Abstract
Characterizing the components of GW/processing bodies is key to elucidating RNA interference and messenger RNA processing pathways. This protocol addresses challenges in isolating a low-abundance protein GW182 and GW body (GWB)-associated proteins by building on previous reports that used polyclonal sera containing autoantibodies to GW/P body components. This protocol uses commercially available monoclonal antibodies to GW182 that are covalently coupled to Protein A or G sepharose beads and then used to immunoprecipitate GW182 and associated proteins from cell extracts. Immunoprecipitates are separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and probed by western blot with antibodies directed to proteins of interest. This protocol, which is expected to take 4–5 d, provides a biochemical approach for detecting GW182 and associated proteins in biological samples and thus facilitates the elucidation of the diverse functions of GWBs. It is expected that this protocol can be adapted to the detection of other RNA-binding complexes.
INTRODUCTION
GW bodies (GWBs) are cytoplasmic foci in mammalian cells, initially recognized by human autoimmune sera and are enriched with a marker protein GW182, which is characterized by multiple glycine (G) and tryptophan (W) repeats and a carboxyl terminal classical RNA-binding domain1. GWBs are thought to be the sites responsible for messenger RNA (mRNA) processing and degradation2,3 and provide the appropriate microenvironment for the RNA-induced silencing complex (RISC) and critical steps in the RNA interference (RNAi) pathway4,5. GW182 has been shown to colocalize with mRNA decay factors Dcp1 and LSm4 in mammalian cells6, and accordingly, GWB are thought to be closely related or identical to processing bodies (P-bodies) that are involved in mRNA storage and decay in Saccharomyces cerevisiae2,3. A number of additional proteins that localize to GWBs have subsequently been described and they include the deadenylase Ccr4, 5′-decapping factor Dcp2, exonuclease XRN1, rck/p54 and the LSm1-7 complex (reviewed in refs. 3,7–9). GWBs may also participate in other mechanisms of mRNA expression control given that GWBs contain the cap-binding protein eIF4E and the related factor eIF4E-transporter (eIF4E-T)10,11. The interaction between these two factors represses mRNA translation and is thought to serve as a prerequisite for the targeting of mRNA to GWB for degradation10,11. Of paramount interest, GWBs contain RNAi pathway components4,5, including Dicer, Ago2 and microRNAs (miRNA), that are incorporated in the effector complex RISC (reviewed in refs. 3,8,12,13), ultimately leading to either cleavage or translational inhibition of the target mRNA. Finally, stress granules are shown to be dependent on and share components with GWBs (see refs. 14,15). Stress granules have been shown to be composed of nontranslating mRNAs that interact with GWBs and may ‘hand off ’ mRNAs to GWBs where they are targeted for repression and/or degradation16–20.
Historically, many laboratories have had difficulties in identifying GW182 and related GWB proteins by conventional western blot (WB), a challenge that may be attributed to a number of features that include their molecular mass, relatively low abundance and/or incompletely understood unique physicochemical properties, such as the high-density hydrophobic glycine(G)/tryptophan(W) domains1. Before examining the biochemical interactions between GW182 and other messenger ribonucleoprotein components as outlined in this protocol, indirect immunofluorescence and colocalization studies are commonly used as a first step to determine whether a protein of interest is located in spatial proximity to GW182/GWB. This paper is focused on GW182 and details a protocol to aid in identifying the diverse functions of GWB, but as discussed below, it can be adapted or suitably modified to study a variety of cellular components such as neuronal granules, RNA granules, messenger ribonucleoprotein and other RNA-binding protein complexes. This protocol first immunoprecipitates the protein complex of interest from the cellular milieu using GWB antibodies covalently coupled to Protein A or G sepharose beads, followed by WB to detect proteins of interest associated with the cognate protein complex of interest (Fig. 1). First, an antibody showing high specificity for the low-abundance target (i.e., GW182) is coupled to a solid-phase matrix (Protein A or G sepharose beads); this then binds protein(s) of interest and adsorbs the protein complex from the cell lysates. Other proteins not bound to the antibody–Protein A or G bead support are washed away and the proteins of interest are eluted from the antibody-coupled beads and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). This is followed by WB to determine the identity of proteins that are associated with the protein of interest. An important advantage of combined immunoprecipitation (IP)–WB reactions over WB alone is their potential to deliver not only the target protein but also other macromolecules that interact with the target protein. The IP–WB protocol published previously by our group21,22 that used human polyclonal sera to detect other proteins associated with RISC, P-bodies and mRNA granules has been modified by adopting the use of monoclonal antibodies raised against GW182 and other key components of GWB (reviewed in ref. 8). Although monoclonal antibodies are probes of choice, they may have limitations in biochemical techniques that can be attributed to low-affinity polyreactivity (particularly IgM antibodies), low concentrations in commercially available preparations and reactivity to restricted epitopes that may be inaccessible when proteins retain their tertiary structures.
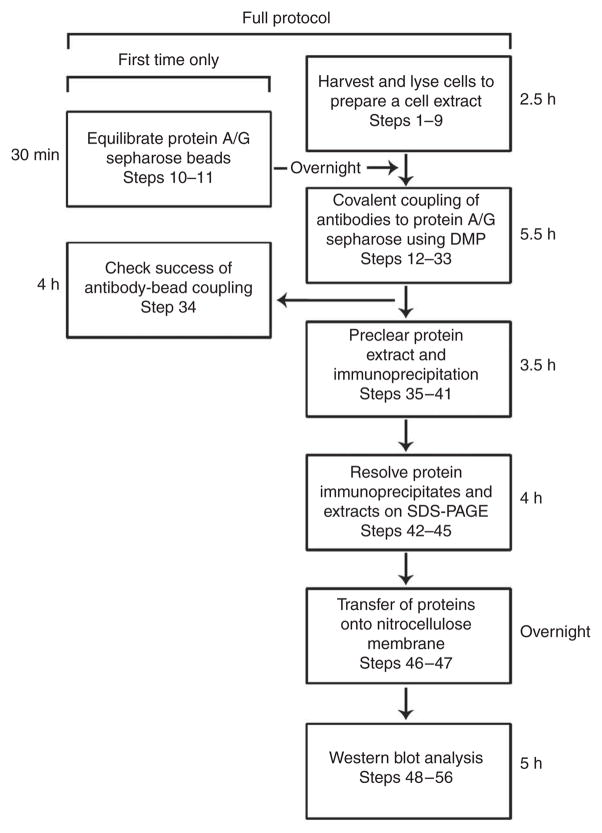
Figure 1.
Summary of the steps involved and the approximate time required in detecting GW182 and associated proteins that are components of GWBs. Note that the steps shown in the boxes on the left are required only the first time if sufficient reagent is prepared for future experiments.
As indicated above, this protocol can most likely be adapted and modified to detect other RNA-binding proteins and mRNA complexes that can lead to a further understanding of the role of GWB components in RNA interference8, mRNA stabilization and transport3,21 and cell stress responses18. These analyses include RT-PCR, which examine interactions of RNA-binding proteins with their cognate mRNAs23, or cDNA arrays, which examine large-scale RNA-binding protein mRNAs24. In addition, this protocol has been adapted to identify mRNAs and miRNAs associated with GW182 in miRNA microarrays as when total RNA was extracted from IPs and analyzed in a protocol that used a monoclonal Ago2 antibody to identify the associated miRNAs by RT-PCR25.
Experimental design
Sample type and preparation
This method was first published using U-87 astrocytoma cells21 and more recently HeLa cells22, but it can be applied to other cell types according to the user’s preference. Protein isolated from tissues may be examined using the methodology outlined in this protocol but has not been examined by the authors. Cell morphology, growth patterns and resulting cell density are well known to differ between cell lines. For example, when examining the difference between HeLa cells and U-87 astrocytoma cells, it is obvious that the latter cells have long, widely spread projections that are remarkably different from the much more compact phenotype seen in HeLa cell cultures. This type of growth pattern, typical of astrocytoma cells, requires a larger number of U-87 cells to obtain the same amount of protein harvested from HeLa cells. It is very important to appreciate that a relatively large amount of protein (~40 μg per lane) is recommended to identify the GW182-associated proteins or other low-abundance proteins. Cell lysis in this protocol is achieved by mechanical methods without the addition of salts or detergents. From experience, mechanical methods for the detection of GW182 are superior to the use of lytic or chaotropic agents. Addition of lysis buffers containing detergents and salts may interfere with native protein conformation, protein–protein interactions and the binding of proteins to antibodies in the subsequent IP. Both lysis approaches must be tested and optimized for particular cells and tissues that may be used in other studies. Comparison of lysis methods are made following IP and WB steps and the choice ultimately rests with the investigator.
Choice of antibodies for IP and WB
First, an important consideration is the choice of antibodies used to immunoprecipitate the protein complex of interest. The chosen antibodies should have proven specificity and minimal cross-reactivity or nonspecific binding. Human serum 18033 was initially used to immunoprecipitate GW182 (see refs. 21,22), but the results have recently been improved with the use of the commercially available mouse monoclonal 4B6 antibody. Human 18033 serum is polyclonal, recognizes multiple GWB-associated proteins, such as GW182, Ago2 and Rap55, and efficiently immunoprecipitates GWB-associated proteins and detects GW182 by WB (Fig. 2). Mouse monoclonal 4B6 was generated by immunization with recombinant GW182 (see ref. 26) and it can be used to immunoprecipitate GW182 from the cytoplasmic protein milieu and to detect GW182 and Ago2 by WB (Fig. 2a,c). As cross-reactivity tends to be more common when polyclonal antibodies are used or when low-avidity IgM monoclonal antibodies are used, it is very important to select antibodies of the highest specificity and affinity for the protein in question. Hence, it is important to test the specificity and binding characteristics of different antibodies in a pilot IP analysis to validate their efficiency and usefulness. For WB analysis, antibodies should be proven acceptable for WB applications, be specific for the protein of interest and their dilution must be optimized to achieve a result that produces low background reactions.
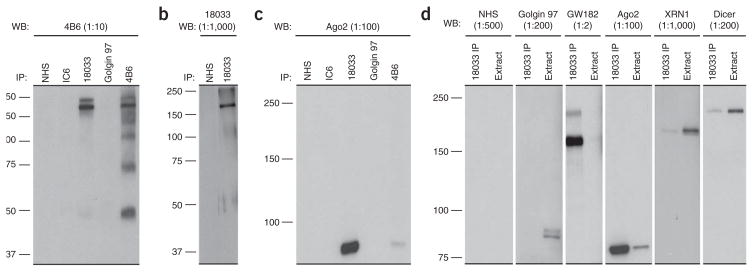
Figure 2.
Antibodies to GW182-immunoprecipitated Ago2, Dicer and XRN1 proteins from HeLa cell extracts as examined by WB analysis. GW182 and associated proteins were immunoprecipitated using human sera 18033 containing antibodies to GW182 coupled with Protein A sepharose beads and by mouse anti-GW182 monoclonal 4B6 coupled to Protein G sepharose beads. Normal human serum (NHS), IC6 human serum and mouse monoclonal antibody to golgin 97 were used as IP controls. Immunopreciptates were carried out with buffer that did not contain detergent. Proteins (40 μg) were resolved on a 10% SDS-PAGE gel and were detected with a 4B6 monoclonal antibody (a) or 18033 human anti-GWB serum (b). Immunopreciptates were carried out with buffer that contained 0.3% NP-40 detergent. Proteins (40 μg) were resolved on a 6.5% SDS-PAGE gel and were detected using a mouse anti-Ago2 monoclonal 4F9 antibody (c). Protein (40 μg) from whole cell extracts or 18033 immunoprecipitates carried out using 0.3% NP-40 buffer were separated on a 6.5% SDS-PAGE gel and probed with NHS or antibodies to golgin 97, GW182, Ago2, XRN1 or Dicer. (d) Mouse monoclonal antibody to Dicer and rabbit anti-XRN1 antibody were purchased from Abcam Inc. (cat. no. ab14601) and Bethyl Laboratories (cat. no. A300-443A), respectively. Mouse monoclonal 4F9 to recombinant hAgo2 was obtained as described previously25.
Control antibodies
Control antibodies must be chosen for IP and WB that do not cross-react with the proteins of interest (i.e., GW182) but are specific for their cognate antigen. For example, mouse monoclonal golgin 97 (CDF4) antibody is specific to the Golgi complex golgin 97 protein (Fig. 2d, extract lane, protein band at ~97 kDa) but does not bind to GW182 or associate with GWB complex proteins (Fig. 2d, 18033 IP lane) in WB analysis. Another example is IC6 human serum, which contains antibodies that recognize the Ge-1/Hedls protein (Fig. 2a,c). Normal human serum (NHS) or sera with an unrelated autoantibody should be used as an additional control (Fig. 2; IP and WB applications). To account for nonspecific binding of the Protein A or G sepharose beads, another control to be considered are IPs in the absence of cell lysate extracts (i.e., no protein, phosphate-buffered saline (PBS) only) or extracts that do not contain the protein targets of interest. However, the two latter controls are not necessary when the cell lysates are precleared by incubation with the beads. Preclearing of the cell lysate normally removes nonspecific, high-affinity proteins that bind to Protein A or G before IP reactions. In addition, a whole cell extract can be included in a parallel SDS-PAGE lane to examine the baseline expression of a particular protein without IP and to check if the antibody is an appropriate reagent for WB. For example, XRN1 and Dicer are two proteins that are enriched in the whole cell extract as compared with the GW182 IP; however, Ago2 and GW182 are enriched in the GW182 IP as compared with the whole cell extract (Fig. 2d).
Optimization of IP conditions
This protocol uses dimethyl pimelimidate dihydrochloride (DMP) to covalently link Protein A sepharose beads to human IgG antibodies and Protein G sepharose beads to mouse IgG antibodies. Protein A and G Sepharose preparations have the cognate immunoglobulin-binding proteins already immobilized on insoluble beads. The A and G proteins immobilized on the sepharose beads bind to the Fc domain of antibodies. As only the Fc antibody region is bound to sepharose, the Fab antibody region is available for antigen binding. Depending on the species from which the primary antibody is derived, the appropriate beads for antibody IP must be chosen by the user. In general, human IgGs bind to both Protein A and G, whereas mouse IgGs show variable binding properties (mouse IgG1 binds very poorly to Protein A, whereas IgG2a and 2b bind to both with equal specificity). Hence, for mouse antibodies, Protein G is the preferred ligand. The option of covalently coupling the antibody with the beads rests with the user, but if the antibodies are not covalently linked to a solid-phase bead matrix, boiling the IP sample in 2× Laemmli sample buffer (LSB) will result in the coelution of the antibody along with the target protein(s) (Fig. 3). If coelution of antibody fragments with the antigen results in bands interfering with detection of any coprecipitated proteins on SDS-PAGE, the antibody should be cross-linked to the beads. Although cross-linking is not entirely efficient and a small amount of IgG may be eluted from the beads inevitably, this method does improve the quality of WB results. The IP buffer should be chosen after considerable planning and can be modified to increase or decrease stringency. Options include increasing salt concentrations to reduce ionic interactions (ranges between 150 mM NaCl and 1,000 mM NaCl; 150 mM NaCl used in this protocol) and/or adding one or more detergents, such as 0.3–0.5% NP-40 or 0.05–0.5% Triton X-100 to increase protein solubility and unfold proteins. IP buffers that do not wash away nonspecific binding proteins will become evident after WB if extra protein bands appear in the IP lane and are similar to what is observed in the whole cell extract.
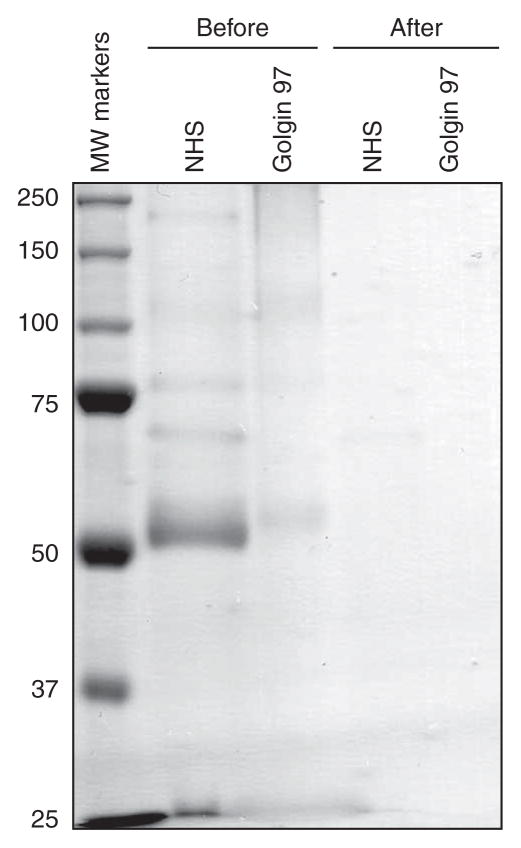
Figure 3.
Example of successful covalent coupling of antibodies to sepharose beads. The ‘Before’ conjugation samples and ‘After’ conjugation samples (NHS-Protein A, golgin 97-Protein G) were resolved on 10% SDS-PAGE and stained with the Colloidal Blue Staining Kit. The antibody light (25-kDa) and heavy (50-kDa) chains are visible on the gel in the ‘Before’ samples, but not in the ‘After’ samples, indicating that covalently coupled antibodies remained bound to the beads after boiling in 2× LSB.
SDS-PAGE considerations
Detection and resolution of GW182 and TNGW1 (a recently characterized longer isoform of GW182 (see ref. 22)) depends upon the resolving power of the SDS-PAGE gel at highmolecular masses (i.e., 182 and 210 kDa, respectively). In these situations, a 6.5% (vol/vol) SDS-PAGE gel is recommended, but if 30–100 kDa proteins are of interest, a 10% (vol/vol) SDS-PAGE gel may be desirable. See REAGENT SETUP for varying percentage resolving gel components and 5% (vol/vol) stacking gel components. Once proteins from SDS-PAGE are transferred onto nitrocellulose or polyvinylidene fluoride membranes, a Ponceau S stain is used as a rapid and reversible stain to ensure transfer of proteins from the SDS gel before WB (Fig. 4). Ponceau S stain is easily reversed by washing the membrane in ddH2O.
Figure 4.
Example of a Ponceau S-stained nitrocellulose membrane containing 40 μg of protein per lane from a HeLa cell lysate extract. The membrane was stained for 1 min with Ponceau S and destained with quick washes in ddH2O until proteins bands became pink and the background became white. Numbers are protein size in kDa.
Membrane choice and transfer
Nitrocellulose membranes are used in this protocol but polyvinylidene fluoride membranes may be used; however, they have not been extensively tested by the authors. Wet transfers at 22–25 V (overnight, 4 °C) have worked best for transfer of proteins from SDS-PAGE (6.5%, 10% or 12% (vol/vol) gel), although semidry transfer has also been used with some success (unpublished data).
MATERIALS
REAGENTS
Cells of choice approximately 1 × 109–1 × 1011 cells (~20 T-150 cm2 U-87 cell flasks to yield 12 mg ml−1 protein and ~10 T-150 cm2 HeLa cell flasks to yield 20 mg ml−1 protein)
PBS (pH 7.4 diluted from 10× PBS pH 7.4 sterile, Rnase-, Dnase- and Protease-free as tested by supplier) (Ambion, cat. no. AM9625)
Complete Mini ethylenediaminetetraacetic acid (EDTA)-free Protease Inhibitor Cocktail Tablets (Roche, cat. no. 04693159001)
Sodium azide (NaN3; VWR, cat. no. CASX 0300-3)
Bicinchoninic Acid (BCA) Protein Assay Reagent kit (Pierce, cat. no. 23227)
Protein A Sepharose CL-4B beads (Amersham/GE Healthcare, cat. no. 17-0780-01)
Protein G Sepharose 4 Fast Flow beads (Amersham/GE Healthcare, cat. no. 17-0618-01)
Primary antibodies of choice to detect GWB or the RISC complex, e.g., Mouse GW182 (4B6) Monoclonal Antibody (Santa Cruz Biotechnology Inc., cat. no. sc-56314) ▴ CRITICAL 4B6 mouse monoclonal antibody has proved to be the best available monoclonal antibody for IP and WB applications. Mouse GW182 (2D6) monoclonal antibody (Santa Cruz Biotechnology Inc., cat. no. sc-sc-56313) may also be used in IP and WB applications; however, this antibody tends to be weaker and more protein must be loaded onto a gel when probing extracts or a greater volume of antibody must be used when coupling antibody to sepharose beads. Human 18033 serum (or equivalent) containing autoantibodies directed to GWB (Mitogen Advanced Diagnostics Laboratory, limited supply available on request to Dr. M. Fritzler: fritzler@ucalgary.ca).
Primary antibodies for controls include the following: mouse anti-human golgin 97 (Golgi complex) monoclonal antibody, Clone CDF4 (Molecular Probes/Invitrogen, cat. no. A21270); NHS (Mitogen Advanced Diagnostics Laboratory)
Ethanolamine (Sigma, cat. no. E9508) ! CAUTION Corrosive and toxic. Harmful if inhaled or in contact with skin. Toxic if ingested. Wear gloves, lab coat, safety glasses and keep the bottle pointed away from user when transferring liquid.
-
Sodium tetraborate or borax anhydrous, pH 9.0 (Sigma, cat. no. 71996)
! CAUTION Harmful with possible risk of impaired fertility and of harm to an unborn child. Wear suitable protective clothing and gloves.
DMP (Sigma, cat. no. D8388) ! CAUTION Irritating to the skin. Avoid contact with skin and eyes by wearing suitable protective clothing and gloves.
Colloidal Blue Staining Kit (Invitrogen, cat. no. LC6025)
Tris-HCl (Trizma; Sigma, cat. no. T-3253)
Tris-base (Bio-Rad, cat. no. 161-0719)
Sodium chloride (NaCl; VWR, cat. no. CA-EM7760)
0.5 M EDTA, pH 8.0 (GIBCO/Invitrogen, cat. no. 15575-020)
NP-40 detergent (Sigma, cat. no. N-6507)
2× LSB (see REAGENT SETUP)
Glycerol (VWR, cat. no. CA-EM4750)
Bromophenol blue (VWR, cat. no. CA-EM2830)
2-Mercaptoethanol (Bio-Rad, cat. no. 161-0710) ! CAUTION Toxic in contact with skin, harmful if swallowed, causes burns and is combustible. Wear protective gloves/protective clothing/eye protection/face protection. Keep away from heat/sparks/open flames/hot surfaces. Handle in a fume hood.
Ammonium persulfate (APS; Sigma, cat. no. A-1433) ! CAUTION Oxidizer. Harmful if swallowed and irritating to the eyes/respiratory system/skin. Wear suitable protective clothing and gloves and avoid contact with combustible materials, as this may cause a fire.
40% acrylamide (wt/vol) (Bio-Rad, cat. no. 161-0140) ! CAUTION Acrylamide is a suspected human carcinogen, severe neurotoxin and causes irritation of the eyes, skin (is readily absorbed) and respiratory tract. Wear gloves, lab coat, safety lab goggles and keep the bottle pointed away from the user when transferring liquid.
2% Bis-acryl (wt/vol) (Bio-Rad, cat. no. 161-0142)
SDS (Bio-Rad, cat. no. 161-0302) ! CAUTION Highly flammable, harmful in contact with skin and irritant to the respiratory tract and eyes. Wear suitable protective clothing, gloves and a mask covering the mouth and nose.
Double-distilled water (ddH2O)
N, N, N′, N′-tetramethyl-ethylenediamine (TEMED; Bio-Rad, cat. no. 161-0800) ! CAUTION Corrosive, highly flammable and harmful by inhalation. Wear suitable protective clothing, gloves and eye/face protection.
Glycine (Sigma, cat. no. G7126)
Methanol (VWR, cat. no. CAMX0485-7)
Running buffer (see REAGENT SETUP)
Protein molecular weight markers of choice
Nitrocellulose membrane (recommend 0.45-μm pore size; Bio-Rad, cat. no. 162-0115)
Transfer buffer (see REAGENT SETUP)
Ponceau Red (Sigma, cat. no. P3504)
Glacial acetic acid (VWR, cat. no. CAAX0073-59)
Ponceau S stain (see REAGENT SETUP)
Skimmed milk powder
PBS with 0.05% (vol/vol) Tween (PBST) (Sigma; cat. no. P1379), pH 7.4
Secondary antibodies of choice conjugated to horseradish peroxidase (HRP) for enhanced chemiluminescence (ECL) visualization, e.g., goat anti-mouse IgG-HRP (Santa Cruz Biotechnology Inc., cat. no. sc-2005); goat anti-human IgG-HRP (Sigma, cat. no. A8667)
Enhanced chemiluminescent (ECL) Western Blotting Detection Reagents (Amersham/GE Healthcare, cat. no. RPN2106)
EQUIPMENT
Cell scrapers (40 cm, VWR, cat. no. CA15621-015)
120 V Ultrasonic water bath sonicator/cleaner (VWR, cat. no. 98000-328)
-
3-ml syringe fitted with a 21 G 1.5-inch needle (BD, cat. no. 305274)
▴ CRITICAL Needle size is critical.
1.5-ml microcentrifuge tubes (VWR, cat. no. 20170-355)
15-ml and 50-ml conical polystyrene centrifuge tubes (BD Falcon, VWR, cat. nos. CA21008-930 and CA21008-938)
Refrigerated microcentrifuge of choice for 1.5-ml/2.0-ml microtubes (VWR, cat. no. 13916-836)
Spectrophotometer of choice with 1.5-ml disposable cuvettes (VWR, cat. no. 97000-586)
Nanodrop 1000 (Thermo Scientific, cat. no. ND-1000) (Optional: if using colorimetric BCA protein assay with spectrophotometer)
Accumet Basic AB15 pH meter (Fisher Scientific, https://www1.fishersci.com/wps/portal/CMSTATIC?href=index.jsp&store=Scientific&segment=scientificStandard, cat. no. 13-636-AB15V)
Nutating mixer (VWR, cat. no. 82007-202)
Microtube rotator (VWR, cat. no. 13916-822)
Rocking platform shaker (VWR, cat. no. 40000-300)
Centrifuge of choice for 15-ml centrifuge tubes (VWR, cat. no. 82017-654)
Protein running gel apparatus of choice (SE 260 Mini-Vertical Unit, Amersham/GE Healthcare, cat. no. 80-6149-35)
Power pack of choice (EPS 301, Amersham/GE Healthcare, cat. no. 18-1130-01)
Wet transfer apparatus of choice (TE 22 Mini Tank Transfer Unit, Amersham/GE Healthcare, cat. no. 80-6204-26)
Film of choice (recommend either Kodak BioMax MR Film, VWR, cat. no. IB-IB8701302 or Amersham Hyperfilm ECL, VWR, cat. no. CA95017-659L)
Automated film developer or dip trays/tanks with fixer and developing reagents (reagents supplied by Christie Group Ltd, http://www.christiegrp.com/english/index.html, cat. nos. V-306A, V-306B and V-307)
REAGENT SETUP
PBS with protease inhibitor
Combine 1 Complete Mini EDTA-free Protease Inhibitor Cocktail Tablet per 10 ml of sterile PBS. Mix until tablet dissolves. This solution is stable for 12 weeks when stored at −20 °C.
10%(wt/vol) Protein A sepharose bead slurry
Wash 0.75 g of Protein A beads two times with 10 ml of sterile PBS. Equilibrate Protein A beads in 25 ml of sterile PBS containing protease inhibitor. Allow the beads to swell overnight at 4 °C; after swelling, the protein A bead slurry corresponds to a 10% (wt/vol) solution. Store at 4 °C for 1 year with the addition of 0.05% (wt/vol) sodium azide.
10% (wt/vol) Protein G sepharose bead slurry
Transfer 5 ml of beads stored in 70% (vol/vol) ethanol to a 50-ml centrifuge tube and wash a total of five times with 20 ml of sterile PBS to remove all traces of ethanol. Rinse out the supply bottle with 5 ml of sterile PBS to collect the remaining beads and add to the 50-ml centrifuge tube. Prepare a 10% (wt/vol) Protein G sepharose slurry by adding 25 ml of sterile PBS containing protease inhibitor to the washed Protein G beads. Allow the beads to swell overnight at 4 °C; after swelling, the protein G bead slurry corresponds to a 10% (wt/vol) solution. Store at 4 °C for 1 year by adding 0.05% (wt/vol) sodium azide.
0.2 M ethanolamine, pH 8.0
Dilute 2.44 ml of ethanolamine with 200 ml of autoclaved double-distilled water. Adjust the pH to 8.0 by adding glacial acetic acid. Store at room temperature (17–24 °C) for a maximum of 2 years.
! CAUTION Use a fume hood if using hydrochloric acid to adjust pH, as this acid will vaporize upon addition to ethanolamine. Hydrochloric acid vapors irritate the nose and respiratory tract. Recommend adjusting pH by adding acetic acid instead.
0.2 M sodium borate buffer (SBB), pH 9.0
Mix 20.12 g of sodium tetraborate anhydrous in 500 ml of autoclaved ddH2O. Adjust pH to 9.0. Filter-sterilize and store at room temperature for a maximum of 2 years. ▴ CRITICAL Difficult to dissolve until at an optimal pH of 9.0.
20 mM DMP
Add 26 mg of DMP powder to 5 ml of 0.2 M SBB, pH 9.0.
▴ CRITICAL Must be made immediately before use because of its instability in solution (hydrolyzes at neutral pH).
1 M Tris-HCl, pH 7.4
Mix 132.2 g of Tris-HCl and 19.4 g of Tris base into 500 ml of ddH2O and adjust the pH to 7.4 if necessary. Bring up to a final volume of 1 liter by adding ddH2O. Store at room temperature for a maximum of 2 years.
NET2F IP buffer
50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, pH 8.0. For greater stringency, add 0.3% (vol/vol) NP-40 detergent and/or 0.3% (vol/vol) Triton X-100 and/or adjust to 500 mM NaCl. Filter-sterilize and store at 4 °C for a maximum of 1 year.
2× LSB
25% (vol/vol) glycerol, 2% (wt/vol) SDS, 250 mM Tris-HCl pH 6.8, 0.1% (wt/vol) bromophenol blue up to desired volume with ddH2O. Without reducing agent, it can be stored for up to 1 year at room temperature. Before adding to protein samples, add 50 μl of reducing agent 2-mercaptoethanol per 950 μl of 2× LSB for a final concentration of 5% (vol/vol) 2-mercaptoethanol. Alternatively, dithiothreitol (DTT) may be used at a final concentration of 350 mM (54 mg ml−1). With the addition of reducing agent, it can be stored for up to 3 months at −20 °C.
1 M Tris-HCl, pH 8.7
Mix 30.0 g of Tris-HCl and 98.0 g of Tris base into 500 ml of ddH2O and adjust the pH to 8.7 if necessary. Bring up to a final volume of 1 liter by adding ddH2O. Store at room temperature for a maximum of 2 years.
1 M Tris-HCl, pH 6.9
Dissolve 121.1 g of Tris-HCl in 500 ml of ddH2O to dissolve, adjust the pH to 6.9 by adding HCl and bring the volume to 1 liter by adding ddH2O. Store at room temperature for a maximum of 2 years.
10% (wt/vol) APS
Dissolve 0.1 g of APS in 1 ml of ddH2O and mix by vortexing. ▴ CRITICAL Best if stored at 4 °C and used within 7 d.
10% (wt/vol) SDS
Dissolve 5.0 g of SDS powder in 30 ml of ddH2O over warm/hot plate and take volume to 50 ml using ddH2O. Store at room temperature for amaximum of 1 year. ! CAUTION Highly flammable, harmful in contact with skin and irritant to the respiratory tract and eyes. Wear a nose and mouth facemask to avoid oral and nasal contact with powder. Do not autoclave.
▴ CRITICAL Do not adjust the pH.
5× Stock running buffer
Dilute 200ml of 5× stock running buffer into 800 ml of ddH2O to make working 1× running buffer. Mix 45 g of Tris base, 282 g of glycine and 15 g of SDS up to 3 liters of ddH2O. Store at 4 °C. Prepared 1× running buffer is best if used within 1 week. ▴ CRITICAL Do not adjust the pH.
SDS-PAGE resolving gels and 5% (vol/vol) stacking gel (for 2 mini gels)
| Materials | 6.5% (vol/vol) resolving | 10% (vol/vol) resolving | 12.5% (vol/vol) resolving | 15% (vol/vol) resolving | 5% (vol/vol) stacking |
|---|---|---|---|---|---|
| 40% (wt/vol) acrylamide | 3.1 ml | 4.8 ml | 6.0 ml | 7.1 ml | 1.2 ml |
| 2% (wt/vol) bis-acryl | 1.7 ml | 2.7 ml | 3.4 ml | 4.0 ml | 0.7 ml |
| 1 M Tris, pH 8.7 | 7.5 ml | 7.5 ml | 7.5 ml | 7.5 ml | — |
| 1MTris, pH6.9 | — | — | — | — | 1.5ml |
| 10% (wt/vol) SDS | 200 μl | 200μl | 200μl | 200μl | 125 μl |
| ddH2O | 7.4 ml | 4.7 ml | 2.8 ml | 1.0 ml | 8.9 ml |
| 10% (wt/vol) APS | 100 μl | 100μl | 100μl | 100μl | 63μl |
| TEMED | 10 μl | 10μl | 10μl | 10μl | 13μl |
! CAUTION Acrylamide is a suspected human carcinogen and a severe neurotoxin and causes irritation of the eyes, skin (is readily absorbed) and respiratory tract. Wear gloves, lab coat, safety lab goggles and keep the bottle pointed away from the user when transferring liquid. ! CAUTION SDS is highly flammable, harmful in contact with skin and irritant to the respiratory tract and eyes. Wear suitable protective clothing, gloves and a mask covering the mouth and nose. ! CAUTION TEMED is corrosive, highly flammable and harmful by inhalation. Wear suitable protective clothing, gloves and eye/face protection.
Mix the top five reagents in a 50-ml centrifuge tube or Erlenmeyer flask to obtain the gel percentage of choice. The components of the 5% (vol/vol) stacking gel are combined in another 50-ml centrifuge tube or Erlenmeyer flask. Add 10% (wt/vol) APS and TEMED to the other reagents immediately before pouring the gel liquid into the gel apparatus. After pouring the resolving gel, layer the top with water-saturated butanol or water. Leave the gel to solidify for 45 min to 1 h. If using water-saturated butanol, rinse the gel surface with ddH2O five times. Add 10% (wt/vol) APS and TEMED to stacking gel mixture and pour on top of the resolving gel. Position combs and let the gel solidify for 45 min to 1 h.
Transfer buffer
Combine 23.2 g of Tris base, 11.6 g of glycine, 1.48 g of SDS and 800 ml of methanol, and add ddH2O up to 4 liters. Store at room temperature for a maximum of 3 months.
Ponceau S stain
Prepare 0.5% (wt/vol) Ponceau Red and 1% (vol/vol) glacial acetic acid in ddH2O. May be reused several times. Store at room temperature indefinitely.
5% (wt/vol) skimmed milk in PBST
Dissolve 5.0 g of skimmed milk powder in 100 ml of PBST. Use immediately or store at 4 °C for up to 2 d.
Stripping buffer
Prepare 2% (wt/vol) SDS, 62.5 mM Tris, pH 6.7, and 100 mM 2-mercaptoethanol in PBST in the fume hood. Store at room temperature for a maximum of 1 year without the addition of 2-mercaptoethanol. Use immediately after the addition of 2-mercaptoethanol.
PROCEDURE
Day 1: cell lysate preparation • TIMING 2.5 h
1| Harvest ~1 × 109 cells from the appropriate number of T-150 cm2 tissue culture flasks by discarding the growth medium and adding 10 ml of ice-cold sterile PBS (protease-free, tested by supplier) to each flask. Scrape the cells with a cell scraper to detach the cells from the flask and transfer to two prechilled 50-ml tubes on ice.
2| Centrifuge for 3 min at 196g and 4 °C.
3| Discard the supernatant and add 5 ml of ice-cold sterile protease-free PBS to each 50-ml tube. Resuspend the pellets and pool into one 15-ml tube.
4| Repeat Step 2 and wash with an additional 5 ml aliquot of ice-cold sterile protease-free PBS.
5| Repeat Step 2 and discard the supernatant. Add an equal pellet volume of ice-cold sterile protease-free PBS with protease inhibitor (~850 μl) to the cell pellet, resuspend the cells and place on ice.
6| Sonicate the cells in the water bath sonicator for 80 s, vortex and place the cells on ice. Repeat after 1 min.
-
7| Shear the viscous lysate by passing four times through a syringe fitted with a 21 G 1.5-inch needle.
▴ CRITICAL STEP Using mechanical lysis with 21 G 1.5-inch needle is critical.
8| Transfer the lysate to a 1.5-ml microfuge tube. Centrifuge the lysate in a tabletop microfuge for 10 min at 16,000g and 4 °C. Collect the supernatant into a new 1.5-ml microfuge tube and place on ice.
-
9| Quantitate the concentration of protein extract in the sample using either the colorimetric BCA protein assay following the manufacturer’s instructions with a spectrophotometer set at 562 nm using bovine serum albumin as standards or the Nanodrop 1,000. The concentration of the protein extract is typically 12–15 μg μl−1.
▪ PAUSE POINT Store the lysate at −20 or −70 °C for short-term (up to 1 week) or long-term (up to 1 year) use, respectively.
? TROUBLESHOOTING
Equilibration of sepharose beads • TIMING Overnight
10| Prepare a 10% (wt/vol) Protein A sepharose slurry by washing 0.75 g of Protein A beads two times with 10 ml of sterile PBS with protease inhibitor. Equilibrate Protein A beads in 25 ml of sterile PBS containing protease inhibitor. Allow the beads to swell overnight at 4 °C to prepare for day 2.
11| Protein G sepharose beads are supplied in ethanol. Transfer 5 ml of beads stored in 70% (vol/vol) ethanol to a 50-ml centrifuge tube. Centrifuge the beads at 196g for 5 min at room temperature and discard the supernatant. Wash a total of five times with 20 ml of sterile PBS to remove all traces of ethanol. Prepare a 10% (wt/vol) Protein G sepharose slurry by adding 25 ml of sterile PBS containing protease inhibitor to the washed Protein G beads. Allow the beads to swell overnight at 4 °C to prepare for day 2.
Day 2: covalent coupling of antibodies to Protein A or G using DMP • TIMING 5.5 h up to pause point; after pause point (Step 34), allow for an additional 4 h
12| Transfer 2 ml of the 10% (wt/vol) suspension of Protein A sepharose beads into two 15-ml centrifuge tubes. Add 150 μl of normal human serum (NHS control) to the bead slurry in one tube and 150 μl of the human serum 18033 to the other tube.
13| Transfer 2 ml of 10% (wt/vol) suspension of Protein G sepharose beads into two 15-ml centrifuge tubes. Add 500 μl of mouse monoclonal golgin 97/CDF4 antibody control to the bead slurry in one tube and 500 μl of mouse monoclonal 4B6 antibody to the other tube.
14| Allow the antibodies to bind to the beads (from Steps 12 and 13) for 2 h at room temperature on a rolling (nutating) mixer.
15| Add 5 ml of SBB to each antibody-bound bead slurry.
16| Centrifuge for 1 min at low speed (196g) at room temperature to pellet the beads; discard the supernatant.
17| Wash the beads once using 5 ml of SBB, centrifuge as in Step 16 and discard the supernatant.
18| To determine if the coupling procedure is successful, resuspend the beads (~200 μl packed bead volume) in 200 μl of SBB to achieve a 50% (wt/vol) bead suspension. Remove a 10 μl aliquot of the beads and label as ‘Before Sample’. Add 10 μl of 2× LSB to this aliquot, mix and store at −20 °C until the coupling procedure is complete.
19| Centrifuge the remaining 50% (wt/vol) bead suspension as in Step 16 and discard the supernatant.
-
20| Dissolve solid DMP in SBB (see REAGENT SETUP) and add 5 ml to each 15-ml tube of beads.
▴ CRITICAL STEP DMP must be prepared in SBB immediately before use because of its instability in solution.
21| Mix the beads by placing the 15-ml tubes on a rolling mixer for 1 h at room temperature.
22| Centrifuge for 1 min at 196g at room temperature to pellet the beads and discard the supernatant.
23| Add 1 ml of 0.2 M ethanolamine, pH 8.0, and mix gently.
24| Repeat Step 22.
25| Repeat Steps 23 and 24.
26| Add 5 ml of ethanolamine and incubate for 2 h at room temperature on a rolling mixer.
27| Centrifuge for 1 min at 196g at room temperature to pellet the beads and discard the supernatant.
28| Add 2 ml of sterile PBS and mix gently.
29| Centrifuge for 1 min at 196g at room temperature and discard the supernatant.
30| Repeat Steps 28 and 29.
31| Resuspend the beads (~200 μl packed bead volume) in 200 μl of sterile PBS to achieve a 50% (wt/vol) bead suspension. Remove a 10-μl aliquot of beads and label as ‘After Sample’. Add 10 μl of 2× LSB to this aliquot, mix and store at −20 °C.
32| Using the remaining 50% (wt/vol) bead solution, repeat Step 29.
-
33| Add 1 ml of sterile PBS with protease inhibitor to each 15-ml tube to achieve a 20% (wt/vol) suspension of beads.
▪ PAUSE POINT Store beads at 4 °C for up to 1 year. For long-term storage, add 0.05% (wt/vol) sodium azide to prevent bacterial contamination. After storage in sodium azide, the beads should be washed three times with sterile PBS and resuspended in sterile PBS with protease inhibitor before use.
-
34| Check the success of the antibody coupling to the beads by analyzing the ‘Before’ and ‘After’ samples using 10% (vol/vol) SDS-PAGE (see REAGENT SETUP). Boil the samples at 95 °C for 5 min and load carefully into wells along with a protein ladder of interest. Separate the proteins with 120 V until the blue bands migrate to the bottom of the gel. Stain the gel with Colloidal Blue Staining Kit as per the manufacturer’s instructions and leave in ddH2O overnight to destain the background (see Fig. 3 for an example of successful covalent coupling of antibodies to sepharose beads).
? TROUBLESHOOTING
Day 3: preclearing protein extracts and immunoprecipitation • TIMING <3.5 h
35| Thaw the protein extract from day 1 (Step 9) on ice and divide into two 1.5-ml microcentrifuge tubes (200 μl to each tube). Label tubes ‘Protein A extract’ and ‘Protein G extract’. Save ~50 μl of protein extract to use as a ‘no IP extract control’ on day 4 (Step 43). Protein extracts have a protein concentration of ~12 μg μl−1; therefore, each tube of 400 μl contains 2,400 μg of protein.
36| To preclear the extract, add 50 μl of 10% (wt/vol) Protein A sepharose beads in sterile PBS with protease inhibitors (prepared in Step 10) to ‘Protein A extract’ and 50 μl of 10% (wt/vol) Protein G sepharose beads in sterile PBS with protease inhibitors (prepared in Step 11) to ‘Protein G extract’. Incubate on a rotator for 30 min up to 1 h at 4 °C. Alternatively, incubate on a rotator at room temperature for 5–10 min.
37| Centrifuge for 1 min at 82g at room temperature with a microcentrifuge and remove the precleared extract into a clean 1.5-ml tube to use for IP.
38| For IP, combine 100 μl of the 20% (wt/vol) suspension of antibody-coupled Protein A or G Sepharose beads (prepared in Step 33; NHS, 18033, golgin 97 and 4B6) with 500 μl of NET2F buffer of choice and 200 μl of precleared protein extract from Step 37 (~1,200 μg) and incubate for 2 h at 4 °C on a rotator. While waiting for the IP incubation, prepare protein SDS-PAGE gels for Step 42.
39| Centrifuge the tubes for 1 min at 82g at room temperature to collect the beads and discard the supernatant.
-
40| Wash the beads with 1 ml of ice-cold NET2F by rotating the tubes for 5 min at 4 °C. Centrifuge for 1 min at 82g at room temperature to collect the beads and discard the supernatant. Wash the beads for a total of five times.
▴ CRITICAL STEP If using a NET2F buffer with a high salt concentration (i.e., 500 mM), wash four times with the same NET2F buffer and wash an additional two times with a NET2F buffer that contains a lower salt concentration (i.e., 150 mM). IPs that are carried out and washed with higher salt concentrations will alter the expected protein migration.
-
41| After the last wash, pellet the beads by centrifugation at 82g for 1 min at room temperature and discard the supernatant. Add an equal volume of 2× LSB to the bead volume (approximately 20–25 μl).
▪ PAUSE POINT Store IPs with 2× LSB at −20 °C overnight or proceed right away to SDS-PAGE described on day 4. Prepared gels may also be stored overnight at 4 °C wrapped in moist paper towel and saran wrap.
Day 4: resolving GW182 and control immunoprecipitates on SDS-PAGE and wet transfer to nitrocellulose membrane • TIMING 1 d
42| Prepare the gel percentage of choice during the 2-h IP incubation in Step 38, set up electrophoresis apparatus and fill with cold 4 °C running buffer.
43| On the basis of the protein concentration of the extract determined in Step 9, dilute the ‘no IP extract control’ (from Step 35) with PBS to load between 30 and 40 μg of protein into gel wells beside the IP samples from Step 41. Add an equal volume of 2× LSB.
44| Boil the ‘no IP extract control’ and the four IP samples for 5 min at 95 °C and load carefully into wells along with a protein ladder of choice.
45| Insert electrodes into power supply and set the gel to run at 120 V (increase to 150 V after 15 min) at room temperature or 4 °C for the desired time. If interested in examining high molecular weight proteins, such as GW182 and TNGW1, run the 6.5% (vol/vol) gel for at least 3 h until the 75-kDa ladder band is at the bottom of the gel.
46| While the protein samples are resolving, set up the wet transfer according to the manufacturer’s instructions for overnight transfer. Remove the gels from the electrophoresis apparatus carefully and place in a dish with Transfer buffer.
47| Transfer the proteins from the SDS-PAGE gel onto a nitrocellulose membrane at 25 V in a wet transfer apparatus overnight (approximately 14–18 h) at 4 °C.
Day 5: detecting GW182, other RISC components or mRNA degradation proteins by WB analysis • TIMING 5+ h
-
48| Remove the membrane from the overnight wet transfer and wash briefly in ddH2O. Stain membranes briefly (~1 min) with Ponceau S stain on a rocking platform shaker and destain with quick washes with ddH2O until proteins bands are pink and the background is nearly white. Dry the membrane at room temperature on a filter paper. Label the membrane as needed.
▪ PAUSE POINT Store the membrane at −20 °C until required between two sheets of filter paper and wrapped in tin foil, or proceed with WB.
? TROUBLESHOOTING
49| Block the nitrocellulose membrane for 1 h at room temperature in 5% (wt/vol) nonfat milk in PBST.
50| Overlay the membrane with the primary antibody of choice at the appropriate dilution in 5% (wt/vol) milk/PBST for 1 h at room temperature. Recommended dilutions for WB using the same antibodies used in the IP, which include 4B6 (1:2 up to 1:10), 18033 (1:1,000), NHS (1:500) and golgin 97 (1:200).
51| Wash the membrane with PBST three times, for 10 min each, on a rocking platform shaker.
52| Overlay the membrane with secondary HRP-conjugated antibodies of choice at their appropriate dilution in 5% (wt/vol) milk/PBST for 1 h at room temperature. Although this may vary from laboratory to laboratory, suggested dilutions for secondary HRP antibodies (see REAGENTS) are goat anti-human (1:20,000) and goat anti-mouse (1:2,000).
53| Repeat Step 51.
54| Detect bound antibody using ECL reagents (mixed in a 1:1 ratio) for 1 min. Remove excess ECL reagent and place in film cassette.
-
55| In the dark room, expose the film to the membrane for the desired length of time (start at 1 min up to a maximum of 15 min depending upon the type of film used). Develop in an automated film developer or in developing reagents (fixer and developer).
? TROUBLESHOOTING
56| To reuse membranes, wash them with three changes of PBST for 10 min each. Strip antibodies from antigens using stripping buffer (see REAGENT SETUP) for 45 min at 50 °C to allow reprobing using different antibodies in further WB analysis. Wash three times for 10 min with PBST and store in fresh PBST at 4 °C.
• TIMING
Steps 1–9, day 1, cell lysate preparation: 2.5 h
Steps 10 and 11, day 1, equilibration of sepharose beads: overnight
Steps 12–34, day 2, covalent coupling of antibodies to Protein A or G using DMP: 9.5 h
Steps 35–41, day 3, preclearing protein extracts and immunoprecipitation: 3.5 h
Steps 42–45, day 4, resolving GW182 and control immunoprecipitates on SDS-PAGE: 4 h
Steps 46 and 47, day 4: wet transfer to nitrocellulose membrane: overnight
Steps 48–56, day 5: detecting GW182, other RISC components or mRNA degradation proteins by WB analysis, film exposure and membrane regeneration: 5+ h
This protocol can be shortened to 4 d. Days 4 and 5 are the only days in the protocol that need to be consecutive.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason(s) | Suggested solution(s) |
|---|---|---|---|
| 9 | No protein detected by BCA protein assay or Nanodrop after the cell lysis procedure | Cell lysis conditions are not strong enough to disrupt the cells | Use a lysis buffer that includes a detergent. Detergents may include 0.5% Triton X-100 or 0.3–0.5% NP-40 |
| 34 and 55 | Heavy (50 kDa) and light (25 kDa) chains appear in gel stained with Colloidal Blue (Step 34) and on WB (Step 55) after coupling with DMP | Cross-linking of antibody to Protein A or G was not effective | Verify that the pH of DMP is between 8 and 9 before and after addition to beads as cross-linking efficiency is greatly reduced outside this pH range |
| Ensure that DMP is prepared in SBB immediately before use because of its instability in solution | |||
| 48 | Protein did not transfer from gel to membrane | Transfer not set up with gel and membrane positioned correctly | Proteins are negatively charged. Ensure correct gel–nitrocellulose orientation in the transfer cassette to allow migration of proteins from gel to nitrocellulose |
| Voltage or time not sufficient for proteins to transfer | Increase the voltage from 25 to 30 V over-night (14 h) and increase the voltage in the morning to 70 V for an additional 30 min to ensure that high molecular weight proteins have transferred. Transfer efficiency can be monitored by examining the molecular weight standard | ||
| Transfer method may have to be changed | If using a wet transfer method, switch to a semidry transfer method or vice versa | ||
| Membrane type may have to be changed | Switch from nitrocellulose membranes to PVDF membranes or vice versa | ||
| 55 | Protein(s) are not immunoprecipitated from the cell lysate | Antibody does not immunoprecipitate the protein of interest | Antibody may not work for IP |
| Obtain an antibody that has been shown to immunoprecipitate the protein of interest | |||
| Interactions between antibodies and proteins were disrupted during IP or wash steps | Use a less stringent IP buffer, i.e., with less salt, less detergent or less EDTA | ||
| Nonspecific proteins are immunoprecipitated from the total cell extract after the IP step | Antibodies bound nonspecifically to other proteins | Decrease the concentration of the primary antibody used. Alternatively, use a different antibody, perhaps a monoclonal antibody. Under certain conditions, a monoclonal antibody may bind a variety of epitopes on unrelated targets that bear similar linear or conformational domains | |
| IP and wash steps did not disrupt nonspecific interactions | Increase the stringency of the IP buffer, i.e., add more salt and gradually increase the number of detergents. Increase the number of wash steps or prolong washes | ||
| Protein bands are too dark on WB | Secondary antibodies may have expired or not stored correctly | Purchase new secondary antibodies | |
| Secondary antibodies may be too concentrated | Dilute the secondary antibody | ||
| Film may be overexposed | Expose film for less time. Usually, exposures are optimal between 1 and 10 min. However, WBs with human antibodies usually require less exposure (<1 min) | ||
| Protein bands are too light on WB | Secondary antibodies may have expired or not stored correctly | Purchase new secondary antibodies | |
| Secondary antibodies are not concentrated enough | Decrease the dilution of the antibody | ||
| Film may be underexposed | Expose film for more time. Some WBs may require exposure times of up to 10 min |
DMP, dimethyl pimelimidate dihydrochloride; EDTA, ethylenediamine tetraacetic acid; IP, immunoprecipitation; PVDF, polyvinylidene fluoride; WB, western blot.
ANTICIPATED RESULTS
The yield of protein from day 1 depends upon the volume of lysis buffer, cell type used and the growth pattern of the cell line. In general, approximately 1 × 109−1 × 1011 cells should be harvested to yield 12–20 μg of protein. Before IP, the success of the coupling procedure should be examined by resolving ‘Before’ coupling and ‘After’ coupling antibody-bead samples in 10% (vol/vol) SDS-PAGE (see example in Fig. 3). The antibody light and heavy chains should be visible on the gel in the ‘Before’ sample, but not in the ‘After’ sample, as the covalently coupled antibodies should remain bound to the beads after boiling in 2× LSB (Fig. 3). Ponceau S staining of nitrocellulose membranes after protein transfer should produce red or pink stained bands (Fig. 4). If the protein of interest is associated with GWB, optimal results would appear as clean, sharp bands in the 4B6 and 18033 IP lanes, with no bands present in the golgin 97, NHS and IC6 IP control lanes. Cell lysate extracts with no IP serve as a control to ensure that the antibody recognizing the protein of interest is working properly by WB analysis (Fig. 2d, extract lanes). Figure 2 shows a variety of typical IP-WBs using HeLa cells to examine the proteins associated with GWB. The antibodies were used as described by Moser et al.21. Figure 2a shows the detection of the 182-kDa GW182 by WB with monoclonal antibody 4B6 in the 18033 and 4B6 IPs but not in the control IPs (NHS, IC6 and golgin 97). This shows that commercially available 4B6 can be used effectively to IP GW182 and detect it by WB. The additional lower molecular weight bands observed with the 4B6 IP (Fig. 2a) is unexplained at this time but may be related to the observation that 4B6 binds to an epitope26 that is not shared by human anti-GWB sera tested to date27. Figure 2b shows the detection of GW182 at 182 kDa by WB with 18033 serum (contains autoantibodies directed to GWB) in the 18033 IP but not in the control NHS IP. This confirms that 18033 antiserum contains GW182 antibodies. Figure 2c shows the detection of Ago2 (the catalytic effector protein of the RISC) at ~95 kDa by WB analysis in GW182 IPs (18033 and 4B6) but not in the control IPs (NHS, IC6, golgin 97). This confirms previous observations that Ago2 is associated with GW182 (see refs. 28–34) and the apparent enrichment of Ago2 in the 18033 IP lane as compared with the 4B6 IP lane is due to the binding of 18033 to the Ago2 protein. Figure 2d shows that GWB contain proteins GW182, Ago2, XRN1 (exonuclease involved in 5′→3′ mRNA degradation) and Dicer (double-stranded RNA-specific endonuclease involved in RNAi). GW182 and Ago2 were enriched in the 18033 IP as compared with the cellular extract (4B6 and Ago2 WB), whereas XRN1 and Dicer were enriched in the cellular extract as compared with the 18033 IPs (XRN1 and Dicer WB). As expected, the 97-kDa golgin 97 protein was present in the extract but not in the 18033 IP. As indicated, control NHS is not expected to react with 18033 IP or the cellular extract.
Acknowledgments
This work was supported in part by the Canadian Institutes for Health Research Grant MOP-57674 and NIH Grant AI47859. M.J.F. holds the Arthritis Society Chair. J.J.M. is supported by a CIHR Doctoral Research Award in the Area of Clinical Research and by an Alberta Heritage Foundation for Medical Research Studentship Award.
References
- 1.Eystathioy T, et al. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol Biol Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 4.Mello CC, Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 5.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 6.Eystathioy T, et al. The GW182 protein co-localizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakymiw A, et al. The role of GW/P bodies in RNA processing and silencing. J Cell Sci. 2007;120:1317–1323. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- 9.Fillman C, Lykke-Andersen J. RNA decapping inside and outside of processing bodies. Curr Opin Cell Biol. 2005;17:326–331. doi: 10.1016/j.ceb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Andrei MA, et al. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HC, Cho H, Kim YK. Ectopic expression of eIF4E-transporter triggers the movement of eIF4E into P-bodies, inhibiting steady-state translation but not the pioneer round of translation. Biochem Biophys Res Commun. 2008;369:1160–1165. doi: 10.1016/j.bbrc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Lian S, et al. Small interfering RNAs-mediated silencing induces target-dependent assembly of GW bodies. Mol Biol Cell. 2007;18:3375–3387. doi: 10.1091/mbc.E07-01-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 14.Lau NC, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 15.Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008;183:441–455. doi: 10.1083/jcb.200807043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson P, Kedersha N. Stressful initiations. J Cell Sci. 2002;115:3227–3234. doi: 10.1242/jcs.115.16.3227. [DOI] [PubMed] [Google Scholar]
- 17.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 18.Kedersha N, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 20.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Moser JJ, Eystathioy T, Chan EKL, Fritzler MJ. Markers of mRNA stabilization and degradation, and RNAi within astrocytoma GW bodies. J Neurosci Res. 2007;85:3619–3631. doi: 10.1002/jnr.21439. [DOI] [PubMed] [Google Scholar]
- 22.Li S, et al. Identification of GW182 and its novel isoform TNGW1 as translational repressors in Ago-2-mediated silencing. J Cell Sci. 2008;121:4134–4144. doi: 10.1242/jcs.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng F, et al. A protocol for PAIR: PNA-assisted identification of RNA binding proteins in living cells. Nat Protoc. 2006;1:920–927. doi: 10.1038/nprot.2006.81. [DOI] [PubMed] [Google Scholar]
- 24.Tenenbaum SA, Carson CC, Lager PJ, Keene JD. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc Natl Acad Sci USA. 2000;97:14085–14090. doi: 10.1073/pnas.97.26.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda K, et al. Detection of the argonaute protein Ago2 and microRNAs in the RNA induced silencing complex (RISC) using a monoclonal antibody. J Immunol Methods. 2006;317:38–44. doi: 10.1016/j.jim.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eystathioy T, et al. A panel of monoclonal antibodies to cytoplasmic GW bodies and the mRNA binding protein GW182. Hybridoma Hybridomics. 2003;22:79–86. doi: 10.1089/153685903321947996. [DOI] [PubMed] [Google Scholar]
- 27.Eystathioy T, et al. Clinical and serological associations of autoantibodies to a novel cytoplasmic autoantigen, GW182 and GW bodies. J Mol Med. 2003;81:811–818. doi: 10.1007/s00109-003-0495-y. [DOI] [PubMed] [Google Scholar]
- 28.Jakymiw A, et al. Disruption of GW bodies impairs mammalianm RNA interference. Nat Cell Biol. 2005;7:1167–1174. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, et al. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7:1161–1166. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillai RS, et al. Inhibition of translational initiation by Let-7microRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 32.Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 34.Eulalio A, Huntzinger E, Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol. 2008;15:346–353. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]