Abstract
There is now conclusive evidence that, as a group, subjects with asthma have lower levels of lung function as compared with their peers and that a significant proportion of subjects with persistent asthma are at risk of developing non–fully reversible airflow limitation, the clinical hallmark of chronic obstructive pulmonary disease. Although at the population level the most conspicuous form of airflow limitation in asthma seems to be that of subjects who wheeze during the first years of life and whose symptoms persist into adult life, asthma-related lung deficits can be related to both acquired deficits in growth of lung function in childhood and steeper decline of lung function in adult life. These trajectories of lung function are likely to differ across subgroups of individuals with asthma, suggesting that different windows of opportunity may exist to modify the natural course of the disease before irreversible deficits are established. These observations indicate the importance of identifying biomarkers that can be used to target children and adults with asthma at increased risk for airflow limitation and determining whether pharmacological interventions can protect these patients from the development of chronic obstructive pulmonary disease.
Keywords: asthma, chronic obstructive pulmonary disease, lung function, airflow limitation, FEV1
Both population-based and clinical studies have now clearly demonstrated that, as a group, subjects with asthma show significant lung function deficits as compared with their peers. These deficits appear within normal limits among most subjects with mild disease, but they may lead to impaired lung function and clinically manifest airflow limitation—as assessed by reductions in FEV1 and in the ratio between FEV1 and FVC—in cases of persistent and severe disease. Indeed, asthma has been shown to account for (or at least contribute to) 30 to 50% of cases of airflow limitation at the population level (1, 2). In addition, in some individuals with asthma lung function deficits appear also to be accompanied by a decrease in bronchodilator-mediated reversibility of airflow limitation over time (3–5). Taken together, these observations indicate that, in the long term, a significant proportion of subjects with persistent asthma may be at risk of developing non–fully reversible airway obstruction, the clinical hallmark of chronic obstructive pulmonary disease (COPD) (6, 7).
Several considerations point toward the importance of understanding the origins of these lung function deficits in asthma. First, although FEV1 levels are not the only factor taken into account to classify disease severity (8), they have long been known to be one of the major predictors of mortality among individuals with asthma (9). Second, lung function deficits, even those with magnitudes insufficient to cause clinically manifest functional impairment, may be related to molecular pathways that, in turn, increase susceptibility to the pulmonary effects of noxious environmental exposures, such as cigarette smoking and occupational hazards. Finally, subjects with asthma who develop non–fully reversible airflow limitation carry a morbidity and mortality burden comparable to that of patients with COPD (10–12), the fourth leading cause of death in the United States and one of the major causes of disability-adjusted life-years lost worldwide.
ASTHMA ACCOUNTS FOR A SUBSTANTIAL PROPORTION OF AIRFLOW LIMITATION IN THE GENERAL POPULATION
Epidemiological studies have consistently shown that in the general population subjects with asthma are more likely to report coexisting COPD clinical phenotypes than subjects with no asthma (13). These findings could be attributed, at least partly, to report or diagnostic bias. However, in a study of a random sample of adults, up to 55% of subjects with objectively assessed irreversible airflow limitation (i.e., FEV1/FVC less than 70% after bronchodilator administration) were found to have asthma, defined as either a postbronchodilator increase in FEV1 greater than 15% or peak flow variability greater than 20% during 1 week of testing, or as a physician diagnosis of asthma plus current symptoms or inhaler use (2). Thus, asthma accounts for (or at least contributes to) a substantial proportion of cases of airflow limitation in the general population. This observation is consistent with previous longitudinal findings demonstrating that subjects with active asthma have a greater than 10-fold increased risk of developing COPD as compared with subjects with no asthma (14). Of note, these cases of coexisting asthma and COPD appear to be associated with particularly elevated disease severity (13, 15), health care costs (11), and mortality risk (10).
LUNG FUNCTION TRAJECTORIES IN ASTHMA: EVIDENCE FROM ADULT COHORTS
These observations highlight the importance of determining the natural course of lung function through which subjects with asthma may develop FEV1 deficits and airflow limitation. Not surprisingly, there is no single answer to this question. Undoubtedly, asthma-related deficits of lung function may develop through different courses in subgroups of patients. The identification of such trajectories is complicated by the fact that, until data from prospective studies that monitored the same subjects from birth (or at least childhood) up to late adult life are available, this topic can be studied only separately in cohorts of children and adults. Thus, a thorough synthesis requires combining evidence and bridging results from both these types of studies.
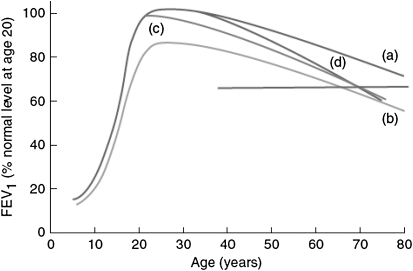
In principle, any subject—with or without asthma—may develop impaired levels of lung function in adult life through two main mechanisms: an incomplete growth of lung function in childhood (line b in Figure 1) or an acceleration of lung function decline in adult life (Figure 1, line d), or a combination of these factors. The possibility of an early start of lung function decline (Figure 1, line c) also exists, but this potential mechanism has not been investigated to the extent of the other two and is not discussed in this review.
Figure 1.
Hypothetical mechanisms that may lead to a critically low level of lung function in adult life and to chronic airway obstruction (horizontal line): line a, normal growth and decline; line b, impaired lung growth with a lower plateau phase but a normal rate of decline compared with line a; line c, normal plateau with rapid initial decline in lung function and a subsequent normal rate of decline; line d, normal plateau with normal initial rate of decline but a subsequent accelerated loss in lung function. Reprinted by permission from Reference 46.
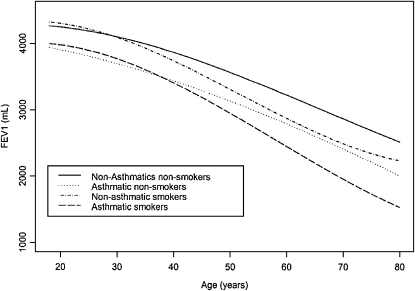
Whether an accelerated decline of lung function occurs in asthma has been the object of extensive research in adult cohorts. This question should be preferentially addressed in population-based studies because clinical studies are likely to enroll subjects with particularly severe and possibly progressive forms of the disease. Thus, although the findings of clinical studies have internal validity within populations of patients they can hardly be representative of the vast majority of asthma cases in the general population. In one of the largest population-based prospective studies, the Copenhagen City Heart Study, subjects who identified themselves as having asthma were found to have an excess FEV1 decline of 16 ml/year over a 15-year follow-up period as compared with subjects with no asthma (16). The magnitude of this effect appears remarkable when considering that the average FEV1 decline among subjects with no asthma was 22 ml/year. A subsequent report from the Busselton Health Study (17) addressed specifically the question of whether asthma is associated with both reduced lung function at the beginning of adult life and an increased rate of decline during adulthood. As compared with the Copenhagen study, asthma was found to be associated with a smaller, although still significant, 4-ml annual excess of FEV1 decline in adult life. In this regard, a few considerations are noteworthy. First, the authors used a somewhat more stringent definition of asthma, which also required the subject to report having been told by a doctor that they have asthma or to have been treated for asthma. Although the issues of report bias and reverse causality between asthma diagnosis and lung function impairment cannot be completely eliminated in this type of study, this approach is likely to reduce the impact of these limitations and the related misclassification between asthma and COPD. Second, in the Busselton study, in addition to an increased rate of FEV1 decline in adult life, asthma was also associated with significant FEV1 deficits that were already established by the beginning of adult life. As clearly shown in Figure 2, at least among males the magnitude of asthma-related FEV1 deficits that were established by age 19 years was greater than the magnitude of the additional deficits that were accumulated over the following 50 years of life as a result of the accelerated FEV1 decline associated with the disease. In other words, according to these findings the greater part of the lung function deficits that a 70-year-old male with asthma has accumulated over his life can be attributed to the tracking of FEV1 deficits that were already established by the time he entered adult life. Interestingly, this proportion appeared substantially smaller for females. Because—as compared with their male counterparts—females are less likely to have severe asthma in childhood but are more susceptible to adult-onset asthma, a possible explanation for these findings is that lung function trajectories in asthma may differ depending on whether the disease has its onset in childhood or becomes clinically manifest only in adult life.
Figure 2.
Decline of FEV1 over the span of adult life in male participants in the Busselton Health Study derived from linear mixed effects models. The continuous line refers to nonsmoking males without asthma; the dotted line to nonsmoking with asthma; the dashed and dotted line to smoking without asthma; and the dashed line to smoking with asthma. Reprinted by Reference 17.
This scenario is consistent with findings from a report from the Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD) (1), in which the natural course of lung function over the span of adult life was studied separately for subjects who developed persistent airflow limitation (i.e., an FEV1/FVC ratio consistently lower than 70% in multiple surveys) in association with no asthma, asthma onset occurring no later than 25 years of age, or asthma onset at greater than 25 years. As expected, the vast majority of subjects who developed persistent airflow limitation without asthma did so as a consequence of a steeper decline in lung function in adult life associated with cigarette smoking. Among these subjects, the excess loss of FEV1 between ages 25 and 75 years (as compared with control subjects) accounted for up to 80% of their lung function deficits at age 75 years. The accelerated loss of FEV1 also accounted for a significant proportion (60%) of lung function deficits shown in late adult life by subjects who developed persistent airflow limitation in association with adult-onset asthma. However, among subjects who developed persistent airflow limitation as a sequela of asthma that had its onset at no more than 25 years of age, the bulk of lung function deficits (∼75%) was already established by the time they entered adult life. These findings have critical implications for the prevention of lung function impairment associated with early-onset asthma because they point toward the importance of characterizing the clinical course of these subjects early in life when in principle it is still possible to modify the natural history of their disease. This has been the subject of several prospective studies from children's cohorts.
LUNG FUNCTION TRAJECTORIES IN ASTHMA: EVIDENCE FROM CHILDREN'S COHORTS
Several long-term studies of either unselected children from general population samples or children classified according to the severity of their asthma symptoms and monitored up to adult life have contributed important new information abut the origins of the deficits in lung function present in patients with asthma. In the Melbourne cohort (18, 19), children with various asthma-like phenotypes and a group of control subjects without asthma were enrolled at age 7 years during the early 1970s and an additional group of children with severe asthma was subsequently added to the cohort at the age of 10 years. At age 42 years, decades later, the deficits in lung function of all groups of subjects who had asthma in childhood, relative to their peers, were not larger that those observed by age 14 years in these same groups. Specifically, the group of subjects who had severe asthma in childhood was still mostly symptomatic at age 42, but their deficits in lung function had tracked with age from childhood and up to mid-adult life. Similar results were reported from the Dunedin birth cohort, in which more than 1,000 unselected newborns were monitored up to age 26 years with spirometric assessments performed every 2–5 years, starting at age 9 years (20). Subjects who had persistent asthma during follow-up or who relapsed after having remitted during adolescence had deficits in lung function that were already present at age 9, and showed little further deterioration thereafter.
If a significant proportion of the deficits in lung function growth in asthma is already present during the early school years, the question that remains unanswered from the previous studies concerns whether children with asthma are born with these deficits or whether they are acquired during the course of the disease. To answer this question, cohorts in which lung function was measured very early in life and before any respiratory events occurred were needed. Data from three such cohorts have become available. Using data from the Tucson Children's Respiratory Study, Morgan and colleagues (21) reported that children who wheezed during the first 3 years of life and were still wheezing at age 6 years (“persistent wheezers”) were also at high risk of continued wheezing beyond that age and up to age 16 years. When compared with children who did not wheeze during the first 6 years of life, their levels of airway function (measured using maximal expiratory flows) were slightly but not significantly lower at birth but became significantly lower at age 6 years, and tracked with age up to age 16. These results thus suggested that the deficits in lung function observed in children with asthma could be partly present at birth, but for the most part were acquired during the first years of life. In apparent contrast with these findings, Håland and colleagues (22) reported that children who had asthma at age 10 had significantly lower respiratory system compliance and lower values for a parameter derived from tidal breathing curves (the fraction of expiratory time to peak tidal expiratory flow to total expiratory time) measured shortly after birth compared with children with no asthma. Moreover, using data from an unselected birth cohort in Perth, Turner and colleagues (23) showed that children who had recent wheeze at 11 years of age had significantly reduced maximal expiratory flow rates at 1 month of age when compared with children with no recent wheeze. Children who wheezed between 4 and 6 years and whose wheezing persisted at 11 years were significantly more likely to have reduced maximal expiratory flows at 1 month than those who did not wheeze at either age period. These findings are consistent with the observation that birth weight, even when standardized for sex and gestational age, is an independent predictor of lung function up to the age of 50 years (24). Taken together, this evidence suggests that the deficits in lung function growth observed in school-age children with asthma are in part congenital but in a substantial proportion are also acquired during the course of the disease in the first years of life.
These studies suggested that most of the deficits in lung function observed in asthma developed very early in life or could even be present at birth in a proportion of subjects, but a legitimate criticism raised against this conclusion has been that these cohorts included a relatively small number of subjects with moderately severe asthma. In contrast, the Childhood Asthma Management Program (CAMP) randomized more than 1,000 children with asthma at a mean age of 8 years into three 4- to 6-year inhaler treatments: budesonide, nedocromil, or placebo (25). After the end of the trial, children were monitored up to a mean age of 16 years and lung function was assessed periodically. Regardless of therapy, 31–35% of participants had an FEV1/FVC ratio that was below the lower limit of normal at age 6–8 years, and this number steadily increased with age, reaching 52% at age 18 years (26). Using data from this same cohort, Covar and colleagues (27) identified a subgroup of patients comprising approximately one fourth of the population that showed at least a 1%/year deficit in growth of postbronchodilator FEV1% predicted during follow-up. These results thus suggest that, although 60% of lung function deficits observed by early adult life in children whose asthma started in early childhood are already present at age 6–8 years, further deterioration may indeed occur in a subgroup of at-risk individuals. Of particular interest was the observation that the subgroup of children with asthma who showed deficits in lung function growth was as likely to have mild asthma at randomization as the group that showed no such deficits. Moreover, there was no difference between the two groups in number of emergency care visits, days receiving inhaled corticosteroid, total inhaled corticosteroid dose, prednisone courses, and prednisone days from randomization to the end of the treatment period (27). Only hospitalization rates were slightly (and paradoxically) lower in children with as compared with those without deficits in lung function growth. Although concurrent exposures to cigarette smoke, allergens, and other environmental triggers may account for part of these differences, these findings support the hypothesis that the molecular determinants of impairment in lung function growth in children with asthma may be different from those that beget asthma symptoms (28). The lack of any effect on lung function impairment of 4–6 years of treatment with inhaled corticosteroids, which was clearly effective in controlling asthma symptoms and decreasing the incidence of asthma exacerbations in these same subjects, also argues in favor of parallel but distinct molecular pathways for asthma symptoms and chronic airflow limitation in asthma.
BEYOND THE NATURAL HISTORY: IDENTIFYING “SUSCEPTIBLE” SUBJECTS
The finding that trajectories of lung function differ across subgroups of subjects with asthma suggests that different windows of opportunity may exist to modify the natural course of the disease before irreversible deficits are established. However, which subjects are prone to develop such irreversible deficits remains largely unknown. Indeed, other than frequency of acute exacerbations (29, 30), a surprisingly small number of factors has been consistently linked to progression of lung function deficits and development of irreversible airflow limitation in subjects with asthma.
Prospective studies have shown that the degree of asthma-related phenotypes, such as eosinophilic inflammation—assessed both in the airways and in blood—bronchial hyperresponsiveness, and bronchodilator response appear positively associated with subsequent low lung function and development of airflow limitation in subjects with asthma and/or persistent wheezing (1, 4, 20). These observations appear in line with findings from cross-sectional studies on clinical cohorts of subjects with asthma that showed associations between eosinophilia, bronchial hyperresponsiveness, and atopic sensitization on the one hand and persistent airflow limitation and air trapping on the other (31–33).
Yet another, apparently conflicting line of evidence supports the notion that subjects with asthma who will go on to develop significant lung function deficits and irreversible airflow limitation have clinical and immunological phenotypes that resemble more closely those associated with COPD rather than those associated with traditional asthma. Elevated degrees of bronchial hyperresponsiveness and bronchodilator reversibility were indeed protective against the development of irreversible airflow limitation in a group of 228 Dutch patients with asthma monitored for 26 years (3). Similarly, among subjects with asthma, bronchial CD8+ T-cell infiltration was associated prospectively with decline in lung function (5) and both airway IL-8 production and neutrophilic inflammation were correlated with disease severity and airflow limitation (34–36).
Taken together, this evidence suggests that different molecular pathways are likely to be involved in determining progressive lung function deficits in asthma. Some of them appear to respond to the degree of atopy-related clinical phenotypes and eosinophilic inflammation and are probably predominant in most cases of severe childhood asthma. Others resemble immunological profiles associated with COPD and may be at work mainly in adult cases of overlapping asthma and COPD. Given this complexity, it is thus not surprising that we still lack established biomarkers to predict the course of lung function in individuals with asthma. Most likely—to be sufficiently specific and sensitive—future biomarkers will need to capture multiple molecular pathways that are involved in the progression of the disease. In line with these considerations, patients with asthma with persistent airflow limitation were found (37) to differ from patients with no airflow limitation by increased sputum levels of both helper T-cell type 2 cytokines, such as IL-13, and helper T-cell type 1 cytokines, such as IL-12 and IFN-γ, the serum levels of which had also been linked to FEV1 decline in a previous study on a representative group of 25 adults with asthma from the Normative Aging Study (38).
CONCLUSIONS
There is now conclusive evidence indicating that airflow limitation in asthma is related to both congenital and acquired deficits in airway function. Acquired deficits in growth in lung function (in the case of children) or steeper decline in lung function (in the case of adults) can occur at any age, but the most conspicuous form of airflow limitation in asthma seems to be that of subjects who wheeze during the first years of life and whose symptoms persist beyond that age and into adult life. Although some studies suggest that inhaled corticosteroids given very early during the course of the disease in older children and adults may prevent the development of airflow limitation in asthma (39), data from several large clinical trials indicate that inhaled corticosteroids have no effect on levels (40) and long-term growth (25) of lung function in asthma and do not change the natural course of the disease (41, 42). Post hoc analyses of the Childhood Asthma Management Program data strongly suggest that there are susceptible patients with asthma who are at high risk for the development of airflow limitation during the course of the disease (27). There is thus an urgent need to identify biomarkers that can be used to identify children and adults at increased risk for airflow limitation in asthma and to test the hypothesis that, analogous to what has been shown for cystic fibrosis (43, 44), nonsteroidal antiinflammatory drugs and therapies that can control neutrophilic and IL-8–mediated inflammation (45) can protect against the development of chronic obstructive pulmonary disease in patients with asthma.
Supported by grants HL064307, HL080083, HL056177, and HL095021 from the National Heart, Lung, and Blood Institute, and by a Parker B. Francis Fellowship (S.G.).
Conflict of Interest Statement: S.G. has received funding from a noncommercial entity, the NIH ($100,001 or more). F.M. has received reimbursement for consultancies with MedImmune ($5,001–$10,000), GlaxoSmithKline ($1,001–$5,000), and for serving on advisory boards with Merck ($5,001–$10,000) and MedImmune ($5,001–$10,000). He has received honoraria for lectures with Merck ($10,001–$50,000), and Genentech ($1,001–$5,000). He has also received funding from a noncommercial entity, the NIH ($100,001 or more).
References
- 1.Guerra S, Sherrill DL, Kurzius-Spencer M, Venker C, Halonen M, Quan SF, Martinez FD. The course of persistent airflow limitation in subjects with and without asthma. Respir Med 2008;102:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, Hansell AL, Nowitz MR, McNaughton AA, Soriano JB, et al. Proportional classifications of COPD phenotypes. Thorax 2008;63:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003;58:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J 1999;14:892–896. [DOI] [PubMed] [Google Scholar]
- 5.van Rensen EL, Sont JK, Evertse CE, Willems LN, Mauad T, Hiemstra PS, Sterk PJ. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med 2005;172:837–841. [DOI] [PubMed] [Google Scholar]
- 6.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2008; accessed August, 2009). Available from: http://www.goldcopd.com
- 8.Global Initiative for Asthma. Global strategy for asthma management and prevention, global initiative for asthma (updated 2008; accessed August, 2009). Available from: http://www.ginasthma.org
- 9.Lange P, Ulrik CS, Vestbo J; Copenhagen City Heart Study Group. Mortality in adults with self-reported asthma. Lancet 1996;347:1285–1289. [DOI] [PubMed] [Google Scholar]
- 10.Meyer PA, Mannino DM, Redd SC, Olson DR. Characteristics of adults dying with COPD. Chest 2002;122:2003–2008. [DOI] [PubMed] [Google Scholar]
- 11.Shaya FT, Dongyi D, Akazawa MO, Blanchette CM, Wang J, Mapel DW, Dalal A, Scharf SM. Burden of concomitant asthma and COPD in a Medicaid population. Chest 2008;134:14–19. [DOI] [PubMed] [Google Scholar]
- 12.Blanchette CM, Gutierrez B, Ory C, Chang E, Akazawa M. Economic burden in direct costs of concomitant chronic obstructive pulmonary disease and asthma in a Medicare Advantage population. J Manag Care Pharm 2008;14:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the UK. Chest 2003;124:474–481. [DOI] [PubMed] [Google Scholar]
- 14.Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004;126:59–65. [DOI] [PubMed] [Google Scholar]
- 15.Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, Aldington S, Beasley R. Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Respir J 2009;34:812–818. [DOI] [PubMed] [Google Scholar]
- 16.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998;339:1194–1200. [DOI] [PubMed] [Google Scholar]
- 17.James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, Musk AW. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med 2005;171:109–114. [DOI] [PubMed] [Google Scholar]
- 18.Oswald H, Phelan PD, Lanigan A, Hibbert M, Carlin JB, Bowes G, Olinsky A. Childhood asthma and lung function in mid-adult life. Pediatr Pulmonol 1997;23:14–20. [DOI] [PubMed] [Google Scholar]
- 19.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol 2002;109:189–194. [DOI] [PubMed] [Google Scholar]
- 20.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med 2003;349:1414–1422. [DOI] [PubMed] [Google Scholar]
- 21.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005;172:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Håland G, Carlsen KC, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, Carlsen KH. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med 2006;355:1682–1689. [DOI] [PubMed] [Google Scholar]
- 23.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Cox M, Young S, Goldblatt J, Landau LI, Le Souef PN. The relationship between infant airway function, childhood airway responsiveness, and asthma. Am J Respir Crit Care Med 2004;169:921–927. [DOI] [PubMed] [Google Scholar]
- 24.Tennant PW, Gibson GJ, Pearce MS. Lifecourse predictors of adult respiratory function: results from the Newcastle Thousand Families Study. Thorax 2008;63:823–830. [DOI] [PubMed] [Google Scholar]
- 25.Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–1063. [DOI] [PubMed] [Google Scholar]
- 26.Strunk RC, Weiss ST, Yates KP, Tonascia J, Zeiger RS, Szefler SJ. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006;118:1040–1047. [DOI] [PubMed] [Google Scholar]
- 27.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the Childhood Asthma Management Program. Am J Respir Crit Care Med 2004;170:234–241. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FD. Asthma treatment and asthma prevention: a tale of 2 parallel pathways. J Allergy Clin Immunol 2007;119:30–33. [DOI] [PubMed] [Google Scholar]
- 29.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007;30:452–456. [DOI] [PubMed] [Google Scholar]
- 30.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med 2009;179:19–24. [DOI] [PubMed] [Google Scholar]
- 31.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med 2001;164:744–748. [DOI] [PubMed] [Google Scholar]
- 32.Bumbacea D, Campbell D, Nguyen L, Carr D, Barnes PJ, Robinson D, Chung KF. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J 2004;24:122–128. [DOI] [PubMed] [Google Scholar]
- 33.Busacker A, Newell JD Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest 2009;135:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 1999;160:1532–1539. [DOI] [PubMed] [Google Scholar]
- 35.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 2007;132:1871–1875. [DOI] [PubMed] [Google Scholar]
- 36.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminska M, Foley S, Maghni K, Storness-Bliss C, Coxson H, Ghezzo H, Lemiere C, Olivenstein R, Ernst P, Hamid Q, Martin J. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol 2009;124:45–51.e1–e4. [DOI] [PubMed] [Google Scholar]
- 38.Litonjua AA, Sparrow D, Guevarra L, O'Connor GT, Weiss ST, Tollerud DJ. Serum interferon-γ is associated with longitudinal decline in lung function among asthmatic patients: the Normative Aging Study. Ann Allergy Asthma Immunol 2003;90:422–428. [DOI] [PubMed] [Google Scholar]
- 39.O'Byrne PM, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, Ullman A, Lamm CJ, Pauwels RA. Effects of early intervention with inhaled budesonide on lung function in newly diagnosed asthma. Chest 2006;129:1478–1485. [DOI] [PubMed] [Google Scholar]
- 40.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet 2006;368:754–762. [DOI] [PubMed] [Google Scholar]
- 41.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–1997. [DOI] [PubMed] [Google Scholar]
- 42.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med 2006;354:1998–2005. [DOI] [PubMed] [Google Scholar]
- 43.Konstan MW. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr Opin Pulm Med 2008;14:567–573. [DOI] [PubMed] [Google Scholar]
- 44.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW III. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290:1749–1756. [DOI] [PubMed] [Google Scholar]
- 45.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med 2008;177:148–155. [DOI] [PubMed] [Google Scholar]
- 46.Guerra S, Martinez FD. Natural history. In: Barnes PJ, Drazen JM, Rennard SI, Thomson NC, editors. Asthma and COPD, 2nd ed. San Diego, CA: Academic Press; 2009. pp. 23–35.