Abstract
Biological signal transduction commonly involves cooperative interactions in the binding of ligands to their receptors. In many cases, ligand concentrations in vivo are close to the value of the dissociation constant of their receptors, resulting in the phenomenon of ligand depletion. Using examples based on rotational bias of bacterial flagellar motors and calcium binding to mammalian calmodulin, we show that ligand depletion diminishes cooperativity and broadens the dynamic range of sensitivity to the signaling ligand. As a result, the same signal transducer responds to different ranges of signal with various degrees of cooperativity according to its effective cellular concentration. Hence, results from in vitro dose-response analyses cannot be applied directly to understand signaling in vivo. Moreover, the receptor concentration is revealed to be a key element in controlling signal transduction and we propose that its modulation constitutes a new way of controlling sensitivity to signals. In addition, through an analysis of the allosteric enzyme aspartate transcarbamylase, we demonstrate that the classical Hill coefficient is not appropriate for characterizing the change in conformational state upon ligand binding to an oligomeric protein (equivalent to a dose-response curve), because it ignores the cooperativity of the conformational change for the corresponding equivalent monomers, which are generally characterized by a Hill coefficient  . Therefore, we propose a new index of cooperativity based on the comparison of the properties of oligomers and their equivalent monomers.
. Therefore, we propose a new index of cooperativity based on the comparison of the properties of oligomers and their equivalent monomers.
Introduction
Dose-response is one of the most common experimental approaches used by biologists to monitor the properties of signaling molecules. The power of this approach arises from the fact that the change in any quantifiable physiological response can be measured as a function of the chemical stimulus responsible. In some cases, the resulting curve is sigmoidal, which generally implies cooperative interactions between the binding sites for the ligand that initiates the response (but other explanations are possible — see below). In general, cooperativity (or ultrasensitivity) arises for numerous biological processes regulated by protein-protein or protein-ligand interactions involving multi-site proteins that transduce signals via conformational isomerization [1]–[3].
Cooperativity has been represented for numerous oligomeric protein systems by the allosteric model of concerted transitions [1]. The model is based on spontaneous transitions between two conformational states, designated  (for “tense”) and
(for “tense”) and 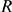 (for “relaxed”). The governing principle of the model is that in the absence of any bound ligands, the
(for “relaxed”). The governing principle of the model is that in the absence of any bound ligands, the 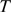 conformation is energetically favored over the
conformation is energetically favored over the 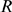 conformation. However, because the
conformation. However, because the 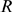 conformation has a higher affinity than the
conformation has a higher affinity than the 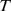 state for a ligand specific for the protein under consideration, the presence of ligand pulls the
state for a ligand specific for the protein under consideration, the presence of ligand pulls the  equilibrium towards the
equilibrium towards the 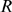 state. Under these conditions, a clear distinction can be made between two mathematical functions that describe the behavior of protein-ligand interactions as a function of ligand concentration: 1) the binding function,
state. Under these conditions, a clear distinction can be made between two mathematical functions that describe the behavior of protein-ligand interactions as a function of ligand concentration: 1) the binding function, 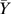 , defined as the fractional occupancy of the ligand binding sites of the protein, taking into account both the
, defined as the fractional occupancy of the ligand binding sites of the protein, taking into account both the 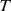 and
and 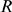 states; and 2) the state function,
states; and 2) the state function, 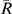 , defined as the fraction of molecules in the
, defined as the fraction of molecules in the 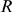 state. The state function
state. The state function 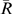 corresponds closely to what is measured by dose-response analysis for an allosteric “receptor” protein. The definitions of
corresponds closely to what is measured by dose-response analysis for an allosteric “receptor” protein. The definitions of 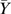 ,
, 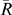 , and various related parameters are summarized in Table 1.
, and various related parameters are summarized in Table 1.
Table 1. Summary of terms for cooperativity and ligand depletion.
| Term | Description | Equation |

|
The concentration of ligand normalized to the affinity of the  state state |
3 |

|
The value of  corresponding to corresponding to 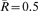
|
16 |
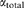
|
The value of  comprising both free and bound ligand comprising both free and bound ligand |
9 |

|
The ratio of ligand dissociation constants for the  and and  states states |
2 |
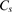
|
The molar concentration of ligand binding sites | 9 |

|
The allosteric constant governing the intrinsic 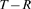 equilibrium equilibrium |
1 |
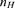
|
The Hill coefficient, defined by slope of loglog plot | 18 |
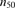
|
The Hill coefficient at 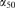
|
— |
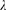
|
The allosteric constant governing the intrinsic equivalent monomers  - -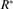 equilibrium equilibrium |
10 |

|
Cooperativity of the state function for an oligomer relative to the equivalent monomer | 15 |

|
The maximal value of  , which occurs at , which occurs at 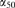
|
16 |

|
The ligand stabilization factor for  over over 
|
6 |

|
The “relaxed” (high affinity) conformational state | 1 |
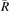
|
Fraction of total molecules ( and and  ) in the ) in the  state as a function of state as a function of 
|
5 |
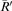
|
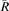 as a function of the total concentration ligand (free and bound) as a function of the total concentration ligand (free and bound) |
9 |
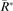
|
The fraction of equivalent monomers (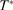 and and 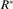 ) in the ) in the 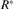 state state |
12 |

|
The “tense” (low affinity) conformational state | 1 |
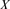
|
Any ligand | 3 |
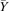
|
Fraction of all binding sites ( and and  ) occupied by ligand ) occupied by ligand |
4 |
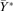
|
Fraction of equivalent monomer binding sites occupied by ligand | 17 |
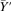
|
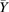 as a function of the total concentration of ligand (free and bound) as a function of the total concentration of ligand (free and bound) |
— |
From its initial application to the sigmoidal oxygen-binding curve of hemoglobin, cooperativity has been conveniently characterized by the Hill coefficient, 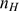 [4], [5]. The value of
[4], [5]. The value of 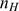 is obtained as the slope of the Hill plot: the logarithm of the ratio of occupied to unoccupied binding sites on the ordinate is given as a function of the logarithm of the ligand concentration on the abscissa. The value of
is obtained as the slope of the Hill plot: the logarithm of the ratio of occupied to unoccupied binding sites on the ordinate is given as a function of the logarithm of the ligand concentration on the abscissa. The value of 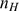 provides an empirical index of cooperativity: its upper limit is the number of interacting sites and its value is directly related to non-cooperative systems, since for a monomeric protein with a single site,
provides an empirical index of cooperativity: its upper limit is the number of interacting sites and its value is directly related to non-cooperative systems, since for a monomeric protein with a single site, 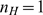 . The Hill coefficient is widely used, including for dose-response curves, but care must be taken in interpreting its value [6]–[8], since kinetic effects can alter apparent cooperativity [9] and even a monomeric enzyme can display cooperative behavior, i.e.
. The Hill coefficient is widely used, including for dose-response curves, but care must be taken in interpreting its value [6]–[8], since kinetic effects can alter apparent cooperativity [9] and even a monomeric enzyme can display cooperative behavior, i.e. 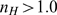 [10], [11].
[10], [11].
Cooperativity can also be generated by relatively simple networks [12], for example through competition between two sets of phosphorylation sites [13], as well as sequestration effects involving an inactive complex [14] or more complex signal transduction cascades [15]. The interpretation of values of 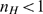 , which can be a sign of negative cooperativity [16], also requires careful attention, since even for hemoglobin, binding curves with
, which can be a sign of negative cooperativity [16], also requires careful attention, since even for hemoglobin, binding curves with 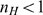 can be generated in the presence of non-stoichiometric concentrations of the positive effector, 2,3-diphosphoglycerate [17].
can be generated in the presence of non-stoichiometric concentrations of the positive effector, 2,3-diphosphoglycerate [17].
In addition to cooperativity, the non-linear properties of ultrasensitive systems define a dynamic range of signal intensities for which the responses vary. The greater the degree of cooperativity for a system with respect to signal changes, the narrower the dynamic range over which the response varies. For highly cooperative systems, such as bacterial chemotaxis, elaborate mechanisms have evolved in order to extent the dynamic range of response to changes in the concentrations of attractants or repellants [18], [19].
For all signal transduction systems considered, a predominant effect under physiological conditions is ligand depletion. When the concentrations of receptors are close to the dissociation constant for the relevant ligand, the free concentration of the ligand falls significantly below the total concentration of ligand, which in fact constitutes the actual input signal. This effect can be particularly important under in vivo conditions, for which most protein concentrations and dissociation constants are within the nano- to micro-molar range. The general principle of ligand depletion has been widely recognized [20]–[22] and various aspects have been considered for biological networks [14], [15], [23]. Here we focus on the consequences of ligand depletion with respect to cooperativity and dynamic range, as visualized for two extreme systems. First, we examine the highly cooperative flagellar motor system [24], [25]. Second, we turn to the minimally cooperative, but ubiquitous example of calmodulin [26], [27] in order to explore the consequences of ligand depletion under diverse conditions that apply in distinct regions of the brain and other organs. Finally, after illustrating why the Hill coefficient is not appropriate for measuring cooperativity of signal transduction, we define a new index of cooperativity,  , as illustrated with the classical example of the allosteric enzyme aspartate transcarbamylase [28], [29]. We show that
, as illustrated with the classical example of the allosteric enzyme aspartate transcarbamylase [28], [29]. We show that  , based on the introduction of an “equivalent monomer” concept, is a reliable measure of cooperativity for dose-response type curves under all conditions.
, based on the introduction of an “equivalent monomer” concept, is a reliable measure of cooperativity for dose-response type curves under all conditions.
Results
Ligand Depletion and Dynamic Range in the Flagellar Motor System
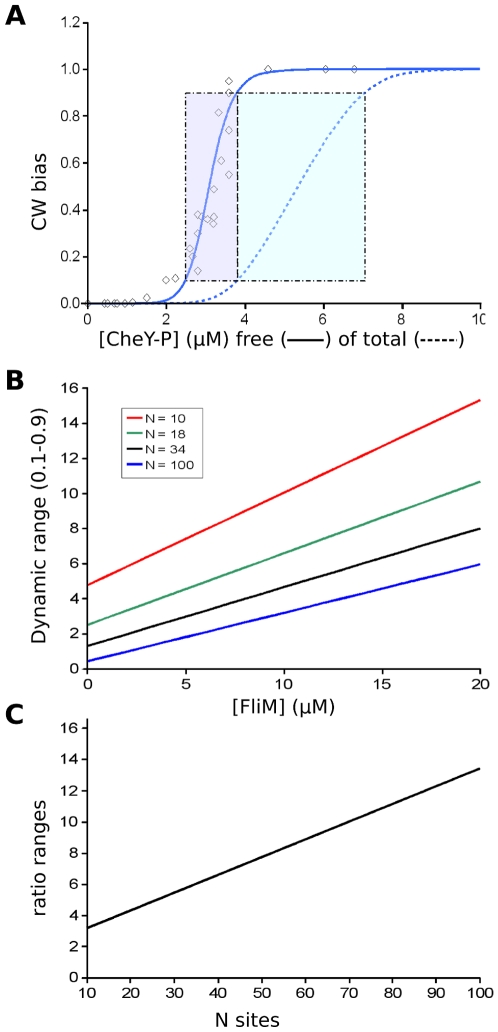
We illustrate the importance of considering ligand depletion with the highly cooperative E. coli flagellar motor system [30], which controls the direction of flagellar rotation in response to the concentration of phosphorylated CheY [31]. The rotational bias of individual motors as a function of CheY-P has been measured using tethered single cells and GFP-CheY [30]. The motor bias reflects a change of rotation from counter-clockwise to clockwise and therefore a change of fractional activation (or state function, 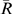 ), which is influenced by the interaction of CheY-P with the 34 units of FliM comprising the motor ring [32]. The data show a high degree of cooperativity, with Hill coefficients of up to 10 reported [30]. In contrast, the fractional occupancy, measured using FRET between CheY and FliM appears to be much less cooperative [31].
), which is influenced by the interaction of CheY-P with the 34 units of FliM comprising the motor ring [32]. The data show a high degree of cooperativity, with Hill coefficients of up to 10 reported [30]. In contrast, the fractional occupancy, measured using FRET between CheY and FliM appears to be much less cooperative [31].
For dose-response measurements it is reasonable to assume equivalence to within experimental errors of the concentrations of the free and total ligand only if the protein to which the ligand is bound is present at sufficiently low concentration compared to the dissociation constant. However, for the measurements of the flagella motor system, free and total ligand were determined directly and were found to be far from equivalent [31]. The free concentration is significantly reduced compared to the total concentration, due to binding to FliM, as well as to CheA and CheZ [33]. In order to characterize this effect, we define 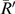 , the response as a function of the total ligand concentration, which is distinct from
, the response as a function of the total ligand concentration, which is distinct from 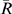 , the response as a function of the free ligand concentration (see Table 1).
, the response as a function of the free ligand concentration (see Table 1).
When ligand-depletion effects are taken into account, the curve for 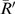 is displaced far to the right of the curve for
is displaced far to the right of the curve for 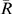 (Figure 1A). In addition,
(Figure 1A). In addition, 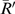 is significantly less steep than
is significantly less steep than 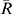 . Moreover, the effect of ligand depletion on response curves is exhibited by all cooperative frameworks based on thermal equilibria, not only strictly concerted-models, such as proposed by Duke et al [34]. Therefore, ligand depletion results in an increase in the dynamic range of signal concentrations sensed by the system, as measured for instance by the differences in total concentration of CheY-P corresponding to
. Moreover, the effect of ligand depletion on response curves is exhibited by all cooperative frameworks based on thermal equilibria, not only strictly concerted-models, such as proposed by Duke et al [34]. Therefore, ligand depletion results in an increase in the dynamic range of signal concentrations sensed by the system, as measured for instance by the differences in total concentration of CheY-P corresponding to 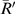 values between 0.1 and 0.9, which increase from
values between 0.1 and 0.9, which increase from 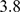 µM to
µM to 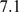 µM for a full change of response in this range. In comparison, the results presented using
µM for a full change of response in this range. In comparison, the results presented using 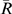 without taking into account ligand depletion could contribute to an underestimation of the dynamic range, since equivalent response changes would be achieved by increase from
without taking into account ligand depletion could contribute to an underestimation of the dynamic range, since equivalent response changes would be achieved by increase from 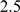 µM to
µM to 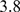 µM. With variations in the concentration of FliM, the dynamic range increases linearly (Figure 1B). More generally for multisite receptors, the dynamic range varies with the number of subunits, as observed for the family of curves in Figure 1B and 1C. Ligand depletion may also account for the discrepancies observed between the results reported by Cluzel et al [30] and other studies [35], [36] showing a much lower apparent cooperativity.
µM. With variations in the concentration of FliM, the dynamic range increases linearly (Figure 1B). More generally for multisite receptors, the dynamic range varies with the number of subunits, as observed for the family of curves in Figure 1B and 1C. Ligand depletion may also account for the discrepancies observed between the results reported by Cluzel et al [30] and other studies [35], [36] showing a much lower apparent cooperativity.
Figure 1. Flagellar motor model.
(A) Curves for 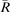 as a function of the concentration of free CheY-P (no ligand depletion: solid blue line) and curves for
as a function of the concentration of free CheY-P (no ligand depletion: solid blue line) and curves for 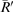 as a function of total CheY-P (with ligand depletion: dashed blue line), with
as a function of total CheY-P (with ligand depletion: dashed blue line), with 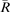 and
and 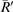 expressed in terms of CW bias, the measured parameter of the flagellar motor corresponding to the fraction of time undergoing clockwise rotation. The dynamic range, defined as the ligand concentration range between values of
expressed in terms of CW bias, the measured parameter of the flagellar motor corresponding to the fraction of time undergoing clockwise rotation. The dynamic range, defined as the ligand concentration range between values of 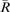 or
or 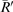 of 0.1 and 0.9, is represented by the shaded rectangles for the curves with and without ligand depletion. The open diamond points correspond to the measurements reported by Cluzel et al. [30]. (B) Variations in the dynamic range due to ligand depletion as a function of the concentration of FliM for values of
of 0.1 and 0.9, is represented by the shaded rectangles for the curves with and without ligand depletion. The open diamond points correspond to the measurements reported by Cluzel et al. [30]. (B) Variations in the dynamic range due to ligand depletion as a function of the concentration of FliM for values of 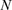 (the number of sites) = 10, 18, 34, and 100. For each value of
(the number of sites) = 10, 18, 34, and 100. For each value of 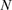 , the curve for
, the curve for 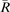 is computed based on an
is computed based on an 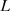 value set by
value set by 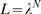 (see Materials and Methods, Eqn 11), where
(see Materials and Methods, Eqn 11), where 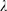 is fixed by the value used for
is fixed by the value used for 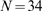 , i.e.
, i.e. 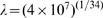 . (C) The ratio of the dynamic range for
. (C) The ratio of the dynamic range for 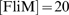 µM to the dynamic range for
µM to the dynamic range for 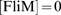 as a function of
as a function of 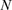 , the number of sites and calculated as in (B). Parameter values used for the curves in (A):
, the number of sites and calculated as in (B). Parameter values used for the curves in (A): 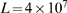 ,
, 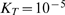 M,
M, 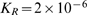 M, and
M, and 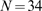 , with a concentration of
, with a concentration of 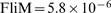 M. Calculation of ligand depletion effects as described in Eqn 9 of the Material and Methods section.
M. Calculation of ligand depletion effects as described in Eqn 9 of the Material and Methods section.
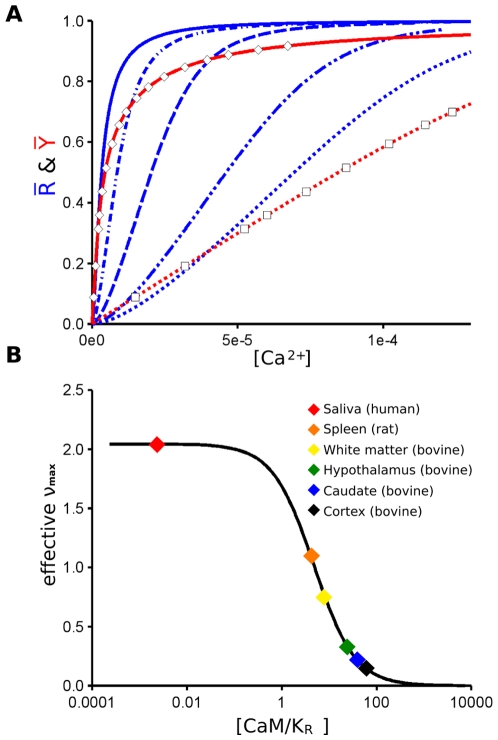
The Effect of Ligand Depletion on the Response Characteristics of Calmodulin
In contrast to the behavior of a system of high cooperativity as described above, we examined the properties of calmodulin, a key molecule of calcium signaling with relatively low cooperativity [37], for which an analysis based on the MWC model has recently been presented [38]. The protein exists as a small monomer (148 residues), with four distinct calcium binding sites, each characterized by specific dissociation constants for calcium that vary between the low-affinity and high-affinity states [38]. Although the reference ligand binding properties that we used for our analysis are free of ligand-depletion effects [39], we have transformed the data to simulate conditions of ligand depletion, with points that fit the curve for 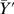 (the fractional occupancy as a function of the total calcium concentration) for calmodulin at
(the fractional occupancy as a function of the total calcium concentration) for calmodulin at 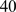 µM (Figure 2A). In addition, we have calculated a series of response curves presented in Figure 2A for the activation of calmodulin by calcium both under conditions with no ligand depletion (
µM (Figure 2A). In addition, we have calculated a series of response curves presented in Figure 2A for the activation of calmodulin by calcium both under conditions with no ligand depletion (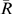 ), as well as under condition with ligand depletion (
), as well as under condition with ligand depletion (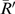 ) corresponding to various concentration of calmodulin found in vivo [40]. The differences between
) corresponding to various concentration of calmodulin found in vivo [40]. The differences between 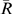 and
and 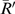 are very clear, including a progressive broadening of the dynamic range, with markedly diminished cooperativity as the concentration of calmodulin increases. The corresponding decreases in cooperativity as a function of calmodulin concentration are presented in Figure 2B, showing a dramatic fall off with concentration from the initial value
are very clear, including a progressive broadening of the dynamic range, with markedly diminished cooperativity as the concentration of calmodulin increases. The corresponding decreases in cooperativity as a function of calmodulin concentration are presented in Figure 2B, showing a dramatic fall off with concentration from the initial value 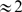 under conditions where Ca2+ is in large excess, to cooperativity values for the highest concentrations approaching zero.
under conditions where Ca2+ is in large excess, to cooperativity values for the highest concentrations approaching zero.
Figure 2. Ligand depletion for calmodulin.
(A) Curves for 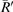 (blue) and
(blue) and 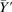 (red) as a function of the calcium concentration. (B) Values of effective cooperativity
(red) as a function of the calcium concentration. (B) Values of effective cooperativity 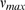 as a function of calmodulin (CaM) concentration/
as a function of calmodulin (CaM) concentration/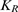 , where
, where 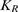 is the affinity of the
is the affinity of the 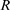 state for calcium. For the curves with solid lines in (A),
state for calcium. For the curves with solid lines in (A), 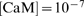 M and no ligand depletion occurs; the dashed curves for
M and no ligand depletion occurs; the dashed curves for 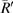 present conditions of ligand depletion based on the bovine brain calmodulin concentrations of white matter:
present conditions of ligand depletion based on the bovine brain calmodulin concentrations of white matter: 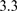 µM (- - - . . - - -); hypothalamus:
µM (- - - . . - - -); hypothalamus: 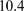 µM (- - - -); caudate nucleus:
µM (- - - -); caudate nucleus: 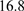 µM ( . . - . . ); and cortex:
µM ( . . - . . ); and cortex: 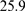 µM ( . . . . ), as reported by Kakiuchi et al. [40] or for
µM ( . . . . ), as reported by Kakiuchi et al. [40] or for 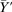 with the concentration of
with the concentration of 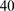 µM used in the measurements by Porumb [39], with data points shown as open squares. Although the calmodulin concentration of
µM used in the measurements by Porumb [39], with data points shown as open squares. Although the calmodulin concentration of 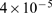 M [39] was close to the in vivo concentration of
M [39] was close to the in vivo concentration of 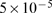 M in dendritic spines [60], the data were obtained by flow dialysis, which relates binding to the free calcium concentration, such that ligand depletion effects can be ignored, but we have transformed the data to simulate conditions of ligand depletion, with experimental points that closely follow the curve for
M in dendritic spines [60], the data were obtained by flow dialysis, which relates binding to the free calcium concentration, such that ligand depletion effects can be ignored, but we have transformed the data to simulate conditions of ligand depletion, with experimental points that closely follow the curve for 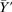 , the fractional occupancy as a function of the total calcium concentration. The same calcium concentrations in (A) are used for the calculations in (B), with the addition of a value for saliva and rat spleen [40], [61]. Other parameter values as published previously [38] obtained using data from several sources. The curves under conditions of ligand depletion in (A) are calculated as described in the legend to Figure 1. Cooperativity in B is expressed in relation to the effective value of the index
, the fractional occupancy as a function of the total calcium concentration. The same calcium concentrations in (A) are used for the calculations in (B), with the addition of a value for saliva and rat spleen [40], [61]. Other parameter values as published previously [38] obtained using data from several sources. The curves under conditions of ligand depletion in (A) are calculated as described in the legend to Figure 1. Cooperativity in B is expressed in relation to the effective value of the index 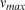 (Table 1), which decreases as a function of the total concentration of CaM.
(Table 1), which decreases as a function of the total concentration of CaM.
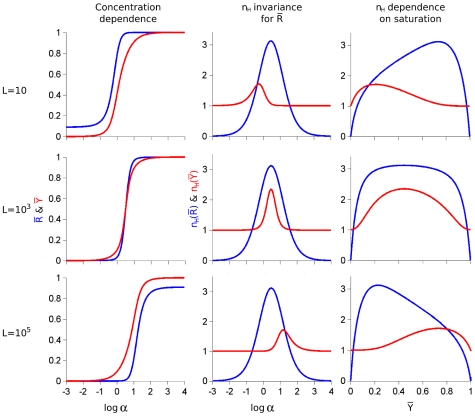
Limitations of the Hill Coefficient for Dose-Response Measurements and Introduction of a New Index Applied to Aspartate Transcarbamylase
Since cooperativity of binding is generally evaluated by the Hill coefficient, 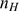 , it is not surprising that the Hill coefficient has also been used to characterize many cooperative biological processes, including the fractional activation of signaling receptors and other proteins. However, as we shall demonstrate here, for conformational isomerization of a multi-site protein,
, it is not surprising that the Hill coefficient has also been used to characterize many cooperative biological processes, including the fractional activation of signaling receptors and other proteins. However, as we shall demonstrate here, for conformational isomerization of a multi-site protein, 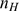 is not a reliable measure of cooperativity. In contrast to the cooperativity of
is not a reliable measure of cooperativity. In contrast to the cooperativity of 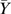 , which varies with the energy difference of the two conformational states, as specified by the conformational isomerization constant,
, which varies with the energy difference of the two conformational states, as specified by the conformational isomerization constant, 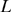 , the value of
, the value of 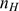 for
for 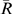 is independent of the value of
is independent of the value of 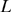 [41], as shown in Figure 3. When conditions of low, intermediate, and high affinity are examined for a hypothetical hexamer (Figure 3, left panels), the corresponding
[41], as shown in Figure 3. When conditions of low, intermediate, and high affinity are examined for a hypothetical hexamer (Figure 3, left panels), the corresponding 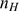 curves for cooperativity (Figure 3, middle panels) change appropriately for
curves for cooperativity (Figure 3, middle panels) change appropriately for 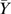 , but are identical for
, but are identical for 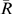 in the three cases. As a result, when cooperativity is examined as a function of
in the three cases. As a result, when cooperativity is examined as a function of 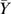 (Figure 3, right panels), the point of maximal cooperativity moves to the right for
(Figure 3, right panels), the point of maximal cooperativity moves to the right for 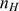 of
of 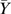 as affinity decreases, but the maximum value
as affinity decreases, but the maximum value 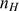 for
for 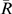 displays the opposite pattern.
displays the opposite pattern.
Figure 3. Dependence of 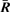 and
and 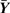 and their respective Hill coefficients (
and their respective Hill coefficients (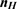 ) on the value of
) on the value of 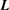 .
.
Three values of 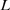 are illustrated, low
are illustrated, low 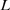 (
( ; top three panels); intermediate
; top three panels); intermediate 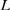 (
(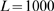 ; middle three panels — this value corresponds to the maximal cooperativity for the value of
; middle three panels — this value corresponds to the maximal cooperativity for the value of  used:
used: 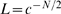 , where
, where 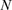 is the number of subunits or binding sites:
is the number of subunits or binding sites: 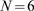 ); and high
); and high 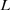 (
(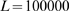 ; lower three panels). For each line of panels, the curves for
; lower three panels). For each line of panels, the curves for 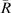 (blue) and
(blue) and 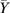 (red) are in the left panels, while the Hill coefficient (
(red) are in the left panels, while the Hill coefficient (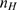 ) is presented as a function of
) is presented as a function of  (middle panels) or of
(middle panels) or of 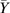 (right panels), in both cases for
(right panels), in both cases for 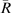 (blue) and
(blue) and 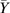 (red). The three panels of the central column illustrate that
(red). The three panels of the central column illustrate that 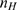 is invariant for
is invariant for 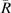 as function of
as function of 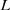 . Therefore, as function of
. Therefore, as function of 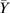 (three panels of the right column), the maximal value of
(three panels of the right column), the maximal value of 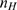 for
for 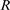 is at a high value of
is at a high value of 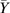 for low
for low 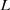 (upper right panel) and at a low value of
(upper right panel) and at a low value of 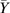 for high
for high 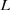 (lower right panel).
(lower right panel).
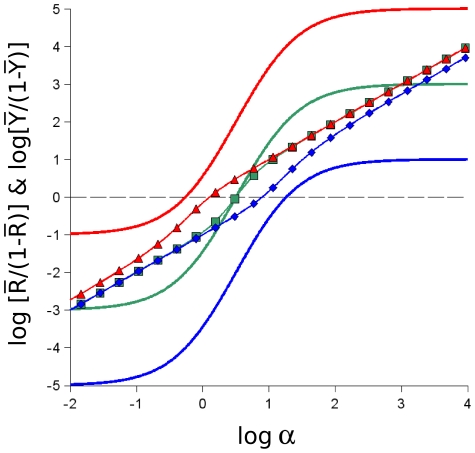
Since 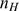 does not vary with the energetic difference of the two states, the shape of the curves for
does not vary with the energetic difference of the two states, the shape of the curves for 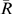 when expressed as Hill plots are invariant for different
when expressed as Hill plots are invariant for different 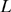 values, as shown in Figure 4. In contrast to the Hill plots of
values, as shown in Figure 4. In contrast to the Hill plots of 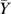 , for which the shape changes as a function of
, for which the shape changes as a function of 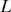 values, the curves for
values, the curves for 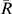 change only vertical position, not shape. Since cooperativity is generally measured around 50% response, correct results are obtained for
change only vertical position, not shape. Since cooperativity is generally measured around 50% response, correct results are obtained for 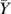 , but the apparent cooperativity of
, but the apparent cooperativity of 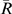 at 50%, i.e.
at 50%, i.e. 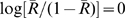 for a Hill plot, depends on the vertical position of the curve for
for a Hill plot, depends on the vertical position of the curve for 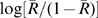 and is only a valid estimate of cooperativity for
and is only a valid estimate of cooperativity for 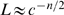 (Figure 4, green curve). The differences in shape between the curves for
(Figure 4, green curve). The differences in shape between the curves for 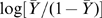 and
and 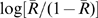 also explain why the cooperativity curves in Figure 3 (middle panels) tend towards
also explain why the cooperativity curves in Figure 3 (middle panels) tend towards 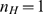 at the extremes for
at the extremes for 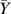 , but towards
, but towards 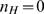 at the extremes for
at the extremes for 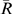 . Values of
. Values of 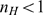 are commonly considered to be characteristic of negative cooperativity rather than the absence of cooperativity, but the properties of
are commonly considered to be characteristic of negative cooperativity rather than the absence of cooperativity, but the properties of 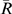 curves represent a special case for which the conventional reasoning does not apply. Overall, the analyses presented in Figures 3 and 4 make clear that as a general parameter to characterize
curves represent a special case for which the conventional reasoning does not apply. Overall, the analyses presented in Figures 3 and 4 make clear that as a general parameter to characterize 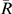 under any conditions, the Hill coefficient is not a reliable measure of cooperativity.
under any conditions, the Hill coefficient is not a reliable measure of cooperativity.
Figure 4. Hill plots for 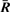 and
and 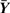 .
.
The data of Figure 3 (left column) are presented converted to the Hill plot, with the ordinate in the form of 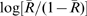 or
or 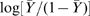 . For the three values of
. For the three values of 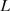 (
(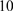 , red curves;
, red curves; 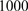 , green curves; or
, green curves; or 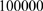 , blue curves) the data for
, blue curves) the data for 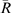 (solid lines) appear as parallel curves displaced vertically as a function of
(solid lines) appear as parallel curves displaced vertically as a function of 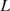 . In contrast, the data for
. In contrast, the data for 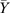 (triangles for
(triangles for  , open squares for
, open squares for 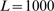 , diamonds for
, diamonds for 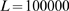 ) vary with the inflection points displaced progressively to the right with increasing magnitude of
) vary with the inflection points displaced progressively to the right with increasing magnitude of 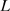 .
.
In order to overcome the limitations of the Hill coefficient applied to 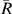 , we reexamined how cooperativity is computed for conformational isomerization using data for the allosteric enzyme aspartate transcarbamylase (ATCase), one of the original examples of allosteric phenomenon [42]. Following the formulation of the two-state MWC model [1], it was recognized that under many conditions,
, we reexamined how cooperativity is computed for conformational isomerization using data for the allosteric enzyme aspartate transcarbamylase (ATCase), one of the original examples of allosteric phenomenon [42]. Following the formulation of the two-state MWC model [1], it was recognized that under many conditions, 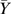 and
and 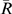 as a function of ligand concentration would not overlap [43]. In a classic study of ATCase, the direct binding of succinate (
as a function of ligand concentration would not overlap [43]. In a classic study of ATCase, the direct binding of succinate (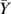 ) was compared to the succinate-dependent conformational change (
) was compared to the succinate-dependent conformational change (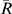 ) as measured by sedimentation or reactivity of protein sulfydryl groups [44], [45]. ATCase was initially characterized as a tetramer, but later studies revealed a hexamer [46], [47] and subsequent structural studies have thoroughly characterized the two hexameric conformational states, T and R, and their concerted interconversion [48], [49]. Using the parameters of the MWC model established for
) as measured by sedimentation or reactivity of protein sulfydryl groups [44], [45]. ATCase was initially characterized as a tetramer, but later studies revealed a hexamer [46], [47] and subsequent structural studies have thoroughly characterized the two hexameric conformational states, T and R, and their concerted interconversion [48], [49]. Using the parameters of the MWC model established for 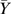 and
and 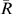 data on the basis of four sites, the theoretical curves were recalculated with six sites, as presented in Figure 5. Under the experimental conditions employed, the curve for
data on the basis of four sites, the theoretical curves were recalculated with six sites, as presented in Figure 5. Under the experimental conditions employed, the curve for 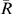 is substantially to the left of the curve for
is substantially to the left of the curve for 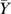 , which constituted strong evidence a conformational equilibrium pre-existing to ligand binding [45]. When the Hill coefficients are determined at 50% for both the
, which constituted strong evidence a conformational equilibrium pre-existing to ligand binding [45]. When the Hill coefficients are determined at 50% for both the 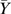 and
and 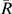 curves, the value of
curves, the value of 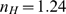 for
for 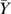 is a reliable measure of the cooperativity, but the value of
is a reliable measure of the cooperativity, but the value of 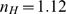 for
for 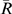 dramatically underestimates the intrinsic cooperativity, as we now demonstrate.
dramatically underestimates the intrinsic cooperativity, as we now demonstrate.
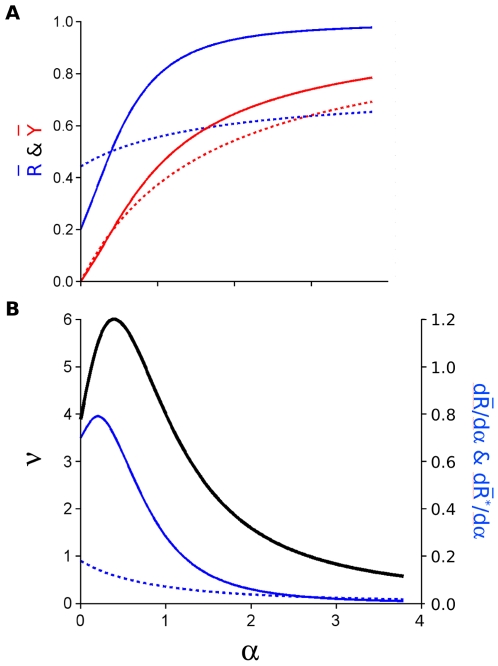
Figure 5. New measure of cooperativity for aspartate transcarbamylase based on an equivalent monomer.
(A) Curves for 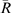 and
and 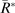 (in blue) and
(in blue) and 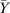 and
and 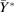 (in red) as a function of
(in red) as a function of  (
(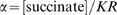 ); the curves for
); the curves for 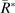 and
and 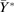 are dashed. (B) Values of
are dashed. (B) Values of  in black corresponding to the left ordinate and values of the derivatives
in black corresponding to the left ordinate and values of the derivatives 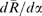 and
and 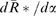 in blue corresponding to the right ordinate, with the latter as a dashed curve. While curves for
in blue corresponding to the right ordinate, with the latter as a dashed curve. While curves for 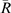 and
and 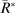 in A cross at
in A cross at 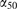 (defined by
(defined by 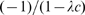 , with a value 0.5 (see Material and Methods, Eqn 16) at this point, the curves for
, with a value 0.5 (see Material and Methods, Eqn 16) at this point, the curves for 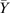 and
and 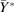 also cross at
also cross at 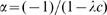 , but their value is
, but their value is 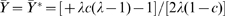 , which only equals 0.5 for
, which only equals 0.5 for 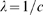 . For the conditions presented here, at the cross point:
. For the conditions presented here, at the cross point: 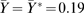 . The original analysis based on the MWC model with four subunits used the values of
. The original analysis based on the MWC model with four subunits used the values of 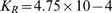 M,
M, 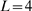 and
and 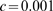 [45]. The model was re-analyzed by generating theoretical curves with the original parameters for a tetramer and performing a least-squares fit to obtain the best parameters for the hexamer, resulting in a change of the value of c to 0.26, when
[45]. The model was re-analyzed by generating theoretical curves with the original parameters for a tetramer and performing a least-squares fit to obtain the best parameters for the hexamer, resulting in a change of the value of c to 0.26, when 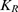 and
and 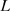 were unchanged. For ATCase, ligand depletion was not considered, since experimental results were obtained at concentrations of the enzyme for which ligand depletion was negligible and even in overproducing strains [62] ligand depletion is only a minor effect in vivo.
were unchanged. For ATCase, ligand depletion was not considered, since experimental results were obtained at concentrations of the enzyme for which ligand depletion was negligible and even in overproducing strains [62] ligand depletion is only a minor effect in vivo.
In order to establish the correct intrinsic cooperativity of an oligomeric protein undergoing conformational isomerization, a reference state is required that corresponds to a hypothetical equivalent monomer, characterized by same intrinsic affinities for ligand of the 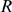 and
and 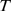 states. A conformational transition of the equivalent monomer as a function of the binding of its ligand can be defined and is represented here by
states. A conformational transition of the equivalent monomer as a function of the binding of its ligand can be defined and is represented here by 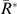 , along with the binding to the equivalent monomer represented by
, along with the binding to the equivalent monomer represented by 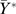 . For an equivalent monomer, the energy difference between the
. For an equivalent monomer, the energy difference between the 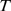 and
and 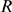 states is postulated to be
states is postulated to be 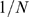 of the energy for the oligomer, since the energy difference for the oligomer is spread equally over the
of the energy for the oligomer, since the energy difference for the oligomer is spread equally over the 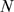 subunits. Therefore, we define
subunits. Therefore, we define 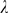 , a conformational isomerization parameter for the equivalent monomer, where
, a conformational isomerization parameter for the equivalent monomer, where 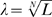 (see also Materials and Methods, Eqn 11). When the curves for
(see also Materials and Methods, Eqn 11). When the curves for 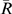 and
and 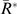 are compared as in Figure 5A, they cross at the value of 0.5 (which is true for all symmetrical MWC-type systems), but the curve for the equivalent monomer is clearly much more shallow.
are compared as in Figure 5A, they cross at the value of 0.5 (which is true for all symmetrical MWC-type systems), but the curve for the equivalent monomer is clearly much more shallow.
With respect to ligand binding, the curves for 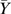 and
and 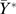 in Figure 5A differ only slightly and are characterized by Hill coefficients of
in Figure 5A differ only slightly and are characterized by Hill coefficients of 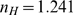 and
and 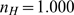 , respectively. In contrast, the
, respectively. In contrast, the 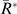 curve, with a Hill coefficient of
curve, with a Hill coefficient of 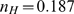 is much less cooperative than the curve for
is much less cooperative than the curve for 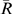 , with
, with 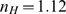 , exactly 6-fold higher than the value for
, exactly 6-fold higher than the value for 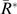 . In general, under virtually all conditions
. In general, under virtually all conditions 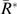 is characterized by a value of the Hill coefficient,
is characterized by a value of the Hill coefficient, 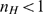 (see Figure 6).
(see Figure 6).
Figure 6. Properties of equivalent monomers.
(A) Dependence of the state function 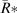 versus
versus  on the value of
on the value of  . Six values of
. Six values of  are presented corresponding to the color code indicated in the inset to the figure. (B) Value
are presented corresponding to the color code indicated in the inset to the figure. (B) Value 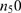 , the Hill coefficient at
, the Hill coefficient at  , as a function of the monomer transition parameter
, as a function of the monomer transition parameter 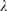 for the six values of
for the six values of  presented in (A) with the same color code.
presented in (A) with the same color code.
In order to overcome the insensitivity of 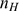 for
for 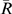 to
to 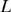 (Figure 3) and to rely on an appropriate reference state corresponding to the equivalent monomer, we propose replacing the Hill coefficient for dose-response type behavior by a new cooperativity index,
(Figure 3) and to rely on an appropriate reference state corresponding to the equivalent monomer, we propose replacing the Hill coefficient for dose-response type behavior by a new cooperativity index,  (Greek letter nu), based on the ratio of the derivatives of the functions for
(Greek letter nu), based on the ratio of the derivatives of the functions for 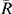 and
and 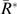 . The function
. The function  therefore corresponds to the ratio of the slopes exhibited by the responses of the cooperative protein and its equivalent non-cooperative monomer. When the new derivative functions are calculated, for
therefore corresponds to the ratio of the slopes exhibited by the responses of the cooperative protein and its equivalent non-cooperative monomer. When the new derivative functions are calculated, for 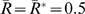 for the ATCase data in Figure 5, the values of the derivatives are 0.710 and 0.118, respectively, with a ratio of 6.0. The new cooperativity index
for the ATCase data in Figure 5, the values of the derivatives are 0.710 and 0.118, respectively, with a ratio of 6.0. The new cooperativity index  can also be computed directly from the definition of
can also be computed directly from the definition of 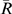 and
and 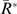 (see Material and Methods, Eqn 15). For ATCase, direct calculation also yields
(see Material and Methods, Eqn 15). For ATCase, direct calculation also yields 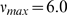 .
.
The revised analysis of ATCase illustrates that the intrinsic cooperativity at 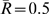 is always maximal, i.e. equal to the number of binding sites (
is always maximal, i.e. equal to the number of binding sites (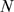 ), when compared to the equivalent monomer reference state for symmetrical oligomeric proteins. In other words, for a multi-site protein that undergoes a concerted conformational transition, as defined by the MWC model [1], the maximal cooperativity is always equivalent to the number of ligand-binding sites present and may be grossly underestimated on the basis of the Hill coefficient. This property reflects the absolute linkage, or infinite junctional energy, between binding sites in the MWC framework [34]. When data for the flagellar motor is re-examined in this context, the ratio of the derivatives of
), when compared to the equivalent monomer reference state for symmetrical oligomeric proteins. In other words, for a multi-site protein that undergoes a concerted conformational transition, as defined by the MWC model [1], the maximal cooperativity is always equivalent to the number of ligand-binding sites present and may be grossly underestimated on the basis of the Hill coefficient. This property reflects the absolute linkage, or infinite junctional energy, between binding sites in the MWC framework [34]. When data for the flagellar motor is re-examined in this context, the ratio of the derivatives of 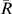 and
and 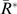 at 50% (Figure 1) corresponds precisely to the value of
at 50% (Figure 1) corresponds precisely to the value of 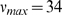 . The value of
. The value of  represents the intrinsic cooperativity of the protein and
represents the intrinsic cooperativity of the protein and 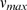 is not affected by ligand-depletion. For various signal transduction systems, the intrinsic cooperativity can, however, be modulated by ligand depletion effects. In order to characterize the effects of ligand depletion on cooperativity we calculated the effective
is not affected by ligand-depletion. For various signal transduction systems, the intrinsic cooperativity can, however, be modulated by ligand depletion effects. In order to characterize the effects of ligand depletion on cooperativity we calculated the effective 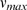 by correcting
by correcting  for the ratio of the slopes of
for the ratio of the slopes of 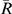 and
and 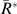 for corresponding fractional activations. As shown for calmodulin (Figure 2B), as for any sensor protein that possesses intrinsic cooperativity, ligand depletion can dramatically reduce the effective cooperativity in a physiological context. Indeed, this effect can bring the effective cooperativity to near 0 (Figure 2B). Because of non-equivalence of the four calmodulin ligand-binding sites, the non-identical dissociation constants for the sites result in the value of
for corresponding fractional activations. As shown for calmodulin (Figure 2B), as for any sensor protein that possesses intrinsic cooperativity, ligand depletion can dramatically reduce the effective cooperativity in a physiological context. Indeed, this effect can bring the effective cooperativity to near 0 (Figure 2B). Because of non-equivalence of the four calmodulin ligand-binding sites, the non-identical dissociation constants for the sites result in the value of 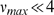 in Figure 2B.
in Figure 2B.
Discussion
Since many cellular control networks involve cooperative interactions among their components, modeling in the context of complete systems requires accurate estimations of the cooperativity of individual reactions. Since ligand depletion can exert an attenuating effect on cooperativity, it is important to have reliable estimates in the absence of ligand depletion. As illustrated in Figure 3, the Hill coefficient as applied to the state function of the MWC model, 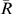 (equivalent to a dose-response curve) clearly does not reflect the correct cooperativity of the response, due to the invariance in the shape, as visualized in the Hill plot presented in Figure 4. As a result, when applied to the classical allosteric enzyme, aspartate transcarbamylase, the difference between the functions for ligand binding (
(equivalent to a dose-response curve) clearly does not reflect the correct cooperativity of the response, due to the invariance in the shape, as visualized in the Hill plot presented in Figure 4. As a result, when applied to the classical allosteric enzyme, aspartate transcarbamylase, the difference between the functions for ligand binding (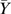 ) and change of conformational state (
) and change of conformational state (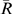 ) are not meaningfully characterized by their respective Hill coefficients. The value of
) are not meaningfully characterized by their respective Hill coefficients. The value of 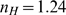 for (
for (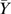 ) accurately reflects the correct degree of cooperative binding, since it contrasts with the non-cooperative case, with
) accurately reflects the correct degree of cooperative binding, since it contrasts with the non-cooperative case, with 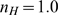 . In comparison, for
. In comparison, for 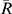 the observed value of
the observed value of  is not meaningful, since the non-cooperative case, as expressed by the corresponding “equivalent monomer,” displays a value of
is not meaningful, since the non-cooperative case, as expressed by the corresponding “equivalent monomer,” displays a value of 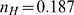 . The correct extent of cooperativity of
. The correct extent of cooperativity of 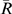 can be calculated from the ratio of these two values, or directly from the new index,
can be calculated from the ratio of these two values, or directly from the new index,  , as defined in Eqn 15, with
, as defined in Eqn 15, with 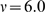 in the case of
in the case of 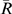 for ATCase.
for ATCase.
The results presented here demonstrate that neither dynamic range nor effective cooperativity are properties of sensing proteins that can be considered to be invariant; rather than are likely to vary according to the organ, tissue, or cell-type. The concentrations of most signaling proteins are similar to their dissociation constants, in the nano- to micro-molar range, as for example in the well-characterized compartment of the PSD signaling complex of dendritic spines [50]. For calmodulin, it is particularly clear that ligand-depletion is common under physiological conditions, as shown in Figure 2, with the exact consequences depending on the tissue. Related examples include the interaction of calmodulin with other downstream components, such as calcineurin in the micromolar range [51]. While dose-response curves provide the basic characterizations of “systems” and therefore lie at the core of pharmacological treatments, in the analyses presented here we show that dose-response parameters cannot be reused directly in models of signaling systems. Instead one needs to build “mechanistic” models and run parameter-fitting approaches for particular conditions. Although we emphasized the effects of ligand depletion using the allosteric model [1], the general conclusions would apply equally well to other mechanistic descriptions, including the classical Adair-Klotz formulation [52].
It is also important to emphasize that cooperativity and dynamic range can change with the level of expression of the sensor. It is known that the available pools of signaling proteins can be quickly modified by segregation, inhibition, or change in expression. Because of the extreme cooperativity of the flagellar motor, ligand depletion dramatically increases the dynamic range of the system, as shown in Figure 1, making this system extremely sensitive to concentration effects. Since flagellar protein concentration will ultimate influence these properties, it is therefore clear that by changing the number of motors, bacterial cells could enhance their adaptation properties. Since the number of flagella per bacterial cell can vary considerably [53], this parameter must be taken into account for any complete characterization of chemotaxis [54]. More generally, the use of ligand depletion could be a widespread physiological mechanism for cells to adapt non-linear properties and sensitivity ranges to evolving environmental conditions. Because ligand depletion can decrease the effective cooperativity of transducers in situ and increases the dynamic range, we propose that modifying the concentration of the sensor may be a powerful way to adapt quickly to a new environment and switch from a measurement mode to a detection mode.
As modeling of biological phenomena encompasses systems of increasing complexity, particularly in efforts to develop realistic models of the nervous system [55]–[59], it is important to represent the underlying molecular processes as accurately as possible. The results presented here, in line with other published findings [14], [15], [20]–[23], emphasize that cooperativity and its consequences, especially dynamic range, cannot be introduced into models as fixed parameters based on Hill coefficients estimated from in vitro studies. Rather, each set of reaction components must be evaluated separately with respect to effects of concentration in the system examined, in order to describe accurately the functional properties that apply.
Materials and Methods
Dose-Response Relationships for an Oligomeric Protein with Two Conformational States
We consider a multisite signaling protein that can interconvert between two functionally distinct conformational states, a more active state (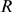 ) with a high affinity for ligand (
) with a high affinity for ligand (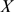 ) and a less active state (
) and a less active state (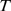 ), with a low affinity for the ligand. The partition between the two states is characterized by
), with a low affinity for the ligand. The partition between the two states is characterized by 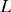 , the relative intrinsic stability of the two states in the absence of ligand:
, the relative intrinsic stability of the two states in the absence of ligand:
| (1) |
The affinities of the 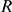 and
and 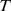 states for the specifically bound ligand are characterized by the intrinsic dissociation constants:
states for the specifically bound ligand are characterized by the intrinsic dissociation constants: 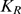 and
and 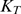 . For convenience, as originally proposed in the MWC model [1], the ratio of affinities is represented by
. For convenience, as originally proposed in the MWC model [1], the ratio of affinities is represented by  :
:
| (2) |
and the parameter  is defined as the normalized ligand concentration:
is defined as the normalized ligand concentration:
| (3) |
Using these parameters [1], for a protein with 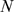 sites, the binding function is given by:
sites, the binding function is given by:
| (4) |
and the state function is given by:
| (5) |
In order to generalize Eqn 5 to multiple ligands, we introduce a new parameter,  , to describe the relative stabilization of the T state by a ligand:
, to describe the relative stabilization of the T state by a ligand:
| (6) |
For a protein with 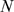 sites, at any concentration of
sites, at any concentration of 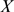 , the state function
, the state function 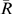 is then given with respect to
is then given with respect to  by:
by:
| (7) |
For  different ligands binding on multiple sites to the same protein,
different ligands binding on multiple sites to the same protein, 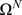 in the above equation is replaced by the product of
in the above equation is replaced by the product of 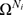 for the respective ligands:
for the respective ligands:
| (8) |
Since 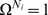 if the number of sites is 0, the concentration of the effector is 0, or the affinities for the
if the number of sites is 0, the concentration of the effector is 0, or the affinities for the 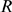 and
and 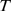 states are identical, this formula actually describes the absolute state function, modulated by any possible effector [43].
states are identical, this formula actually describes the absolute state function, modulated by any possible effector [43].
Calculation of Ligand Depletion
Under conditions of significant ligand depletion, i.e. ligand concentrations in the same range as dissociation constant, the degree of ligand binding to its receptor cannot be calculated directly from the total concentration, because only a fraction of this concentration is “free” and available to participate in the binding equilibrium. For any total concentration, the corresponding free concentration can be calculated with respect to a given receptor concentration as one of the roots of the appropriate second-order equation [22]. However, a simpler approach was used here. We define the parameter 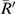 to define
to define 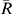 as a function of the total concentration. For each value of
as a function of the total concentration. For each value of 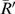 , the corresponding value of the total concentration, expressed as
, the corresponding value of the total concentration, expressed as 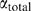 total, is calculated from the equation:
total, is calculated from the equation:
| (9) |
where 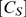 is the concentration of ligand binding sites. Multiplying
is the concentration of ligand binding sites. Multiplying 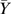 by
by 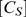 therefore provides a correction factor that when added to
therefore provides a correction factor that when added to 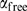 gives
gives 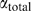 .
.
The Index of Cooperativity,  , for an Oligomer with Respect to Its Equivalent Monomer
, for an Oligomer with Respect to Its Equivalent Monomer
In order to evaluate the cooperativity of 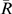 versus
versus  , it must be compared to the properties of a single-site “equivalent monomer.” For any conditions of
, it must be compared to the properties of a single-site “equivalent monomer.” For any conditions of 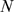 ,
, 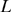 , and
, and  , we postulate an equivalent monomer with transitions between monomeric states
, we postulate an equivalent monomer with transitions between monomeric states 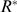 and
and 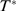 defined by:
defined by:
| (10) |
where
| (11) |
For a symmetrical system composed of identical ligand-binding sites, the fraction of monomers in the 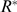 state is given by:
state is given by:
| (12) |
In this case, the curves for 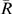 and
and 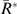 as a function of
as a function of  cross at
cross at 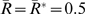 . The slopes of
. The slopes of 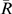 and
and 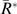 versus
versus  are obtained from, respectively, the following derivatives:
are obtained from, respectively, the following derivatives:
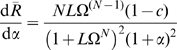 |
(13) |
and
| (14) |
The intrinsic cooperativity or amplification of the signal reflected by the properties of 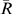 can then be obtained by a new parameter, represented by the coefficient
can then be obtained by a new parameter, represented by the coefficient  (the Greek letter nu) and calculated from the ratio of the two derivatives above (
(the Greek letter nu) and calculated from the ratio of the two derivatives above (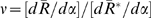 ) which simplifies to the equation:
) which simplifies to the equation:
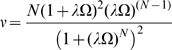 |
(15) |
The coefficient  gives the cooperativity of the oligomeric protein for the state function
gives the cooperativity of the oligomeric protein for the state function 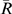 in a manner analogous to
in a manner analogous to 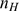 (the Hill coefficient) for the binding function (
(the Hill coefficient) for the binding function (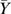 ), which describes cooperativity with respect to a monomer that in every case displays a value of
), which describes cooperativity with respect to a monomer that in every case displays a value of 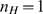 . In contrast, when applied to
. In contrast, when applied to 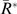 , the Hill coefficient is likely to be substantially less than 1 (see Figure 6B), demonstrating why the Hill coefficient is inappropriate for estimating the cooperativity of
, the Hill coefficient is likely to be substantially less than 1 (see Figure 6B), demonstrating why the Hill coefficient is inappropriate for estimating the cooperativity of  . For a given value of
. For a given value of 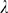 the lower limit of
the lower limit of 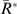 is given by
is given by 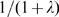 and the upper limit of
and the upper limit of 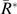 is given by
is given by 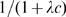 , with the curves for
, with the curves for 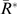 as a function of
as a function of  described in Figure 6A. The intersection of the curves for
described in Figure 6A. The intersection of the curves for 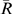 and
and 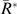 at 0.5 corresponds to the value of
at 0.5 corresponds to the value of  defined as
defined as 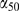 and is given by:
and is given by:
| (16) |
Under these conditions, 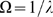 and the
and the 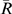 cooperativity parameter is at its maximal value:
cooperativity parameter is at its maximal value: 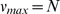 (whereas
(whereas 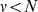 for all other values of
for all other values of  ).
).
Derivation of the Hill Coefficient for an Equivalent Monomer
With respect to ligand binding, compared to Eqn 4 for fractional ligand binding, 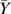 , within the context of the two state MWC model [1], the corresponding equation for fractional binding to the equivalent monomer,
, within the context of the two state MWC model [1], the corresponding equation for fractional binding to the equivalent monomer, 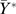 , is given by:
, is given by:
| (17) |
The Hill coefficient, 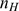 , is defined by the derivative:
, is defined by the derivative:
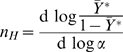 |
(18) |
Substituting Eqn 17 for 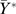 yields:
yields:
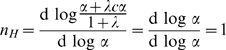 |
(19) |
In contrast, 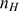 for
for 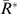 as defined by Eqn 12 yields the derivative:
as defined by Eqn 12 yields the derivative:
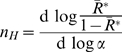 |
(20) |
Substituting Eqn 12 for 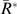 yields:
yields:
| (21) |
Therefore, since 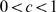 and
and 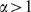 , it is clear that:
, it is clear that:
| (22) |
and hence for 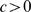 , the Hill coefficient for
, the Hill coefficient for 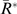 must be
must be 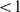 (additional details in M. Stefan, Thesis, University of Cambridge, 2009).
(additional details in M. Stefan, Thesis, University of Cambridge, 2009).
Acknowledgments
The authors are grateful to Philippe Cluzel and Victor Sourjik for their raw data and enlightening discussions and to Rava A. da Silveira and Nick Goldman and his group for help with the mathematics.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: S.E. acknowledges support from the Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 2.Bray D, Duke T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu Rev Biophys Biomol Struct. 2004;33:53–73. doi: 10.1146/annurev.biophys.33.110502.132703. [DOI] [PubMed] [Google Scholar]
- 3.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–8. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 4.Hill A. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 1910;40:4–7. [Google Scholar]
- 5.Edelstein S. Cooperative interactions of hemoglobin. Annu Rev Biochem. 1975;44:209–232. doi: 10.1146/annurev.bi.44.070175.001233. [DOI] [PubMed] [Google Scholar]
- 6.Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. a possible mechanism for phosphofructokinase. Biochem J. 1966;98:278–83. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehead EP. Co-operativity and the methods of plotting binding and steady-state kinetic data. Biochem J. 1978;171:501–4. doi: 10.1042/bj1710501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss JN. The hill equation revisited: uses and misuses. Faseb J. 1997;11:835–41. [PubMed] [Google Scholar]
- 9.Qian H. Cooperativity and specificity in enzyme kinetics: a single-molecule time-based perspective. Biophys J. 2008;95:10–7. doi: 10.1529/biophysj.108.131771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004;12:429–38. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim YB, Kalinowski SS, Marcinkeviciene J. A pre-steady state analysis of ligand binding to human glucokinase: evidence for a preexisting equilibrium. Biochemistry. 2007;46:1423–31. doi: 10.1021/bi0617308. [DOI] [PubMed] [Google Scholar]
- 12.Goldbeter A, Koshland JDE. An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci U S A. 1981;78:6840–4. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Ferrell JJE. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell. 2007;128:1133–45. doi: 10.1016/j.cell.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Buchler NE, Louis M. Molecular titration and ultrasensitivity in regulatory networks. J Mol Biol. 2008;384:1106–19. doi: 10.1016/j.jmb.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 15.Bluthgen N, Bruggeman FJ, Legewie S, Herzel H, Westerhoff HV, et al. Effects of sequestration on signal transduction cascades. FEBS J. 2006;273:895–906. doi: 10.1111/j.1742-4658.2006.05105.x. [DOI] [PubMed] [Google Scholar]
- 16.Koshland JDE. The structural basis of negative cooperativity: receptors and enzymes. Curr Opin Struct Biol. 1996;6:757–61. doi: 10.1016/s0959-440x(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 17.Kister J, Poyart C, Edelstein S. An expanded two-state allosteric model for interactions of human hemoglobin A with nonsaturating concentrations of 2,3-diphosphoglycerate. J Biol Chem. 1987;262:12085–12091. [PubMed] [Google Scholar]
- 18.Rao CV, Glekas GD, Ordal GW. The three adaptation systems of bacillus subtilis chemotaxis. Trends Microbiol. 2008;16:480–7. doi: 10.1016/j.tim.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen CH, Endres RG, Wingreen NS. Chemotaxis in Escherichia coli: a molecular model for robust precise adaptation. PLoS Comput Biol. 2008;4:e1. doi: 10.1371/journal.pcbi.0040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein A, Barrett RW. Ligand dissociation constants from competition binding assays: errors associated with ligand depletion. Mol Pharmacol. 1987;31:603–9. [PubMed] [Google Scholar]
- 21.Swillens S. Interpretation of binding curves obtained with high receptor concentrations: practical aid for computer analysis. Mol Pharmacol. 1995;47:1197–203. [PubMed] [Google Scholar]
- 22.Martinez K, Corringer P, Edelstein S, Changeux JP, Merola F. Structural differences in the two agonist binding sites of the torpedo nicotinic acetylcholine receptor revealed by time-resolved fluorescence spectroscopy. Biochemistry. 2000;39:6979–90. doi: 10.1021/bi992811p. [DOI] [PubMed] [Google Scholar]
- 23.Legewie S, Bluthgen N, Herzel H. Quantitative analysis of ultrasensitive responses. FEBS J. 2005;272:4071–9. doi: 10.1111/j.1742-4658.2005.04818.x. [DOI] [PubMed] [Google Scholar]
- 24.Kentner D, Sourjik V. Spatial organization of the bacterial chemotaxis system. Curr Opin Microbiol. 2006;9:619–24. doi: 10.1016/j.mib.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Belas R, Zhulin IB, Yang Z. Bacterial signaling and motility: sure bets. J Bacteriol. 2008;190:1849–56. doi: 10.1128/JB.01943-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–76. doi: 10.1038/nrn1647. [DOI] [PubMed] [Google Scholar]
- 27.Gsponer J, Christodoulou J, Cavalli A, Bui JM, Richter B, et al. A coupled equilibrium shift mechanism in calmodulin-mediated signal transduction. Structure. 2008;16:736–46. doi: 10.1016/j.str.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schachman H. Can a simple model account for the allosteric transition of aspartate transcarbamoylase? J Biol Chem. 1988;263:18583–18586. [PubMed] [Google Scholar]
- 29.Lipscomb W. Aspartate transcarbamylase from Escherichia coli: activity and regulation. Adv Enzymol Relat Areas Mol Biol. 1994;68:67–151. doi: 10.1002/9780470123140.ch3. [DOI] [PubMed] [Google Scholar]
- 30.Cluzel P, Surette M, Leibler S. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science. 2000;287:1652–5. doi: 10.1126/science.287.5458.1652. [DOI] [PubMed] [Google Scholar]
- 31.Sourjik V, Berg HC. Binding of the Escherichia coli response regulator CheY to its target measured in vivo by fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 2002;99:12669–74. doi: 10.1073/pnas.192463199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DR, Morgan DG, DeRosier DJ. Rotational symmetry of the C ring and a mechanism for the flagellar rotary motor. Proc Natl Acad Sci U S A. 1999;96:10134–9. doi: 10.1073/pnas.96.18.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEvoy MM, Bren A, Eisenbach M, Dahlquist FW. Identification of the binding interfaces on CheY for two of its targets, the phosphatase CheZ and the flagellar switch protein fliM. J Mol Biol. 1999;289:1423–33. doi: 10.1006/jmbi.1999.2830. [DOI] [PubMed] [Google Scholar]
- 34.Duke TA, Le Novère N, Bray D. Conformational spread in a ring of proteins: a stochastic approach to allostery. J Mol Biol. 2001;308:541–53. doi: 10.1006/jmbi.2001.4610. [DOI] [PubMed] [Google Scholar]
- 35.Alon U, Camarena L, Surette MG, Aguera y Arcas B, Liu Y, et al. Response regulator output in bacterial chemotaxis. EMBO J. 1998;17:4238–48. doi: 10.1093/emboj/17.15.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharf BE, Fahrner KA, Turner L, Berg HC. Control of direction of flagellar rotation in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1998;95:201–6. doi: 10.1073/pnas.95.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Stefan MI, Edelstein SJ, Le Novère N. An allosteric model of calmodulin explains differential activation of PP2B and CaMKII. Proc Natl Acad Sci U S A. 2008;105:10768–73. doi: 10.1073/pnas.0804672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porumb T. Determination of calcium-binding constants by flow dialysis. Anal Biochem. 1994;220:227–37. doi: 10.1006/abio.1994.1332. [DOI] [PubMed] [Google Scholar]
- 40.Kakiuchi S, Yasuda S, Yamazaki R, Teshima Y, Kanda K, et al. Quantitative determinations of calmodulin in the supernatant and particulate fractions of mammalian tissues. J Biochem. 1982;92:1041–8. doi: 10.1093/oxfordjournals.jbchem.a134019. [DOI] [PubMed] [Google Scholar]
- 41.Karlin A. On the application of “a plausible model” of allosteric proteins to the receptor of acetylcholine. J Theor Biol. 1967;16:306–320. doi: 10.1016/0022-5193(67)90011-2. [DOI] [PubMed] [Google Scholar]
- 42.Gerhart J, Pardee A. The enzymology of control by feedback inhibition. J Biol Chem. 1962;237:891–896. [PubMed] [Google Scholar]
- 43.Rubin M, Changeux JP. On the nature of allosteric transitions: Implications of non-exclusive ligand binding. J Mol Biol. 1966;21:265–274. doi: 10.1016/0022-2836(66)90097-0. [DOI] [PubMed] [Google Scholar]
- 44.Gerhart J, Schachman H. Allosteric interactions in aspartate transcarbamylase. II. evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968;7:538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- 45.Changeux JP, Rubin M. Allosteric interactions in aspartate transcarbamylase. III. interpretations of experimental data in terms of the model of monod, wyman, and changeux. Biochemistry. 1968;7:553–561. doi: 10.1021/bi00842a601. [DOI] [PubMed] [Google Scholar]
- 46.Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968;218:1116–9. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- 47.Wiley D, Lipscomb W. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968;218:1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- 48.Kantrowitz ER, Lipscomb WN. Escherichia coli aspartate transcarbamoylase: the molecular basis for a concerted allosteric transition. Trends Biochem Sci. 1990;15:53–9. doi: 10.1016/0968-0004(90)90176-c. [DOI] [PubMed] [Google Scholar]
- 49.Fetler L, Kantrowitz ER, Vachette P. Direct observation in solution of a preexisting structural equilibrium for a mutant of the allosteric aspartate transcarbamoylase. Proc Natl Acad Sci U S A. 2007;104:495–500. doi: 10.1073/pnas.0607641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–70. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- 51.Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res. 1986;397:161–72. doi: 10.1016/0006-8993(86)91381-8. [DOI] [PubMed] [Google Scholar]
- 52.Stefan MI, Edelstein SJ, Le Novère N. Computing phenomenologic Adair-Klotz constants from microscopic MWC parameters. BMC Syst Biol. 2009;3:68. doi: 10.1186/1752-0509-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvetti S, Ghelardi E, Celandroni F, Ceragioli M, Giannessi F, et al. FlhF, a signal recognition particle-like GTPase, is involved in the regulation of flagellar arrangement, motility behaviour and protein secretion in Bacillus cereus. Microbiology. 2007;153:2541–52. doi: 10.1099/mic.0.2006/005553-0. [DOI] [PubMed] [Google Scholar]
- 54.Endres RG, Oleksiuk O, Hansen CH, Meir Y, Sourjik V, et al. Variable sizes of Escherichia coli chemoreceptor signaling teams. Mol Syst Biol. 2008;4:211. doi: 10.1038/msb.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markram H. The blue brain project. Nat Rev Neurosci. 2006;7:153–60. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- 56.Le Novere N. The long journey to a systems biology of neuronal function. BMC Syst Biol. 2007;1:28. doi: 10.1186/1752-0509-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izhikevich EM, Edelman GM. Large-scale model of mammalian thalamocortical systems. Proc Natl Acad Sci U S A. 2008;105:3593–8. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giegling I, Hartmann AM, Genius J, Benninghoff J, Moller HJ, et al. Systems biology and complex neurobehavioral traits. Pharmacopsychiatry. 2008;41(Suppl 1):S32–6. doi: 10.1055/s-2008-1081200. [DOI] [PubMed] [Google Scholar]
- 59.Villoslada P, Steinman L, Baranzini SE. Systems biology and its application to the understanding of neurological diseases. Ann Neurol. 2009;65:124–39. doi: 10.1002/ana.21634. [DOI] [PubMed] [Google Scholar]
- 60.Gamble E, Koch C. The dynamics of free calcium in dendritic spines in response to repetitive synaptic input. Science. 1987;236:1311–5. doi: 10.1126/science.3495885. [DOI] [PubMed] [Google Scholar]
- 61.Henkin RI. The concentration of calmodulin present in parotid cells. J Dent Res. 1997;76:1430. doi: 10.1177/00220345970760080201. [DOI] [PubMed] [Google Scholar]
- 62.Gerhart JC, Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967;242:2886–92. [PubMed] [Google Scholar]