Abstract
The bronchial epithelium is the barrier to the external environment and plays a vital role in protection of the internal milieu of the lung. It functions within the epithelial-mesenchymal trophic unit to control the local microenvironment and help maintain tissue homeostasis. However, in asthma, chronic perturbation of these homeostatic mechanisms leads to alterations in the structure of the airways, termed remodeling. Damage to the epithelium is now recognized to play a key role in driving airway remodeling. We have postulated that epithelial susceptibility to environmental stress and injury together with impaired repair responses results in generation of signals that act on the underlying mesenchyme to propagate and amplify inflammatory and remodeling responses in the submucosa. Many types of challenges to the epithelium, including pathogens, allergens, environmental pollutants, cigarette smoke, and even mechanical forces, can elicit production of mediators by the epithelium, which can be translated into remodeling responses by the mesenchyme. Several important mediators of remodeling have been identified, most notably transforming growth factor-β, which is released from damaged/repairing epithelium or in response to inflammatory mediators, such as IL-13. The cross talk between the epithelium and the underlying mesenchyme to drive remodeling responses is considered in the context of subepithelial fibrosis and potential pathogenetic mechanisms linked to the asthma susceptibility gene, a disintegrin and metalloprotease (ADAM)33.
Keywords: epithelium, transforming growth factor, fibrosis, ADAM33, angiogenesis
AIRWAY REMODELING IN ASTHMA
Asthma is an inflammatory diseases associated with alterations in the normal architecture of the airway walls, termed airways remodeling (1). These structural changes are characterized by epithelial goblet cell hyperplasia and metaplasia, an increase in bronchial smooth muscles and new blood vessels, and deposition of interstitial collagens that extends beyond the thickened lamina reticularis to involve the entire inner airway wall in proportion to disease severity. Tissue remodeling is an early and consistent component of childhood asthma with several studies describing increased collagen deposition and thickening of the lamina reticularis, increased smooth muscle, and angiogenesis (2–4). Although no reticular basement membrane thickening can be found in wheezing infants (5, 6), the airway wall has been reported to be abnormal in infants who subsequently develop asthma (7).
THE EPITHELIAL MESENCHYMAL TROPHIC UNIT
The concept of the epithelial mesenchymal trophic unit (EMTU) was introduced by Plopper and Evans (8) and referred to the involvement of the airways structural cells in controlling the airway microenvironment during key processes, such as lung development, repair of damaged tissue, and regulation of the inflammatory response. They highlighted the role of the attenuated fibroblast sheath, a layer of large, flat stellate cells in close proximity to the epithelial/environmental interface that could respond in a local manner to various stimuli. These fibroblastic cells have the ability to differentiate into myofibroblasts that secrete extracellular matrix (ECM) proteins as well as proinflammatory mediators. The ECM influences cell behavior and contributes to structure and compartmentalization in the airways, and its composition determines the mechanical properties and elasticity of the tissue. Because the ECM compartment is dynamic, reflecting the net balance of synthesis and degradation, a shift in this balance toward increased matrix deposition can result in fibrosis leading to altered structure and abnormal mechanical properties. In asthma, we have postulated that susceptibility to injury and aberrant repair responses result in persistent activation of the EMTU leading to tissue remodeling and altered airway function (9, 10).
EPITHELIAL REPAIR
The bronchial epithelium is pivotally involved in provision of chemical, physical, and immunological barriers to the inhaled environment (11). These barriers serve to maintain tissue homeostasis, but when compromised the immunological barrier becomes activated to protect the internal milieu of the lung. This leads to an acute inflammatory response that can become pathological in the face of chronic activation. Thus, a rapid repair response is essential for restoration of tissue homeostasis.
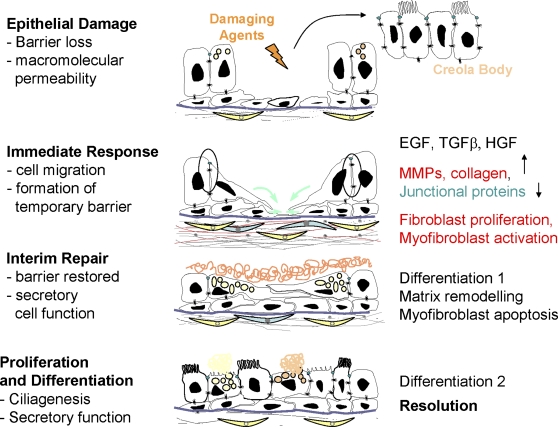
After epithelial injury, the process of epithelial repair can be considered to occur in a series of stages (Figure 1). The immediate response involves induction of cell migration leading to formation of a temporary barrier. Epithelial cells become migratory in response to growth factors, such as transforming growth factor (TGF)-β or epidermal growth factor (EGF), by undergoing an epithelial-to-mesenchymal transition (EMT) characterized by down-regulation of tight junctions and increased expression of matrix metalloproteases and ECM components. In other lung diseases, EMT has been attributed to pathological changes associated with tissue fibrosis (12); however, markers such as α-smooth muscle actin or vimentin are not expressed in asthmatic airway epithelium (13) and there is currently no evidence that epithelial cells invade the basement membrane and contribute to the mesenchymal compartment in asthma.
Figure 1.
Schematic representation of the stages of epithelial repair in asthma. EGF = epidermal growth factor; HGF = hepatocyte growth factor; MMP = matrix metalloprotease; TGF = transforming growth factor.
In parallel with epithelial activation, the underlying attenuated fibroblast sheath responds to epithelial injury by synthesis and deposition of a provisional matrix that helps seal the temporary barrier while the epithelium is compromised. This involves increased proliferation of fibroblasts and their differentiation into myofibroblasts. Both of these processes have been observed in asthma (14). As soon as the barrier has been reformed, the epithelial cells divide to replace cells that have been lost and these must undergo differentiation. This initially involves goblet cell differentiation allowing restoration of secretory function, as the secretions provide additional protection to the airways, and is followed by ciliogenesis to restore mucociliary clearance. As the epithelium recovers normal function, the provisional matrix that was laid down in the early stages of repair must be remodeled and degraded to restore normal tissue architecture. During this process, the myofibroblasts should undergo apoptosis to return the submucosal fibroblast number to normal. These processes eventually progress toward resolution and return of the tissue to its normal structure.
PRODUCTION OF MEDIATORS BY REPAIRING EPITHELIAL CELLS AND ACTIVATION OF THE EMTU
In asthma there is evidence that epithelial injury and repair are abnormal. Several studies have reported increased susceptibility to injury (15–17) and abnormal repair responses, including increased expression of the EGF receptor (EGFR) in bronchial biopsies from adults (18) and children (4) with asthma, as well as expression of the cyclin-dependent kinase inhibitor, p21waf1 (4, 19). Furthermore, in cultures of epithelial cells from children, the asthmatic airway epithelium displays a dysregulated repair response taking longer to repair mechanically induced wounds (20) and undergoing a more extensive EMT in response to TGF-β than cultures from donors without asthma (13).
To investigate the functional consequences of abnormal epithelial repair responses, early work by our group using bronchial epithelial cells damaged by scrape wounding showed that TGF-β2 was released as an early response to injury and that inhibition of repair using an EGFR tyrosine kinase inhibitor–augmented TGF-β2 release (18). More recent studies using differentiated epithelial cultures have confirmed that damage causes release of TGF-β and have shown that coculture of epithelial cells and fibroblasts results in sustained TGF-β release (21). In this coculture model, there was also marked synthesis of interstitial collagen, which was deposited in close proximity in basal surface of the epithelium, closely mirroring the thickening of the lamina reticularis seen in asthmatic bronchial biopsies. Other studies have used different forms of epithelial damage, including pneumatic compression (22, 23) or dynamic lateral compression (24), to induce epithelial damage and fibroblast activation, measured as increased proliferation, myofibroblast differentiation, or synthesis of extracellular matrix components.
The in vitro studies of the EMTU appear to model some of the changes seen in vivo after allergen challenge. In these studies, bronchial biopsies taken 7 days after allergen exposure showed significant increases in collagen deposition in the lamina reticularis, whereas the inflammatory response, which had increased 1 day after allergen exposure, had subsided (25). The collagen deposition might be explained by epithelial stress or injury caused by bronchoconstriction, as epithelial expression of TGF-β2 has been shown to increase after allergen challenge (26). However, it cannot be excluded that the allergen-induced remodeling changes are, in part, a consequence of mediators produced by inflammatory cells such as eosinophils. The persistence of remodeling changes after resolution of the inflammatory response might reflect different time courses for these processes. However, the marked increase in collagen deposition suggests that active remodeling and degradation of the surplus ECM is a relatively slow process and may be impaired in asthma. This aspect of the tissue repair and remodeling response merits further investigation.
A DISINTEGRIN AND METALLOPROTEASE 33 AND ACTIVATION OF THE EMTU
In addition to stimulation of ECM synthesis, TGF-β can elicit other responses in bronchial fibroblasts, including stimulating their proliferation and synthesis of a range of growth factors (27). It also has effects on the asthma susceptibility gene, a disintegrin and metalloprotease (ADAM)33, which has been implicated as an asthma remodeling gene.
ADAM33 was the first asthma susceptibility gene to be identified by positional cloning, showing associations with asthma and bronchial hyperresponsiveness (BHR) but not atopy (28). Replication of this association has been demonstrated in ethnically diverse populations (29–32) and a metaanalysis (33). ADAM33 polymorphisms have also been associated with more rapid decline in lung function in the general population (34), in asthma (35), and in COPD (34, 36). Furthermore, asthma-related single nucleotide polymorphisms in ADAM33 predict reduced lung function in young children (37), suggesting that the influences of ADAM33 commence early in life.
ADAM33 comprises 22 exons that encode a full-length molecule of 813 amino acids comprising several functional domains, including the pro-, metalloprotease (MP), disintegrin, cysteine-rich, EGF, transmembrane, and cytoplasmic domains (28). ADAM33 belongs to the ADAM family of multifunctional membrane-anchored glycoproteins whose unique structural organization enables their involvement in cell surface remodeling and ectodomain shedding and in mediating cell–cell and cell–matrix interactions (38, 39). ADAM33 protein is present in mesenchymal progenitor cells in developing lungs, whereas in adults its expression localizes predominantly to smooth muscle bundles in the conducting airways (40). This cellular provenance is consistent with the genetic association of ADAM33 with BHR. Although some studies have reported that ADAM33 is also expressed in airway epithelial cells (41, 42), these findings are controversial. We have found that the gene is silenced in epithelial cells because of DNA methylation of the ADAM33 promoter and we have failed to find evidence of ADAM33 mRNA expression in epithelial cells (43).
Although ADAM33 has been characterized at molecular and structural levels (44–46) and knockout mice generated (47), none of these studies has led to identification of a function for ADAM33 or elucidation of its contribution to asthma pathogenesis. However, discovery of a 55-kD soluble form of ADAM33 (sADAM33) containing the MP domain in bronchoalveolar lavage fluid (BALF) of subjects with asthma, but not healthy subjects, and demonstration that levels of sADAM33 were inversely correlated with lung function provided the first clue of a disease-related effect (48). As there was no evidence that it arose by alternative splicing (40, 45), we investigated whether sADAM33 could arise by ectodomain shedding as observed with other ADAMs (49). This led to the discovery that shedding of sADAM33 could be time- and dose-dependently enhanced by TGF-β50. Because release of sADAM33 might allow the MP enzyme access to substrates that would not normally be available to it when membrane-anchored, ectodomain shedding might give rise to a gain of function that contributes to structural remodeling in asthma.
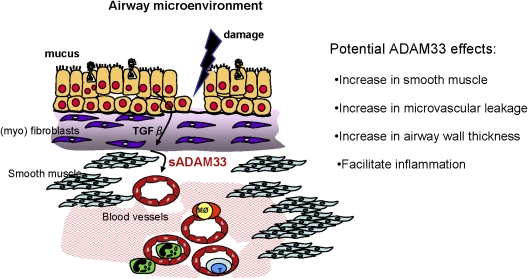
Using highly purified recombinant proteins, we have demonstrated that the purified MP domain or sADAM33 are proangiogenic using either in vitro, ex vivo, or in vivo assays (50), providing for the first time a biological function for ADAM33. This observation, together with the fact that TGF-β2 promotes ectodomain shedding, supports our concept of the importance of cell–cell communication and activation of the EMTU (Figure 2). Because in both children and adults angiogenesis is increased in asthma and correlates with reduced lung function (3, 51), the ability of sADAM33 to promote vessel formation may not only contribute to eventual inflammation in the lung but may also provide a source of nutrients for the developing smooth muscle.
Figure 2.
Model for the impact of the epithelial mesenchymal trophic unit on the production and function of the soluble form of a disintegrin and metalloprotease (sADAM)33 in asthma.
ADAM33 IN THE EARLY LIFE ORIGINS OF ASTHMA
Because most asthma has its origins early in life, we have also investigated the expression and function of ADAM33 in developing lungs and the impact of environmental factors linked to maternal allergy. We have found that Adam33/ADAM33 expression increases during early branching morphogenesis and again after birth when air breathing starts (52). To model potential interactions between the Adam33 locus and maternal allergy in vivo, we have used ova-sensitized allergic A/J mice (bhr1 locus-positive, which is syntenic to ADAM33). This revealed that maternal allergy significantly suppressed Adam33 mRNA in lungs of newborn pups, although processed Adam33 protein increased and several smaller isoforms, similar to sADAM33, were detected (52). If these effects of maternal allergy on Adam33 translate into human asthma, the presence of sADAM33 in amniotic fluid or BALF of genetically susceptible young children might reflect the early life influences of the maternal allergic environment on ADAM33 and may be a predictor for persistent wheezing in young children.
In summary, the cross talk between the epithelium and the underlying mesenchyme appears to be central in driving remodeling responses in asthma. The expression of the asthma susceptibility gene ADAM33 in the EMTU and its involvement with airway remodeling helps place these processes at the center of asthma pathogenesis. Further understanding of the function of this and other asthma genes and their interaction with environmental factors within the EMTU may help to identify novel therapies close to the origin of the disease.
Supported by the Medical Research Council UK, the Wellcome Trust, the Rayne Foundation, the Asthma Allergy and Inflammation Research Charity, and the Roger Brooke Charitable Trust.
Conflict of Interest Statement: D.E.D. served as a consultant for Synairgen Research Ltd, receiving $10,001–$50,000, and owns stocks or options of Synairgen plc.
References
- 1.Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:176–183. [DOI] [PubMed] [Google Scholar]
- 2.Cokugras H, Akcakaya N, Seckin I, Camcioglu Y, Sarimurat N, Aksoy F. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax 2001;56:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med 2006;174:975–981. [DOI] [PubMed] [Google Scholar]
- 4.Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax 2005;60:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med 2005;171:722–727. [DOI] [PubMed] [Google Scholar]
- 6.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med 2007;176:858–864. [DOI] [PubMed] [Google Scholar]
- 7.Pohunek P, Warner JO, Turzikova J, Kudrmann J, Roche WR. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol 2005;16:43–51. [DOI] [PubMed] [Google Scholar]
- 8.Evans MJ, van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol 2000;21:655–657. [DOI] [PubMed] [Google Scholar]
- 9.Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol 2000;105:193–204. [DOI] [PubMed] [Google Scholar]
- 10.Holgate ST, Davies DE. Airway inflammation and remodelling in asthma—cause and effect? The Immunologist 2001;8:131–135. [Google Scholar]
- 11.Swindle EJ, Collins JE, Davies DE. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J Allergy Clin Immunol 2009;124:23–34. [DOI] [PubMed] [Google Scholar]
- 12.Hodge S, Holmes M, Banerjee B, Musk M, Kicic A, Waterer G, Reynolds PN, Hodge G, Chambers DC. Posttransplant bronchiolitis obliterans syndrome is associated with bronchial epithelial to mesenchymal transition. Am J Transplant 2009;9:727–733. [DOI] [PubMed] [Google Scholar]
- 13.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am J Respir Crit Care Med 2009;180:122–133. [DOI] [PubMed] [Google Scholar]
- 14.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1990;3:507–511. [DOI] [PubMed] [Google Scholar]
- 15.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Howarth PH, Djukanovic R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol 2002;27:179–185. [DOI] [PubMed] [Google Scholar]
- 16.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 2007;120:1233–1244. [DOI] [PubMed] [Google Scholar]
- 18.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000;14:1362–1374. [DOI] [PubMed] [Google Scholar]
- 19.Puddicombe SM, Torres-Lozano C, Richter A, Bucchieri F, Lordan JL, Howarth PH, Vrugt B, Albers R, Djukanovic R, Holgate ST, et al. Increased expression of p21(waf) cyclin dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol 2003;28:61–68. [DOI] [PubMed] [Google Scholar]
- 20.Stevens PT, Kicic A, Sutanto EN, Knight DA, Stick SM. Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin Exp Allergy 2008;38:1901–1910. [DOI] [PubMed] [Google Scholar]
- 21.Thompson HG, Mih JD, Krasieva TB, Tromberg BJ, George SC. Epithelial-derived TGF-beta2 modulates basal and wound-healing subepithelial matrix homeostasis. Am J Physiol Lung Cell Mol Physiol 2006;291:L1277–L1285. [DOI] [PubMed] [Google Scholar]
- 22.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 2000;162:357–362. [DOI] [PubMed] [Google Scholar]
- 23.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol 2003;28:142–149. [DOI] [PubMed] [Google Scholar]
- 24.Choe MM, Sporn PH, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol 2006;35:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kariyawasam HH, Aizen M, Barkans J, Robinson DS, Kay AB. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med 2007;175:896–904. [DOI] [PubMed] [Google Scholar]
- 26.Torrego A, Hew M, Oates T, Sukkar M, Fan CK. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax 2007;62:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter A, Puddicombe SM, Lordan JL, Bucchieri F, Wilson SJ, Djukanovic R, Dent G, Holgate ST, Davies DE. The contribution of interleukin (IL)-4 and IL-13 to the epithelial- mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol 2001;25:385–391. [DOI] [PubMed] [Google Scholar]
- 28.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, Torrey D, Pandit S, McKenny J, Brausnschweiger K, et al. Association of the ADAM-33 gene with asthma and bronchial hyper-responsiveness. Nature 2002;418:426–430. [DOI] [PubMed] [Google Scholar]
- 29.Howard T, Postma D, Jongepier H, Moore W, Koppelman G, Zheng S, Xu J, Bleecker E, Meyers D. Association of a disintegrin and metalloprotease 33 (ADAM33) gene with asthma in ethnically diverse populations. J Allergy Clin Immunol 2003;112:717–722. [DOI] [PubMed] [Google Scholar]
- 30.Sakgami T, Hasegawa T, Yoshizawa H, Suzuki E, Koshino T, Gejyo F. ADAM33 Polymorphisms are Associated with Aspirin Intolerant Asthma in Japanese population. Am J Respir Crit Care Med 2003;167:A750. [Google Scholar]
- 31.Werner M, Herbon N, Gohlke H, Altmuller J, Knapp M, Heinrich J, Wjst M. Asthma is associated with single-nucleotide polymorphisms in ADAM33. Clin Exp Allergy 2004;34:26–31. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Park HS, Park SW, Jang AS, Uh ST, Rhim T, Park CS, Hong SJ, Holgate ST, Holloway JW, et al. ADAM33 polymorphism: association with bronchial hyper-responsiveness in Korean asthmatics. Clin Exp Allergy 2004;34:860–865. [DOI] [PubMed] [Google Scholar]
- 33.Blakey J, Halapi E, Bjornsdottir US, Wheatley A, Kristinsson S, Upmanyu R, Stefansson K, Hakonarson H, Hall IP. Contribution of ADAM33 polymorphisms to the population risk of asthma. Thorax 2005;60:274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Diemen CC, Postma DS, Vonk JM, Bruinenberg M, Schouten JP, Boezen HM. A Disintegrin A Metalloprotease 33 polymorphisms and lung function decline in the general population. Am J Respir Crit Care Med 2005;172:329–333. [DOI] [PubMed] [Google Scholar]
- 35.Jongepier H, Boezen HM, Dijkstra A, Howard TD, Vonk JM, Koppelman GH, Zheng SL, Meyers DA, Bleecker ER, Postma DS. Polymorphisms of the ADAM33 gene are associated with accelerated lung function decline in asthma. Clin Exp Allergy 2004;34:757–760. [DOI] [PubMed] [Google Scholar]
- 36.Gosman MM, Boezen HM, van Diemen CC, Snoeck-Stroband JB, Lapperre TS, Hiemstra PS, Ten Hacken NH, Stolk J, Postma DS. A Disintegrin and Metalloproteinase 33 and chronic obstructive pulmonary disease pathophysiology. Thorax 2007;62:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson A, Maniatis N, Jury F, Cakebread JA, Lowe LA, Holgate ST, Woodcock A, Ollier WER, Collins A, Custovic A, et al. Polymorphisms in A Disintegrin and Metalloprotease 33 (ADAM33) predict impaired early-life lung function. Am J Respir Crit Care Med 2005;172:55–60. [DOI] [PubMed] [Google Scholar]
- 38.Black RA, White JM. ADAMs: focus on the protease domain. Curr Opin Cell Biol 1998;10:654–659. [DOI] [PubMed] [Google Scholar]
- 39.Blobel CP. Remarkable roles of proteolysis on and beyond the cell surface. Curr Opin Cell Biol 2000;12:606–612. [DOI] [PubMed] [Google Scholar]
- 40.Haitchi HM, Powell RM, Shaw TJ, Howarth PH, Wilson SJ, Wilson DI, Holgate ST, Davies DE. ADAM33 expression in asthmatic airways and human embryonic lungs. Am J Respir Crit Care Med 2005;171:958–965. [DOI] [PubMed] [Google Scholar]
- 41.Foley SC, Mogas AK, Olivenstein R, Fiset PO, Chakir J, Bourbeau J, Ernst P, Lemiere C, Martin JG, Hamid Q. Increased expression of ADAM33 and ADAM8 with disease progression in asthma. J Allergy Clin Immunol 2007;119:863–871. [DOI] [PubMed] [Google Scholar]
- 42.Dijkstra A, Postma DS, Noordhoek JA, Lodewijk ME, Kauffman HF, Ten Hacken NH, Timens W. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virchows Arch 2009;454:441–449. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Haitchi HM, Cakebread J, Sammut D, Harvey A, Powell RM, Holloway JW, Howarth P, Holgate ST, and Davies DE. Epigenetic mechanisms silence a disintegrin and metalloprotease 33 expression in bronchial epithelial cells. J Allergy Clin Immunol 2008;121:1393–1399. [DOI] [PubMed] [Google Scholar]
- 44.Zou J, Zhu F, Liu J, Wang W, Zhang R, Garlisi CG, Liu YH, Wang S, Shah H, Wan Y, et al. Catalytic activity of human ADAM33. J Biol Chem 2004;279:9818–9830. [DOI] [PubMed] [Google Scholar]
- 45.Powell RM, Wicks J, Holloway JW, Holgate ST, Davies DE. The splicing and fate of ADAM33 transcripts in primary human airways fibroblasts. Am J Respir Cell Mol Biol 2004;31:13–21. [DOI] [PubMed] [Google Scholar]
- 46.Orth P, Reichert P, Wang W, Prosise WW, Yarosh-Tomaine T, Hammond G, Ingram RN, Xiao L, Mirza UA, Zou J, et al. Crystal structure of the catalytic domain of human ADAM33. J Mol Biol 2004;335:129–137. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Huang X, Sheppard D. ADAM33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol Cell Biol 2006;26:6950–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JY, Park SW, Chang HK, Kim HY, Rhim T, Lee JH, Jang AS, Koh ES, Park CS. A disintegrin and metalloproteinase 33 protein in patients with asthma: relevance to airflow limitation. Am J Respir Crit Care Med 2006;173:729–735. [DOI] [PubMed] [Google Scholar]
- 49.Gaultier A, Cousin H, Darribere T, Alfandari D. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J Biol Chem 2002;277:23336–23344. [DOI] [PubMed] [Google Scholar]
- 50.Puxeddu I, Pang YY, Harvey A, Haitchi HM, Nicholas B, Yoshisue H, Ribatti D, Clough G, Powell RM, Murphy G, et al. The soluble form of a disintegrin and metalloprotease 33 promotes angiogenesis: implications for airway remodeling in asthma. J Allergy Clin Immunol 2008;121:1400–1406. [DOI] [PubMed] [Google Scholar]
- 51.Vrugt B, Wilson S, Bron A, Holgate ST, Djukanovic R, Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J 2000;15:1014–1021. [DOI] [PubMed] [Google Scholar]
- 52.Haitchi H-M, Bassett D, Bucchieri F, Gao X, Powell R, Hanley NA, Wilson DI, Holgate ST, Davies DE. Induction of ADAM33 during embryonic lung development and the influence of interleukin-13 or maternal allergy. J Allergy Clin Immunol 2009;124:590–597. [DOI] [PubMed] [Google Scholar]