Abstract
Cerebral amyloid angiopathy (CAA) is an age-associated condition and a common finding in Alzheimer’s disease in which amyloid-β (Aβ) vascular deposits are featured in >80% of the cases. Familial Aβ variants bearing substitutions at positions 21–23 are primarily associated with CAA, although they manifest with strikingly different clinical phenotypes: cerebral hemorrhage or dementia. The recently reported Piedmont L34V Aβ mutant, located outside the hot spot 21–23, shows a similar hemorrhagic phenotype, albeit less aggressive than the widely studied Dutch E22Q variant. We monitored the apoptotic events occurring after stimulation of human brain microvascular endothelial and smooth muscle cells with nonfibrillar structures of both variants and wild-type Aβ40. Induction of analogous caspase-mediated mitochondrial pathways was elicited by all peptides, although within different time frames and intensity. Activated pathways were susceptible to pharmacological modulation either through direct inhibition of mitochondrial cytochrome c release or by the action of pan- and pathway-specific caspase inhibitors, giving a clear indication of the independent or synergistic engagement of both extrinsic and intrinsic mechanisms. Structural analyses of the Aβ peptides showed that apoptosis preceded fibril formation, correlating with the presence of oligomers and/or protofibrils. The data support the notion that rare genetic mutations constitute unique paradigms to understand the molecular pathogenesis of CAA.—Fossati, S., Cam, J., Meyerson, J., Mezhericher, E., Romero, I. A., Couraud, P. O., Weksler, B. B., Ghiso, J., Rostagno, A. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-β variants in cells composing the cerebral vessel walls.
Keywords: cerebral amyloid angiopathy, Alzheimer’s disease, endothelial cells, smooth muscle cells, oligomerization
Cerebral amyloid angiopathy (CAA), the deposition of amyloid in vessel walls of the central nervous system, is an age-associated condition and a common feature of Alzheimer’s disease (AD), in which it is present in ∼80% of the patients. CAA is the most frequent condition associated with focal ischemia, cerebral hemorrhage, and neurovascular dysfunction. It compromises medium- and small-sized arteries and arterioles as well as capillary endothelium, leading to endothelial degeneration, decreased cerebral blood flow, and ischemic metabolic changes (1, 2). The most frequent form of CAA is related to amyloid-β (Aβ) deposition in sporadic AD. In these cases, the vascular deposits, albeit highly heterogeneous, are largely constituted by the 40-residue peptide Aβ40, in contrast with the parenchymal plaques, which are predominantly associated with deposition of Aβ42 (2). Familial types of CAA, although not common, are typically associated with a diverse group of mutated proteins ranging from genetic variants of Aβ to totally unrelated proteins (1,2,3).
Mutations in the Aβ precursor protein concentrated in the amino acid cluster comprising Aβ residues 21–23 are primarily associated with the development of CAA, although they present with dissimilar clinical manifestations. One of the most aggressive clinical phenotypes described in familial AD is associated with a Glu to Gln substitution at residue 22 (E22Q) in a disorder known as hereditary cerebral hemorrhage with amyloidosis Dutch type (HCHWA-D; ref. 4). The disease is characterized by recurrent strokes and vascular dementia in the absence of neurofibrillar pathology, with fatal cerebral bleeding resulting from the massive amyloid deposition in leptomeningeal and cortical vessels (5). Parenchymal mature plaques characteristic of AD are rare in this kindred, while diffuse preamyloid deposits are relatively frequent, particularly in younger patients (6, 7). Recently, a new intra-Aβ mutation manifesting with comparable, albeit less aggressive, features has been found in Piedmont, Italy, in a family with autosomal dominant, recurrent intracerebral hemorrhages (8). The mutation, a Leu to Val substitution at residue 34 (L34V), is exclusively associated with CAA. The neuropathological features are similar to those in HCHWA-D, but the striking vascular compromise is even more accentuated by the complete absence of parenchymal Aβ deposits of any type, diffuse or mature plaques, as well as dystrophic neurites and neurofibrillary tangles.
In general, Aβ mutants exert stronger toxicity than the wild-type (WT) counterparts in both neuronal (9) and cerebrovascular cells (10, 11), albeit striking differences exist among the variants themselves. Differences in toxicity likely correlate with the distinct structural properties conferred by the specific amino acid substitutions, generally translating into enhanced fibrillogenic properties (12), that, in turn, may well influence the onset and aggressiveness of the clinical phenotypes (2). The data presented herein provide for the first time a structural analysis of the aggregation/fibrillization properties of the new L34V mutant in comparison with E22Q and the WT-Aβ40 and Aβ42 counterparts. The studies monitored the apoptotic events occurring after stimulation of brain microvascular endothelial cells (ECs) and smooth muscle cells (SMCs) with both vasculotropic variants and evaluated the cellular mechanisms involved, highlighting the participation of caspase-mediated mitochondrial pathways. Overall, our results suggest a deep correlation between the aggregation properties of the different amyloid subunits and their proapoptotic effects on vessel wall cells.
MATERIALS AND METHODS
Peptide synthesis
Synthetic homologues of WT Aβ40 and Aβ42 as well as the Aβ40-genetic variants containing the E22Q and L34V substitutions were synthesized using N-tert-butyloxycarbonyl chemistry by James I. Elliot at Yale University (New Haven, CT, USA) and purified by reverse phase-high performance liquid chromatography on a Vydac C4 column (Western Analytical, Murrieta, CA, USA). Molecular masses were corroborated by matrix-assisted laser desorption ionization time-of-flight (MALDI-ToF) mass spectrometry, and concentrations were assessed by amino acid analysis.
Peptide aggregation
Synthetic Aβ homologues were dissolved to 1 mM in hexafluoroisopropanol (HFIP; Sigma Chemical Co., St. Louis, MO, USA), a pretreatment that breaks down β-sheet structures and disrupts hydrophobic forces leading to monodisperse Aβ preparations (13). After 1 h incubation and lyophilization to remove HFIP, peptides were thoroughly dissolved to 5 mM in dimethyl sulfoxide followed by the addition of deionized water and an equal volume of 2×-concentrated PBS, pH 7.4, to a final concentration of 1 mg/ml in 1× PBS. Peptides were either incubated at 37°C for up to 6 d for the aggregation studies or diluted into culture medium at the required concentrations for the cell culture experiments. For the aggregation studies, structural properties of the different peptides at any given time point were assessed by Western and dot-blot analysis, circular dichroism (CD) spectroscopy, and Thioflavin T binding, as described below.
Peptide structural analysis
Native gel electrophoresis and Western blot analysis
Electrophoretic analysis for the assessment of peptide aggregation was performed under native conditions using 10–30% gradient polyacrylamide gels, in the absence of SDS, and 25 mM Tris/glycine buffer, pH 8.8, as running buffer. Markers for molecular mass consisted of proteins with acidic pI: human albumin, ovoalbumin, soybean trypsin inhibitor, lactoglobulin, and insulin. The relative mobility of the standards was analyzed by nonlinear regression and sigmoideal curve fitting, as described previously (14, 15), with the aid of GraphPad Prism software (GraphPad, La Jolla, CA, USA). Protein bands were visualized either by direct gel staining with GelCode Blue (Pierce, Rockford, IL, USA) or by subsequent Western blot analysis. For the latter, after the electrophoretic separation, proteins were electrotransferred to nitrocellulose membranes (0.45 μm pore size; Hybond-ECL, GE Healthcare Life Sciences, Piscataway, NJ, USA) at 400 mA for 2.5 h, using 10 mM 3-cyclohexylamino-1-propanesulfonic acid (Sigma) buffer, pH 11.0, containing 10% (v/v) methanol. After blocking with 5% nonfat milk in PBS containing 0.1% Tween 20, the membranes were immunoreacted with a combination of mouse monoclonal anti-Aβ antibodies 4G8 (epitope: residues Aβ18–22) and 6E10 (epitope: residues Aβ3–8), both from Covance (Princeton, NJ, USA), at a 1:3000 dilution each, followed by incubation with horseradish peroxidase (HRP)-labeled F(ab′)2 anti-mouse IgG (1:5000; GE Healthcare). Fluorograms were developed by ECL with ECL Western blotting detection reagent (GE Healthcare) and exposed to Hyperfilm ECL (GE Healthcare).
Dot-blot analysis
Oligomer formation during the peptide aggregation experiments was assessed by dot-blot analysis using rabbit polyclonal A11 anti-oligomer antibodies (Invitrogen, Carlsbad, CA, USA; ref. 16). Briefly, 800-ng aliquots of each of the aggregation data point samples were loaded on a nitrocellulose membrane assembled into a Bio-Dot Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA) and left to diffuse passively for ∼30 min before vacuum application. The membrane was then blocked in situ for 1 h with 1% nonfat milk in Tris-buffered saline, pH 7.4, containing 0.1% Tween 20 (TBST), followed by vacuum application and 2 subsequent washes with TBST. After removal from the dot-blot apparatus and further blocking with 5% milk in TBST (1 h, room temperature), the membrane was incubated overnight with A11 antibody (1:1000) followed by HRP-conjugated anti-rabbit secondary antibody. Immunoreactivity was assessed by ECL as above.
As a positive control for oligomer formation and immunoreactivity with A11, samples for each of the peptides at 1- and 3-d aggregation time points were subjected to size-exclusion chromatography (SEC) on Sephadex G-75 (10/300 GL, GE Biosciences) under isocratic conditions (PBS, pH 7.4; flow rate 0.5 ml/min), as described previously (17). Equal protein load (500 ng) of each of the SEC oligomer peaks (retention time 20 min) was subjected to dot-blot analysis and probed with A11 antibody as above.
CD spectroscopy
Changes in the secondary structure of the different Aβ peptides were estimated by CD spectroscopy, as described previously (10). Spectra in the far-ultraviolet light (190–260 nm; bandwidth 1 nm; intervals 1 nm; scan rate 60 nm/min) yielded by the different peptides at each time point of aggregation were recorded at 24°C with a Jasco J-720 spectropolarimeter (Jasco Inc., Easton, MD, USA), using a 0.2-mm-path quartz cell and a peptide concentration of 1 mg/ml. For each sample, 15 consecutive spectra were obtained and averaged, and baseline was subtracted. Results are expressed in terms of mean residue ellipticity (deg·cm2·dmol−1; ref. 18).
Thioflavin T binding assay
Thioflavin T binding was monitored as described previously (19). Briefly, 6-μl aliquots of each of the peptide aggregation time-point samples were added to 10 μl of 0.1 mM Thioflavin T (Sigma) and 50 mM Tris-HCl buffer, pH 8.5, to a final volume of 200 μl. Fluorescence was recorded after 300 s in a LS-50B luminescence spectrometer (Perkin Elmer, Waltham, MA, USA) with excitation and emission wavelengths of 435 and 490 nm (slit width 10 nm), respectively, as described previously (18, 20). Each sample was analyzed in duplicate.
Electron microscopy
Aliquots (3 μl)of each of the peptide aggregation time-point samples were placed onto carbon-coated 400-mesh Cu/Rh grids (Ted Pella, Inc., Redding, CA, USA) and stained with 1% uranyl acetate in distilled water (Polysciences, Inc., Warrington, PA, USA). Stained grids were examined in a Philips CM-12 transmission electron microscope and photographed with a Gatan (4k×4k) digital camera at the Image Core Facility of the Skirball Institute of Biomedical Medicine (New York University School of Medicine, New York, NY, USA), as described previously (18).
Cell cultures
Immortalized human brain microvascular ECs hCMEC/D3 (D3; ref. 21) were cultured in complete EBM-2 medium (Lonza, Allendale, NJ, USA) containing growth supplements and 2.5% FBS. This cell line retains the morphological characteristics of primary brain ECs and expresses specific brain endothelial markers and cell surface adhesion molecules (21).
Human brain vascular SMCs were purchased from ScienCell (San Diego, CA, USA) and grown in SMC medium with 10% FBS in accordance with the manufacturer’s specifications.
Evaluation of apoptosis induction by Aβ-variant peptides
Cell-death ELISA
The extent of apoptosis caused by the different Aβ peptides was assessed by quantitation of nucleosome formation with the Cell Death ELISAplus kit (Roche Applied Science, Indianapolis, IN, USA). Briefly, 2 × 104cells/well were seeded on 24-well plates and allowed to rest for 1 d before treatment with the different Aβ peptides. WT, E22Q, and L34V peptides, pretreated in HFIP and solubilized as above, were diluted to a 50 μM final concentration in EBM-2/1% FBS medium for EC challenge and in SMC medium without FBS for SMC treatment. This peptide concentration had proved to induce maximum nucleosome formation in primary EC cultures (Cell Systems, Kirkland, WA, USA) subjected to 24-h treatment with 5–50 μM E22Q (17). After amyloid incubation for 1–3 d for ECs, and up to 6 d for SMCs, plates were centrifuged in a Beckman J-6B centrifuge (10 min, 1000 rpm; Beckman Instruments, Fullerton, CA, USA) to collect floaters, cells were lysed, and DNA-histone complexes (nucleosomes) were quantitated by cell-death ELISA (Roche Applied Science), as described by the manufacturer. For >3-d peptide treatments, with high apoptotic levels, a double volume of lysis buffer was used to avoid subsequent saturation of the colorimetric system.
Phase-contrast microscopy
Morphological changes induced by incubation with the different amyloid subunits were evaluated by phase-contrast microscopy. ECs and SMCs, grown on 24-well plates and subjected to amyloid challenge as above, were visualized in a Nikon Eclipse TE300 inverted epifluorescence microscope (Nikon, Tokyo, Japan) using Spot software (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) for image acquisition.
Annexin V staining
Detection of apoptotic cells exhibiting phosphatidylserine membrane translocation was performed in adherent cells using the Annexin V-FLUOS kit (Roche Applied Science). Cells were plated on glass coverslips precoated with type I collagen (BD Biosciences, Bedford, MA, USA) or poly-d-lysine (Sigma) for ECs or SMCs, respectively, and cultured for 1 d in the pertinent complete medium. Challenge with the different amyloid peptides, pretreated and dissolved as above, was carried out for the same time lengths as for the ELISA experiments. After treatment, cells were washed with warm PBS and incubated with annexin V reagent as suggested by the manufacturer. After 2 washes with PBS and a brief rinse with distilled water, coverslips were mounted onto microscope slides with aqueous-based mounting medium containing DAPI (Vectashield, Burlingame, CA, USA). Fluorescence signals were visualized with a Nikon Eclipse E 800 deconvolution microscope using Image-Pro Plus software (Media Cybernetics Inc., Bethesda, MD, USA) for image acquisition and processing and Autodeblur (Media Cybernetics) for 3-D deconvolution.
Immunocytochemical evaluation of mitochondrial cytochrome c release
ECs and SMCs were plated on glass chamber slides (Thermo Fisher Scientific, Rochester, NY, USA), precoated with collagen or with poly-d-lysine, respectively. After seeding, cells were allowed to rest for 1 d, then were treated with the different peptides, as above, for 1–4 d. After being washed with cold PBS, cells were fixed with 4% paraformaldehyde (10 min, room temperature) and preincubated for 1 h with PBS containing 0.3% Triton X-100 (PBST) and 20 mg/ml BSA. Slides were further incubated with monoclonal anti-cytochrome c (CytC) antibody (BD Biosciences; 1:200 in PBST containing 5 mg/ml BSA; 2 h, room temperature) followed by Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen; 1:200 in PBST with 5 mg/ml BSA; 1 h, room temperature). Slides were mounted and images were acquired and deconvolved as above. Specificity of immune detection was corroborated by omission of the primary antibody in the immunostaining procedure.
Pharmacologic inhibition of Aβ-mediated apoptosis
CytC release inhibition
Confirmation of the involvement of CytC in the downstream activation of cell-death pathways was achieved through the use of methazolamide, an inhibitor of the mitochondrial protein release and therefore a protective agent against apoptosis. Aβ peptides were incubated with the EC and SMC cultures under the same conditions listed above, in the presence and absence of methazolamide (Sigma; 100 and 300 μM), as described previously (22). The length of incubation with the different peptides was selected to yield maximum apoptosis in the absence of inhibitor and therefore varied depending on the peptides’ inherent proapoptotic capabilities for the respective cell types, as described above. The most aggressive peptide, E22Q, required shorter challenge (1 d for both ECs and SMCs), while for the less aggressive ones, longer incubation times were needed (L34V and WT-Aβ40: 3 d for ECs, 5 d for SMCs). In all cases, induction of apoptosis was evaluated by cell-death ELISA. Results are expressed as percentage of apoptosis, with 100% the increase in nucleosome formation yielded by Aβ peptides in the absence of methazolamide compared with no-peptide controls.
Caspase inhibition
Determination of caspase participation in amyloid-induced apoptosis was assessed through the use of both specific and pan-caspase inhibitors. Briefly, the respective Aβ peptides were incubated with ECs and SMCs in the presence and absence of the pan-caspase inhibitor Z-VAD-FMK (Promega Corp., Madison, WI), and the specific caspase-8 (Z-IETD-FMK) or caspase-9 (Z-LEHD-FMK) inhibitors (R&D Systems, Minneapolis, MN, USA), all at 100 μM concentration. The length of incubation with the different peptides, as in the case of the inhibition of CytC release, was selected for maximum apoptosis in the absence of the inhibitors (E22Q: 1 d for ECs, 3 d for SMCs; L34V and WT-Aβ40: 3 d for ECs, 4 d for SMCs). In all cases, apoptosis was evaluated by cell-death ELISA as above.
Statistical analysis
ANOVA for comparison of multiple groups with Bonferroni or Tukey post hoc tests was performed using GraphPad InStat (GraphPad). Values of P < 0.05 were considered significant.
RESULTS
Structural analysis of Aβ-genetic variants
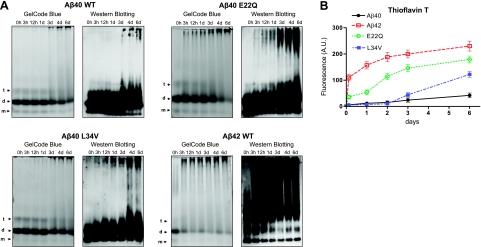
The aggregation pattern of the different Aβ peptides, pretreated, solubilized, and aggregated as described in Materials and Methods, was analyzed via nondenaturing electrophoresis and Western blot. GelCode Blue staining (Fig. 1A, left panels) revealed that immediately after solubilization, all peptides were predominantly dimeric, as previously confirmed for E22Q and WT peptides by SEC in a Superdex 75 column (PBS, pH 7.4; flow rate 0.5 ml/min; ref. 17). In all cases, very faint monomeric and tetrameric components were also noticeable, while only the highly aggregation-prone Aβ42 exhibited a faint smear of larger-molecular-mass components at time 0. Western blot probed with anti-Aβ antibodies, due to its higher sensitivity, highlighted in all cases the presence of additional species (Fig. 1A, right panels). Aβ42, as expected due to its high tendency to aggregate, started to form high-order molecular-mass assemblies by 3 h. On the contrary, the less aggregation-prone WT-Aβ40 began exhibiting high-molecular-mass species only after 3 d, and under our experimental conditions, it never reached the highly aggregated stage of Aβ42. The aggressive E22Q showed faster polymerization than its WT-Aβ40 counterpart. High-molecular-mass species became noticeable at 3 h and steadily increased with continued incubations but without reaching the high aggregation levels of Aβ42. L34V exhibited a lag phase comparable with that of WT-Aβ40 presenting an increase in complex structures only after 3 d incubation at 37°C under physiological salt concentrations. However, the formation of high-mass-order oligomers appears to occur slightly faster than for the WT peptide, achieving intermediate levels between Aβ40 and E22Q.
Figure 1.
Comparative study of structural properties of Aβ genetic variants. HFIP-treated peptides at 1 mg/ml in PBS, prepared as described in Materials and Methods, were incubated at 37°C for up to 6 d, and their aggregation/fibrillization propensity was analyzed by native gel electrophoresis (A) and Thioflavin T binding (B). A) Left panels: GelCode Blue protein staining after separation on nondenaturing 10–30% gradient polyacrylamide gels. Arrows indicate the positions of monomers (m), dimers (d), and tetramers (t). Right panels: Western blot analysis subsequent to nondenaturing electrophoresis probed with a mixture of 4G8 and 6E10 antibodies. B) Thioflavin T binding assay. Fluorescence evaluation (excitation and emission wavelengths 435 and 490 nm, respectively) of Thioflavin T binding assay of the samples collected at the different time points during the 6-d duration of the experiments was performed as described in Materials and Methods. Data are means ± sd of duplicate independent experiments. AU, arbitrary units.
Secondary structure analysis by CD spectroscopy (Supplemental Fig. 1) confirmed the different structural properties of the peptides.
Thioflavin T binding, a property associated with the presence of β-sheet structures and typically correlating with the existence of fibrillar and/or protofibrillar components (19), also varied among the different peptides. In accordance with its known fibrillogenic propensity, large content of β-structure, and elevated concentration of high-order aggregates, Aβ42 exhibited the highest fluorescence values (Fig. 1B). WT-Aβ40 thioflavin levels, also consistent with the CD and Western blot data, remained almost negligible even at 6 d of incubation. E22Q, although showing a longer lag phase than Aβ42, showed thioflavin-fluorescence values significantly elevated with respect to WT-Aβ40 even at 3 h. These values continued to steadily increase throughout the aggregation time but never reached the Aβ42 levels. L34V exhibited fluorescence values that practically did not differ from the WT peptide until d 2. However, fluorescence started to increase by d 3 and reached high values, albeit of lower intensity than those of Aβ42 and E22Q, by d 6, correlating with the magnitude of the high-order molecular-mass aggregates visualized by native Western blot.
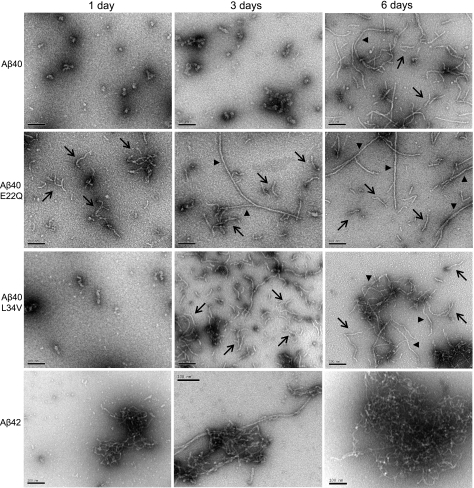
The formation of oligomeric assemblies during the aggregation experiments was monitored by dot-blot analysis using A11 anti-oligomer antibody (Supplemental Fig. 2), and final assessment of the conformational structures was evaluated by transmission electron microscopy (18). After 24-h incubation at physiological salt concentration, WT-Aβ40 and L34V mainly exhibited small globular structures, which typically precede the formation of protofibrils (23), whereas E22Q showed abundant protofibrillar components (Fig. 2). The short string-like structures exhibited by WT-Aβ40 and L34V elongated with time, and, in the latter, protofibrillar and fibrillar structures became abundant by d 6. E22Q protofibrils also elongated with time but at a faster pace, with long fibrillar components detectable at 3 d and more abundant at 6 d, albeit still coexisting with protofibrillar structures. Aβ42, our positive control for aggregation/fibrillization, showed some fibrillar components populating the sample already at d 1. These elements increased with time in length and abundance, and by d 6, clusters of mature fibrils were the only components.
Figure 2.
Electron microscopy study of oligomerization/fibrillization properties of Aβ variants. Transmission electron microscope analysis at each of the aggregation time points was performed, after negative staining with 1% uranyl acetate, using a Philips CM-12 microscope equipped with a Gatan (4k×4k) digital camera. Figure depicts the conformational assemblies of WT-Aβ40, E22Q, L34V, and Aβ42 after incubation under physiological salt concentration for 1, 3, and 6 d. Scale bars = 100 nm. Arrows indicate protofibrillar structures; arrowheads indicate fibrillar elements.
Differential induction of apoptosis in brain microvascular cells by Aβ40 variants
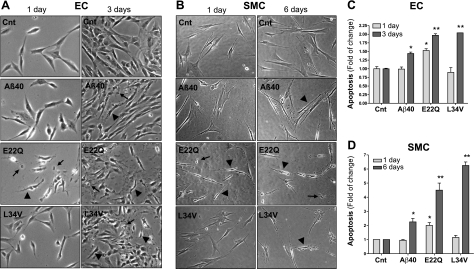
Our studies evaluated the apoptotic effect of L34V, in comparison with E22Q and WT-Aβ40, on brain ECs and SMCs. After 1 d, E22Q induced apoptosis in both cells, as indicated by abnormal cell morphology, evidence of cell shrinking, and appearance of apoptotic bodies in phase-contrast microscopy (Fig. 3A, B). On the contrary, cells treated with Aβ40 and L34V appeared normal. After more prolonged treatment (3 d for ECs, 6 d for SMCs), the detrimental effect of L34V and, to a lower extent Aβ40, also became evident (Fig. 3A, B). To confirm and quantitate the apoptotic response induced by the respective amyloid peptides, we evaluated nucleosome generation by ELISA. After 1 d of stimulation of ECs and SMCs with E22Q, nucleosome formation increased 1.5- and 2-fold, respectively, compared with the levels of no-peptide controls, while the other peptides had no effect (Fig. 3C, D). The predominant apoptotic effect of E22Q on ECs at this time point was corroborated in primary human brain microvascular ECs (Cell Systems; not shown), further confirming that the immortalized EC line retains the functional properties of the primary cerebral cell counterpart. At 3 d, apoptosis induced by L34V in D3 cells was comparable with that of E22Q, reaching ∼2-fold the nucleosome levels of the no-peptide controls, while WT-Aβ40, albeit also proapoptotic, exhibited a milder effect (Fig. 3C). Similarly, L34V toxicity for SMCs increased with more prolonged incubation, reaching values even higher than those of E22Q peptide after 6 d (>6-fold increase). Aβ40 showed also a modest proapoptotic capability in SMCs compared with the other peptides (Fig. 3D), as seen in ECs.
Figure 3.
Apoptosis induction by Aβ peptides in cerebral ECs and SMCs. ECs and SMCs were challenged with WT-Aβ40, E22Q, and L34V (50 μM) as described in Materials and Methods, and apoptosis was evaluated by phase-contrast microscopy and cell-death ELISA. A) Phase-contrast images of ECs both under control conditions and stimulated with Aβ peptides for 1 and 3 d. B) Phase-contrast images of SMCs both under control (Cnt) conditions and after challenge with Aβ peptides for 1 and 6 d. Arrowheads indicate cell shrinking; arrows indicate apoptotic bodies. C) Nucleosome formation by ECs challenged for 1 and 3 d with the respective amyloid molecules at 50 μM concentration, as evaluated by cell-death ELISA. D) Cell-death ELISA of SMCs treated for 1 and 6 d with 50 μM Aβ peptides. Apoptosis is expressed as fold of change compared with no-peptide controls. Data represent means ± sd of 3 independent experiments performed in triplicate. *P < 0.05, **P < 0.001 vs. respective no-peptide controls.
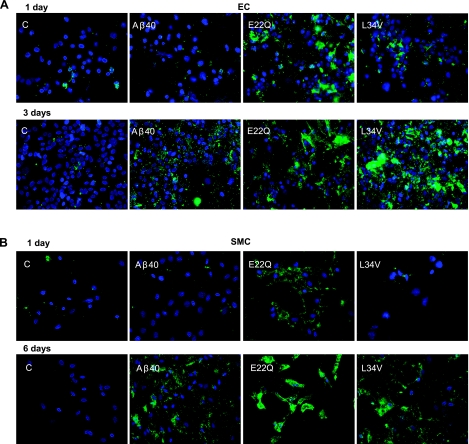
Results obtained by ELISA were confirmed by annexin V staining, which determines the extent of phosphatidylserine exposure on the external side of the cell membranes occurring during apoptosis. The fluorescent signal was clearly evident in ECs treated 24 h with E22Q (Fig. 4A), correlating with the ELISA data and the phase-contrast images. L34V-challenged ECs started showing few annexin-V-positive cells at 24 h and were highly positive at 3 d (Fig. 4A), reaching levels comparable with those of E22Q within the same time frame. In contrast, WT-Aβ40-treated cells only showed annexin-V-positive signals at 3 d. Similar results were obtained for SMCs treated for 1 and 6 d with the respective peptides (Fig. 4B).
Figure 4.
Annexin V immunofluorescence in Aβ-challenged cerebral EC and SMC cultures. EC and SMC cultures were treated with WT-Aβ40, E22Q, and L34V (50 μM) as indicated in Materials and Methods and phosphatidylserine exposure on the outer plasma membrane, a marker of apoptosis, evaluated via annexin V binding. Cells maintained under identical culture conditions but not treated with amyloid peptides were used as negative controls. Green, annexin-V-positive staining; blue, nuclei counterstained with DAPI. A) ECs challenged with various Aβ peptides at 50 μM concentration for 1 and 3 d. B) SMCs treated with different Aβ homologues at 50 μM concentration for 1 and 6 d. Scoring the percentage of annexin-V-positive cells (Supplemental Fig. 3) indicated that both the time frame and the relative capability of the different Aβ peptide to induce phosphatidylserine exposure in ECs and SMCs was comparable with the evaluation of apoptosis by cell-death ELISA illustrated in Fig. 3.
Aβ40 variants induce CytC release and caspase activation in cerebral ECs and SMCs
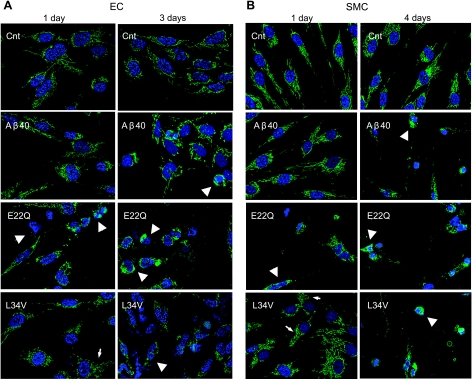
To explore the cellular mechanisms involved in the induction of apoptosis by L34V, in comparison with E22Q and WT-Aβ40, CytC release from mitochondria was analyzed by immunocytochemistry. Figure 5 illustrates the release of CytC in both cell types after 24-h treatment with E22Q, while Aβ40-treated cells showed a preserved CytC mitochondrial localization, comparable with no-peptide controls (Fig. 5A, B, left panels). Interestingly, although CytC remained associated with the mitochondria in both ECs and SMCs stimulated 24 h with L34V, there were, in contrast to the no-peptide controls, subtle changes in the intracellular organelle pattern, with perinuclear localization and fragmented mitochondrial chains, indicating that the peptide had already started to affect the mitochondrial structure, although to a lower degree than E22Q. Notably, alterations in the cable-like morphology exhibited by functionally normal mitochondria, similar to the ones observed in our data and typically reflecting impaired fission/fusion mechanisms, have emerged over the past few years as early markers of neurodegenerative disorders (24). The release of CytC increased with time, being highest for ECs at 3-d challenge with E22Q (strong cytosolic staining), followed by L34V (loss of fluorescent signal associated with mitochondrial chains); only a partial release could be observed in cells triggered with WT-Aβ40 (Fig. 5A, right panel). Similarly, mitochondrial CytC release in SMCs increased with the time of peptide incubation and, although only positive at 24 h with the aggressive E22Q, became highly evident after 4 d with all 3 peptides (Fig. 5B, right panel).
Figure 5.
Induction of CytC release by Aβ peptides. Immunocytochemical evaluation of CytC in ECs (A) and SMCs (B) treated with WT-Aβ40, E22Q, and L34V (50 μM) as described in Materials and Methods. Green fluorescence indicates CytC localization after immunostaining with the pertinent primary antibody followed by anti-mouse IgG conjugated with Alexa Fluor-488; blue fluorescence indicates nuclear DNA counterstained with DAPI. Arrows indicate CytC signal colocalizing with mitochondria showing early changes in organelle pattern, perinuclear distribution, and fragmented mitochondrial chains, typically preceding alterations in membrane permeability and subsequent release of CycC to the cytosol. Arrowheads indicate cells showing CytC cytosolic localization after release from mitochondria.
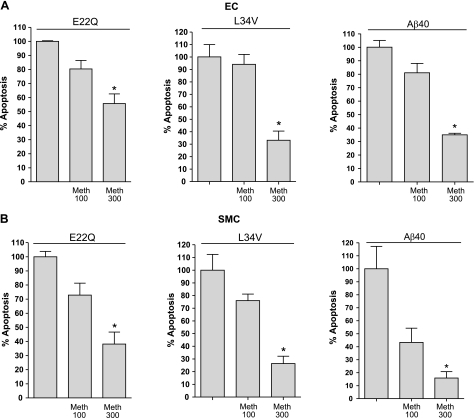
Confirming the mitochondrial involvement, pharmacological inhibition of CytC release resulted in a significant decrease in nucleosome formation. As illustrated in Fig. 6, coincubation of amyloid peptides with ECs and SMCs in the presence of methazolamide resulted in a dose-response inhibition of apoptosis in all cases. For ECs (Fig. 6A), the inhibition of nucleosome formation with 300 μM methazolamide ranged from ∼40% for E22Q to 65% for WT-Aβ40. In the case of SMCs (Fig. 6B), reduction of apoptosis was even higher than in ECs, with levels ranging from 60% inhibition for E22Q to 83% for WT-Aβ40. The differential levels of CytC-susceptible apoptosis achieved in ECs and SMCs suggests a likely dissimilar contribution of the cellular apoptotic pathways, intrinsic vs. extrinsic, to the toxic responses elicited by the peptides in each cell type. Alternatively, the different inhibitory values could relate to the specific stage of the apoptotic cascade at the time of cell-death evaluation. Notably, although the reduction achieved statistical significance for all the peptides, total inhibition of apoptosis was not achieved in any case, suggesting the existence of additional nonmitochondrial paths.
Figure 6.
Effect of pharmacological inhibition of CytC release on Aβ-induced apoptosis. ECs (A) and SMCs (B) were treated with the different Aβ peptides at 50 μM concentration in the presence and absence of methazolamide, an inhibitor of mitochondrial CytC release, at 100 and 300 μM concentrations. Length of incubation with the different peptides was selected to yield maximum apoptosis in the absence of inhibitor and therefore varied depending on the peptides’ inherent proapoptotic capabilities for the respective cell types, as described in Materials and Methods. Apoptosis induction in all cases was evaluated by cell-death ELISA. Results are expressed as percentage of apoptosis, with 100% the increase in nucleosome formation yielded by Aβ peptides in the absence of methazolamide compared with no-peptide controls. Data represent means ± sd of 3 independent experiments performed in duplicate. *P < 0.05.
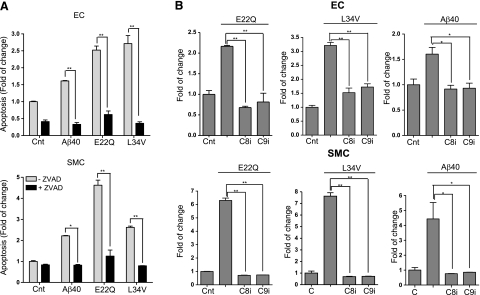
Further biochemical characterization of the apoptotic paths elicited by the respective amyloid peptides was assessed by evaluation of apoptosis protection by the pan-caspase inhibitor Z-VAD. As indicated in Fig. 7A, nucleosome formation elicited by all Aβ peptides was completely abolished in both ECs and SMCs in the presence of Z-VAD, a clear indication of the involvement of caspase-mediated mechanisms. Of note, the low level of spontaneous apoptosis observed in ECs during the experimental time frame under no-peptide control conditions was also sensitive to the presence of Z-VAD. Incubation of ECs and SMCs (Fig. 7B) with E22Q, L34V, and WT-Aβ40, in the presence of either Z-IETD-FMK (caspase-8 inhibitor) or Z-LEHD-FMK (caspase-9 inhibitor), resulted in complete blocking of the proapoptotic effect of the peptides. This action was not a consequence of a direct effect of the inhibitors on the polymerization of the Aβ peptides, since their aggregation profiles in the presence of the various caspase inhibitors remained unchanged (Supplemental Figs. 4 and 5), thereby confirming the participation of both intrinsic and extrinsic apoptotic pathways, either independently or synergistically.
Figure 7.
Induction of caspase mediated apoptotic pathways by Aβ peptides. ECs and SMCs were treated with the different Aβ peptides at 50 μM concentration, in the presence and absence of either pan-caspase or specific caspase-8 or caspase-9 inhibitors, as indicated in Materials and Methods. Apoptosis induction in the presence and absence of the different pharmacological inhibitors was evaluated by cell-death ELISA, and results are expressed as fold of change compared with the no-peptide controls in absence of the inhibitors. A) Nucleosome formation in the presence or absence of the pan-caspase inhibitor Z-VAD (100 μM). Data represent 3 independent experiments. B) Nucleosome formation in the presence or absence of the specific caspase-8 (Z-IETD-FMK) or caspase-9 (Z-LEHD-FMK) inhibitors (both at 100 μM). Top panel: ECs; bottom panel: SMCs. Results represent means ± sd of 3 independent experiments performed in duplicate.*P < 0.05; **P < 0.001.
DISCUSSION
Cerebral amyloid diseases are considered to be part of the emerging group of chronic and progressive entities collectively known as “disorders of protein folding” (25, 26). It is now considered that in all of these diseases, the transition from soluble monomeric species normally present in body fluids to the oligomeric, protofibrillar, and endpoint fibrillar assemblies contributes significantly to disease pathogenesis. In AD, intermediate oligomeric and protofibrillar forms seem to display the most potent effects in neuronal cells, inducing synaptic disruption and neurotoxicity (27, 28). A similar dependence on the aggregation state of the amyloidogenic peptides appears to exist also for toxicity in cerebral ECs, as reported in our recent studies (18, 20). Detailed characterization of the peptide assemblies triggering EC apoptosis indicated a dependence on the formation of intermediate-sized oligomeric assemblies, an event exacerbated in E22Q in comparison with WT-Aβ40 (18, 20). Based on the clinical and neuropathological similarities between the familial AD forms associated with vascular deposition of E22Q and the more recently discovered L34V, our studies compare the in vitro aggregation/fibrillization kinetics of both variants, providing a relative assessment of their proapoptotic properties for cerebral microvascular ECs and SMCs in comparison with the WT-Aβ40 counterpart.
The limited degree of oligomerization/fibrillization observed in L34V, which exhibited a behavior mostly comparable with WT-Aβ40, contrasted with the aggressive E22Q. Our data on the latter aggregation-prone peptide correlate well with the findings from nuclear magnetic resonance spectroscopy and molecular dynamics simulations, which highlight the effect of mutations at position 22 in fibril stabilization (29). These studies indicate that WT-Aβ, although mostly unstructured in solution, bears some regions of structural order. The protease-resistant segment comprising residues 21–30 adopts a bend structure in solution that serves as the folding nucleation site (30, 31). This turn is stabilized by hydrophobic interactions between Val24 and Lys28, as well as, by long-range electrostatic interactions between Lys28 and either Glu22 or Asp23. Computational studies predict that the E22Q mutation would translate into destabilization of the turn and enhanced oligomerization propensity, consistent with our data. Unlike E22Q, L34V has an unchanged Glu at position 22, which would allow the formation of the salt bridge and result in additional stabilization to a structure more resistant to aggregation, in agreement with our experimental findings. It has also been shown that the WT-Aβ region comprising residues 30–40 adopts β-strand conformations and forms parallel β sheets through intermolecular hydrogen bonding (32), similarly to the region composed of aa 12–24. Side chains of L34, as well as those of Ala30, Ile32, Val36, and Val40, form a hydrophobic face, while other charged and polar side chains are distributed on the opposite face of the molecule (32). The similar behavior of L34V to WT-Aβ40, at least on the first days of aggregation, is consistent with the conservative nature of the amino acid substitution. Whether the replacement of Leu for Val, despite the similarities between both residues, introduces modifications in the stability of the final structures that could account for the different behavior of WT and L34V at more prolonged incubations remains to be determined, since no structural studies currently exist on this variant peptide.
Analysis of the conformational state of the amyloid peptides triggering cell-death mechanisms indicated that in all cases, the early stages of apoptosis preceded fibril formation, correlating with the presence of intermediate-sized oligomeric assemblies. The apoptotic effect was fastest and most prominent with E22Q, which showed accelerated formation of oligomeric/protofibrillar assemblies. L34V exhibited a delay in apoptosis induction but achieved similar levels to E22Q with more prolonged incubation and after the peptide had formed comparable structural assemblies. WT-Aβ40 had a lower and even more delayed effect but was capable, nonetheless, of exerting deleterious effects on vessel wall cells after prolonged treatment, suggesting a potential contribution of apoptotic mechanisms to sporadic AD pathogenesis.
Increasing evidence suggests that apoptotic biochemical cascades play pivotal roles in the neuronal dysfunction and death observed in AD (33). Two main pathways, extrinsic and intrinsic, lead to apoptosis in mammalian cells. The latter, modulated by the Bcl-2 family of proteins and typically initiated by oxidative stress and calcium dysregulation, involves mitochondrial outer membrane permeabilization allowing the release of proteins, including CytC, to the cytoplasm. These events, in turn, facilitate downstream cell-death cascades, leading to the sequential activation of caspase-9, followed by the effector caspase-3, DNA fragmentation, and formation of apoptotic bodies. The extrinsic path, normally activated through specific cell receptors, involves multiple partners and complex mechanisms and is typically centered in the initiator caspase-8 before the downstream activation of effector caspases common to both intrinsic and extrinsic pathways (33). Both mechanisms are not completely independent, and, once activated, caspase-8 can also result in the involvement of the mitochondrial path through its proteolytic effect on Bid, leading to Bax translocation, oligomerization, and insertion onto the mitochondria, with subsequent leakage of CytC. Our data clearly demonstrate that the vasculotropic Aβ variants studied herein, albeit requiring different time frames for maximal effect, are strong inducers of the apoptotic mitochondrial pathway and highlight their role in various downstream cascades. In fact, E22Q and L34V, and to a lesser extent WT-Aβ40, all induce CytC release to the cytosol, triggering caspase-9 activation and downstream DNA fragmentation. The additional participation of caspase-8, suggested by the protective effect achieved by its specific inhibitor, indicates an additional participation of receptor-mediated pathways, as also suggested for nonhuman ECs challenged with a nonphysiologic Aβ-truncated fragment (34). The present data indicate the Aβ-mediated induction of analogous cell-death mechanisms in vascular cells as those described in neurons in which, in addition to an active mitochondrial participation (33, 35), apoptotic mechanisms engaging cell-death receptors have also been postulated (36). Whether in our experimental design both intrinsic and extrinsic pathways are simultaneously activated or the mitochondrial dysfunction is a mere result of a primary involvement of cell-death receptors is currently being elucidated.
Markers of apoptosis have not been investigated in familial AD cases related to E22Q and L34V deposition; however, it is noteworthy that studies in sporadic AD have demonstrated alterations in the expression of apoptosis-related genes (33), suggesting that this process takes place in vivo. Active forms of effector caspases were found colocalizing with neurofibrillary tangles, senile plaques, and dystrophic neurites (33, 37), while mRNA evaluation corroborated the up-regulation in the AD temporal cortex of caspase-3 and caspase-7, as well as of the death receptor-related caspase-8 (38), in agreement with our own findings in cerebrovascular cells. Whether independently initiated or triggered through caspase-8 activation, the mitochondrial apoptosis pathway seems to have active participation in AD and other neurodegenerative disorders (33, 35, 36). Many of the apoptosis-associated genes transcriptionally up-regulated in AD are active participants in the mitochondrial cascade, e.g., elevated levels of Bax and Bak coexist with down-regulated expression of the antiapoptotic Bcl-2 family members in the brains of affected individuals (33, 39).
Irrespective of its initiation by either the mitochondrial or receptor-mediated pathways, CytC leakage to the cytoplasm with its downstream effects is a critical step within the cell-death program. A number of drugs are currently available with the capacity to inhibit this process, among them methazolamide, a currently FDA-approved compound for the treatment of glaucoma, which is well tolerated by patients and able to cross the blood-brain barrier (22). Confirming the crucial role of CytC release in amyloid-induced apoptosis, our results clearly demonstrated that specific inhibition of the process by methazolamide exerts a dose-dependent protective effect ameliorating EC and SMC toxicity. Whether the agent will also display beneficial effects in animal models exhibiting Aβ vascular deposition is currently under investigation. It should be noted that methazolamide has been successfully employed in a transgenic mouse model of Huntington disease widely used for studying novel drug therapies for the human disease (40). Intraperitoneal inoculation of methazolamide resulted in a significant dose-dependent delay of disease onset and mortality (22), highlighting the importance of this therapeutic strategy in neurodegenerative disorders.
CONCLUSIONS
Our data link the induction of Aβ-mediated vessel wall cell apoptosis to the peptides’ structural stability and propensity for oligomerization and protofibril formation, paralleling the in vivo clinical phenotypes that exhibit an earlier onset of the disease in the Dutch than in the Piedmont kindred. The activation of analogous cell-death pathways elicited by the E22Q and L34V variants as well as the WT-Aβ40 peptide, albeit at different time frames and with different intensity, supports the notion that genetic mutations, although rare, through their accelerated effect and enhanced response constitute interesting and unique paradigms to help understand how amyloid affects vascular cell functionality.
Supplementary Material
Acknowledgments
This work was partially funded by U.S. National Institutes of Health grants NS-051715 and AG-30539, the Alzheimer’s Association, and the American Heart Association.
References
- Revesz T, Holton J, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B, Revesz T, Vidal R, Holton J, Lashley T, Houlden H, Wood N, Rostagno A, Plant G, Ghiso J. Familial cerebral amyloid angiopathy related to stroke and dementia. Amyloid. 2001;8:36–42. [PubMed] [Google Scholar]
- Rostagno A, Tomidokoro Y, Lashley T, Ng D, Plant G, Holton J, Frangione B, Revesz T, Ghiso J. Chromosome 13 dementias. Cell Mol Life Sci. 2005;62:1814–1825. doi: 10.1007/s00018-005-5092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman M D, Fernandez Madrid I J, Power M D, Lieberburg I, van Duinen S G, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Zhang-Nunes S X, Maat-Schieman M, van Duinen S, Roos R, Frosch M P, Greenberg S M. The cerebral β-amyloid angiopathies: Hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat-Schieman M, Yamaguchi H, van Duinen S, Natte R, Roos R A. Age-related plaque morphology and C-terminal heterogeneity of amyloid β in Dutch-type hereditary cerebral hemorrhage with amyloidosis. Acta Neuropathol. 2000;99:409–419. doi: 10.1007/s004010051143. [DOI] [PubMed] [Google Scholar]
- Maat-Schieman M, Roos R, van Duinen S. Hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neuropathology. 2005;25:288–297. doi: 10.1111/j.1440-1789.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, Ferrari G, Coverlizza S, Merlini G. A novel AbetaPP mutation exclusively associated with cerebral amyloid angiopathy. Ann Neurol. 2005;58:639–644. doi: 10.1002/ana.20571. [DOI] [PubMed] [Google Scholar]
- Murakami K, Irie K, Morimoto A, Ohigashi H, Shindo M, Nagao M, Shimizu T, Shirasawa T. Neurotoxicity and physicochemical properties of Abeta mutant peptides from cerebral amyloid angiopathy: implication for the pathogenesis of cerebral amyloid angiopathy and Alzheimer’s disease. J Biol Chem. 2003;278:46179–46187. doi: 10.1074/jbc.M301874200. [DOI] [PubMed] [Google Scholar]
- Miravalle L, Tokuda T, Chiarle R, Giaccone G, Bugiani O, Tagliavini F, Frangione B, Ghiso J. Substitution at codon 22 of Alzheimer’s Aβ peptide induce diverse conformational changes and apoptotic effects in human cerebral endothelial cells. J Biol Chem. 2000;275:27110–27116. doi: 10.1074/jbc.M003154200. [DOI] [PubMed] [Google Scholar]
- Davis J B, van Nostrand W E. Enhanced pathologic properties of Dutch-type mutant amyloid beta-protein. Proc Natl Acad Sci U S A. 1996;93:2996–3000. doi: 10.1073/pnas.93.7.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Ghiso J, Frangione B. Peptides homologous to the amyloid protein of Alzheimer’s disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun. 1991;179:1247–1254. doi: 10.1016/0006-291x(91)91706-i. [DOI] [PubMed] [Google Scholar]
- Stine W B J, Dahlgren K N, Krafft G A, LaDu M J. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Hames D. One-dimensional polyacrylamide gel electrophoresis. Hames B D, Rickwood D, editors. New York: Oxford University Press; Gel Electrophoresis of ProteinsA Practical Approach. 1990:1–147. [Google Scholar]
- Gallagher S. Native discontinuous electrophoresis. Coligan J E, editor. New York: John Wiley & Sons; Current Protocols in Protien Science. 1995:10.13.11–10.13.11. [Google Scholar]
- Kayed R, Head E, Thompson J L, McIntire T M, Milton S C, Cotman C W, Glabe C G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Cam J, Meyerson J L, Frangione B, Ghiso J, Rostagno A. Oligomeric assemblies of the Aβ Dutch mutant induce the formation of nucleosomes in primary cerebral endothelial cells. Iqbal K, Winblad B, Avila J, editors. Bologna, Italy: Medimond International Proceedings; Alzheimer’s DiseaseNew Advances. 2006:397–402. [Google Scholar]
- Solito R, Corti F, Fossati S, Mezhericher E, Donnini S, Ghiso J, Giachetti A, Rostagno A, Ziche M. Dutch and Arctic mutant peptides of beta amyloid(1–40) differentially affect the FGF-2 pathway in brain endothelium. Exp Cell Res. 2009;315:385–395. doi: 10.1016/j.yexcr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D M, Hartley D M, Kusumoto Y, Fezoui Y, Condron M M, Lomakin A, Benedek G B, Selkoe D, Teplow D. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofribrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- Viana R J, Nunes A F, Castro R E, Ramalho R M, Meyerson J, Fossati S, Ghiso J, Rostagno A, Rodrigues C M. Tauroursodeoxycholic acid prevents E22Q Alzheimer’s Abeta toxicity in human cerebral endothelial cells. Cell Mol Life Sci. 2009;66:1094–1104. doi: 10.1007/s00018-009-8746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B B, Subileau E A, Perrière N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male D K, Roux F, Greenwood J, Romero I A, Couraud P O. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Pei Z, Drozda M, Stavrovskaya I G, Del Signore S J, Cormier K, Shimony E M, Wang H, Ferrante R J, Kristal B S, Friedlander R M. Inhibitors of cytochrome c release with therapeutic potential for Huntington’s disease. J Neurosci. 2008;28:9473–9485. doi: 10.1523/JNEUROSCI.1867-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J D, Wong C W, Lieber C M, Lansbury P T. Assembly of Aβ amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- Knott A B, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J P, Hardy J, Fischbeck K H. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–1995. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- Dobson C M. Protein folding and its links with human disease. Biochem Soc Symp. 2001;68:1–26. [PubMed] [Google Scholar]
- Caughey B, Lansbury P T J. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the inocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Walsh D M, Selkoe D J. A beta oligomers–a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Zheng J, Jang H, Ma B, Tsai C-J, Nussinov R. Modeling the Alzheimer Aβ17–42 fibril architecture: Tight intermolecular sheet-sheet association and intramolecular hydrated cavities. Biophys J. 2007;93:3046–3057. doi: 10.1529/biophysj.107.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone M G, Baumketner A, Bernstein S L, Wyttenbach T, Lazo N D, Teplow D, Bowers M T, Shea J E. Effects of familial Alzheimer’s disease mutations on the folding nucleation of the amyloid beta-protein. J Mol Biol. 2008;381:221–228. doi: 10.1016/j.jmb.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M A, Lazo N D, Lomakin A, Condron M M, Arai H, Yamin G, Rigby A C, Teplow D. Familial Alzheimer’s disease mutations alter the stability of the amyloid beta-protein monomer folding nucleus. Proc Natl Acad Sci U S A. 2007;104:16522–16527. doi: 10.1073/pnas.0705197104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova A T, Ishii Y, Balbach J J, Antzutkin O N, Leapman R D, Delaglio F, Tycko R. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci U S A. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M P. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen S, Ku G, Ahmed S H, Xu J, Chen H, Hsu C Y. Amyloid beta peptide-induced cerebral endothelial cell death involves mitochondrial dysfunction and caspase activation. J Cereb Blood Flow Metab. 2001;21:702–710. doi: 10.1097/00004647-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Takuma K, Yan S-D, Stern D, Yamada K. Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J Pharmacol Sci. 2005;97:312–316. doi: 10.1254/jphs.cpj04006x. [DOI] [PubMed] [Google Scholar]
- Folin M, Baiguera S, Fioravanzo L, Conconi M T, Grandi C, Nussdorfer G G, Parnigotto P P. Caspase-8 activation and oxidative stress are involved in the cytotoxic effect of beta-amyloid on rat brain microvascular endothelial cells. Int J Mol Med. 2006;17:431–435. [PubMed] [Google Scholar]
- Cribbs D H, Poon W W, Rissman R A, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer’s disease. Am J Pathol. 2004;165:353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Ramasamy K, Ingelsson M, Fukumoto H, Conrad C, Frosch M P, Irizarry M, Yuan J, Hyman B T. Coordinated expression of caspase 8, 3 and 7 mRNA in temporal cortex of Alzheimer disease: relationship to formic acid extractable Aβ levels. J Neuropathol Exp Neurol. 2006;65:508–515. doi: 10.1097/01.jnen.0000229238.05748.12. [DOI] [PubMed] [Google Scholar]
- Sajan F D, Martiniuk F, Marcus D L, Frey W H, Hite R, Bordayo E Z, Freedman M L. Apoptotic gene expression in Alzheimer’s disease hippocampal tissue. Am J Alzheimers Dis Other Dement. 2007;22:319–328. doi: 10.1177/1533317507302447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Ona V O, Li M, Ferrante R J, Fink K B, Zhu S, Bian J, Guo L, Farrell L A, Hersch S M, Hobbs W, Vonsattel J P G, Cha J H, Friedlander R M. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.