Abstract
Emerging results suggest that ceramides with different fatty acid chain lengths might play distinct functions in the regulation of tumor growth and therapy. Here we report that de novo-generated C18- and C16-ceramides by ceramide synthases 1 and 6 (CerS1 and CerS6) play opposing proapoptotic and prosurvival roles, respectively, in human head and neck squamous cell carcinomas (HNSCCs). Unexpectedly, knockdown of CerS6/C16-ceramide using small interfering RNA induced endoplasmic reticulum (ER)-stress-mediated apoptosis. Reconstitution of C16-ceramide generation by induced expression of wild-type CerS6, but not its catalytically inactive mutant, protected cells from cell death induced by knockdown of CerS6. Moreover, using molecular tools coupled with analysis of sphingolipid metabolism showed that generation of C16-ceramide, and not dihydro-C16-ceramide, by induced expression of CerS6 rescued cells from ER stress and apoptosis. Mechanistically, regulation of ER-stress-induced apoptosis by CerS6/C16-ceramide was linked to the activation of a specific arm, ATF6/CHOP, of the unfolded protein response pathway. Notably, while expression of CerS1/C18-ceramide inhibited HNSCC xenograft growth, CerS6/C16-ceramide significantly protected ER stress, leading to enhanced tumor development and growth in vivo, consistent with their pro- and antiapoptotic roles, respectively. Thus, these data reveal an unexpected and novel prosurvival role of CerS6/C16-ceramide involved in the protection against ER-stress-induced apoptosis and induction of HNSCC tumor growth.—Senkal, C. E., Ponnusamy, S., Bielawski, J., Hannun, Y. A., Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways.
Keywords: sphingolipid, cell death, head and neck cancer, unfolded protein response
The bioactive sphingolipid ceramide mediates antiproliferative signaling in response to various stress stimuli (1). Many anticancer agents elevate endogenous long-chain ceramide either via the hydrolysis of sphingomyelin (SM; ref. 1) or the de novo pathway, which then leads to growth arrest, and/or apoptosis in various human cancer cells, including human head and neck squamous cell carcinomas (HNSCCs; refs. 2, 3). Alterations of ceramide metabolism and/or transport have also been linked to resistance to anticancer agents (4). Recently, human dihydro(dh)-ceramide synthases 1-6, dh-CerS1-6, identified as yeast homologues of the longevity assurance gene 1-6 (LASS1-6), have been discovered (5, 6). Notably, CerS/LASS proteins (CerS1-6) regulate the de novo generation of ceramides with different fatty acid chain lengths; for example, whereas CerS1 preferentially generates dihydroceramides with intermediate (C18-C20) chain lengths, CerS5 and CerS6 prefer substrates with short chain lengths (C12-C16) to generate C12-C16-dh-ceramides (7,8,9). These dh-ceramides are then desaturated to ceramides (with their distinct fatty acid chain lengths) by dh-ceramide desaturase (Des). CerS proteins are localized mainly to the endoplasmic reticulum (ER) but with some differences in membrane orientation (9). In addition, desaturation of dh-ceramides also occurs in the ER.
In general, the ER is a critical organelle in the induction of apoptosis and is responsible for intracellular Ca2+ storage. More important, the ER provides a topologically distinct membranous network for protein modifications such as glycosylation, disulfide bond formation, and proper protein folding and assembly. Thus, a significant number of ER resident proteins either sequester Ca2+ or function as molecular chaperones to monitor proper protein folding. Therefore, failure of this machinery to fold newly synthesized ER client proteins, or perturbation of the ER-Ca2+ equilibrium, presents unique dangers to the cell, which disrupts normal cellular functions and is termed as “ER stress” (10, 11). A complex homeostatic mechanism, known as the unfolded protein response (UPR), has evolved to minimize ER stress by increasing the protein folding capacity of ER, decreasing the rate of secretory protein synthesis, and/or increasing the chaperone capacity in cells (10, 11). Thus, both transcriptional and translational signals play a role in UPR. This concerted and complex UPR is mediated through three ER transmembrane proteins: inositol-requiring enzyme 1 (IRE1), pancreatic ER kinase (PKR)-like ER kinase (PERK), and activating transcription factor 6 (ATF6; refs. 12,13,14). In resting cells, all three ER stress proteins are maintained in an inactive state by their association with the ER chaperone GRP78 (also known as Bip) (15). On accumulation of unfolded proteins, GRP78 dissociates from these stress receptors, leading to their activation, which triggers the UPR. Therefore, UPR is essentially a prosurvival response to reduce the accumulation of unfolded proteins, or protein aggregates, and to restore normal ER homeostasis.
However, if protein unfolding or aggregation is persistent and cannot be resolved, then UPR signaling switches from prosurvival to proapoptosis mode (16, 17). It is known that signaling through PERK, IRE1, and ATF6 can trigger apoptosis during persistent ER stress, with an activation of their downstream targets, mainly the CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP), JNK, and Bcl-2 family proteins, which are involved in the commitment phase of ER-stress-mediated apoptosis (18,19,20,21). Although CHOP generally induces gene expression, it down-regulates the transcription of Bcl-2 (22,23,24), leading to cell death. Recently, the roles of ceramides in the induction of ER stress and apoptosis have been reported in various human cancer cells (25, 26). However, the mechanisms by which de novo-generated ceramides by CerS1-6 regulate ER stress are still enigmatic.
Interestingly, our recent preclinical and clinical data (3, 27, 28) suggested that de novo-generated C18- and C16-ceramides might play distinct roles in the regulation of apoptosis in HNSCC cells and tumors. The data presented here reveal that CerS1/C18-ceramide induces apoptosis, whereas, unexpectedly, CerS6-generated C16-ceramide plays opposing roles and protects HNSCC cells from ER-stress-induced cell death both in situ and in vivo. Moreover, in this study, the mechanisms by which CerS6/C16-ceramide regulates ER-stress-induced apoptosis and the metabolic requirements for this novel role of ceramide in the protection of HNSCC cells from apoptosis were also determined.
MATERIALS AND METHODS
Cell lines and culture conditions
HNSCC cell lines UM-SCC-1 (retromolar trigone/floor of the mouth), UM-SCC-14A (SCC of anterior floor of the mouth), and UM-SCC-22A (SCC of hypopharynx) were obtained from Dr. Thomas Carey (Department of Otolaryngology/Head and Neck Surgery, University of Michigan, Flint, MI, USA). Cells were cultured as described previously (27). Possible mycoplasma contaminations were monitored regularly by the MycoAlert mycoplasma detection kit (Cambrex, East Rutherford, NJ, USA) and treated with Plasmocin (InvivoGen; San Diego, CA, USA) when necessary. Caspase inhibitors were purchased from R&D Systems (Minneapolis, MN, USA). Tauroursodeoxycholic acid (TUDCA), CA074-ME, pepsitatin A, and MG-132 were purchased from Calbiochem (San Diego, CA, USA). All other reagents used were obtained from Sigma (St. Louis, MO, USA).
Cloning, site-directed mutagenesis, and generation of Tet-inducible cell lines
CerS6 cDNA was amplified with an N-terminal FLAG tag (underscored) containing 5′-XhoI and 3′-EcoRI restriction sites (italicized) using the forward primer 5′-CCCTCGAGGGATGGATTACAAGGATGACGACGATAAGATGGCAGGGATCTTAGCCTGG-3′, and the reverse primer 5′-CGGAATTCCGTTAATCATCCATGGAGCAGGA-3′. The resulting DNA was purified, digested with XhoI and EcoRI, and ligated into pCMVexSVneo mammalian expression plasmid.
Catalytically inactive CerS6 bearing a point mutation, H212A, was generated using the Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) as described by the manufacturer, with forward primer 5′-GACTTTGGCATTATGTTCCTGCACGCCCTTGTATCTATTTTCTTGATTAC-3′ and reverse primer 5′-GTAATCAAGAAAATAGATACAAGGGCGTGCAGGAACATAATGCCAAAGTC-3′.
Stable expression of Tet-inducible CerS1 and CerS6 in UM-SCC-22A cells was performed using the ViraPower T-Rex Lentiviral Expression System (Invitrogen, Carlsbad, CA, USA) as described by the manufacturer.
Western blotting and antibodies
Western blotting was performed as described previously (3). The antibodies used in the study were as follows: anti-CerS1 and anti-CerS6 (mouse polyclonal; Abnova, Taipei City, Taiwan); anti-V5 (Invitrogen); rabbit polyclonal anti-actin (Sigma); anti-caspase 9, anti-Bip, anti-IRE1 (Cell Signaling Technology, Danvers, MA, USA); anti-Bcl-2, anti-calnexin, anti-ATF6, anti-CHOP (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and anti-calreticulin (Upstate Biotechnology, Lake Placid, NY, USA). The blots were visualized using ECL chemiluminescence reagents (GE Healthcare, Piscataway, NJ, USA), and the signals were quantitated using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Detection of caspase activation by fluorometry
The activation of caspase 3 was measured by fluorometry using a caspase 3 activity assay kit (R&D Systems) as described by the manufacturer.
Small interfering (si)RNAs and siRNA transfections
Knockdown of proteins was performed using siRNAs (Qiagen, Valencia, CA, USA) with the following targeting sequences: CerS1, AAGGTCCTGTATGCCACCAGT; CerS2, AAGCCTCAGATCTCTATATCA; CerS6 siRNA-1, AAGGTCTTCACTGCAATTACA; and CerS6 siRNA-2, AACGCTGGTCCTTTGTCTTCA. Nontargeting siRNA and Smart pool siRNAs that target CHOP, ATF4, ATF6, IRE1, GCS, and GM3 synthase were from Dharmacon (Lafayette, CO, USA). siRNA against Des was from Ambion (Austin, TX, USA). Transfections of siRNAs were performed using Oligofectamine (Invitrogen) as described by the manufacturer.
Quantitative real-time PCR (Q-RT-PCR) and conventional RT-PCR
Total RNA was isolated using the RNeasy RNA isolation kit (Qiagen) according to the manufacturer’s instructions with on-column DNase treatment. One microgram of total RNA was used in reverse transcription reactions using a reverse transcription kit (Promega, Madision, WI, USA). The resulting cDNA was then used in the Q-RT-PCR to measure the mRNA levels using a TaqMan gene expression kit (Applied Biosystems, Foster City, CA, USA) with an ABI 7300 Q-PCR system (Applied Biosystems). The mRNA levels of β-actin and rRNA were used as internal controls. Conventional RT-PCR was performed as described previously (3). Primer sequences and PCR conditions are available on request.
Detection of cell survival
Cells were transfected with siRNAs in 6-well plates in triplicates, and after the indicated time points, the inhibition of cellular growth was determined by 3-(4,5-dimethyl-2-thizolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, as described previously (27).
Measurement of endogenous ceramide levels using high-performance liquid chromatography/mass spectroscopy (LC/MS)
Cellular levels of endogenous ceramides were measured by LC/MS, as described previously (30). Ceramide levels were normalized to total inorganic phosphate levels.
Analysis of XBP1 mRNA splicing
Loss of PstI restriction site on splicing of XBP1 mRNA was determined using RT-PCR for the analysis of XBP1 mRNA splicing. After isolation of total RNA and synthesis of cDNA, XBP1 mRNA was amplified using the forward primer 5′-GGAGTTAAGACAGCGCTTGG-3′ and the reverse primer 5′-TGAGAGGTGCTTCCTCGATT-3′. The resulting PCR product was purified, digested with PstI, and separated on 1.5% agarose gel. DNA was then visualized by ethidium bromide staining under ultraviolet light using the Gel-Doc system (Bio-Rad, Hercules, CA, USA).
Detection of mitochondrial membrane potential
Changes in the mitochondrial membrane potential were detected by flow cytometry using the JC-1 mitochondrial membrane potential detection kit (Cell Technology, Mountain View, CA, USA), as described previously (27). JC-1 dye accumulates in the mitochondria as red aggregates in healthy cells; in apoptotic cells, the mitochondrial potential collapses, and JC-1 remains in the cytoplasm as a green fluorescent monomeric form.
Immunoprecipitation
Anti-FLAG immunoprecipitation was carried out using anti-FLAG antibody conjugated agarose beads (Sigma) as described by the manufacturer. Proteins were separated by SDS-PAGE and detected by Western blotting.
Determination of HNSCC tumor growth in vivo
Xenografts were generated by injection of UM-SCC-22A control cells or UM-SCC-22A cells stably expressing CerS1 or CerS6 under the regulation of a Tet-inducible promoter in the flanks of the SCID mice. After tumors were grown to at least 25 mm3 (∼2 wk after implantation), Tet (1 mg/ml) was added to the drinking water of the mice to turn on the gene expression. Tumor size was measured every 3 d, and tumor volumes were calculated as described previously (29).
Statistical analysis
Statistical significance of the data from the xenografts was analyzed using Tukey’s Student range test, as described previously (29). Treatment of animals was performed according to the procedures approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. For rest of the experiments, Student’s t test was used. Values of P < 0.05 were considered significant.
RESULTS
Knockdown of CerS6 using siRNAs induces apoptosis in HNSCC cells
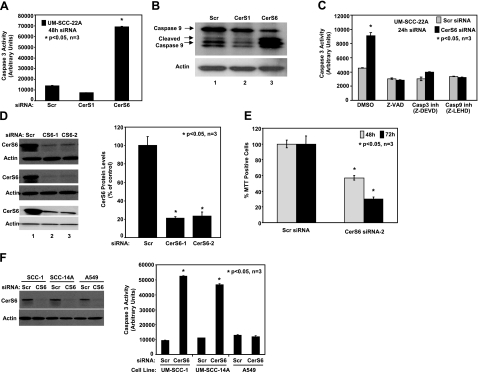
Our previous studies (3, 27, 28) have suggested that de novo-generated C18- and C16-ceramides play distinct roles in the regulation of cell death and/or the pathogenesis of HNSCCs. To determine the roles of dh-C18- and dh-C16-ceramides generated by CerS1 and CerS6, respectively (9, 31), in the regulation of cell death, the expression of CerS1 and CerS6 was down-regulated using siRNAs. Next, the activation of caspases 3 and 9 in response to their knockdown was examined, as compared with controls (treated with nontargeting scrambled siRNA) in UM-SCC-22A cells. To our surprise, inhibition of only CerS6, and not CerS1, resulted in the induction of apoptosis via the activation of caspase 3 and caspase 9 in these cells (Fig. 1A, B). The activation of caspase 3 in response to siRNA-mediated knockdown of CerS6 was completely prevented by a pan-caspase inhibitor z-VAD or inhibitors of caspases 3 and 9 (Fig. 1C). The specific effects of these siRNAs on down-regulation of their target CerS genes, which caused ∼75–80% inhibition of their expression, were confirmed by Q-RT-PCR (Supplemental Fig. S1A, B).
Figure 1.
Knockdown of CerS6 induces caspase activation and cell death. A, B) UM-SCC-22A cells were transfected with either scrambled (Scr), CerS1, or CerS6 siRNAs at 50 nM for 48 h, and their effects on the activation of caspase 3 (A) or caspase 9 (B) were measured. C) Roles of caspase inhibitors Z-VAD, Z-DEVD, and Z-LEHD in the prevention of caspase 3 activation in response to knockdown of CerS6 were examined. D) Left panel: down-regulation of CerS6 using siRNAs was confirmed by Western blotting in 3 independent experiments. Right panel: CerS6 protein levels were quantified from the blots and normalized to the levels of actin. E) Effects of knockdown of CerS6 using CerS6 siRNA-2 on UM-SCC-22A cell growth were measured by MTT after 48 and 72 h of transfection. F) UM-SCC-1, UM-SCC-14A, and A549 cells were transfected with either Scr or CerS6 siRNA (50 nM, 48 h); down-regulation of CerS6 was confirmed by Western blotting (F, left panel); and caspase 3 activity was measured (F, right panel). Data represent 3 independent studies. Bars represent means ± sd from 3 independent experiments. *P < 0.05.
To confirm the specificity of the roles of knockdown of CerS6 using siRNA (CerS6 siRNA-1) in the induction of apoptosis, another CerS6 siRNA, targeting a distinct sequence (CerS6 siRNA-2), was used. Data indicated that both CerS6 siRNA-1 and -2 inhibited CerS6 expression (∼80%), compared with scrambled siRNA, as determined by Western blot analysis using a mouse polyclonal antibody that recognizes CerS6 (Fig. 1D). More important, these data revealed that treatment of cells with CerS6 siRNA-2 inhibited growth by ∼40–60% at 48 and 72 h after transfection, respectively, compared with controls (Fig. 1E). In addition, partial inhibition of CerS6 decreased the generation of C16-ceramide and increased the levels of C22- and C24-ceramides in these cells when compared with controls (Supplemental Fig. S1C, D), while dihydrosphingosine and sphingosine levels did not change significantly by down-regulation of CerS6 (data not shown). Interestingly, while down-regulation of CerS6, as determined by Western blotting (Fig. 1F, left panel), induced apoptosis in two additional HNSCC cell lines, representing different anatomical structures of the oral cavity, UM-SCC-1 and UM-SCC-14A, it did not alter caspase activation in A549 human lung adenocarcinoma cells (Fig. 1F, right panel) or MCF-7 breast cancer cells (data not shown). Thus, these data indicate that siRNA-mediated knockdown of CerS6/C16-ceramide inhibits growth, and induces caspase activation preferentially in HNSCC cells.
Knockdown of CerS6 via siRNA induces ER-stress-mediated apoptosis
We next investigated how knockdown of CerS6 induces apoptosis. We hypothesized that inhibition of CerS6 expression and C16-ceramide generation might mediate ER stress, leading to apoptosis in these cells. The possible involvement of CerS6/C16-ceramide in ER stress was prompted by several lines of evidence: 1) CerS6 is an ER resident protein, and de novo synthesis of ceramide occurs in the ER; therefore, attenuation of the generation of a major ER residing protein and/or lipid molecule (C16-ceramide) might affect ER homeostasis; and 2) HNSCC cells are sensitive to ER stress, which can be activated by conventional chemotherapeutic agents, leading to apoptosis (32, 33).
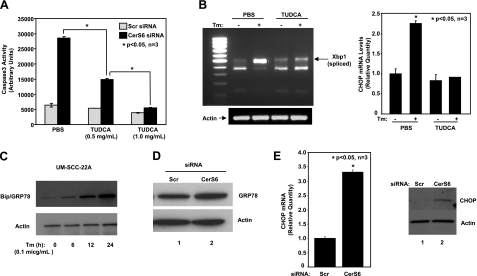
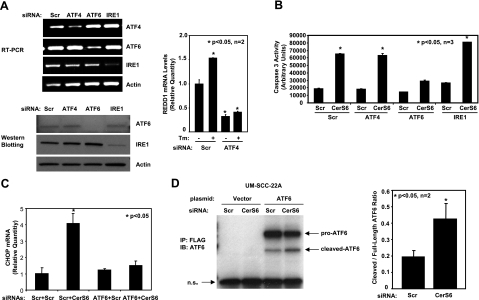
To determine whether down-regulation of CerS6/C16-ceramide induces ER stress, which then results in apoptosis, we treated UM-SCC-22A cells with CerS6 siRNA in the presence or absence of TUDCA, a recently described ER stress blocker (34), and then assessed its roles in the activation of caspase 3. Remarkably, the data showed that pretreatment with TUDCA significantly prevented the activation of caspase 3 (in a dose-dependent manner) in response to CerS6 siRNA-1 (Fig. 2A). The prevention of ER stress by TUDCA at doses used in our experiments was also confirmed by examining its effects on tunicamycin-induced XBP splicing/activation and CHOP mRNA induction, known markers of ER stress. As expected, treatment with TUDCA significantly prevented XBP splicing in response to tunicamycin in these cells, as determined by RT-PCR (Fig. 2B, left panel). In addition, TUDCA pretreatment also prevented tunicamycin-induced elevation of CHOP mRNA levels (Fig. 2B, right panel), demonstrating that TUDCA can effectively prevent ER stress. As a positive control, increased expression levels of Bip/GRP78 in response to ER stress induced by tunicamycin at 6–24 h were examined in these cells by Western blotting (Fig. 2C). The activation of ER stress by CerS6 siRNA was further supported by the increased expression of the Bip/GRP78 protein (Fig. 2D, lanes 2 and 1, respectively) and induced mRNA and protein levels of CHOP compared with scrambled siRNA (Fig. 2E). Thus, these novel data suggest that inhibition of CerS6 expression and down-regulation of C16-ceramide, or dh-C16-ceramide generation, mediate ER-stress-induced apoptosis in these cells.
Figure 2.
Down-regulation of CerS6 induces ER-stress-mediated apoptosis. A) UM-SCC-22A cells were transfected with either Scr or CerS6 siRNA (50 nM, 48 h) in the presence of either vehicle (PBS) or TUDCA (0.5 and 1 mg/ml), and caspase 3 activity was measured. Bars represent means ± sd from 3 independent experiments. *P < 0.05. B) UM-SCC-22A cells were pretreated with TUDCA (1 mg/ml, 2 h) and treated with tunicamycin (Tm; 0.1 μg/ml, 2 h). After treatments, XBP-1 mRNA splicing was analyzed using RT-PCR (left panel); CHOP mRNA levels were measured by Q-RT-PCR (right panel). C) UM-SCC-22A cells were treated with tunicamycin at 0.1 μg/ml for indicated time points, and its effects on Bip/Grp78 and actin protein levels were examined by Western blotting. D, E) UM-SCC-22A cells were transfected with either Scr or CerS6 siRNA (50 nM, 48 h), and protein levels of Bip/GRP78 were detected by Western blotting (D), or mRNA and protein levels of CHOP were measured using Q-RT-PCR (E, left panel) and Western blotting (E, right panel), respectively.
Reconstitution of C16-ceramide generation by induced expression of wild-type (WT) CerS6 protects HNSCC cells from ER-stress-mediated apoptosis
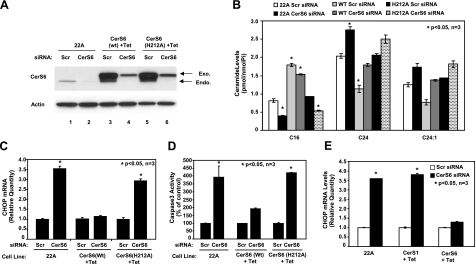
To determine the role of dh-C16-ceramide generation by CerS6 in the regulation of ER stress and apoptosis, we stably expressed a WT and a catalytically inactive mutant of CerS6, containing the H212A mutation in its catalytic domain (35, 36), using the tetracycline (Tet)-inducible lentiviral expression system in UM-SSC-22A cells. First, equal expression of WT and the mutant CerS6 in the presence of Tet was confirmed by Western blotting using a polyclonal antibody that recognizes both endogenous and exogenous (lentiviral) CerS6 proteins (Fig. 3A, lanes 3 and 5, top and bottom bands, respectively).
Figure 3.
Prevention of CHOP mRNA induction by reconstitution of C16-dh-ceramide generation via expression of wt-CerS6. A–D) Control UM-SCC-22A (22A) cells (lanes 1, 2), and Tet-induced (+Tet) UM-SCC-22A cells expressing either WT (lanes 3, 4) or catalytically inactive mutant (H212A) CerS6 (lanes 5, 6) were transfected with either Scr or CerS6 siRNA (50 nM) for 48 h, and expression levels of CerS6 or actin proteins (A) were detected by Western blotting. Then, their effects on ceramide levels were measured by LC/MS (B), CHOP mRNA was measured by Q-PCR (C), and activation of caspase 3 was measured by activity assay (D). E) Tet-induced cells expressing CerS1 or CerS6 were transfected with siRNA as in A, and CHOP mRNA was measured by Q-PCR. Bars represent means ± sd from 3 experiments. *P < 0.05.
Next, the possible roles of inducible expression of WT vs. the mutant CerS6 in the protection of ER stress and apoptosis mediated by siRNA against CerS6 were examined. Treatment with CerS6 siRNA down-regulated the expression of the endogenous protein significantly (∼90%), as compared with scrambled siRNA-treated cells (Fig. 3A, bottom bands in lanes 2 and 1, respectively). However, overexpressed WT and the mutant CerS6 proteins (in the presence of Tet) exhibited some resistance against CerS6 siRNA when compared with controls (Fig. 3A, top bands, lanes 4, 6 and 3, 5, respectively). In addition, ceramide measurement by LC/MS showed that while knockdown of CerS6 decreased C16-ceramide generation by ∼50%, overexpression of WT-CerS6 reconstituted the levels of C16-ceramide, whereas as expected expression of the mutant CerS6 did not affect C16-ceramide levels (Fig. 3B). More importantly, while down-regulation of endogenous CerS6 using siRNA induced ER stress and apoptosis, as determined by increased CHOP mRNA (Fig. 3C) and caspase 3 activation (Fig. 3D), overexpression of WT-CerS6, and not its mutant form, almost completely prevented the induction of CHOP mRNA expression and caspase 3 activation in response to CerS6 siRNA. On the other hand, overexpression of CerS1, which specifically generates C18-ceramide (Supplemental Fig. S1E, F), did not prevent induction of CHOP mRNA on CerS6 knockdown (Fig. 3E). These data demonstrate that increased generation of C16-ceramide by CerS6 plays an important role in the protection against ER stress and apoptosis in HNSCC cells.
Dissection of CerS6-generated C16-ceramide metabolism involved in the protection of ER-stress-mediated apoptosis
Nonetheless, the data presented above also suggest that various sphingolipid molecules, such as generation of dh-C16-ceramide or C16-ceramide in response to alterations of CerS6 expression, might be involved in the regulation of ER-stress-induced apoptosis.
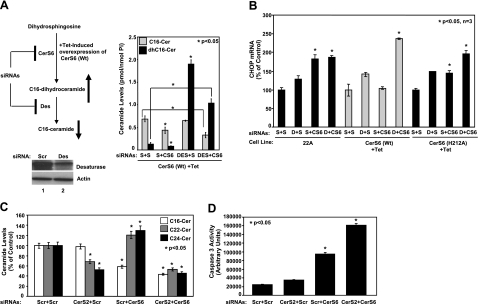
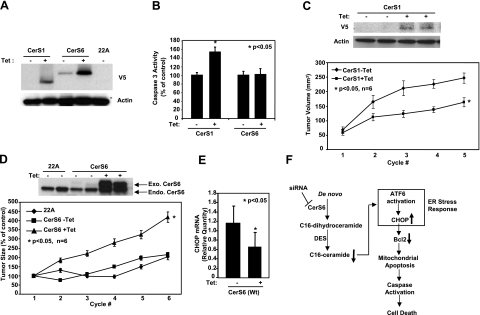
To dissect the roles of CerS6-mediated generation of C16-ceramide as compared with dh-C16-ceramide in the regulation of ER stress, we took advantage of the down-regulation of dh-ceramide Des, which is required for the conversion of dh-ceramide to ceramide, using siRNA. As summarized in Fig. 4A, top left panel, down-regulation of Des when combined with overexpression of WT CerS6 helped selectively accumulate dh-C16-ceramide compared with controls in the absence of CerS6 siRNA. In the presence of CerS6 siRNA, dh-C16-ceramide levels were still elevated, while C16-ceramide levels were decreased (Fig. 4A, right panel). Then, whether dh-C16-ceramide plays a protective role against CerS6 siRNA-induced ER stress was examined. Treatment of UM-SCC-22A cells with CerS6 siRNA-1 significantly induced the mRNA levels of CHOP in the absence or presence of Des siRNA in control UM-SCC-22A cells (Fig. 4B). As expected, induced expression of WT-CerS6 almost completely prevented CHOP activation in response to CerS6 siRNA. Interestingly, when Des expression was down-regulated by Des siRNA, as confirmed by Western blotting using the anti-Des antibody (Fig. 4A, bottom left panel), overexpression of WT-CerS6 was no longer protective against CerS6 siRNA-mediated CHOP activation (Fig. 4B). Thus, these data indicate that while dh-C16-ceramide is dispensable, generation of C16-ceramide by functions of CerS6 and Des is necessary for the protection of ER stress and apoptosis in these cells.
Figure 4.
Dissection of the roles of dihydroceramide or ceramide in the regulation of ER stress. A) Left panel: schematic representation of the experiments carried out to dissect the roles of C16- or dh-C16-ceramides in the prevention of CHOP activation and ER stress. Right panel: C16- and dh-C16-ceramide levels of Tet-induced UM-SCC-22A cells expressing CerS6 (WT) after transfections with Scr, Des, or CerS6 siRNAs were measured by LC/MS. Down-regulation of Des using siRNA in UMSCC-22A cells compared with nontargeting scrambled siRNA-treated cells was confirmed by Western blotting (bottom panel, lanes 2 and 1, respectively). Actin levels were used as controls. B) Control UM-SCC-22A (22A) cells and Tet-induced (+Tet) UM-SCC-22A cells expressing either WT or catalytically inactive mutant (H212A) CerS6 were transfected with Scr (S), Des (D), or CerS6 (CS6) siRNAs (20 nM) alone or in combination (S+S, D+S, S+CS6, and D+CS6) for 48 h, and their roles in the regulation of CHOP mRNA levels were measured by Q-PCR. C, D) Roles of down-regulation of CerS2 and CerS6, alone or in combination, in the regulation of ceramide levels (C) and activation of caspase 3 (D) were detected by LC/MS and caspase activity assay, respectively. Bars represent means ± sd from 2 experiments done in duplicates. *P < 0.05.
In addition, as presented in Supplemental Fig. S1C, D, siRNA-mediated down-regulation of CerS6 not only decreased the generation of C16-ceramide, it also caused an increase in C22- and C24-ceramide levels. To investigate the possibility that activation of caspase 3 in response to knockdown of CerS6 might be due to increased C22- and C24-ceramide levels, down-regulation of CerS6 was carried out in the presence of CerS2 siRNA to down-regulate C22- and C24-ceramides (37). After siRNA transfections, down-regulation of both CerS2 and CerS6 was confirmed by Q-RT-PCR (Supplemental Fig. S2A, B), and increased generation of C22- and C24-ceramides was prevented in response to CerS6 siRNA by down-regulation of CerS2, while C16-ceramide levels were still decreased (Fig. 4C). More importantly, down-regulation of CerS2 did not prevent the activation of caspase 3 on CerS6 siRNA treatment (Fig. 4D), suggesting that induction of apoptosis by knockdown of CerS6 is due to decreased levels of C16-ceramide and not due to increased levels of CerS2-generated C22- and C24-ceramides in these cells. On the contrary, down-regulation of CerS2 further enhanced caspase 3 activation in response to down-regulation of CerS6 (Fig. 4D).
Down-regulation of CerS6 expression results in the activation of ER-stress-mediated apoptosis via selective induction of the ATF6/CHOP arm of UPR
To determine the mechanisms by which down-regulation of C16-ceramide induces ER stress, activation of the 3 arms of the ER-stress-response pathways was examined after knockdown of CerS6. Expression levels of ATF6, IRE1, or ATF4 were down-regulated using siRNAs, compared with controls. Down-regulation of the genes was confirmed by RT-PCR (Fig. 5A, top left panel) and Western blotting (Fig. 5A, bottom left panel). Down-regulation of ATF4 was also confirmed functionally by measuring REDD1, a known ATF4 target (38), mRNA levels in the absence and presence of ER stress induced by tunicamycin (Fig. 5A, right panel). Then, the roles of the 3 arms in the activation of CHOP expression in response to knockdown of CerS6 were determined. The results revealed that down-regulation of ATF6 almost completely prevented activation of caspase 3 and induction of CHOP expression in response to Cer6 siRNA, as compared to controls (Fig. 5B, C, respectively). However, down-regulation of IRE1 or ATF4 did not significantly activate caspase 3 in these cells (Fig. 5B). In addition, cleavage and nuclear localization of ATF6 on knockdown of CerS6 were confirmed by immunoprecipitation (Fig. 5D). Moreover, as expected, down-regulation of CerS6 in A549 lung adenocarcinoma cells, which did not result in apoptosis, did not induce ATF6 cleavage or CHOP mRNA induction (data not shown). Hence, these data suggest a novel mechanism for the regulation of a specific effector arm, ATF6/CHOP, of the ER stress response, leading to apoptosis by down-regulation of CerS6/C16-ceramide in HNSCC but not in A549 cells.
Figure 5.
Down-regulation of CerS6 specifically activates the ATF6/CHOP arm of ER-stress response pathways. A) Left panels: specific knockdown of ATF4, ATF6, and IRE1 using siRNAs was confirmed using RT-PCR (top panel) and by Western blotting (bottom panel). Right panel: functional down-regulation of ATF4 in response to siRNA transfection was confirmed by analysis of REDD1 mRNA levels by Q-RT-PCR in the absence and presence of tunicamycin (Tm). B, C) Roles of ATF4, ATF6, and IRE1 in the regulation of caspase 3 activation (B) or CHOP mRNA (C) in response to down-regulation of CerS6 were examined. D) Roles of down-regulation of CerS6 in the regulation of ATF6 activation in UM-SCC-22A cells were examined, following transfections with N-terminal FLAG-tagged ATF6 and/or CerS6 siRNA. Cleaved and uncleaved ATF6 were detected by anti-FLAG immunoprecipitation followed by immunoblotting (left panel). Data represent 3 independent experiments. Band intensities were quantified and plotted as cleaved to full-length ATF6 ratio (right panel). Bars represent means ± sd from at least 2 experiments. *P < 0.05.
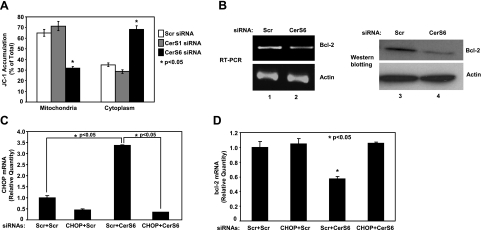
Then, to determine whether activation of ATF6/CHOP in response to down-regulation of CerS6 leads to mitochondrial apoptosis, the roles of knockdown of CerS6 and CerS1 using siRNAs in the alterations of mitochondrial membrane potential were measured using JC-1, a fluorescently labeled compound that accumulates in healthy mitochondria. The data showed that down-regulation of CerS6, and not CerS1, by siRNA resulted in a loss of mitochondrial membrane potential, as detected by decreased levels of mitochondrial JC-1 using flow cytometry in UM-SCC-22A cells (Fig. 6A). Since one of the downstream mechanisms of the ATF6/CHOP arm of ER-stress-mediated mitochondrial apoptosis is the down-regulation of Bcl-2 mRNA (22), we then examined the roles of CerS6 knockdown in the regulation of Bcl-2 expression. The data showed that down-regulation of CerS6/C16-ceramide resulted in a significant decrease in Bcl-2 mRNA and protein, detected by RT-PCR and Western blotting, respectively, when compared with controls (Fig. 6B).
Figure 6.
Knockdown of CerS6 down-regulates Bcl-2 via activation of CHOP and induces loss of mitochondrial membrane potential. A) UM-SCC-22A cells were transfected with Scr, CerS1, or CerS6 siRNA (50 nM, 48 h), and their effects on the loss of mitochondrial membrane potential were detected by JC-1 staining followed by flow cytometry. B) UM-SCC-22A cells were transfected with Scr or CerS6 siRNA (50 nM, 48 h), and their effects on the mRNA and protein levels of Bcl-2 were examined by RT-PCR and Western blotting. C, D) Roles of CerS6 and CHOP in the down-regulation of Bcl-2 mRNA by RT-PCR were examined in UM-SCC-22A cells, after knockdown of CerS6 and/or CHOP, alone or in combination using siRNAs. CHOP (C) and Bcl-2 (D) mRNA levels were measured by Q-RT-PCR. Results represent 2 independent experiments performed in duplicate. *P < 0.05.
Next, we examined whether activation of the ATF6/CHOP arm of the ER stress pathway is mechanistically involved in the down-regulation of Bcl-2 in response to down-regulation of CerS6 in the presence/absence of siRNA against CHOP (Fig. 6C). Notably, these data revealed that down-regulation of CHOP completely prevented the CerS6 siRNA-mediated decrease in Bcl-2 mRNA expression when compared with controls (Fig. 6C), demonstrating the role of CHOP mRNA induction and its activation by knockdown of CerS6 in the repression of Bcl-2 expression as its downstream target. Taken together, these results reveal a novel role for CerS6/C16-ceramide in the regulation of the ATF6/CHOP axis of the ER stress, which then results in the down-regulation of Bcl-2 (Fig. 6D), leading to the activation of caspase 3 in these cells. In summary, these data suggest that down-regulation of CerS6/C16-ceramide induces ER stress via the activation of the ATF6/CHOP axis selectively, which then results in decreased expression of Bcl-2, resulting in the loss of mitochondrial membrane potential, caspase activation, and apoptosis, responses that are prevented by the reconstitution of CerS6 expression and C16-ceramide generation.
Endogenous C18- and C16-ceramides generated by CerS1 and CerS6, respectively, play distinct roles in the regulation of HNSCC tumor growth in vivo
To determine whether de novo-generated C18- and C16-ceramides play distinct roles in the regulation of apoptosis, we first examined the effects of Tet-induced expression of CerS1 and CerS6 (Fig. 7A), which preferentially generate C18- and C16-ceramides (shown in Supplemental Fig. S1E, D), respectively, on caspase activation in UM-SCC-22A cells. As expected, induction of CerS1/C18-ceramide significantly increased caspase 3 activity by ∼60% (P<0.05; n=3) when compared with controls (−Tet; Fig. 7B). Interestingly, induced expression of CerS6 did not increase caspase activity in these cells (Fig. 7B).
Figure 7.
Roles of CerS1 and CerS6 in the regulation of HNSCC tumor growth. A) UM-SCC-22A cells expressing C-terminal V5-tagged CerS1 or CerS6 under a Tet-inducible promoter were either noninduced (−Tet) or induced with Tet (1 μg/ml; +Tet) for 48 h, and CerS1 and CerS6 protein expression was detected by Western blotting using V5 antibody. Untransfected UM-SCC-22A cells (22A) were used as controls. B) Activation of caspase 3 was measured without or with Tet induction (48 h) in cells expressing CerS1 or CerS6. Bars represent means ± sd from 3 experiments. C–E) HNSCC xenografts were generated on the flanks of SCID mice using control UM-SCC-22A (22A) cells and UM-SCC-22A cells expressing CerS1 or CerS6 under a Tet-inducible promoter, as described in Materials and Methods. Sizes of the xenografts were measured every 3 d with calipers. Mice with the described xenografts were sacrificed, and CerS1 and actin protein (C), CerS6 (D), and CHOP (E) levels of the xenografts were detected by Western blotting and by Q-PCR. Bars represent means ± sd. *P < 0.05. F) Summary of data presented in this study. Results revealed that knockdown of CerS6 using siRNA initiates a specific arm of the ER stress response through the ATF6/CHOP pathway, which then leads to induction of caspase-dependent apoptosis.
Next, to test the roles of CerS1 and CerS6 in the regulation of tumor growth in vivo, we implanted UM-SCC-22A cells that stably express C-terminal V5-tagged WT CerS1 or WT CerS6 in response to Tet induction into the flanks of SCID mice (n=6 mice/group). Then, the effects of CerS1 and CerS6 overexpression on HNSCC tumor growth, in the absence or presence of Tet, were determined by measuring tumor volumes every 3 d for 5–7 cycles. Remarkably, the data showed that while expression of CerS1 inhibited tumor growth significantly (P<0.05; n=6), overexpression of WT CerS6 induced HNSCC tumor growth (∼2.5-fold) as compared with controls (P<0.05; n=6), which were xenografts generated using uninduced (−Tet) or vector-only transfected cells (Fig. 7C, D). After tumor size measurements were completed, tumors were surgically removed, and the expression of CerS1 and CerS6 in two of the tumor tissues for each group was examined by Western blotting using anti-V5 and anti-CerS6 antibodies, respectively. The results confirmed that both CerS1 and CerS6 were effectively overexpressed in response to Tet induction (Fig. 7C, D) in vivo, which was provided to the animals in their drinking water, and there was no significant exogenous CerS1 or CerS6 expression in the absence of Tet. In addition, induction of CerS6 expression was concomitant with decreased levels of CHOP mRNA in these xenograft tumors compared with controls (Fig. 7E). Therefore, these results indicate that up-regulation of CerS6/C16-ceramide plays prosurvival and/or protective roles in HNSCC cells and induces the growth of HNSCC xenograft tumors, in stark contrast to the proapoptotic/growth inhibitory role of CerS1/C18-ceramide both in situ and in vivo.
DISCUSSION
The data presented here demonstrate the following: 1) selective functions of individual CerS genes in the regulation of apoptosis and tumor growth in HNSCCs; 2) a prosurvival role for CerS6 in HNSCCs (and notably, these results not only reveal clearly distinct functions for C16-ceramide, as opposed to ceramides of other chain lengths, they also raise the intriguing, but real, possibility that C16-ceramide formed via CerS6 exerts functions distinct from CerS1-generated C18-ceramide); 3) loss of CerS6 initiates a specific arm of the ER stress response through the ATF6/CHOP pathway, which then leads to induction of caspase-dependent apoptosis; and 4) while CerS1/C18-ceramide inhibits HNSCC tumor growth, CerS6/C16-ceramide plays a protective role against ER stress and promotes survival of HNSCC tumors in vivo. These findings are summarized in Fig. 7E.
Recent data revealed that defects in the CerS1-dependent generation of C18-ceramide play important roles in HNSCC pathogenesis (27, 28) and/or response to therapy (3, 38). In addition, a role for CerS1 in the regulation of cisplatin sensitivity has been demonstrated previously (39), confirming the antiproliferative roles of CerS1-generated C18-ceramide in various cancer cells. Interestingly, our recent data (27, 28) suggested that whereas levels of C18-ceramide are low, C16-ceramide is significantly up-regulated in the majority (∼80%) of tumor tissues of HNSCC patients when compared with adjacent normal tissues (P<0.001; n=45 pairs). Increased C16-ceramide in HNSCC tumor tissues was also associated with increased expression of CerS6 (28), one of the two synthases (CerS5 and CerS6) responsible for C16-ceramide synthesis (7,8,9). In contrast, treatment of HNSCC cells or xenografts with the chemotherapeutic agents gemcitabine and doxorubicin in combination, which inhibited growth and caused tumor suppression in situ and in vivo, resulted in the down-regulation of CerS6 expression and inhibition of C16-ceramide generation (3). Collectively, these data are in agreement with data presented herein, supporting the novel view that while CerS1/C18-ceramide exerts proapoptotic functions, CerS6/C16-ceramide plays opposing roles and protects cell from ER stress and apoptosis at least in human head and neck cancer models.
The results presented here are also consistent with a recently published report (40) that demonstrated that while functions of some CerS homologues such as hyl-1 and lagr-1 are necessary for induction of radiation-induced apoptosis in the germ line of Caenorhabditis elegans, another CerS homologue, hyl-2, might not play a role in the induction of apoptosis. Notably, recent data (41), which showed that loss of hyl-2- and C20–22-ceramides results in sensitivity to stress (anoxia and hyperthermia)-induced necrosis/death in C. elegans, are in agreement with the results presented here, suggesting a role for ceramide in the prevention of stress-induced death and/or growth inhibition in various conditions. These studies, collectively, argue against the dogma for antiproliferative and prodeath roles of all endogenous ceramides and support the novel view that ceramides with different fatty acid chain lengths, such as C18- and C16-ceramides generated by CerS1 or CerS6, respectively, play distinct roles in the regulation of cell death and might be conserved, at least from worms to humans.
ER stress conditions have been observed in various diseases, including Alzheimer’s disease, Creutzfeldt-Jakob disease, and Huntington’s disease, as well as in cardiovascular disease and human cancers, indicating that ER-stress-induced cell responses and apoptosis are integral to various pathophysiological conditions (42,43,44). For example, hypoxia, which is important for response to therapy and overall survival of cancers, induced PERK/eIF2/ATF4-mediated UPR, leading to thee promotion of cancer cell survival (45), increased tolerance for hypoxic stress, and increased tumor growth (46). In contrast, recent data demonstrated that the proteasome inhibitor PS-341 (32) and the retinoid N-(4-hydroxyphenyl)retinamide (4-HPR) (33) induce ER stress, leading to apoptosis in HNSCC cells. This suggests that the components of UPR might be attractive targets for antitumor modalities against HNSCCs. Possible roles of ceramide in the activation of ER stress have been indicated recently (25, 26). The link between UPR and ceramide/sphingolipid turnover has been recently implicated in Sec14-Tgl2 mutants in yeast (47). However, the regulation of ER stress and apoptosis by de novo-generated ceramides in human cancer models has not been reported previously. Interestingly, our data are contrary to the conventional view about the proapoptotic role of ceramide (48) and suggest that down-regulation of CerS6 and C16-ceramide induces apoptosis via activation of the ATF6/CHOP arm of UPR in HNSCC cells. To our knowledge, this is the first report to demonstrate the regulation of a specific arm of the UPR, ATF6/CHOP, by ceramide signaling in human cancer cells. Interestingly, ATF6 activation could not be achieved either by down-regulation of CerS6 or treatment with tunicamycin in A549 cells, suggesting that activation of ATF6 might be one of the determinants for ceramide’s distinct actions in different cell types. However, the precise mechanisms by which C16-ceramide, and not C16-dihydro-ceramide, generation by CerS6 regulates ATF6 activation and induction of CHOP expression in various cancer types are still unknown and need to be determined. It should be noted here that further metabolism of CerS6-generated C16-ceramide to complex sphingolipids, such as glucosylceramide (Glc-Cer) and/or SM, might play roles in the regulation of ATF6/CHOP-mediated ER stress and apoptosis. Although knockdown of CerS6 only slightly reduced the total levels of C16-Glc-Cer or SM compared with controls and did not have any detectable effect on S1P levels (data not shown), the roles of these sphingolipids (SM, Glc-Cer, or S1P), which are known to have prosurvival roles in cancer cells, in the regulation of ATF6/CHOP axis of the UPR need to be further evaluated in future studies.
There are also important implications of these data. For example, targeting CerS6 could present a novel strategy for treatment of head and neck cancers, which have been shown to be sensitive to ER-stress-mediated apoptosis in response to various anticancer agents, such as 4-HPR, combination of O6-benzylguanine and cisplatin, or proteasome inhibitor PS341 (Bortezomib; refs. 32, 33, 49). Indeed, it has been reported that treatment of human melanoma cells with PS341 induced the activation of ATF6 and CHOP, with minimal effects on XBP1 (50), which is consistent with data presented in this study. Therefore, identifying cancer cells or types that appear to be “addicted” to CerS6-generated C16-ceramide, such as HNSCCs, and that would be sensitive to CerS6 knockdown for the induction of apoptosis would be valuable. Moreover, determining whether the protective roles of CerS6/C16-ceramide against ER-stress-mediated apoptosis via controlling ATF6 is dependent on the squamous nature of these HNSCC cells would be intriguing. However, the reasons why HNSCC cells are selectively sensitive to CerS6 knockdown for the induction of ER stress and apoptosis are still unknown and need to be determined. Interestingly, recent data (51) suggest that elevated levels of C16-ceramide associate with a positive lymph node status in breast cancer patients, indicating the metastatic potential of C16-ceramide in the clinic. These data are also in agreement with results presented here, suggesting that prosurvival roles of C16-ceramide might not be limited only to HNSCCs but can be relevant in other human cancers also.
CONCLUSIONS
The results presented here suggest for the first time that endogenous ceramides with different chain lengths, such as C18- and C16-ceramides generated by CerS1 and CerS6, respectively, play distinct and opposing roles in the regulation of apoptosis. Specifically, CerS1/C18-ceramide is proapoptotic, and inhibits HNSCC tumor growth. On the other hand, CerS6-generated C16-ceramide is a prosurvival molecule that plays a key role in the protection against ER-stress-induced apoptosis via selective regulation of the ATF6/CHOP axis and induces the growth of HNSCC tumor xenografts in vivo.
Supplementary Material
Acknowledgments
We thank Dr. Jennifer Schnellmann for critically reviewing the manuscript and the members of the B.O. laboratory for helpful discussions. The Flow Cytometry and Cell Sorting, and Lipidomics Core Laboratories are located in a facility constructed with support from the National Institutes of Health (C06 RR-015455). This work was supported by National Institutes of Health grants CA-88932, DE-016572, and CA-97132.
References
- Ogretmen B, Hannun Y A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Mehta S, Blackinton D, Omar I, Kouttab N, Myrick D, Klostergaard J, Wanebo H. Combined cytotoxic action of paclitaxel and ceramide against the human Tu138 head and neck squamous carcinoma cell line. Cancer Chemother Pharmacol. 2000;46:85–92. doi: 10.1007/s002800000140. [DOI] [PubMed] [Google Scholar]
- Senkal C E, Ponnusamy S, Rossi M J, Bialewski J, Sinha D, Jiang J C, Jazwinski S M, Hannun Y A, Ogretmen B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- Swanton C, Marani M, Pardo O, Warne P H, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed A A, Brenton J D, Downward J, Nicke B. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Guillas I, Kirchman P A, Chuard R, Pfefferli M, Jiang J C, Jazwinski S M, Conzelmann A. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 2001;20:2655–2665. doi: 10.1093/emboj/20.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillas I, Jiang J C, Vionnet C, Roubaty C, Uldry D, Chuard R, Wang J, Jazwinski S M, Conzelmann A. Human homologues of LAG1 reconstitute acyl-CoA-dependent ceramide synthesis in yeast. J Biol Chem. 2003;278:37083–370891. doi: 10.1074/jbc.M307554200. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Riebeling C, Bodennec J, Riezman H, Allegood J C, Sullards M C, Merrill A H, Jr, Futerman A H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- Riebeling C, Allegood J C, Wang E, Merrill A H, Jr, Futerman A H. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue S E, Gorman A M, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X Z, Harding H P, Zhang Y, Jolicoeur E M, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H P, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman R J, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 2000;275:27013–27920. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- Dorner A J, Wasley L C, Kaufman R J. Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 1992;11:1563–1571. doi: 10.1002/j.1460-2075.1992.tb05201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge D G, Germain M, Mathai J P, Nguyen M, Shore G C. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Oakes S A, Lin S S, Bassik M C. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr Mol Med. 2006;6:99–109. doi: 10.2174/156652406775574587. [DOI] [PubMed] [Google Scholar]
- Deniaud A, Sharaf El, Dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Liu H, Bowes R C, 3rd, van de Water B, Sillence C, Nagelkerke J F, Stevens J L. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot R T, Remotti H, Stevens J L, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S, Sledge G W, Jr, Nakshatri H. Repression of GADD153/CHOP by NF-kappaB: a possible cellular defense against endoplasmic reticulum stress-induced cell death. Oncogene. 2001;20:2178–2185. doi: 10.1038/sj.onc.1204292. [DOI] [PubMed] [Google Scholar]
- McCullough K D, Martindale J L, Klotz L O, Aw T Y, Holbrook N J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J L, Smyth C A, Bilbao G, Eckstein C, Young C J, Thompson J A, Curiel D T, Eckhoff D E. Coupling endoplasmic reticulum stress to cell death program in isolated human pancreatic islets: effects of gene transfer of Bcl-2. Transpl Int. 2003;16:537–542. doi: 10.1007/s00147-003-0619-x. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Park M A, Zhang G, Martin A P, Hamed H, Mitchell C, Hylemon P B, Graf M, Rahmani M, Ryan K, Liu X, Spiegel S, Norris J, Fisher P B, Grant S, Dent P. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2beta)1-mediated ceramide generation plays a key role in the crosstalk between the ER and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koybasi S, Senkal C E, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day T A, Jiang J C, Jazwinski S M, Hannun Y A, Obeid L M, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- Karahatay S, Thomas K, Koybasi S, Senkal C E, Elojeimy S, Liu X, Bielawski J, Day T A, Gillespie M B, Sinha D, Norris J S, Hannun Y A, Ogretmen B. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): Attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256:101–111. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkal C E, Ponnusamy S, Rossi M J, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day T A, Obeid L M, Hannun Y A, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- Bielawski J, Szulc Z M, Hannun Y A, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Futerman A H. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J Biol Chem. 2005;280:33735–33738. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- Fribley A, Zeng Q, Wang C Y. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadara H, Lacroix L, Lotan D, Lotan R. Induction of endoplasmic reticulum stress by the pro-apoptotic retinoid n-(4-hydroxyphenyl)-retinamide via a reactive oxygen species-dependent mechanism in human head and neck cancer cells. Cancer Biol Ther. 2007;6:705–711. doi: 10.4161/cbt.6.5.3963. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith R O, Gorgun C Z, Hotamisligil G S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama-Yahara N, Riezman H. Transmembrane topology of ceramide synthase in yeast. Biochem J. 2006;398:585–593. doi: 10.1042/BJ20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassieva S, Seo J G, Jiang J C, Bielawski A, Alvarez-Vasquez F, Jazwinski S M, Hannun Y A, Obeid L M. Necessary role of the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem. 2006;281:33931–33938. doi: 10.1074/jbc.M608092200. [DOI] [PubMed] [Google Scholar]
- Laviad E L, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill A H, Jr, Futerman A H. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- Whitney M L, Jefferson L S, Kimball S R. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem Biophys Res Commun. 2009;379:451–455. doi: 10.1016/j.bbrc.2008.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven P P, Alexander H, Futerman A H, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- Deng X, Yin X, Allan R, Lu D D, Maurer C W, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz V, Howell K S, Gentina S, Epstein S, Riezman I, Fornallaz-Mulhauser M, Hengartner M O, Gomez M, Riezman H, Martinou J C. Protection of C. elegans from anoxia by Hyl-2 ceramide synthase. Science. 2009;324:381–384. doi: 10.1126/science.1168532. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner B A, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res. 2006;3:269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- Lee A S, Hendershot L M. ER stress and cancer. Cancer Biol Ther. 2006;5:721–722. doi: 10.4161/cbt.5.7.3120. [DOI] [PubMed] [Google Scholar]
- Hamanaka R B, Bobrovnikova-Marjon E, Ji X, Liebhaber S A, Diehl J A. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28:910–920. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman R J, Bell J, Ron D, Wouters B G, Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousley C J, Tyeryar K, Ile K E, Schaaf G, Brost R L, Boone C, Guan X, Wenk M R, Bankaitis V A. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol Biol Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Gilbertson S, Mullen T, Senkal C, Lu P, Ogretmen B, Obeid L, Voelkel-Johnson C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene. 2009;28:1132–1141. doi: 10.1038/onc.2008.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabik C A, Fishel M L, Holleran J L, Kasza K, Kelley M R, Egorin M J, Dolan M E. Enhancement of cisplatin [cis-diammine dichloroplatinum (II)] cytotoxicity by O6-benzylguanine involves endoplasmic reticulum stress. J Pharmacol Exp Ther. 2008;327:442–452. doi: 10.1124/jpet.108.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport E L, Moore H E, Dunlop A S, Sharp S Y, Workman P, Morgan G J, Davies F E. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- Schiffman S, Sandner J, Birod K, Wobst I, Angioni C, Ruckhaberle E, Kaufmann M, Ackermann H, Lotsch J, Schmidt H, Geisslinger G, Grosch S. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis. 2009;30:745–752. doi: 10.1093/carcin/bgp061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.