Abstract
The smaller airways (<2 mm in diameter) offer little resistance in normal lungs but become the major site of obstruction in chronic obstructive pulmonary disease (COPD). We examined bronchiolar remodeling in COPD by combining quantitative histology, micro-computed tomography (CT), and gene expression studies. Volumes of bronchiolar tissue, total collagen, collagen-1, and collagen-3 were measured in lung tissue from 52 patients with different levels of COPD severity. Micro-CT was used to measure the number and lumen area of terminal bronchioles in four lungs removed before lung transplantation and in four donor lungs that served as controls. Laser capture microdissection provided 136 paired samples of bronchiolar and surrounding lung tissue from 63 patients and the gene expression of a cluster of tissue repair genes was compared. This study shows that total bronchiolar tissue decreased with progression of COPD and was associated with a reduction in total collagen and relative increase in collagen-3 over collagen-1. The micro-CT studies showed a 10-fold reduction in terminal bronchiolar number and a 100-fold reduction in lumen area. Interestingly, most genes associated with tissue accumulation during repair decreased their expression in both airways and in the surrounding lung as FEV1 declined, but eight genes previously associated with COPD increased expression in the surrounding lung tissue. Our study shows that small airway remodeling is associated with narrowing and obliteration of the terminal bronchioles that begins before emphysematous destruction in COPD and in relation to differential expression of tissue repair genes in the airways and surrounding lung.
Keywords: bronchiolitis, small airway obstruction, emphysema, COPD
Tissue remodeling can be defined by changes in quantity, composition, and organization of its structure and is a common feature of the repair of tissue damage (1). The primary lesions responsible for the airflow limitation that defines chronic obstructive pulmonary disease (COPD) obstruct the small conducting airways and result in the emphysematous destruction of the gas-exchanging tissues (2). The inhalation of toxic particles and gases, primarily but not exclusively as a result of inhaling tobacco smoke, is the major risk factor for the development of COPD (2, 3). This type of insult induces an inflammatory response in both animal (4) and human lungs (5–8), and in humans who have been chronically exposed over many years it persists long after they have stopped smoking (8–10). It is now known that everyone who smokes develops inflammation in the lung and that infiltration of the lung tissue by inflammatory immune cells increases in both extent and severity as COPD progresses (2, 8). Persistent inflammation is a cardinal feature of repetitive tissue injury at any site (1) and in the lung it is associated with secretion of mucus and squamous cell metaplasia of the airway epithelium, enlargement of the bronchial mucous glands, obstruction in the smaller conducting airways, and emphysematous destruction of gas-exchanging tissues of the lung (2). This presentation focuses on preliminary data from three separate sets of experiments that concern the remodeling of bronchiolar tissue, the changes that occur in terminal bronchiolar number and their minimal cross-sectional area as a result of this remodeling process, and some of the local differences in the expression of genes associated with tissue repair that might drive this process are discussed. These studies are ongoing in nature and are being conducted in collaboration with investigators at the University of Pittsburgh (Pittsburgh, PA, Washington University St. Louis, MO), and the University of Pennsylvania (Philadelphia, PA) (a list of collaborators is included at the end of the article).
THE NATURE OF BRONCHIOLAR REMODELING IN COPD
The preliminary data on the first of these three studies were obtained from lung tissue removed from persons who required surgery for either small lung tumors, lung volume reduction for emphysema, or lung transplantation for severe COPD under conditions that have been described in detail elsewhere (8). Those with normal spirometry (n = 10) served as control subjects for samples of lung from persons with mild (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 1, n = 11), moderate (GOLD stage 2, n = 11), severe (GOLD stage 3, n = 10), and very severe (GOLD stage 4, n = 10) COPD. The total volume of bronchiolar tissue and its collagen content was measured using the multilevel sampling design of Cruz-Orive and Weibel (11). That was modified by substituting high-resolution computed tomography (HRCT) to estimate total lung, tissue, and gas volumes (8). The volume of small airway tissue was obtained by determining the volume fraction (Vv) of the bronchiolar tissue by point counting the percentage of bronchiolar tissue in whole-mount histological sections prepared from samples of these lungs and multiplying this fraction by total tissue volume measured using the electronic record of the HRCT. The Vv of collagen within the bronchiolar tissue was similarly determined by point counting serial sections cut from the same tissue blocks that were stained with picrosirius red to identify all the collagen types (12) and multiplying the Vv picrosirius red–stained bronchiolar tissue by the Vv total bronchiolar tissue and by the same HRCT reference tissue volume. The Vv values of subsequent sections that took up immunostains for either collagen-1 or collagen-3 were then used in a similar manner to determine their contribution to total bronchiolar collagen.
These data showed a trend for the total volume of bronchiolar tissue to increase from 13.5 ± 2.6 ml in control cases to 20.0 ± 2.3 ml in moderately severe (GOLD stage 2) COPD. That was interrupted by a statistically significant decline to 11.8 ± 2.3 ml in very severe (GOLD stage 4) COPD (P = 0.016). The total volume of tissue stained by picrosirius red, which stains all forms of collagen, followed a similar pattern (5.3 ± 0.9 ml in control samples, and 8.3 ± 1.2 and 3.1 ± 0.6 in GOLD stage 2 and stage 4 COPD samples, respectively). However, this decline in total collagen was associated with a relative increase in the combined contribution of collagen-1 and -3 to the total collagen from 49% in control subjects to 84% in GOLD stage 4 subjects and a sharp decrease in other forms of collagen. Moreover, the ratio of collagen-1 to collagen-3 shifted from close to 1 in the control subjects to 0.44 in the GOLD stage 4 cases of COPD (P = 0.02), indicating a relative increase in collagen-3 over collagen-1 during this process. These data clearly show that the remodeling process changes the quantity, composition, and organization of the bronchiolar tissue in human lungs at different levels of COPD severity. The initial increase in bronchiolar tissue between control and moderate (GOLD stage 2) COPD was attributed to the generalized thickening of existing airways, due to the fact that their total number is complete by the first trimester of intrauterine life (13), making it unlikely that they would increase in number in adult life. In contrast, the decline in bronchiolar tissue volume observed in very severe (GOLD stage 4) COPD might well be explained by an obliterative process that removes these airways. Furthermore, the reduction in total tissue and collagen with a relative increase in collagen-3 compared with collagen-1 at the expense of all other forms of collagen is consistent with replacement of normal bronchiolar tissue by fibrosis (1).
EFFECT OF TISSUE REMODELING ON THE NUMBER AND CALIBER OF TERMINAL BRONCHIOLES
The second set of experiments was undertaken to test the hypothesis that removal of bronchioles accounted for the reduction in bronchiolar tissue observed in very severe (GOLD stage 4) COPD. This issue was previously addressed by Matsuba and Thurlbeck (14), who reported a small reduction in both number and lumen cross-sectional area of airways less than 2 mm in diameter in lungs with emphysema compared with those of control subjects. However, their report preceded the realization that counting the number of objects distributed in three-dimensional space, using the two-dimensional information provided by histology, requires the application of stereological principles (15, 16); the third dimension is obtained by cutting two sections a distance apart that is equal to 20–30% of the maximal height of the object under study. This procedure was developed to avoid the older “brute force” method of examining known volumes of tissue by serial histological sections (16). The introduction of micro-CT made it possible to examine relatively large volumes of tissue with sufficient resolution to observe and measure the histology of the human lung for the first time (17). In simple terms, micro-CT combines a microfocused X-ray source with planar X-ray detectors, with improved resolution obtained by arranging all pixels present in the detector onto a small area. For practical reasons the microsource X-ray tube and detectors remain fixed while the specimen placed between them is rotated to obtain the images required for CT. This procedure allows either small animals or small volumes of human tissue to be examined with increased resolving power. Therefore, we further modified the multilevel sampling design by using micro-CT to serially examine samples of human lung tissue of known volume to identify terminal bronchioles and count their number per milliliter of lung and measure the minimal cross-sectional area of these airways at the narrowest point (18, 19).
To date we have obtained data from four donor lungs that became available for research when no suitable recipient was identified within the required time frame. These lungs served as controls for four patients with very severe (GOLD stage 4) COPD, from whom lung was removed in preparation for treatment by lung transplantation. The mainstem bronchus of each specimen was first cannulated (20) and the lung was gently inflated to total lung capacity, using a constant flow of air and an underwater seal to slowly increase transpulmonary pressure from 0 to 30 cm H2O. The lung was then deflated to a transpulmonary pressure of 10 cm H2O and held in this position on the deflation limb of the pressure–volume curve while it was frozen solid by surrounding it in liquid nitrogen vapor. The lung was kept frozen by placing it in a Styrofoam box containing dry ice and a volumetric HRCT scan was obtained by passing the box containing the lung through the scanner. The specimen was maintained on dry ice while it was cut into 2-cm-thick slices, using a band saw, and these slices were sampled with a sharp metal cylinder precooled to dry ice temperature. Between 8 and 20 samples, 1 cm in diameter and 2 cm in length, were obtained from each lung for micro-CT examination. Each of these tissue cores was then fixed at −80°C in a solution of pure acetone and 1% glutaraldehyde (freezing point, less than −90°C) warmed to room temperature overnight, washed, and then postfixed in osmium and critical-point dried. The micro-CT scans were performed on these dried specimens at the University of Pennsylvania, using an eXplore Locus SP micro-CT scanner (GE Healthcare, Piscataway, NJ) at 16-μm voxel resolution with 1,000 contiguous slices per core. The image stack was then examined to identify and count terminal bronchioles by observing the point at which a purely conducting bronchiole branched into respiratory bronchioles and express this number of terminal bronchioles per milliliter of lung examined. A multiplanar reconstruction using DICOM (Digital Imaging and Communications in Medicine) imaging software (21) was used to reorient each terminal bronchioles in the x–y–z planes to allow examination of its lumen along its entire length and measure the cross-sectional area at its narrowest point. The total number of airways was calculated by multiplying the average number of terminal bronchioles per milliliter of lung by the total lung volume calculated from the HRCT and their total lumen cross-sectional area was determined by multiplying the average minimal lumen cross-sectional area by the computed number of terminal bronchioles in the lung.
The results obtained by micro-CT differ substantially from the earlier report by Matsuba and Thurlbeck (14) in that we found an approximate 10-fold reduction in terminal bronchiolar number and a 100-fold reduction in minimal terminal bronchiolar diameter in very severe (GOLD stage 4) COPD compared with control lungs (18, 19). However, the micro-CT results fit much better with physiological observations that the small airways cause little resistance to flow in the normal lungs but become the major site of airway obstruction in COPD (22–24). Because the bronchioles are arranged in parallel the total resistance of the bronchioles is the sum of the inverse of the resistance of the individual branches (i.e., 1/R = 1/R1 + 1/R2 + … + 1/Rn) and therefore one half the total number of airways must be removed simply to double bronchiolar resistance. On the other hand, a generalized narrowing of the lumen of these airways combined with their gradual removal, as suggested by our results, could easily explain the large increases in peripheral airway resistance measured in the late stages of COPD, because the change in resistance would be in proportion to the change in the radius of the airways raised to the fourth power. Therefore, on the basis of the micro-CT data we conclude that the remodeling of bronchiolar tissue in COPD results from a process that first narrows and then removes a large number of bronchioles from the lung. Moreover, we have postulated that the previously reported association between the thickening of airways less than 2 mm in diameter and a decline in FEV1 results from the removal of many airways, with a trend toward leaving behind those that have thickened their walls (8).
COMPARISON OF TISSUE REPAIR GENE EXPRESSION IN SMALL AIRWAYS AND SURROUNDING LUNG TISSUE
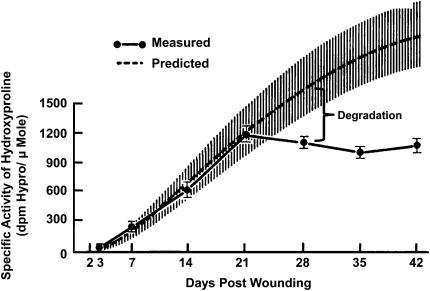
The concept that emphysematous destruction begins in terminal and preterminal bronchioles and spreads into the respiratory bronchioles was first suggested in the classic description of centrilobular emphysema by Leopold and Gough (25). Their analysis of 90 individual centrilobular lesions reconstructed from serial histological sections showed that the preterminal and terminal bronchioles leading into the destroyed respiratory bronchioles were infiltrated by a mixture of lymphocytes and plasma cells in 86% (78 of 90) of lesions and that connective tissue deposited within this inflammatory process thickened the walls and narrowed the lumen in 60% (54 of 90) of these airways. A more recent report has also shown that more of the variance in the association between airway pathology and decline in FEV1 was explained by thickening of small bronchi and bronchioles than by infiltration of any inflammatory cell types (8). Moreover, the micro-CT data presented previously show that the narrowing and removal of terminal bronchioles precede the onset of emphysematous destruction. Collectively, these data suggest that the close proximity of airway thickening to emphysematous destruction in COPD might result from a shift in the balance between synthesis and degradation that controls collagen deposition during tissue repair. The observation that collagen deposition in tissue undergoing repair depends on the balance between synthesis and degradation was first developed in studies of wound healing, in which radiolabeled proline was administered to animals followed by measurement of its incorporation into the hydroxyproline present in collagen deposited in test wounds. An early report from Madden and Peacock (26) using this technique showed (Figure 1) that collagen synthesis begins within a few days of injury and continues to be incorporated at a constant rate long after the total amount of collagen has stabilized within the wound. This indicates that total collagen deposited during the repair of damaged tissue is maintained at a constant level by continuous turnover that is based on a balance between synthesis and degradation of collagen. Similar observations have been made in lung tissue undergoing fibrosis (27) and in mice in which both wall thickness and hydroxyproline content in bronchoalveolar lavage fluid increase after chronic exposure to tobacco smoke (28, 29). An important difference between single and repetitive forms of injury is that the inflammatory process begins to disappear at about the same time that collagen synthesis starts after a single injury but persists in association with repetitive injury.
Figure 1.
The remodeling of repetitively damaged tissue. Shown is a comparison of collagen accumulation in a wound predicted from measurements of the rate of labeled hydroxyproline incorporation (upper curve) and actual values (lower curve). Difference between curves (brackets) indicates that deposition is determined by a balance between synthesis and degradation. Adapted by permission from Reference 26.
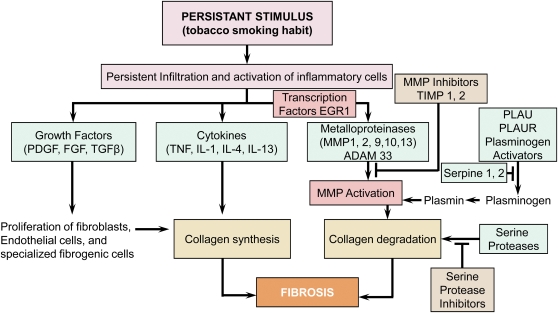
Figure 2 is modified from a standard textbook of pathology (1) to show some of the major genes controlling collagen deposition in repetitively damaged tissue. Figure 2 and the third set of experiments to be discussed here were designed to compare the pattern of expression of a signature set of wound-healing genes in the bronchiolar tissue with that present in the tissue immediately surrounding these airways. These experiments were based on lung tissue collected from 63 persons in a manner that has been previously described in detail (30). These subjects included 16 with normal lung function who served as control subjects for 18 with mild (GOLD stage 1), 17 with moderate (GOLD stage 2), and 12 with either severe (GOLD stage 3) or very severe (GOLD stage 4) COPD. All these tissues were filled with cryomatrix and rapidly frozen, and samples cut from them were used to prepare histological sections. Laser capture microdissection was then performed on these frozen sections to remove all the bronchioles present on each slide and collect them into a test tube. Each of these samples was then paired with the remaining lung tissue by simply scraping the remainder into a separate test tube. This procedure provided a total of 136 paired samples of small airways and surrounding lung tissue from these 63 persons; 35 from the 16 control cases, 37 from the 18 cases with GOLD stage 1, 36 from the 17 cases with GOLD stage 2, and 28 from the 12 cases with either GOLD stage 3 or GOLD stage 4 COPD. RNA isolated from these 136 paired samples was then amplified, converted to cDNA, and stored for subsequent examination of gene expression by real-time polymerase chain reaction. The expression of 54 genes that have been associated with the repair of repetitively damaged tissue (Figure 2) was then measured in each of these samples (30).
Figure 2.
The repair of repetitively damaged tissue. Shown is a diagram modified from Robbins and Cotran (1) to indicate that tobacco smoking is similar to other forms of repetitive injury in that it results in a persistent inflammatory response. Moreover, the cells participating in the persistent inflammatory process generate the transcription factors, growth factors, cytokines, and enzymes required to create the balance between collagen synthesis and degradation that determines the deposition of collagen in the tissue undergoing repair growth. The essence of our working hypothesis is that in the peripheral lung this balance favors deposition of collagen in the damaged bronchiolar tissue in the early stages of chronic obstructive pulmonary disease. However, this balance shifts toward degradation of the terminal bronchioles, leaving relatively thickened airways behind as the process spreads into the respiratory bronchioles to initiate emphysematous destruction.
The FEV1 was compared with the measured level of expression of each of these genes from each patient, using linear regression analysis (31). After corrections for false discovery rates, 8 of 12 genes that remained statistically significantly related to FEV1 increased their expression in the tissue surrounding the bronchioles as FEV1 declined. Interestingly, these eight genes (early growth response-1 [EGR1], matrix metalloproteinase [MMP]-1, MMP-9, MMP-10, urokinase plasminogen activator [PLAU], urokinase plasminogen activator receptor [PLAUR], tumor necrosis factor [TNF], and IL-13) have all been previously implicated in the pathogenesis of emphysema either in animal models or in human lung tissue. For example, the mouse model of emphysema produced by overexpression of IL-13 by Elias and colleagues has been shown to be caused by MMP-9 under the control of the transcription factor EGR1 (32, 33). Moreover, Ning and colleagues (34) also reported that EGR1 is increased in human lung tissue from patients with moderately severe COPD, and D'Armiento and colleagues (35, 36) reported that MMP-1 overexpression produced emphysema in both genetically manipulated mice and lung tissue from patients with COPD. Pathway analysis of a signature set of 203 differentially regulated genes by Wang and colleagues (37) identified PLAU and PLAUR expression as important in the progression of COPD. Furthermore, TNF has also been implicated in the pathogenesis of COPD in a variety of studies, but the enthusiasm for this concept has been dampened by the failure of anti-TNF therapy to modify the progression of COPD, suggesting that its role may not be central to the pathogenesis of this disease (38). Finally, Pinto-Plata and colleagues (39) identified MMP-10 as a possible biomarker for COPD, but excluded it from subsequent analysis because it lacked biological plausibility in COPD pathogenesis.
In sharp contrast, examination of the same gene set in the paired samples of bronchiolar tissue showed that 8 of the 10 genes that remained significantly related to the decline in FEV1 decreased their expression as COPD became more severe (31), suggesting that the genes associated with tissue repair during wound healing nearly all shut down. This analysis also failed to identify any associations between a decline in FEV1 and the expression of transforming growth factor (TGF)-β1 and/or other growth factors such as connective tissue growth factor, platelet-derived growth factor, and fibroblast growth factor that are downstream in this cascade (40). The failure to identify increased TGF-β1 expression as COPD becomes more severe was especially surprising considering the substantial literature implicating it in the pathogenesis of COPD (40–42). However, this shutdown of TGF-β1 and its downstream cascade becomes easier to understand if it were associated with both the disappearance of bronchioles and the onset of the emphysematous destruction as the quantitative histology and micro-CT studies presented earlier in this presentation suggest.
In summary, these preliminary results provide quantitative histological data showing that progression of COPD is associated with an overall reduction in bronchiolar tissue. In association with the micro-CT evidence, this reduction in bronchial tissue can be attributed to a remodeling process that first narrows and then removes a large number of bronchioles, leaving relatively thickened airways behind. Moreover, the micro-CT studies also show that this reduction in the terminal bronchioles begins before the onset of emphysematous destruction, indicating that it may begin early in the natural history of COPD. Finally, this modest survey of the expression of tissue repair genes during wound healing shows that a cluster of genes that have already been implicated in the pathogenesis of tissue destruction in COPD in both humans and in animal models increase their expression in the tissue surrounding the small airways as FEV1 declines. In contrast, most genes associated with tissue accumulation decreased their expression in bronchiolar tissue and showed a similar trend in the surrounding lung tissue. Collectively, these observations fit with the hypothesis originally put forward by Leopold and Gough (25) that centrilobular emphysema actually begins in the terminal and preterminal bronchioles before spreading into the respiratory bronchioles, but also extend their data by providing quantitative histology, micro-CT, and preliminary gene expression data showing that in addition to the thickening and narrowing of terminal bronchioles that they observed, the majority of the terminal bronchioles are removed before the onset of emphysematous destruction. Future studies of genome-wide expression in human lung tissue from patients with COPD could improve our understanding of these issues, especially if emphysematous destruction and airway narrowing and obliteration are used as the outcome variables rather than FEV1. Although we have made a modest beginning in this area with A. Spira and the Boston group (43), the results are too preliminary to discuss at this time.
Supported by the National Heart, Lung, and Blood Institute (NHLBI) (HL084922 and HL084948) and by the Canadian Institutes of Health Research (CIHR).
Conflict of Interest Statement: J.H. has received reimbursement for consultancies with Nycomed ($1,001–$5,000), and for research with GSK/CIHR ($50,001–$100,000). J.H. has also received funding for research through a noncommercial entity: the NIH ($100,001 or more). J.E.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.V.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
List of Collaborators by Institution: University of Pennsylvania: Pablo G. Sanchez, M.D., Joel D. Cooper, M.D., Alex C. Wright, Ph.D., Debra Horng, B.Sc., Warren B. Gefter, M.D., Leslie Litsky, M.D. Washington University in St. Louis: Richard Pierce, Ph.D., John A. Pierce, M.D., Jason Woods, Ph.D. University of Pittsburgh: Frank Sciurba, M.D., Steve Duncan, M.D. University of British Columbia: Ren Yuen, M.D., Ph.D., Masaru Susuki, M.D., Ph.D., Harvey Coxson, Ph.D., Peter D. Pare, M.D., Don Sin, M.D.
References
- 1.Robbins SL, Cotran R. Tissue renewal and repair. In: Pathologic basis of disease, 7th ed. New York: Elsevier Saunders; 2005. pp. 87–118.
- 2.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009;4:435–459. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al.; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev 2009;12:45–64. [DOI] [PubMed] [Google Scholar]
- 5.Di Stefano A, Turato G, Maestrelli P, Mapp CE, Ruggieri MP, Roggeri A, Boschetto P, Fabbri LM, Saetta M. Airflow limitation in chronic bronchitis is associated with T-lymphocyte and macrophage infiltration of the bronchial mucosa. Am J Respir Crit Care Med 1996;153:629–632. [DOI] [PubMed] [Google Scholar]
- 6.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med 1997;155:852–857. [DOI] [PubMed] [Google Scholar]
- 7.Retamales I, Elliott WM, Meshi B, Coxson HO, Pare PD, Sciurba FC, Rogers RM, Hayashi S, Hogg JC. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med 2001;164:469–473. [DOI] [PubMed] [Google Scholar]
- 8.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 9.Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis 1983;127:474–477. [DOI] [PubMed] [Google Scholar]
- 10.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koëter GH, Timens W. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000;55:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Orive LM, Weibel ER. Sampling designs for stereology. J Microsc 1981;122:235–257. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 1994;89:397–410. [DOI] [PubMed] [Google Scholar]
- 13.Bucher U, Reid L. Development of the intrasegmental bronchial tree: the pattern of branching and development of cartilage at various stages of intra-uterine life. Thorax 1961;16:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuba K, Thurlbeck WM. The number and dimensions of small airways in emphysematous lungs. Am J Pathol 1972;67:265–275. [PMC free article] [PubMed] [Google Scholar]
- 15.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 1984;134:127–136. [DOI] [PubMed] [Google Scholar]
- 16.Howard CV, Reed MG. Number estimation. In: Unbiased stereology: three dimensional measurement in microscopy. New York: Springer-Verlag; 1998. pp. 69–106.
- 17.Watz H, Breithecker A, Rau WS, Kriete A. Micro-CT of the human lung: imaging of alveoli and virtual endoscopy of an alveolar duct in a normal lung and in a lung with centrilobular emphysema—initial observations. Radiology 2005;236:1053–1058. [DOI] [PubMed] [Google Scholar]
- 18.McDonough JE, Sanchez PG, Cooper JD, Yuan R, Coxson HO, Elliott WM, Naiman D, Pochettino M, Horng D, Gefter WB, et al. The quantification of lung structure in COPD using micro-CT [abstract]. Am J Respir Crit Care Med 2008;179:A659. [Google Scholar]
- 19.McDonough JE, Sanchez PG, Elliott WM, Horng D, Gefter WB, Wright AC, Cooper JD, Hogg JC. Small airway obstruction in COPD [abstract]. Am J Respir Crit Care Med 2009;179:A2970. [Google Scholar]
- 20.Choong CK, Haddad FJ, Martinez C, Hu DZ, Pierce JA, Meyers BF, Patterson GA, Cooper JD. A simple, reproducible, and inexpensive technique in the preparation of explanted emphysematous lungs for ex vivo studies. J Thorac Cardiovasc Surg 2005;130:922–923. [DOI] [PubMed] [Google Scholar]
- 21.OsiriX Medical Image software, version 2.7.5. OsiriX Foundation, Geneva, Switzerland. Available from: www.osirix-viewer.com
- 22.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med 1968;278:1355–1360. [DOI] [PubMed] [Google Scholar]
- 23.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol 1983;55:1733–1742. [DOI] [PubMed] [Google Scholar]
- 24.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol 1992;72:1016–1023. [DOI] [PubMed] [Google Scholar]
- 25.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax 1957;12:219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden JW, Peacock EE Jr. Studies on the biology of collagen during wound healing. 3. Dynamic metabolism of scar collagen and remodeling of dermal wounds. Ann Surg 1971;174:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurent GJ. Lung collagen: more than scaffolding. Thorax 1986;41:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churg A, Tai H, Coulthard T, Wang R, Wright JL. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. Am J Respir Crit Care Med 2006;174:1327–1334. [DOI] [PubMed] [Google Scholar]
- 29.Gaschler GJ, Skrtic M, Zavitz CC, Lindahl M, Onnervik PO, Murphy TF, Sethi S, Stämpfli MR. Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am J Respir Crit Care Med 2009;179:666–675. [DOI] [PubMed] [Google Scholar]
- 30.Gosselink JV, Hayashi S, Chau E, Cooper J, Elliott WM, Hogg JC. Evaluation of small sample cDNA amplification for microdissected airway expression profiling in COPD. COPD 2007;4:91–105. [DOI] [PubMed] [Google Scholar]
- 31.Gosselink JV, Hayashi S, Elliott WM, Chan B, Sin DD, Pare PD, Pierce JA, Pierce RA, Patterson A, Cooper J, et al. Gene expression profiling of peripheral lung remodeling in COPD [abstract]. Am J Respir Crit Care Med 2008;179:A659. [Google Scholar]
- 32.Cho SJ, Kang MJ, Homer RJ, Kang HR, Zhang X, Lee PJ, Elias JA, Lee CG. Role of early growth response-1 (EGR-1) in interleukin-13–induced inflammation and remodeling. J Biol Chem 2006;281:8161–8168. [DOI] [PubMed] [Google Scholar]
- 33.Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13–induced inflammation and remodeling. J Clin Invest 2002;110:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 2004;101:14895–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell 1992;71:955–961. [DOI] [PubMed] [Google Scholar]
- 36.Foronjy RF, Okada Y, Cole R, D'Armiento J. Progressive adult-onset emphysema in transgenic mice expressing human MMP-1 in the lung. Am J Physiol Lung Cell Mol Physiol 2003;284:L727–L737. [DOI] [PubMed] [Google Scholar]
- 37.Wang IM, Stepaniants S, Boie Y, Mortimer JR, Kennedy B, Elliott M, Hayashi S, Loy L, Coulter S, Cervino S, et al. Gene expression profiling in patients with chronic obstructive pulmonary disease and lung cancer. Am J Respir Crit Care Med 2008;177:402–411. [DOI] [PubMed] [Google Scholar]
- 38.Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, Mahler D, Saadeh C, Siler T, Snell P, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:926–934. [DOI] [PubMed] [Google Scholar]
- 39.Pinto-Plata V, Toso J, Lee K, Park D, Bilello J, Mullerova H, De Souza MM, Vessey R, Celli B. Profiling serum biomarkers in patients with COPD: associations with clinical parameters. Thorax 2007;62:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59–69. [DOI] [PubMed] [Google Scholar]
- 41.Celedon JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, Reilly JJ, Kwiatkowski DJ, Chapman HA, Laird N, et al. The transforming growth factor-β1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD). Hum Mol Genet 2004;13:1649–1656. [DOI] [PubMed] [Google Scholar]
- 42.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor β1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1951–1957. [DOI] [PubMed] [Google Scholar]
- 43.Zeskind J, McDonough JE, Sanchez PG, Zhang S, Cooper JD, Liu G, Horng D, Wright AC, Hayashi S, Hogg JC, et al. Changes in gene and microRNA expression with progression of emphysematous destruction in COPD [abstract]. Am J Respir Crit Care Med 2009;179:A3001. [Google Scholar]