Abstract
The prevalence of marijuana abuse and dependence disorders has been increasing among adults and adolescents in the United States. This paper reviews the problems associated with marijuana use, including unique characteristics of marijuana dependence, and the results of laboratory research and treatment trials to date. It also discusses limitations of current knowledge and potential areas for advancing research and clinical intervention.
Marijuana remains the most widely used illicit substance in the United States and Europe (European Monitoring Centre for Drugs and Drug Addiction, 2006; Substance Abuse and Mental Health Services Administration (SAMHSA), 2007). Although some people question the concept of marijuana dependence or addiction, diagnostic, epidemiological, laboratory, and clinical studies clearly indicate that the condition exists, is important, and causes harm (Budney, 2006; Budney and Hughes, 2006; Copeland, 2004; Roffman and Stephens, 2006). Marijuana dependence as experienced in clinical populations appears very similar to other substance dependence disorders, although it is likely to be less severe. Adults seeking treatment for marijuana abuse or dependence average more than 10 years of near-daily use and more than six serious attempts at quitting (Budney, 2006; Copeland et al., 2001; Stephens et al., 2002). They continue to smoke the drug despite social, psychological, and physical impairments, commonly citing consequences such as relationship and family problems, guilt associated with use of the drug, financial difficulties, low energy and self-esteem, dissatisfaction with productivity levels, sleep and memory problems, and low life satisfaction (Gruber et al., 2003; Stephens et al., 2002). Most perceive themselves as unable to stop, and most experience a withdrawal syndrome upon cessation.
Approximately half of the individuals who enter treatment for marijuana use are under 25 years of age. These patients report a distinctive profile of associated problems, perhaps due to their age and involvement in other risky behaviors (Tims et al., 2002). Adolescents who smoke marijuana are at enhanced risk of adverse health and psychosocial consequences, including sexually transmitted diseases and pregnancy, early school dropout, delinquency, legal problems, and lowered educational and occupational aspirations.
Some 4.3 percent of Americans have been dependent on marijuana, as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000), at some time in their lives. Marijuana produces dependence less readily than most other illicit drugs. Some 9 percent of those who try marijuana develop dependence compared to, for example, 15 percent of people who try cocaine and 24 percent of those who try heroin. However, because so many people use marijuana, cannabis dependence is twice as prevalent as dependence on any other illicit psychoactive substance (cocaine, 1.8 percent; heroin, 0.7 percent; Anthony and Helzer, 1991; Anthony, Warner, and Kessler, 1994).
During the past decade, marijuana use disorders have increased in all age groups. Contributing factors may include the availability of higher potency marijuana and the initiation of use at an earlier age. Among adults, marijuana use disorders increased despite stabilization of rates of use. An increased prevalence of disorders among young adult African-American and Hispanic men and African-American women appears to account for the overall rise among youth (Compton, 2004). The reasons for the upward trend in disorders among minority young people are not clear. Speculation has pointed to the deleterious effects of acculturation on Hispanic youth; growing numbers of minority youth attending college, where they may experience increased exposure to marijuana use; and environmental and economic factors. For example, young people may turn to marijuana abuse when they have difficulty obtaining tobacco and alcohol, and recent higher prices and stricter governmental policies may restrict minorities’ more than Caucasians’ access to legal psychoactive substances.
Paralleling the rise in marijuana use disorders, treatment admissions for primary marijuana dependence have increased both in absolute numbers and as a percentage of total admissions, from 7 percent in 1993 to 16 percent in 2003 (SAMHSA, 2004). The extent of marijuana use and its associated consequences clearly indicate a public health problem that requires systematic effort focused on prevention and intervention.
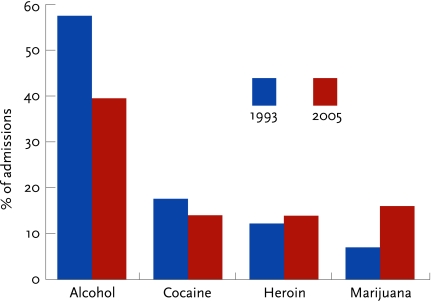
FIGURE 1.
The percentage of substance abuse treatment admissions that were due to marijuana nearly doubled from 1993 to 2005 (SAMHSA, 2006b)
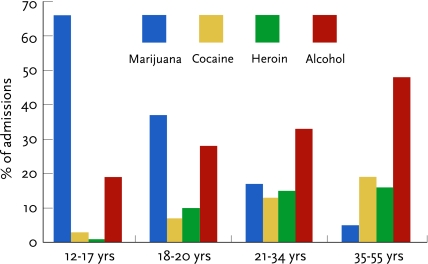
FIGURE 2.
Marijuana accounts for most adolescent drug treatment admissions and progressively smaller proportions of admissions in each successive higher age group (SAMHSA, 2006b)
TREATMENT EFFICACY RESEARCH
Systematic research on psychosocial treatments for marijuana abuse or dependence began approximately 20 years ago, yet the number of controlled studies remains small. Behavioral treatments, such as motivational enhancement therapy (MET), cognitive-behavioral therapy (CBT), and contingency management (CM), as well as family-based treatments have been carefully evaluated and have shown promise. Outpatient treatments for marijuana abuse among adolescents have recently received increasing attention in the scientific literature.
Adults
Seven published, randomized efficacy trials for primary adult marijuana abuse and dependence have consistently demonstrated that outpatient treatments can reduce marijuana consumption and engender abstinence. The most commonly tested interventions are adaptations of interventions initially developed to treat alcohol or cocaine dependence, in particular MET and CBT (also known as coping skills training). Recently, trials have examined the use of CM to enhance the potency of MET- and CBT-based treatments. The cumulative findings indicate that (1) each of these interventions represents a reasonable and efficacious treatment approach; (2) the combination of MET and CBT is probably more potent than MET alone; and (3) an intervention that integrates all three approaches—MET, CBT, and CM— is most likely to produce positive outcomes, especially as measured by rates of abstinence from marijuana.
WEB LINKS TO TREATMENT MANUALS.
Adult Treatment Manuals From the Marijuana Treatment Project Research Group Study (Marijuana Treatment Project Research Group, 2004):
Brief Counseling for Marijuana Dependence (Steinberg et al., 2005) kap.samhsa.gov/products/brochures/pdfs/bmdc.pdf.
A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction (Budney and Higgins, 1998) www.nida.nih.gov/TXManuals/CRA/CRA6.html.
Adolescent Treatment Manuals From the Cannabis Youth Treatment Study (Dennis et al., 2004):
The Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users: 5 Sessions,Volume 1. NCADI number BKD384.
The Motivational Enhancement Therapy and Cognitive Behavioral Therapy Supplement: 7 Sessions of Cognitive Behavioral Therapy for Adolescent Cannabis Users,Volume 2.
Family Support Network for Adolescent Cannabis Users,Volume 3.
The Adolescent Community Reinforcement Approach for Adolescent Cannabis Users,Volume4.
Multidimensional Family Therapy for Adolescent Cannabis Users,Volume 5. ncadistore.samhsa.gov/catalog/Product Details.aspx?ProductID=15868.
Multisystemic Therapy for Adolescents (Henggeler et al., 2006) www.mstservices.com.
MET addresses ambivalence about quitting and seeks to strengthen motivation to change. A typical MET regimen consists of one to four 45- to 90-minute individual sessions. Therapists use a nonconfrontational counseling style to guide the patient toward commitment to and action toward change. Therapeutic techniques include using strategic expression of empathy, reflecting, summarizing, affirming, reinforcing self-efficacy, exploring pros and cons of drug use, rolling with resistance, and forging goals and plans to achieve them. An online manual, Brief Counseling for Marijuana Dependence, describes the use of MET intervention with adult marijuana users.
CBT focuses on teaching patients skills relevant to quitting marijuana and avoiding or managing other problems that may interfere with good outcomes. Patients learn functional analysis of marijuana use and cravings, self-management planning to avoid or cope with drug use triggers, drug refusal skills, problem-solving skills, and lifestyle management. CBT for marijuana dependence is typically delivered in 45- to 60-minute, weekly individual or group counseling sessions; tested CBT interventions have ranged from 6 to14 sessions. Each session involves analysis of recent marijuana use or cravings, development of planned responses to situations that may trigger use or craving, brief training on a coping skill, role-playing or other interactive exercises, and practice assignments. Brief Counseling for Marijuana Dependence describes the content and conduct of CBT sessions in detail (Steinberg et al., 2005; see “Web Links to Treatment Manuals”).
A series of four trials demonstrated the efficacy of both CBT and MET for adult marijuana dependence (Table 1). After an initial trial showed promising results for a CBT group intervention (Stephens, Roffman, and Simpson, 1994), a second trial tested a 14-session group CBT intervention against 2 individual MET sessions or a delayed treatment control (DTC) condition (Stephens, Roffman, and Curtin, 2000). At the 4-month followup, the CBT and MET groups had achieved significantly greater rates of abstinence than the DTC group. Days of use, number of uses per day, dependence symptoms, and problems related to use also fell significantly compared with those measures in the DTC group, and gains were generally maintained throughout the 16-month followup. No significant differences were observed between CBT and MET conditions on any of these outcome measures, suggesting that brief motivational interventions may be as effective as longer CBT interventions. However, this study confounded treatment modality (group vs. individual) and therapist experience (provision of MET by more experienced therapists) with treatment length. A similar study showed that a six-session CBT and a one-session MET treatment, both delivered in individual therapy sessions, produced greater rates of abstinence than DTC, but again little difference was observed between the active treatment groups (Copeland et al., 2001). A positive relation between therapist experience and outcome was reported across both treatment conditions.
TABLE 1.
Randomized Trials for Adult Marijuana Treatment
| AUTHOR(S) | N | INTERVENTION | OUTCOME |
|---|---|---|---|
| MET and CBT | |||
| Stephens, Roffman, and Simpson, 1994 | 212 | CBT vs. social support group discussion intervention | Both groups had significant reductions in marijuana use. No significant differences between groups. |
| Stephens, Roffman, and Curtin, 2000 | 291 | 14-session CBT group treatment vs. 2-session MET treatment vs. DTC | Treatment groups showed greater improvement than DTC. No differences in outcomes between treatment groups. |
| Copeland et al., 2001 | 229 | 6-session MET vs. 1-session MET vs. DTC | Both treatment groups reported better outcomes (higher rates of abstinence, fewer marijuana-related problems) than DTC. |
| Marijuana Treatment Project Research Group, 2004 | 450 | 9-session MET-CBT vs. 2-session M ET vs. DTC | Both treatment groups reported better outcomes than DTC. 9-session MET-CBT engendered greater long-term abstinence and reductions in frequency of use than brief MET. |
| Stephens et al., 2006 | 87 | 9-session MET-CBT vs. 4-session M ET-CBT + pro re nata (PRN; continuing care) | No between-condition outcome differences observed. Only 37 percent of PRN subjects used continuing care sessions; suggestive evidence that use of PRN increased abstinence. |
| CM (Abstinence-Based Vouchers) | |||
| Budney et al., 2000 | 60 | 4-session MET vs. 14-session MET-CBT vs. 14-session MET-CBT + CM | No differences in abstinence between MET and MET-CBT. MET-CBT+CM engendered greater abstinence during and at the end of treatment than M ET or MET-CBT. |
| Budney et al., 2006 | 90 | 14-session MET-CBT vs. MET-CBT + CM vs. CM alone | Two CM conditions engendered better abstinence outcomes during treatment than MET-CBT. MET-CBT+CM had better post-treatment abstinence rates than the other groups. |
| Kadden et al., 2007 | 240 | 9-session MET-CBT vs. M ET-CBT + CM vs. CM alone vs. case management | Two CM conditions engendered better abstinence outcomes, with only the MET-CBT+CM showing superior abstinence rates during the 1-year followup. |
The most comprehensive trial (n = 450) of MET and CBT compared nine sessions of combined MET-CBT with a two-session MET-only intervention and with a DTC (Marijuana Treatment Project Research Group, 2004). MET-CBT and MET-only again produced better abstinence outcomes than DTC. However, in this trial, MET-CBT was associated with significantly greater long-term abstinence and greater reductions in frequency of marijuana use compared with MET alone. Findings generalized across three sites and were not dependent on ethnicity or gender.
In an effort to enhance outcomes further, researchers have begun to examine the efficacy of CM for treating marijuana dependence (Budney et al., 2001). The marijuana CM intervention adapts the abstinence-based voucher approach originally developed and demonstrated effective for treating cocaine dependence (Budney and Higgins, 1998; Higgins et al., 1994). The vouchers are contingent on marijuana abstinence, confirmed by twice-weekly drug testing, and their value escalates with each consecutive negative drug test. Patients exchange them for prosocial retail items or services that, it is hoped, will serve as alternatives to marijuana use.
An initial trial of CM for adult marijuana dependence compared a 4-session MET, a 14-session combined MET-CBT, and a 14-session MET-CBT plus CM (Budney et al., 2000). Individuals could earn up to $570 in vouchers if they provided consistently negative urine samples throughout treatment weeks 3 through 14. The MET-CBT plus CM condition produced the highest abstinence rate during treatment. In a second trial conducted to extend these findings (Budney et al., 2006), 90 adults received MET-CBT, MET-CBT plus CM, or CM alone (no counseling). The magnitude of the CM incentives was identical to that used in the prior study. The MET-CBT-alone intervention differed from the initial study in one regard: vouchers ($5) contingent on providing a urine specimen as scheduled (twice per week) were provided to ensure equivalent retention and treatment contact. This trial produced three notable outcomes. First, MET-CBT plus CM and CM alone both engendered greater initial rates of abstinence than MET-CBT. Second, MET-CBT plus CM produced outcomes that were similar to those of CM alone during treatment, but superior post-treatment.
A recent study by another research group found similar results with a modified CM program (weekly urine testing, $385 maximum voucher earnings for complete abstinence) in a more diverse (40 percent minority) and larger sample (n = 240; Kadden et al., 2007). During 7 weeks of treatment, MET-CBT plus CM and CM alone produced continuous abstinence outcomes that were similar to each other and superior to those seen with MET-CBT. During the following year, the MET-CBT plus CM patient group sustained overall positive outcomes somewhat better than those of the CM group, although differences in abstinence rates were not statistically significant at later followups. As in the previous CM trials, patients in the CM and non-CM conditions self-reported similar rates of marijuana use throughout, illustrating the importance of obtaining subjective and objective indices of use. In summary, MET, CBT, and CM each has empirical support for its efficacy, and CM in combination with MET-CBT has demonstrated the most potency in outpatient treatment for adult marijuana dependence, particularly for engendering longer periods of abstinence.
Recognizing that many people overcome dependence only after multiple treatment exposures, Stephens and Roffman (2005) developed and initially tested a creative, chronic care model of treatment that they termed “marijuana dependence treatment PRN.” Following an initial four sessions of MET-CBT, participants were given the option of determining the number and schedule of treatment sessions they would attend over a 28-month period. The comparison condition in this trial was the same fixed-dose nine-session MET-CBT intervention used in the large multisite trial mentioned earlier (Marijuana Treatment Project Research Group, 2004). There were three key findings from this trial: (1) A relatively small percentage of participants (37 percent) made use of the continuing care sessions, and (2) the PRN condition overall was not more efficacious than the fixed-dose condition, although (3) the few individuals who attended the greatest number of continuing care sessions (mean of 13.4 sessions) had a high level of 90-day abstinence (approximately 60 percent) at followup.
Adolescents and Young Adults
Most information on marijuana treatment efficacy among young people derives from trials that have included users of various drugs and have not focused specifically on marijuana use. Nevertheless, most patients in these studies have been primary marijuana users. Empirical support for group or individual CBT and family-based treatments has begun to emerge (Waldron and Kaminer, 2004). The CBT interventions studied have been similar to those studied for adults in scope and duration. Specific forms of family-based treatment that have been tested include functional family therapy (Waldron et al., 2001), multidimensional family therapy (MDFT; Liddle et al., 2001), multisystemic therapy (Henggeler et al., 2006), family support network intervention (Dennis et al., 2004), and brief strategic family therapy (Azrin et al., 1994; Santisteban et al., 2003). Description of these models is beyond the scope of this paper. However, they each involve structured, skills-based interventions for family members and are well described in their respective manuals.
The largest clinical trial of outpatient treatment for adolescent substance abuse focused on marijuana use (Dennis et al., 2004). Five treatment models were tested in a multisite study: MET-CBT 5 (2 individual and 3 group sessions), MET-CBT 12 (2 individual and 10 group sessions), MET-CBT 12 plus family support network (6 parent education group sessions, 4 home visits, and case management), the community reinforcement approach (10 individual sessions focused on behavioral change in drug use and lifestyle change, and 4 parent sessions focused on effective parenting, communication, and problem solving), and MDFT (12 to 15 family systems-focused sessions: 6 individual, 3 with parents alone, and 6 with family). Significant decreases in drug use and symptoms of dependence were observed following each of the treatments. However, robust between-treatment differences in outcomes were not observed, which unfortunately precludes drawing strong conclusions about their efficacy. Although results were promising compared with prior treatment studies, two-thirds of the youth continued to experience significant substance-related symptoms, suggesting that adolescent treatments can be improved and alternative treatment models should be explored (Compton and Pringle, 2004).
As they are doing with treatments for adults, researchers are attempting to enhance youths’ outcomes by adding a CM intervention to MET-CBT-type interventions. Positive results were observed in an initial pilot study of MET-CBT plus a CM intervention that incorporated an abstinence-based voucher program and parent-based CM (Kamon, Budney, and Stanger, 2005). The voucher program was of the same schedule and magnitude as that used in the previously mentioned adult trials by Budney and colleagues. However, participants could earn vouchers only if urine toxicology screens were negative for all drugs tested and if parents reported that, to their knowledge, the adolescent had not used any drugs or alcohol. The parenting intervention included a contract that directed parents to provide tangible incentives for abstinence and to deliver negative consequences for continued use. Parents also participated in a weekly behavioral training program called Adolescent Transitions (Dishion and Kavanagh, 2003), a treatment of choice for adolescents with conduct disorder. Preliminary data from an initial randomized trial suggest that the MET-CBT plus CM improved rates of marijuana abstinence and effectively maintained abstinence post-treatment compared with MET-CBT combined with weekly parent psychoeducational counseling. The rates of abstinence achieved appeared greater than those reported in prior studies; however, comparison across trials is problematic because of differences in patient characteristics and differences in the way outcomes are measured.
Two other tests of CM with adolescents and young adults have produced promising results. A CM abstinence-based voucher program enhanced drug use outcomes and abstinence when added to a potent outpatient therapy (i.e., multisystemic therapy) among juvenile offenders enrolled in drug court (Henggeler et al., 2006). Lastly, adding incentives for treatment attendance to MET increased treatment participation by young adult marijuana abusers involved with the judicial system, but did not lead to increased marijuana abstinence (Sinha et al., 2003). In summary, a number of behaviorally based interventions appear efficacious for treating adolescent marijuana abuse, and combining interventions like MET, CBT, CM, and family-based programs is likely to enhance efficacy.
Effectiveness
Sufficient evidence has accumulated to conclude that behaviorally based interventions can help many of those who seek treatment for marijuana use disorders. Unfortunately, as with treatment for other dependencies, the rates of “success” are modest. Even with MET-CBT plus CM, the most highly efficacious treatment for adults, only about one-half of those who enroll in treatment achieve an initial 2-week period of abstinence, and among those who do, approximately one-half resume use within a year (Budney et al., 2006; Kadden et al., 2007). Across studies, 1-year abstinence rates have ranged between 19 and 29 percent for MET-CBT, and between 9 and 28 percent for MET. An additional percentage of adults report a reduction in use and in problems associated with use; however, many adults show no evidence of progress.
The treatment outcome data for adolescents paint a similar picture. For example, in the large Cannabis Youth Treatment study, abstinence rates at the end of treatment were only 11 to 15 percent (Dennis et al., 2004; see also the preliminary findings of Dennis and colleagues reported at www.chestnut.org/LI/cyt/findings/index.html), and rates at 12 months post-treatment, defined by self-report of no substance use in the prior month, were 17 to 34 percent across the five treatments. Clearly, there remains much room for improvement in marijuana outpatient treatment.
CLINICAL ISSUES
Most clinical issues in treatment for marijuana use disorders parallel those that arise in treatments for other drug use disorders, though sometimes with distinctive aspects. Among the clinical features that distinguish marijuana dependence are the drug’s relatively mild withdrawal effects and marijuana users’ frequent desire to pursue a goal of reducing—rather than abstaining from—use.
Marijuana as a Secondary Drug of Abuse
In addition to being the illicit drug most commonly used by the general population, marijuana is also the most common “other drug” used by those seeking treatment for stimulant or opiate dependence. Such secondary marijuana use is commonly viewed as a significant risk factor for relapse or treatment failure, although the empirical support for this is equivocal (Epstein and Preston, 2003).
Many individuals who enter treatment for heroin/opiate dependence or cocaine dependence do not consider their marijuana use problematic; thus, their readiness to quit or reduce their marijuana use is low. Some investigators have explored CM-based approaches targeting marijuana use in this clinical population, reasoning that explicit reinforcement or penalty interventions tied to marijuana use may motivate and prompt change in individuals not currently interested in changing.
Calsyn and Saxon (1999) devised a marijuana CM program to function as an adjunct to an existing CM program that required 6 months of urinalysis-confirmed abstinence from all drugs, except for cannabis, in order to earn methadone take-home privileges twice a week. The new intervention simply increased the requirement for obtaining twice-weekly take-home status to include marijuana-negative urinalysis results. In this small study, 50 percent of the participants responded to the contingency by stopping their marijuana use, while the other 50 percent accepted curtailment of their take-home privileges and continued to use marijuana.
Kidorf and colleagues (2007) tested a similar “motivated stepped care” approach to reducing cannabis use in methadone maintenance patients. Fifteen patients who tested positive only for marijuana during a 6-month baseline period were informed that, from then on, a positive test for marijuana (or any other substance) would increase their counseling requirements from 1 hour per week to 4. Ten of the patients discontinued marijuana use when informed about the new counseling rule. The other five—who were among the heaviest users—continued to test positive for marijuana and were required to attend the additional counseling sessions. Of those, four responded to the intensified counseling, eventually discontinuing use and returning to the lower-level schedule. One patient did not respond and dropped out of treatment.
In the cocaine clinic, where many patients do not endorse a goal of stopping marijuana use, the clinician must decide how best to approach this issue without adversely affecting treatment for cocaine dependence (Budney, Higgins, and Wong, 1996). One study of a small number of patients explored a sequential strategy of initially targeting abstinence from cocaine with an abstinence-based voucher CM program, then targeting marijuana once cocaine abstinence had been achieved (Budney et al., 1991). The rationale for this approach was that the experience of achieving cocaine abstinence and the associated positive effects might increase awareness of how marijuana use negatively affects a prosocial lifestyle. Moreover, an initial success with a voucher program for cocaine might motivate participation in a similar program that targets marijuana. In this study, two participants quit using cocaine during a 12-week voucher program, but continued to use marijuana regularly despite counseling that encouraged abstinence. Both entered a second 12-week program that required abstinence from cocaine and marijuana to earn vouchers. Both achieved abstinence from the two drugs and stayed off cocaine throughout a 5-month followup period. Unfortunately, both resumed marijuana use during the followup.
These studies demonstrate how systematic approaches to secondary marijuana abuse can be implemented without having significant adverse effects on treatment for primary opiate or cocaine abuse. Using stepped care or sequential CM approaches appears effective for initiating abstinence among those ambivalent about stopping their marijuana use. However, longer term contingencies or additional interventions may be needed to obtain enduring effects (Kidorf et al., 2007).
Marijuana Withdrawal
As noted earlier, many people question whether one can truly become dependent on marijuana. The basis for skepticism is typically doubt that marijuana use can produce “physiological” dependence—i.e., that cessation of use produces a withdrawal syndrome. A review of the literature relevant to this issue is beyond our scope here. However, research over the past 10 to 15 years has (1) established a neurobiological basis for a marijuana withdrawal syndrome via an endogenous cannabinoid system in the central nervous system; (2) established the reliability, validity, and time course of a marijuana withdrawal syndrome through human laboratory research and clinical studies; and (3) demonstrated the potential clinical importance of the withdrawal syndrome (Budney et al., 2004; Budney and Hughes, 2006).
The marijuana withdrawal syndrome resembles those associated with other drugs, particularly tobacco. Patients experience irritability, anger, depression, difficulty sleeping, craving, and decreased appetite. Many indicate that these symptoms adversely impact their attempts to quit and motivate use of marijuana or other drugs for relief (Copersino et al., 2006). Most symptoms begin within 24 to 48 hours of abstinence, peak within 4 to 6 days, and last from 1 to 3 weeks, although significant individual differences occur in withdrawal expression.
The marijuana withdrawal syndrome does not appear to include major medical or psychiatric consequences and may be considered mild compared with heroin and severe alcohol withdrawal syndromes. Nonetheless, myriad patient reports suggest that additional research to understand and develop effective clinical responses to the withdrawal syndrome may enhance outcomes and promote successful cessation attempts.
Pharmacotherapy
To date, a handful of human laboratory studies and one small clinical trial on potential pharmacotherapies for marijuana dependence have appeared in the literature (Hart, 2005). The majority of these efforts have targeted the marijuana withdrawal syndrome. Bupropion, divalproex, naltrexone, nefazodone, and orally administered Δ9-tetrahydrocannabinol (THC) have all been evaluated in studies with marijuana-dependent participants who were not seeking treatment or planning to quit. Divalproex has also been evaluated in an outpatient placebo-controlled trial (Levin et al., 2004). Only orally given THC and, to a lesser extent, nefazodone have shown promise. THC reduced craving and ratings of anxiety, feelings of misery, difficulty sleeping, and chills (Haney et al., 2004). In addition, participants could not distinguish active THC from placebo. These findings were replicated in an outpatient study, which found that a moderate oral dosage of THC (10 mg, three times daily) suppressed many marijuana withdrawal symptoms and that a higher dosage (30 mg, three times daily) almost completely abolished withdrawal symptoms (Budney et al., 2007). Nefazodone decreased ratings of some withdrawal symptoms (anxiety and muscle pain), but other ratings (irritability, feelings of misery, and difficulty sleeping) remained high (Haney et al., 2003).
In summary, the developing literature on pharmacotherapy for marijuana dependence supports further testing of THC, an approach that parallels the use of agonist medications such as methadone and the nicotine patch. Continued exploration of compounds that target mood, sleep difficulty, craving, and appetite appears warranted given the potent and reliable symptoms observed in withdrawal studies. Other promising strategies for pharmacotherapies include targeting the underlying physiology of withdrawal—specifically, the decreases in dopamine activity in the mesolimbic dopamine pathway—and treating comorbid disorders such as depression or anxiety. Researchers also are exploring the possibility of medications to help abstinent individuals avoid relapse by blocking marijuana’s rewarding effects. One such compound, the cannabinoid receptor antagonist SR141617A (rimonabant), has been shown to block the drug’s subjective and physiological effects (Huestis et al., 2001).
Tobacco Smoking Among Marijuana Users
Like users of other drugs of abuse, regular marijuana users have a higher rate of tobacco use than the general population; approximately 50 percent of heavy cannabis users also smoke tobacco (Ford, Vu, and Anthony, 2002; Moore and Budney, 2001). Moreover, many adolescents and, to a lesser extent, adults use tobacco and marijuana together, either mixing the substances, smoking blunts (hollowed out cigars filled with marijuana), or smoking one immediately after the other.
At least one study suggests that, among cannabis-dependent individuals, tobacco smokers have worse psychosocial problems and poorer cannabis cessation outcomes (Moore and Budney, 2001). Whether this indicates that treatments for marijuana dependence should simultaneously address tobacco smoking is not clear. No clinical studies have focused on this issue. However, research suggests that treatment that promotes smoking cessation does not disrupt alcohol abstinence and may actually enhance the likelihood of longer-term sobriety (Gulliver, Kamholz, and Helstrom, 2006).
One laboratory study compared withdrawal symptoms during simultaneous cessation of marijuana and tobacco to withdrawal from each substance alone (Vandrey et al., 2007). Withdrawal was more severe during simultaneous cessation, but the differences were of short duration and not robust, and substantial individual differences were noted. Interestingly, five participants rated dual abstinence as the most difficult of the three conditions; four rated cannabis abstinence and three rated tobacco abstinence as the most difficult. The reason simultaneous abstinence was not uniformly experienced as most severe may be that both substances are smoked. Individuals quitting one drug might have had withdrawal intensified by the smoking cues associated with continuing use of the other, while individuals quitting both were spared such cues.
Should we encourage individuals trying to quit marijuana use to try also to quit tobacco? Certainly we should discuss this option with clients, as tobacco abstinence may make marijuana abstinence easier and increase chances of maintaining marijuana abstinence for a longer term. However, as with treatments for other substance dependence disorders, mandating tobacco cessation as a treatment goal might create a barrier to treatment seeking or trigger treatment dropout.
Treatment Goals: Abstinence or Moderation?
Because marijuana is perceived as less harmful than cocaine or heroin, some people suggest that use reduction, instead of abstinence, may be an acceptable clinical goal. Indeed, many individuals who enter treatment are ambivalent about giving up marijuana completely.
The only published study (n = 291) that systematically assessed the goals of adults enrolling in marijuana treatment reported that 71 percent sought abstinence, 28 percent wanted to moderate their use to 3 days or less per week, and 1 percent wished only to incur fewer adverse consequences from their smoking (Lozano, Stephens, and Roffman, 2006). Patient goals were measured again at the end of treatment and repeatedly during a 12-month followup period. Ultimately, the portion desiring to be abstinent declined to 49 percent, while those wishing only for fewer negative effects increased to 26 percent. Most notably, patient goals predicted outcomes: 40 to 65 percent of those aiming for abstinence or moderation had achieved their desired outcome at the following assessment. The second most frequent outcome among those with abstinence goals was moderation, while the second most frequent outcome among those with moderation goals was continued problematic use. In summary, abstinence goals predicted better outcomes. That said, because the focus of treatment in this study was abstinence, those with moderation goals were not necessarily provided with treatment that best matched their goals.
Little is known about what constitutes nonharmful use of marijuana, and whether and when moderation may be an appropriate clinical goal for treatment. Clinical epidemiological studies clearly demonstrate that many individuals experiment with marijuana, and some even use the drug regularly without reporting significant consequences. This finding clearly parallels what is observed with alcohol use. The sparse data available on goals discussed earlier are fairly consistent with what is observed in the alcohol treatment literature—that is, patients who aim for abstinence appear to obtain better outcomes. Some individuals who make moderation their objective can achieve it, but the likelihood of failing is greater with this goal. Moderation-focused treatments for marijuana have yet to be tested. Thus, no guidelines or predictors exist concerning which patients might succeed with this approach. Moreover, marijuana’s illegality complicates any consideration of treatment goals other than abstinence.
Early Intervention and Secondary Prevention
Although more people are seeking help for problems with marijuana today, they still represent only a small percentage of those who may benefit from treatment. Of the approximately 4 million persons in the United States who reported problems consistent with a marijuana use disorder in a 2005 survey (SAMHSA, 2006a), only about 7 to 8 percent received treatment. Adolescents who report signs of problematic use—a relatively small percentage—seldom present for treatment. Those who do almost never self-refer; they are typically “forced” into treatment by parents, the juvenile justice system, or their school administration, and most do not admit that their use is problematic (Diamond et al., 2006). Responding to this situation, one group of researchers recently developed “check-up” interventions to reach marijuana users who have not sought treatment, either because they are ambivalent about stopping or do not perceive their use to be a problem, or at least not a problem severe enough to warrant treatment (Stephens et al., 2004; Walker et al., 2006).
The Teen Marijuana Check-Up (TMCU), designed for delivery in high schools, is advertised as an opportunity to “take stock” of marijuana use and is intended to facilitate a candid, in-depth evaluation of a teen’s use. The program features a brief MET intervention, consisting of a computerized assessment and two 30-minute sessions, which encourages participation by demanding minimal effort. The program treats adolescents as experts and decision makers regarding their marijuana use, does not label marijuana users as having a problem, and views ambivalence about the drug as normal. An initial randomized trial conducted in four high schools compared the TMCU with a delayed treatment condition (Walker et al., 2006). Teens in both conditions significantly reduced their marijuana use over a 3-month period; however, no significant between-group differences were observed. Despite the absence of a clear effect of the TMCU, this study showed that adolescents using marijuana would volunteer to participate in an intervention provided at their school, a response that holds promise for reducing problematic levels of marijuana use.
A similar Marijuana Check-Up (MCU) for adults was designed to reach marijuana users who were experiencing adverse consequences, but were ambivalent about change and not likely to enter treatment (Stephens et al., 2004). Marijuana users called the clinic in response to advertisements stating that objective, up-to-date information on marijuana use and its effects was available. Upon contact, callers were told that this was not a treatment study and were invited to the clinic for an assessment that would then be followed by a one-session personalized feedback session, a one-session therapist-guided multimedia session (documentary and slide show providing objective information on marijuana and its effects), or a session (MET or multimedia) delayed by 7 weeks. Respondents to the advertisements were near-daily marijuana users, two-thirds of whom were in the precontemplation or contemplation stage of change. Over 12 months, the MCU condition resulted in greater reductions in marijuana use and in associated problems than the multimedia condition; however, absolute levels of change were relatively small. Nonetheless, like the TMCU for adolescents, this study showed that this intervention model attracted a “unique” sample of ambivalent marijuana users who may be ideal candidates for secondary prevention interventions like the MCU. Continued exploration of more potent MCU models may yield a method for reaching marijuana users who would otherwise not contact the typical treatment system, at least not at this stage of their use.
FUTURE DIRECTIONS FOR TREATMENT RESEARCH
Over the last 15 years, we have witnessed great advances in the empirical base for treatment approaches to marijuana use disorders. Clear evidence has accumulated for the efficacy of behavioral treatments similar to those used for disorders involving alcohol and other drugs of abuse. The goals for future research are more potent treatment approaches and intervention strategies.
IS MARIJUANA UNIQUE?
A large part of the general population has had personal experience with marijuana, and most have not become addicted. Many find it perplexing to contemplate how someone else could become addicted to a drug they themselves have tried and can easily set aside or stop using. Accordingly, they think marijuana dependence must qualitatively differ from dependence on other drugs, such as heroin and cocaine, and require unique treatment approaches.
People who develop problems with marijuana may indeed be different from those who do not, but this phenomenon has been observed with other substances of abuse. A comparison with alcohol use and dependence provides a case in point. The great majority of Americans have tried alcohol and continue to drink alcoholic beverages regularly. However, only an estimated 10 to 15 percent of alcohol drinkers develop problems, and only some of these problem drinkers seek treatment. This is also true of those who have tried cocaine or heroin (Anthony, Warner, and Kessler, 1994).
That said, the experience of dependence on marijuana tends to be less severe than that observed with cocaine, opiates, and alcohol (Budney, 2006; Budney et al., 1998). On average, individuals with marijuana dependence meet fewer DSM dependence criteria; the withdrawal experience is not as dramatic; and the severity of the associated consequences is not as extreme. However, the apparently less severe nature of marijuana dependence does not necessarily mean that marijuana addiction is easier to overcome. Many factors besides a drug’s physiological effects—including availability, frequency and pattern of use, perception of harm, and cost—can contribute to cessation outcomes and the strength of addiction. The low cost of marijuana, the typical pattern of multiple daily use by those addicted, the less dramatic consequences, and ambivalence may increase the difficulty of quitting. Although determining the relative difficulty of quitting various substances of abuse is complex, the treatment literature reviewed here suggests that the experience of marijuana abusers rivals that of those addicted to other substances.
We have argued elsewhere and reiterate here that animal and human experiments, as well as the epidemiological and clinical literature, clearly indicate that marijuana dependence is much more similar to than different from other substance dependencies (Budney, 2006; Budney and Hughes, 2006). As with other substances, sociodemographic, environmental, genetic, and perhaps neurocognitive factors contribute to the risk of marijuana abuse. Reasons for treatment seeking related to marijuana also appear similar to those for other substances (Budney et al., 1998; Dennis et al., 2002; Stephens, Roffman, and Simpson, 1993), and the rate of response to treatments appears similar to that observed for other types of substance dependence (McRae, Budney, and Brady, 2003).
A better understanding of the mechanisms of action of marijuana treatments and predictors of outcome will lead, it is hoped, to innovations that can better match individuals to specific therapeutic modalities or result in modifications to approaches that deliver more of the “active ingredients” necessary for change. For example, with CM interventions, factors related to the frequency, duration, and magnitude of the incentive schedule used to reinforce abstinence are likely to affect the potency of the intervention and influence outcome (Lussier et al., 2006). In addition, researchers need to continue exploring the potential use of pharmacotherapies as a primary or secondary treatment approach. Recent advances in the understanding of the neurobiology of marijuana’s actions make this a very promising area of investigation. Continued development of cost-effective interventions remains a priority. Other areas that warrant focus include continuing care protocols to prevent or reduce the severity of lapses or relapses, exploration of different magnitude and schedules of reinforcement in CM interventions, and use of innovative technologies—such as computers and the Internet—to assist in delivery of treatment or continued care.
Equally important to treatment development research is the pressing need to tackle issues related to dissemination of effective treatments. Unfortunately, the substance abuse services delivery system continues to lag far behind research advances that delineate effective treatment approaches. Serious challenges related to access and cost impede adoption of important scientific advances in drug dependence treatment in general. The current treatment system experiences difficulty recruiting, training, and retaining treatment staff; inadequate financing to provide treatment; insufficient treatment availability to meet demand; and slow adoption of research-based treatment innovations—all of which contribute to limited access to the most effective treatments (Carroll and Rounsaville, 2007; McLellan, Carise, and Kleber, 2003). The availability of the proven treatments for marijuana disorders—MET, CBT, and CM—is low, even though evidence of their efficacy with substance dependence problems other than marijuana dependence has been documented for many years. Although the three treatments are mainstream among treatment researchers, few community-based substance abuse counselors are currently trained to provide quality MET-CBT, and treatment providers remain ambivalent about CM interventions because of their cost and CM’s basic premise of providing incentives for abstinence (Kirby et al., 2006; Ritter and Cameron, 2007). Treatment services research must continue to investigate novel, efficient, and effective methods for treatment dissemination and implementation.
The good news is that the increased recognition that marijuana can cause addiction and significant negative consequences in a subset of users has prompted the development of marijuana-specific interventions and treatment materials paralleling those for other substance use disorders. These advances have increased users’ and caregivers’ perceptions that it is acceptable to seek and provide treatment for marijuana use and have contributed to an increase in the number of individuals requesting help. Optimistic expectations for enhancements to current treatment approaches appear warranted, as our growing understanding of the principles underlying behavioral treatments continues to produce innovative applications that demonstrate incremental gains in efficacy. Rapid advances in the neurobiology associated with marijuana and the cannabinoid system provide further hope for increasingly effective treatment options. As well, check-up interventions hold promise both for preventing more severe cases of marijuana dependence and for increasing therapeutic contacts with marijuana abusers who might benefit from treatment.
ACKNOWLEDGMENTS
Preparation of this paper was supported by research grants from the National Institute on Drug Abuse (R01DA12471, R01DA15186, and R01DA23526) and in part by the Arkansas Biosciences Institute, the major research component of the Arkansas Master Tobacco Settlement.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. Text Revision, DSM-IV-TR. [Google Scholar]
- Anthony JC, Helzer JE. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America. New York: Free Press; 1991. pp. 116–154. [Google Scholar]
- Anthony JV, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2:244–268. [Google Scholar]
- Azrin NH, et al. Youth drug abuse treatment: A controlled outcome study. Journal of Child & Adolescent Substance Abuse. 1994;3:1–16. [Google Scholar]
- Budney AJ. Are specific dependence criteria necessary for different substances: How can research on cannabis inform this issue? Addiction. 2006;101 (Suppl 1):125–133. doi: 10.1111/j.1360-0443.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, et al. Contingent reinforcement of abstinence with individuals abusing cocaine and marijuana. Journal of Applied Behavior Analysis. 1991;24(4):657–665. doi: 10.1901/jaba.1991.24-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, et al. Adults seeking treatment for marijuana dependence: A comparison to cocaine-dependent treatment seekers. Experimental and Clinical Psychopharmacology. 1998;6(4):419–426. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Budney AJ, et al. Adding voucher-based incentives to coping-skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting and Clinical Psychology. 2000;68(6):1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Budney AJ, et al. Contingency management: Using science to motivate change. In: Coombs RH, editor. Addiction Recovery Tools: Using Science to Motivate Change. Thousand Oaks, CA: Sage; 2001. pp. 147–172. [Google Scholar]
- Budney AJ, et al. A review of the validity and significance of the cannabis withdrawal syndrome. American journal of Psychiatry. 2004;161(11):1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, et al. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74(2):307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, et al. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug and Alcohol Dependence. 2007;86(1):22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Bethesda, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- Budney AJ, Higgins ST, Wong CJ. Marijuana use and treatment outcome in cocaine-dependent patients. Experimental and Clinical Psychopharmacology. 1996;4:396–403. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Current Opinion in Psychiatry. 2006;19(3):233–238. doi: 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Calsyn DA, Saxon AJ. An innovative approach to reducing cannabis use in a subset of methadone clients. Drug and Alcohol Dependence. 1999;53(2):167–169. doi: 10.1016/s0376-8716(98)00121-5. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ. A vision of the next generation of behavioral therapies research in the addictions. Addiction. 2007;102(6):850–862. doi: 10.1111/j.1360-0443.2007.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, et al. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. Journal of the American Medical Association. 2004;291(17):2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Compton WM, Pringle B. Services research on adolescent drug treatment. Commentary on “The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27(3):195–196. doi: 10.1016/j.jsat.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Copeland J. Developments in the treatment of cannabis use disorder. Current Opinion in Psychiatry. 2004;17(3):161–167. [Google Scholar]
- Copeland J, et al. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;21(2):55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;20(1):45–52. doi: 10.1016/s0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Copersino ML, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. American Journal on Addictions. 2006;15(1):8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Dennis M, et al. Changing the focus: The case for recognizing and treating cannabis use disorders. Addiction. 2002;97(Suppl 1):4–15. doi: 10.1046/j.1360-0443.97.s01.10.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, et al. The Cannabis Youth Treatment (CYT) Study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27(3):197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Diamond G, et al. The Cannabis Youth Treatment Study: The treatment models and preliminary findings. In: Roffman RA, Stephens RS, editors. Cannabis Dependence: Its Nature, Consequences and Treatment. Cambridge, United Kingdom: Cambridge University Press; 2006. pp. 247–274. [Google Scholar]
- Dishion T, Kavanagh K. Intervening in Adolescent Problem Behavior A Family-Centered Approach. New York: Guilford Press; 2003. [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98(3):269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Annual Report 2006: The State of the Drug Problem in Europe. Luxembourg: Office for Official Publications of the European Communities; 2006. [Google Scholar]
- Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug and Alcohol Dependence. 2002;67(3):243–248. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, et al. Attributes of long-term heavy cannabis users: A case-control study. Psychological Medicine. 2003;33(8):1415–1422. doi: 10.1017/s0033291703008560. [DOI] [PubMed] [Google Scholar]
- Gulliver SB, Kamholz BW, Helstrom AW. Smoking cessation and alcohol abstinence: What do the data tell us? Alcohol Research & Health. 2006;29(3):208–212. [PMC free article] [PubMed] [Google Scholar]
- Haney M, et al. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165(2):157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, et al. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Hart CL. Increasing treatment options for cannabis dependence: A review of potential pharmacotherapies. Drug and Alcohol Dependence. 2005;80(2):147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, et al. Juvenile drug court: Enhancing outcomes by integrating evidence-based treatments. Journal of Consulting and Clinical Psychology. 2006;74(1):42–54. doi: 10.1037/0022-006X.74.1.42. [DOI] [PubMed] [Google Scholar]
- Higgins ST, et al. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51 (7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Huestis MA, et al. Blockade of effects of smoked marijuana by the CB1-sective cannabinoid receptor antagonist SR141716. Archives of General Psychiatry. 2001;58(4):322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Kadden RM, et al. Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors. 2007;32(6):1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamon JL, Budney AJ, Stanger C. A contingency management intervention for adolescent marijuana abuse and conduct problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):513–521. doi: 10.1097/01.chi.0000159949.82759.64. [DOI] [PubMed] [Google Scholar]
- Kidorf M, et al. A stepped care approach for reducing cannabis use in opioid-dependent outpatients. Journal of Substance Abuse Treatment. 2007;32(4):341–347. doi: 10.1016/j.jsat.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kirby KC, et al. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug and Alcohol Dependence. 2006;85(1):19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Levin FR, et al. Pharmacotherapy for marijuana dependence: A double-blind, placebo-controlled pilot study of divalproex sodium. American Journal on Addictions. 2004;13(1):21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Liddle HA, et al. Multidimensional family therapy for adolescent drug abuse: Results of a randomized clinical trial. American Journal of Drug and Alcohol Abuse. 2001;27(4):651–688. doi: 10.1081/ada-100107661. [DOI] [PubMed] [Google Scholar]
- Lozano BE, Stephens RS, Roffman RA. Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction. 2006;101(11):1589–1597. doi: 10.1111/j.1360-0443.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- Lussier JP, et al. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology. 2004;72(3):455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? Journal of Substance Abuse Treatment. 2003;25(2):117–121. [PubMed] [Google Scholar]
- McRae AL, Budney AJ, Brady KT. Treatment of marijuana dependence: A review of the literature. Journal of Substance Abuse Treatment. 2003;24(4):369–376. doi: 10.1016/s0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Tobacco smoking in marijuana-dependent outpatients. Journal of Substance Abuse. 2001;13(4):583–596. doi: 10.1016/s0899-3289(01)00093-1. [DOI] [PubMed] [Google Scholar]
- Ritter A, Cameron J. Australian clinician attitudes towards contingency management: Comparing Down Under with America. Drug and Alcohol Dependence. 2007;87(2–3):312–315. doi: 10.1016/j.drugalcdep.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Roffman RA, Stephens RS. Cannabis Dependence: Its Nature, Consequences and Treatment. Cambridge, United Kingdom: Cambridge University Press; 2006. [Google Scholar]
- Santisteban DA, et al. Efficacy of brief strategic family therapy in modifying Hispanic adolescent behavior problems and substance abuse. Journal of Family Psychology. 2003;17(1):121–133. doi: 10.1037/0893-3200.17.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, et al. Engaging young probation-referred marijuana-abusing individuals in treatment: A pilot trial. American Journal on Addictions. 2003;12(4):314–323. [PubMed] [Google Scholar]
- Steinberg KL, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. DHHS Publication No SMA 05–4022. [Google Scholar]
- Stephens RS, et al. The Marijuana Treatment Project: Rationale, design, and participant characteristics. Addiction. 2002;97(Suppl 1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Stephens RS, et al. The Marijuana Check-Up: Reaching users who are ambivalent about change. Addiction. 2004;99(10):1323–1332. doi: 10.1111/j.1360-0443.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- Stephens RS, et al. Marijuana dependence treatment for adults, PRN. Symposium conducted at the Eleventh International Conference on Treatment of Addictive Behavior; Santa Fe, NM. 2006. [Google Scholar]
- Stephens RS, Roffman RA. Marijuana dependence treatment PRN. Paper presented at the College of Problems of Drug Dependence; Orlando, FL. 2005. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Adult marijuana users seeking treatment. Journal of Consulting and Clinical Psychology. 1993;61(6):1100–1104. doi: 10.1037//0022-006x.61.6.1100. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting and Clinical Psychology. 1994;62(1):92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS) National Admissions to Substance Abuse Treatment Services. Rockville, MD: Department of Health and Human Services; 2004. pp. 1992–2002. DHHS Publication No SMA 04–3965. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Department of Health and Human Services; 2006a. Treatment Episode Data Set (TEDS):Highlights - 2005. DASIS Series: S-36, DHHS Publication No. SMA 07–4229. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06–4194. Rockville, MD: Department of Health and Human Services; 2006b. Results from the 2005 National Survey on Drug Use and Health: National Finding. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07–4293. Rockville, MD: Department of Health and Human Services; 2007. Results from the 2006 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Tims FM, et al. Characteristics and problems of 600 adolescent cannabis abusers in outpatient treatment. Addiction. 2002;97(Suppl 1):46–57. doi: 10.1046/j.1360-0443.97.s01.7.x. [DOI] [PubMed] [Google Scholar]
- Vandrey RG, et al. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence. 2007 July 21; doi: 10.1016/j.drugalcdep.2007.06.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HB, et al. Treatment outcomes for adolescent substance abuse at 4- and 7-month assessments. Journal of Consulting and Clinical Psychology. 2001;69(5):802–813. [PubMed] [Google Scholar]
- Waldron HB, Kaminer Y. On the learning curve: The emerging evidence supporting cognitive-behavioral therapies for adolescent substance abuse. Addiction. 2004;99 (Suppl 2):93–105. doi: 10.1111/j.1360-0443.2004.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DD, et al. Motivational enhancement therapy for adolescent marijuana users: A preliminary randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74(3):628–632. doi: 10.1037/0022-006X.74.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]