (n = 45) | Endometrioid (n = 24) | Mucinousa (n = 17) | |
| Reproductive/hormonal characteristics | ||||||||||
| Mean | ||||||||||
| Age, years | 42 | 45 | 45 | 44 | 44 | 35 | 37 | 38 | 36 | 35 |
| Duration of oral contraceptive use, monthsb | 47 | 44 | 44 | 36 | 38 | 53 | 49 | 39 | 62 | 57 |
| Duration of estrogen use, monthsb | 34 | 44 | 43 | 75 | 20 | 15 | 0 | 0 | 0 | 0 |
| Parity among parous women, no. | 3.1 | 3.0 | 3.2 | 2.9 | 2.9 | 2.1 | 2.0 | 2.2 | 1.8 | 1.8 |
| Duration of breastfeeding, monthsc | 6 | 4 | 4 | 4 | 2 | 13 | 8 | 11 | 10 | 7 |
| Ovulatory years, no.d | 24 | 27 | 28 | 27 | 27 | 17 | 20 | 21 | 18 | 17 |
| Percentage of the population | ||||||||||
| Ever used oral contraceptives | 48 | 38 | 35 | 38 | 43 | 83 | 85 | 87 | 83 | 82 |
| Parous | 94 | 90 | 91 | 82 | 95 | 70 | 63 | 67 | 67 | 53 |
| Tubal ligation | 13 | 8 | 9 | 7 | 10 | 16 | 13 | 18 | 4 | 6 |
| Hysterectomy | 13 | 14 | 18 | 10 | 6 | 4 | 6 | 7 | 8 | 0 |
| Other characteristics | ||||||||||
| Mean | ||||||||||
| Body mass index, kg/m2 | 24 | 24 | 24 | 24 | 23 | 24 | 26 | 24 | 29 | 24 |
| Physical activity, MET-hours/weeke | 13 | 14 | 15 | 13 | 9 | 21 | 22 | 25 | 18 | 17 |
| Percentage of the population | ||||||||||
| Genital talc use >once/weekf | 28 | 29 | 29 | 30 | 40 | |||||
| Past smoker | 23 | 27 | 29 | 17 | 26 | 21 | 22 | 23 | 8 | 20 |
| Current smoker | 33 | 31 | 29 | 33 | 44 | 13 | 12 | 16 | 8 | 13 |
| Family history of breast cancer | 6 | 8 | 7 | 12 | 8 | 6 | 13 | 20 | 21 | 7 |
| Family history of ovarian cancerg | 3 | 5 | 6 | 0 | 19 | 2 | 1 | 4 | 0 | 0 |
Abbreviations: MET, metabolic equivalent task; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Includes borderline and invasive tumors.
Among ever users of oral contraceptives or postmenopausal unopposed estrogens; in the NHSII, only 32 women had used unopposed estrogens at baseline.
Total duration among parous women in 1986 for the NHS and 1993 for the NHSII.
Current age (if premenopausal) or age at natural menopause minus (age at menarche + duration of oral contraceptive use in years + parity).
Physical activity from 1986 for the NHS and 1989 for the NHSII; 3 MET-hours is equivalent to walking at an average pace of 2.0–2.9 miles/hour for 1 hour (1 mile = 1.6 km).
Use among NHS participants only; collected in 1982.
First collected in 1992 in the NHS and 1993 in the NHSII.
When we compared baseline characteristics of women subsequently diagnosed with a serous invasive, endometrioid, or mucinous tumor (Table 1), we found that serous invasive cases were slightly older, had higher parity, and were more physically active than endometrioid/mucinous cases. Endometrioid cases had a longer mean duration of estrogen use (NHS only) and a higher mean body mass index (NHSII only), were less likely to be parous (NHS only) or to have smoked, and were more likely to have a family history of breast cancer. Mucinous cases had a shorter mean duration of estrogen use (NHS only) and breastfeeding and were less physically active, less likely to have had a hysterectomy, and were more likely to have regularly used talc or to currently smoke (NHS only).
The associations with age (P-heterogeneity <0.001), duration of breastfeeding (P-heterogeneity = 0.03), age at natural menopause (P-heterogeneity = 0.05), and duration of estrogen use (P-heterogeneity = 0.009) differed significantly by subtype, whereas other exposures (e.g., oral contraceptive use) exhibited similar associations across the 3 subtypes (Table 2). Age among women less than 50 years was more strongly associated with serous invasive (incidence rate ratio (RR) = 1.15 per year, 95% confidence interval (CI): 1.10, 1.19) and endometrioid (RR = 1.12 per year, 95% CI: 1.06, 1.17) tumors than mucinous tumors. Among women aged 50 years or older, age was associated with a modest increase in risk of serous invasive cancers, was associated with a modest decrease in risk of endometrioid tumors, and was unassociated with mucinous cancers. Duration of breastfeeding was inversely associated with all 3 subtypes, but the association was strongest for mucinous tumors (RR = 0.43 per year). Age at natural menopause was positively associated with the endometrioid subtype only (RR = 1.13 per year, 95% CI: 1.04, 1.22). Duration of estrogen use was associated with a strong increase in risk of endometrioid cancers (RR = 1.87 per 5-year increase, 95% CI: 1.52, 2.31) and a weaker increase in risk of the other subtypes.
Table 2.
Association Between Reproductive/Hormonal Exposures and Risk of Epithelial Ovarian Cancer, by Histologic Subtype, Among 108,870 Women in the NHS From 1976 to 2006 and 112,996 Women in the NHSII From 1989 to 2005a
| All Epithelial (n = 924) |
Serous Invasive (n = 496) |
Endometrioid (n = 139) |
Mucinous (n = 86)b |
P-Heterogeneityc | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Age among women <50 years, (per 1-year increase)d | 1.11 | 1.09, 1.14 | 1.15 | 1.10, 1.19 | 1.12 | 1.06, 1.17 | 1.06 | 1.00, 1.12 | <0.001 |
| Age among women ≥50 years, (per 1-year increase)e | 1.02 | 1.01, 1.04 | 1.04 | 1.02, 1.06 | 0.97 | 0.94, 1.00 | 1.00 | 0.96, 1.04 | |
| Parousf | 0.71 | 0.57, 0.89 | 0.73 | 0.53, 1.02 | 0.61 | 0.37, 1.03 | 1.17 | 0.56, 2.47 | 0.09 |
| Parity among parous womenf | 0.94 | 0.89, 0.99 | 1.00 | 0.94, 1.06 | 0.85 | 0.74, 0.99 | 0.95 | 0.81, 1.13 | |
| Breastfeeding (per 1-year increase)g | 0.82 | 0.74, 0.91 | 0.84 | 0.73, 0.96 | 0.74 | 0.55, 1.00 | 0.43 | 0.25, 0.74 | 0.03 |
| Oral contraceptive use (per 5-year increase) | 0.84 | 0.75, 0.93 | 0.78 | 0.66, 0.91 | 0.77 | 0.58, 1.02 | 0.84 | 0.60, 1.17 | 0.91 |
| Tubal ligation | 0.68 | 0.56, 0.84 | 0.83 | 0.63, 1.09 | 0.59 | 0.34, 1.02 | 0.50 | 0.25, 1.01 | 0.26 |
| Hysterectomy | 0.69 | 0.52, 0.91 | 0.86 | 0.61, 1.20 | 0.68 | 0.39, 1.17 | 0.45 | 0.20, 0.98 | 0.20 |
| Age at natural menopause (per 1-year increase) | 1.03 | 1.00, 1.05 | 1.02 | 0.99, 1.06 | 1.13 | 1.04, 1.22 | 1.01 | 0.93, 1.10 | 0.05 |
| Estrogen use (per 5-year increase)h | 1.37 | 1.25, 1.50 | 1.28 | 1.14, 1.44 | 1.87 | 1.52, 2.31 | 1.31 | 0.89, 1.93 | 0.009 |
Abbreviations: CI, confidence interval; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RR, incidence rate ratio.
Estimates were adjusted for all variables in the table, plus cohort (NHS or NHSII), menopausal status (postmenopause vs. premenopause/perimenopause), missing data on breastfeeding duration (yes/no) because of noncompletion of questionnaire, and missing age at natural menopause (yes/no) because of hysterectomy prior to menopause.
Includes borderline and invasive tumors.
P value from likelihood ratio test comparing, for each covariate, the model with separate estimates for the serous invasive, endometrioid, and mucinous histologic subtypes with the model with a single estimate across the 3 subtypes.
RR for each 1-year increase in age prior to age 50 years.
RR for each 1-year increase in age at age 50 years or older.
Parous: RR for 1 versus 0 children; parity among parous women: RR for each additional child after the first.
Breastfeeding duration first collected in 1986 in the NHS and 1993 in the NHSII.
Duration of postmenopausal use of unopposed estrogens.
Although not statistically significant, there was some evidence of heterogeneity by subtype for parity, tubal ligation, and hysterectomy; the inverse association for oral contraceptive use was similar across subtypes. A first birth was associated with a borderline significant decrease in risk of serous invasive and endometrioid cancers but was unassociated with mucinous tumors. Each additional birth significantly decreased risk of the endometrioid subtype only (RR = 0.85, 95% CI: 0.74, 0.99). In general, tubal ligation and hysterectomy were more strongly inversely associated with endometrioid and mucinous cancers.
In an alternative reproductive model with ovulatory years and duration of menopause, associations with number of ovulatory years (P-heterogeneity = 0.04), duration of menopause (P-heterogeneity <0.001), and duration of breastfeeding (P-heterogeneity = 0.03) differed significantly by subtype (Table 3). Each 1-year increase in the number of ovulatory years was associated with a significant 8% increase in risk of serous invasive and endometrioid tumors but only a 3% increase in risk of mucinous tumors.
Table 3.
Association Between Ovulatory Years and Other Reproductive/Hormonal Exposures and Risk of Epithelial Ovarian Cancer, by Histologic Subtype, Among 107,352 Women in the NHS From 1976 to 2006 and 112,632 Women in the NHSII From 1989 to 2005a,b
| All Epithelial (n = 767) |
Serous Invasive (n = 397) |
Endometrioid (n = 118) |
Mucinousc (n = 80) |
P-Heterogeneityd | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Ovulatory years (per 1-year increase)e | 1.07 | 1.05, 1.08 | 1.08 | 1.06, 1.10 | 1.08 | 1.05, 1.11 | 1.03 | 1.00, 1.07 | 0.04 |
| Duration of menopause (per 1-year increase) | 1.02 | 1.01, 1.04 | 1.04 | 1.02, 1.06 | 0.96 | 0.93, 0.99 | 1.00 | 0.97, 1.04 | <0.001 |
| Breastfeeding (per 1-year increase)f | 0.80 | 0.71, 0.89 | 0.85 | 0.73, 0.98 | 0.68 | 0.49, 0.94 | 0.45 | 0.27, 0.77 | 0.03 |
| Tubal ligation | 0.69 | 0.55, 0.85 | 0.86 | 0.65, 1.16 | 0.57 | 0.32, 1.00 | 0.51 | 0.25, 1.04 | 0.21 |
| Hysterectomy | 0.69 | 0.52, 0.92 | 0.77 | 0.53, 1.13 | 0.78 | 0.42, 1.44 | 0.57 | 0.23, 1.42 | 0.81 |
| Estrogen use (per 5-year increase)g | 1.36 | 1.13, 1.64 | 1.45 | 1.16, 1.81 | 2.33 | 1.53, 3.53 | 0.93 | 0.38, 2.26 | 0.08 |
Abbreviations: CI, confidence interval; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RR, incidence rate ratio.
Estimates were adjusted for all variables in the table, plus cohort (NHS or NHSII), parous (yes/no), menopausal status (postmenopause vs. premenopause/perimenopause), and missing data on breastfeeding duration (yes/no) because of noncompletion of questionnaire.
Model excludes women with missing age at natural menopause because of hysterectomy prior to menopause.
Includes borderline and invasive tumors.
P value from likelihood ratio test comparing, for each covariate, the model with separate estimates for the serous invasive, endometrioid, and mucinous histologic subtypes with the model with a single estimate across the 3 subtypes.
Current age (if premenopausal) or age at natural menopause minus (age at menarche + duration of oral contraceptive use in years + parity).
Breastfeeding duration first collected in 1986 in the NHS and 1993 in the NHSII.
Duration of postmenopausal use of unopposed estrogens.
Building on the final reproductive model, the associations with several nonreproductive exposures appeared to differ by subtype, but only smoking differed significantly (P-heterogeneity = 0.03) (Table 4). Past smoking was associated with decreased risk of endometrioid tumors (RR = 0.59, 95% CI: 0.39, 0.90), whereas past/current smoking was associated with a nonsignificant increased risk of mucinous cancers. Body mass index was positively associated with the endometrioid subtype (RR = 1.18 per 5 kg/m2, 95% CI: 1.02, 1.38) but was unassociated with the other subtypes (P-heterogeneity = 0.06). There also were nonsignificant positive associations between physical activity and serous invasive cancers and between talc use and mucinous tumors. The results for physical activity were unchanged when 1986 was used as the baseline (results not shown).
Table 4.
Association Between Nonreproductive Exposures and Risk of Epithelial Ovarian Cancer, by Histologic Subtype, Among 108,446 Women in the NHS From 1976 to 2006 and 112,054 Women in the NHSII From 1989 to 2005a
| All Epithelial (n = 876) |
Serous Invasive (n = 468) |
Endometrioid (n = 134) |
Mucinousb (n = 84) |
P-Heterogeneityc | |||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Body mass index (per 5-kg/m2 increase) | 1.05 | 0.98, 1.12 | 0.97 | 0.88, 1.07 | 1.18 | 1.02, 1.38 | 0.90 | 0.72, 1.13 | 0.06 |
| Activity (per 15-MET-hour/week increase)d | 1.05 | 0.98, 1.13 | 1.08 | 0.98, 1.19 | 0.94 | 0.76, 1.16 | 0.82 | 0.61, 1.10 | 0.11 |
| Talc use (≥once/week vs. <once/week)e | 1.06 | 0.89, 1.28 | 1.06 | 0.84, 1.35 | 1.06 | 0.66, 1.69 | 1.50 | 0.84, 2.66 | 0.55 |
| Past smoker | 1.05 | 0.91, 1.22 | 1.09 | 0.89, 1.34 | 0.59 | 0.39, 0.90 | 1.54 | 0.94, 2.53 | 0.03 |
| Current smoker | 1.11 | 0.92, 1.35 | 1.14 | 0.88, 1.49 | 0.93 | 0.59, 1.47 | 1.52 | 0.85, 2.74 | |
| Family history of breast cancer | 1.29 | 1.07, 1.56 | 1.34 | 1.04, 1.73 | 1.94 | 1.24, 3.03 | 1.42 | 0.76, 2.63 | 0.38 |
| Family history of ovarian cancerf | 1.75 | 1.19, 2.57 | 1.85 | 1.13, 3.03 | 0.47 | 0.07, 3.39 | 4.50 | 1.76, 11.51 | 0.06 |
Abbreviations: CI, confidence interval; MET, metabolic equivalent task; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RR, incidence rate ratio.
Estimates were adjusted for all variables in the table, plus all covariates in the final reproductive model (Table 2) and variables for missing data on talc use or family history of ovarian cancer (yes/no).
Includes borderline and invasive tumors.
P value from likelihood ratio test comparing, for each covariate, the model with separate estimates for the serous invasive, endometrioid, and mucinous histologic subtypes with the model with a single estimate across the 3 subtypes.
Cumulative average physical activity beginning in 1986 for the NHS and 1989 for the NHSII.
Information on regular genital talc use available for NHS participants only; collected in 1982.
Information on family history of ovarian cancer first collected in 1992 in the NHS and 1993 in the NHSII.
For the association with all epithelial cancers, the AUC for the reproductive model (AUC = 0.624) was slightly higher than that for the ovulatory years model (AUC = 0.617), indicating that these models have similar discriminatory ability (Table 5). The goodness of fit for the reproductive model was highest for the endometrioid subtype (AUC = 0.714), intermediate for the mucinous subtype (AUC = 0.678), and lowest for the serous invasive subtype (AUC = 0.614). Adding the nonreproductive exposures improved the goodness of fit overall and for each subtype. Although the AUC for each model was based on a slightly different study population, the results were similar when we used the same population for all models (results not shown).
Table 5.
AUC for Total Epithelial Ovarian Cancer and Each Histologic Subtype Among Women in the NHS From 1976 to 2006 and the NHSII From 1989 to 2005
| Model | All Epithelial |
Serous Invasive |
Endometrioid |
Mucinousa |
||||
| No. of Cases | AUC | No. of Cases | AUC | No. of Cases | AUC | No. of Cases | AUC | |
| Reproductive (Table 2) | 924 | 0.624 | 496 | 0.614 | 139 | 0.714 | 86 | 0.678 |
| Ovulatory years (Table 3)b | 767 | 0.617 | 397 | 0.616 | 118 | 0.703 | 80 | 0.650 |
| Reproductive + nonreproductive exposures (Table 4) | 876 | 0.645 | 468 | 0.644 | 134 | 0.748 | 84 | 0.744 |
| Ovulatory years + nonreproductive exposuresb,c | 731 | 0.643 | 378 | 0.652 | 114 | 0.746 | 78 | 0.719 |
Abbreviations: AUC, area under the receiver operating characteristic curve; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Includes borderline and invasive tumors.
Excludes women with missing age at natural menopause because of hysterectomy prior to menopause.
Results from this model are not shown.
All results were essentially unchanged when we restricted analyses to the NHS only or excluded primary peritoneal cases (results not shown). In analyses of serous versus nonserous cancers, there were significant differences for the associations with age, parity, tubal ligation, and duration of breastfeeding but no differences for nonreproductive exposures (results not shown). When mucinous cancers were compared with nonmucinous cancers, the associations with only age, duration of breastfeeding, and number of ovulatory years differed significantly (results not shown). When we included clear-cell cancers in the reproductive model, the associations with age, parity, duration of estrogen use, and duration of breastfeeding differed significantly across the 4 subtypes (results not shown).
DISCUSSION
These results suggest that associations with several ovarian cancer risk factors differ by histologic subtype. We observed significant heterogeneity across the serous invasive, endometrioid, and mucinous subtypes for associations with both reproductive and nonreproductive exposures, including age, duration of breastfeeding, duration of estrogen use, and smoking status. There was some evidence of heterogeneity by subtype for several other exposures, including parity and body mass index, but these differences were not statistically significant.
Previous epidemiologic studies have reported differences in the risk factors for each histologic subtype of ovarian cancer, although most studies were retrospective and few reported a statistical test of differences in risk across subtypes. In a pooled analysis, parity and oral contraceptive use were inversely associated with all 4 major subtypes, although parity was most protective for endometrioid and clear-cell tumors, and breastfeeding was inversely associated with the serous, endometrioid, and mucinous subtypes but was most protective for mucinous cancers (6). These results, as well as the pooled associations for family history, body mass index, and smoking, were consistent with our study (6). Tubal ligation was inversely associated with serous and clear-cell cancers in the pooled analysis (6), but other studies have reported inverse associations for tubal ligation or hysterectomy and risk of endometrioid and/or mucinous tumors (8, 13, 14, 21). Age at menopause was associated with an increased risk of endometrioid tumors in a small study (n = 41 endometrioid cases) (22) but not in 2 other studies (7, 23), and estrogen use was more strongly positively associated with endometrioid cancers in some (24–26) but not all (13, 27) previous studies. Three studies of ovulatory years reported a positive association with nonmucinous cancers but no association with the mucinous subtype (9, 10, 14), similar to our study.
Among the nonreproductive exposures, recent physical activity was inversely associated with risk of all 4 histologic subtypes in one study, although the association was statistically significant for serous cancers only (28). Similarly, another study noted inverse associations with risk of serous, endometrioid, and mucinous tumors (29). However, prospective studies, including ours (30), generally have observed null or positive associations (31–33). Several previous studies of genital talc use, including an analysis in the NHS (34), observed a stronger positive association with serous or serous invasive cancers (35–38), although 2 studies reported no difference by subtype (39, 40) and 1 reported a positive association with mucinous tumors (38). Although our results generally are consistent with the existing literature, apparent differences, such as those for talc use, may be due to the limited number of cases of endometrioid or mucinous histology.
At one time, it was believed that the majority of epithelial ovarian cancers, regardless of histology, arose through transformation of the ovarian surface epithelium. However, growing evidence suggests a varied origin of these cancers; for example, high-grade serous carcinomas may arise in the distal fallopian tube (41–43). Morphologically, serous tumors resemble normal fallopian tube epithelium, endometrioid tumors resemble normal endometrium, and mucinous tumors resemble benign intestinal mucosa or cervical epithelium (4). In addition, there are similarities in gene expression between each subtype and its corresponding normal tissue (5).
The risk factor profiles we observed are consistent with evidence that each subtype resembles a different normal tissue. For example, parity, duration of breastfeeding, and smoking were inversely associated with risk of endometrioid tumors, whereas duration of estrogen use and body mass index were positively associated with risk. This pattern of risk factors is similar to that for endometrial cancer, which is influenced by estrogens and is positively associated with hormone-related exposures, most notably obesity and estrogen use (44). For the mucinous subtype, our results suggest that exposure to carcinogens and other chemicals (e.g., tobacco smoke or talc) may increase risk, whereas surgical procedures that decrease ovarian exposure to exogenous agents (e.g., tubal ligation or hysterectomy) may be protective. Although these results generally are not consistent with known risk factors for colon or cervical cancer (45, 46), evidence exists that smoking (47, 48) and exposure to certain chemicals (49–51) may increase risk of these cancers. The serous invasive subtype was associated with reproductive and hormonal exposures, including parity, duration of oral contraceptive use, and duration of estrogen use. Limited data are available on risk factors for fallopian tube carcinoma, although parity and tubal ligation appear to be protective (52). Information on the epidemiology of serous ovarian tumors may be informative for future research of fallopian tube primary carcinomas.
Strengths of our study include the prospective data with repeated measures for most exposures and the large combined study population. In addition, methods used in this analysis allowed for estimation of separate associations with each subtype simultaneously, as well as formal tests for differences across subtypes.
Although our analysis included a large number of epithelial cases, we had a limited number of cases with certain subtypes (e.g., clear-cell and noninvasive serous cancers). Furthermore, we classified histologic subtype based on a review of pathology reports rather than a central pathology review or immunostaining. Although this categorization likely resulted in some misclassification of histologic subtype, a validation study within the NHS found that histologic subtype based on central pathology review corresponded to the pathology report for a high percentage of cases (17). The incomplete data for a few exposures, in particular talc use and family history of ovarian cancer, also are weaknesses because the limited data may have influenced the observed associations for these exposures. The association with talc use in our analysis differed from the association in a previous analysis of the NHS cohort (34), possibly because of a greater degree of exposure misclassification over 24 years of follow-up. However, the suggestive positive association with the mucinous subtype may reflect a longer latency period between talc exposure and development of mucinous tumors. Finally, the use of a single summary measure for certain exposures, such as physical activity, also may have limited our ability to detect an association. Additional analyses of different types/intensities of physical activity and risk of each subtype would help clarify this association.
In summary, our study provides additional evidence that associations with several ovarian cancer risk factors differ by histologic subtype and that these differences are consistent with known similarities between each subtype and a corresponding normal tissue. Differences in risk by subtype may help explain variability in the association with certain exposures across study populations, because the observed associations may differ depending on the distribution of the exposure and histologies. Future epidemiologic studies of ovarian cancer therefore should examine the histologic subtypes separately to determine whether heterogeneity in the association exists across subtypes. Analyses not taking into account differences in ovarian cancer risk by histologic subtype could be misleading.
Acknowledgments
Author affiliations: Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Margaret A. Gates, Bernard A. Rosner, Shelley S. Tworoger); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Margaret A. Gates, Shelley S. Tworoger); Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts (Bernard A. Rosner); and Department of Pathology, Beth Israel Deaconess Medical Center, Boston, Massachusetts (Jonathan L. Hecht).
This work was supported by research grants (P01CA87969, R01CA50385, and P50CA105009) and training grants (R25CA098566 and T32CA009001 to M. G.) from the National Cancer Institute, National Institutes of Health.
The authors thank Dr. Susan Hankinson for her valuable contributions to this study.
Conflict of interest: none declared.
Glossary
Abbreviations
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- NHS
Nurses’ Health Study
- NHSII
Nurses’ Health Study II
- RR
incidence rate ratio
References
- 1.McCluggage WG. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61(2):152–163. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 2.Köbel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies [electronic article] PLoS Med. 2008;12:5. doi: 10.1371/journal.pmed.0050232. e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(suppl 2):S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 4.Crum CP. The female genital tract. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 1059–1118. [Google Scholar]
- 5.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11(17):6116–6126. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 6.Kurian AW, Balise RR, McGuire V, et al. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol. 2005;96(2):520–530. doi: 10.1016/j.ygyno.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Chiaffarino F, Parazzini F, Bosetti C, et al. Risk factors for ovarian cancer histotypes. Eur J Cancer. 2007;43(7):1208–1213. doi: 10.1016/j.ejca.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Risch HA, Marrett LD, Jain M, et al. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144(4):363–372. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 9.Purdie DM, Webb PM, Siskind V, et al. The different etiologies of mucinous and nonmucinous epithelial ovarian cancers. Gynecol Oncol. 2003;88(1 pt 2):S145–S148. doi: 10.1006/gyno.2002.6706. [DOI] [PubMed] [Google Scholar]
- 10.Soegaard M, Jensen A, Høgdall E, et al. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1160–1166. doi: 10.1158/1055-9965.EPI-07-0089. [DOI] [PubMed] [Google Scholar]
- 11.Jordan SJ, Whiteman DC, Purdie DM, et al. Does smoking increase risk of ovarian cancer? A systematic review. Gynecol Oncol. 2006;103(3):1122–1129. doi: 10.1016/j.ygyno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Tworoger SS, Gertig DM, Gates MA, et al. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer. 2008;112(5):1169–1177. doi: 10.1002/cncr.23275. [DOI] [PubMed] [Google Scholar]
- 13.Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Ann Epidemiol. 2001;11(8):568–574. doi: 10.1016/s1047-2797(01)00213-7. [DOI] [PubMed] [Google Scholar]
- 14.Tung KH, Goodman MT, Wu AH, et al. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003;158(7):629–638. doi: 10.1093/aje/kwg177. [DOI] [PubMed] [Google Scholar]
- 15.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 16.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 17.Gates MA, Tworoger SS, Hecht JL, et al. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121(10):2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 19.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 20.Rosner B, Glynn RJ. Power and sample size estimation for the Wilcoxon rank sum test with application to comparisons of C statistics from alternative prediction models. Biometrics. 2009;65(1):188–197. doi: 10.1111/j.1541-0420.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- 21.Wittenberg J, Cook LS, Rossing MA, et al. Reproductive risk factors for mucinous and non-mucinous epithelial ovarian cancer. Epidemiology. 1999;10(6):761–763. [PubMed] [Google Scholar]
- 22.Parazzini F, Chiaffarino F, Negri E, et al. Risk factors for different histological types of ovarian cancer. Int J Gynecol Cancer. 2004;14(3):431–436. doi: 10.1111/j.1048-891x.2004.14302.x. [DOI] [PubMed] [Google Scholar]
- 23.Riman T, Dickman PW, Nilsson S, et al. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case-control study. Am J Epidemiol. 2002;156(4):363–373. doi: 10.1093/aje/kwf048. [DOI] [PubMed] [Google Scholar]
- 24.Weiss NS, Lyon JL, Krishnamurthy S, et al. Noncontraceptive estrogen use and the occurrence of ovarian cancer. J Natl Cancer Inst. 1982;68(1):95–98. [PubMed] [Google Scholar]
- 25.Risch HA. Estrogen replacement therapy and risk of epithelial ovarian cancer. Gynecol Oncol. 1996;63(2):254–257. doi: 10.1006/gyno.1996.0315. [DOI] [PubMed] [Google Scholar]
- 26.Danforth KN, Tworoger SS, Hecht JL, et al. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. 2007;96(1):151–156. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riman T, Dickman PW, Nilsson S, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst. 2002;94(7):497–504. doi: 10.1093/jnci/94.7.497. [DOI] [PubMed] [Google Scholar]
- 28.Riman T, Dickman PW, Nilsson S, et al. Some life-style factors and the risk of invasive epithelial ovarian cancer in Swedish women. Eur J Epidemiol. 2004;19(11):1011–1019. doi: 10.1007/s10654-004-1633-8. [DOI] [PubMed] [Google Scholar]
- 29.Pan SY, Ugnat AM, Mao Y. Physical activity and the risk of ovarian cancer: a case-control study in Canada. Int J Cancer. 2005;117(2):300–307. doi: 10.1002/ijc.21157. [DOI] [PubMed] [Google Scholar]
- 30.Bertone ER, Willett WC, Rosner BA, et al. Prospective study of recreational physical activity and ovarian cancer. J Natl Cancer Inst. 2001;93(12):942–948. doi: 10.1093/jnci/93.12.942. [DOI] [PubMed] [Google Scholar]
- 31.Olsen CM, Bain CJ, Jordan SJ, et al. Recreational physical activity and epithelial ovarian cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2321–2330. doi: 10.1158/1055-9965.EPI-07-0566. [DOI] [PubMed] [Google Scholar]
- 32.Lahmann PH, Friedenreich C, Schulz M, et al. Physical activity and ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(1):351–354. doi: 10.1158/1055-9965.EPI-08-0958. [DOI] [PubMed] [Google Scholar]
- 33.Leitzmann MF, Koebnick C, Moore SC, et al. Prospective study of physical activity and the risk of ovarian cancer. Cancer Causes Control. 2009;20(5):765–773. doi: 10.1007/s10552-008-9291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertig DM, Hunter DJ, Cramer DW, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000;92(3):249–252. doi: 10.1093/jnci/92.3.249. [DOI] [PubMed] [Google Scholar]
- 35.Cook LS, Kamb ML, Weiss NS. Perineal powder exposure and the risk of ovarian cancer. Am J Epidemiol. 1997;145(5):459–465. doi: 10.1093/oxfordjournals.aje.a009128. [DOI] [PubMed] [Google Scholar]
- 36.Cramer DW, Liberman RF, Titus-Ernstoff L, et al. Genital talc exposure and risk of ovarian cancer. Int J Cancer. 1999;81(3):351–356. doi: 10.1002/(sici)1097-0215(19990505)81:3<351::aid-ijc7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Merritt MA, Green AC, Nagle CM, et al. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 38.Mills PK, Riordan DG, Cress RD, et al. Perineal talc exposure and epithelial ovarian cancer risk in the Central Valley of California. Int J Cancer. 2004;112(3):458–464. doi: 10.1002/ijc.20434. [DOI] [PubMed] [Google Scholar]
- 39.Chang S, Risch HA. Perineal talc exposure and risk of ovarian carcinoma. Cancer. 1997;79(12):2396–2401. doi: 10.1002/(sici)1097-0142(19970615)79:12<2396::aid-cncr15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Wong C, Hempling RE, Piver MS, et al. Perineal talc exposure and subsequent epithelial ovarian cancer: a case-control study. Obstet Gynecol. 1999;93(3):372–376. doi: 10.1016/s0029-7844(98)00439-6. [DOI] [PubMed] [Google Scholar]
- 41.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 43.Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100(1):58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 44.Akhmedkhanov A, Zeleniuch-Jacquotte A, Toniolo P. Role of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectives. Ann N Y Acad Sci. 2001;943:296–315. doi: 10.1111/j.1749-6632.2001.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 45.Potter JD, Hunter D. Colorectal cancer. In: Adami HO, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. New York, NY: Oxford University Press; 2002. pp. 188–211. [Google Scholar]
- 46.Stuver S, Adami HO. Cervical cancer. In: Adami HO, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. New York, NY: Oxford University Press; 2002. pp. 340–358. [Google Scholar]
- 47.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124(10):2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 48.Franco EL, Schlecht NF, Saslow D. The epidemiology of cervical cancer. Cancer J. 2003;9(5):348–359. doi: 10.1097/00130404-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Koutros S, Lynch CF, Ma X, et al. Heterocyclic aromatic amine pesticide use and human cancer risk: results from the U.S. Agricultural Health Study. Int J Cancer. 2009;124(5):1206–1212. doi: 10.1002/ijc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44(1):44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 51.Wartenberg D, Reyner D, Scott CS. Trichloroethylene and cancer: epidemiologic evidence. Environ Health Perspect. 2000;108(suppl 2):161–176. doi: 10.1289/ehp.00108s2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riska A, Leminen A. Determinants of incidence of primary fallopian tube carcinoma (PFTC) Methods Mol Biol. 2009;472:387–396. doi: 10.1007/978-1-60327-492-0_18. [DOI] [PubMed] [Google Scholar]


