Abstract
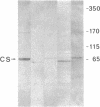
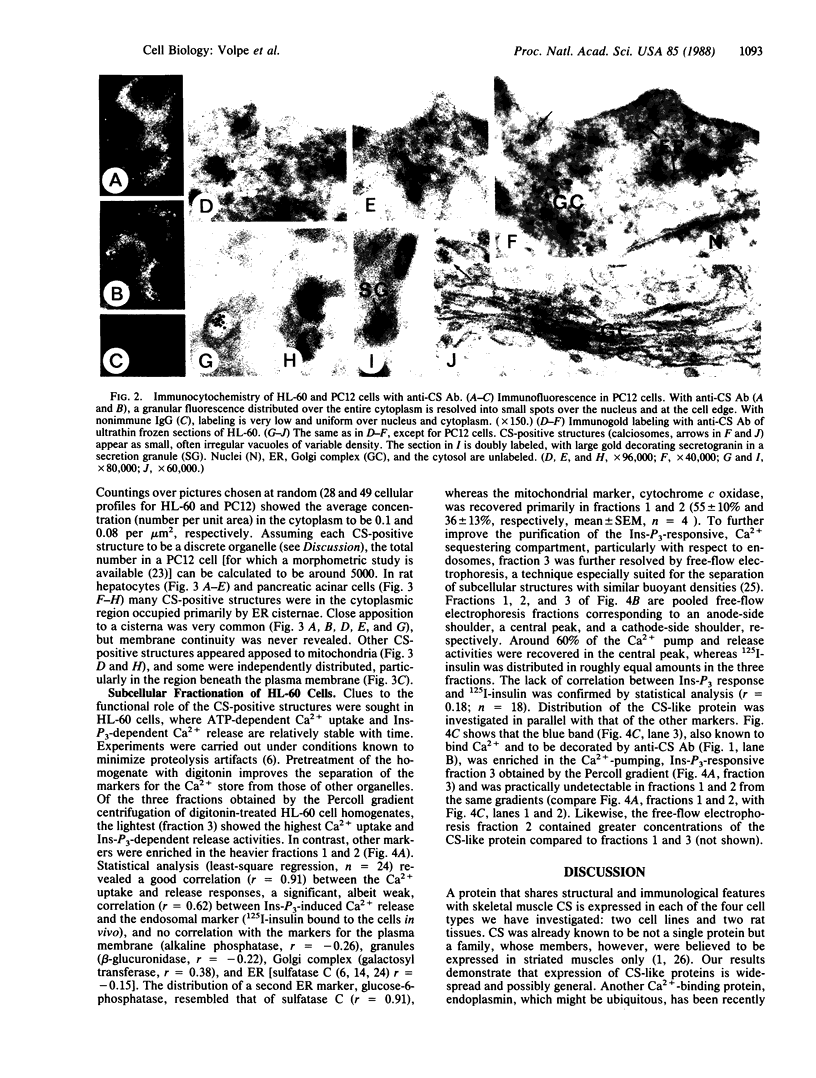
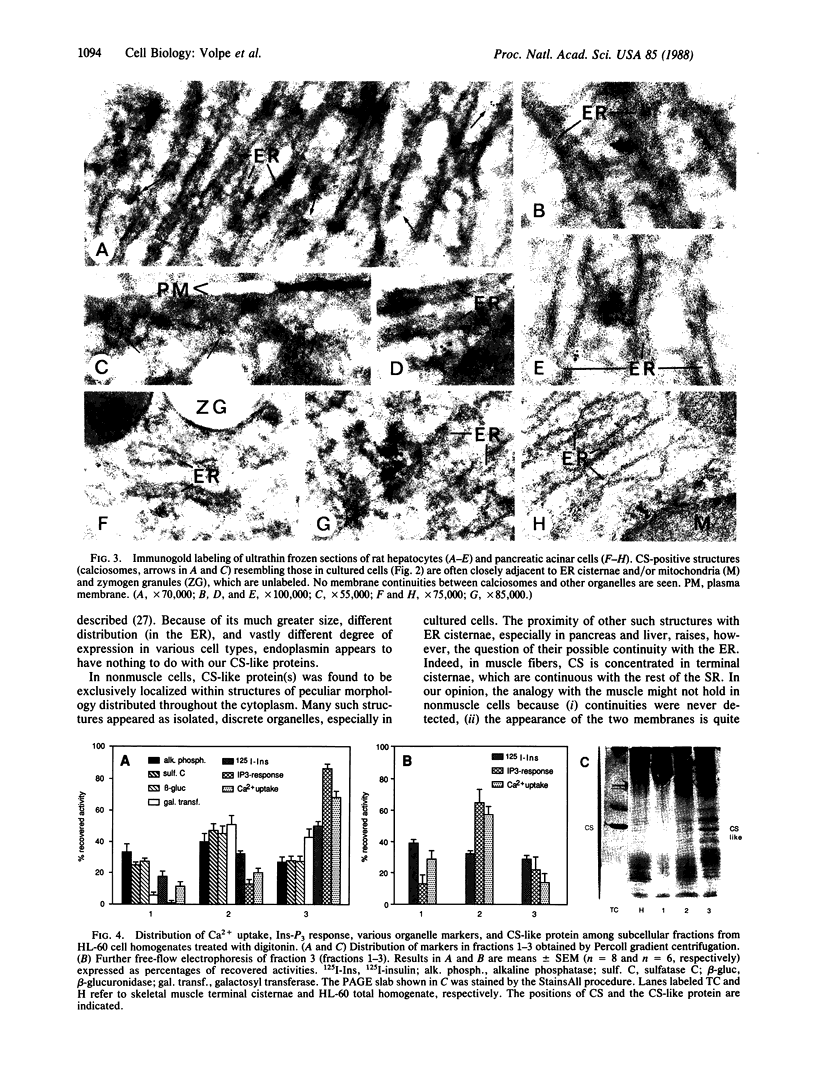
Calsequestrin (CS) is the protein responsible for the high-capacity, moderate affinity binding of Ca2+ within the terminal cisternae of the sarcoplasmic reticulum, believed up to now to be specific for striated muscle. The cells of two nonmuscle lines (HL-60 and PC12) and of two rat tissues (liver and pancreas) are shown here to express a protein that resembles CS in many respects (apparent mass and pH-dependent migration in NaDodSO4/PAGE; blue staining with StainsAll dye; Ca2+ binding ability) and is specifically recognized by affinity-purified antibodies against skeletal muscle CS. In these cells, the CS-like protein is shown by immunofluorescence and immunogold procedures to be localized within peculiar, heretofore unrecognized structures distributed throughout the cytoplasm. These structures appear to be discrete organelles, which we propose to be named "calciosomes." By cell fractionation (Percoll gradient and free-flow electrophoresis), the CS-like protein of HL-60 cells is shown to copurify with the markers of the inositol 1,4,5-trisphosphate (Ins-P3)-sensitive Ca2+ store, whereas the markers of other organelles (endoplasmic reticulum, Golgi complex, mitochondria, endosomes) and of the plasma membrane do not. Calciosome might thus be the intracellular target of Ins-P3--i.e., the source of the Ca2+ redistributed to the cytosol following receptor-triggered generation of the messenger.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayerdörffer E., Streb H., Eckhardt L., Haase W., Schulz I. Characterization of calcium uptake into rough endoplasmic reticulum of rat pancreas. J Membr Biol. 1984;81(1):69–82. doi: 10.1007/BF01868811. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bisson R., Schiavo G. Two different forms of cytochrome c oxidase can be purified from the slime mold Dictyostelium discoideum. J Biol Chem. 1986 Apr 5;261(10):4373–4376. [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Canonico P. G., Beaufay H., Nyssens-Jadin M. Analytical fractionation of mouse peritoneal macrophages: physical and biochemical properties of subcellular organelles from resident (unstimulated) and cultivated cells. J Reticuloendothel Soc. 1978 Aug;24(2):115–138. [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Davis F. E., Palade G. E. Protein blotting in uniform or gradient electric fields. Anal Biochem. 1985 Jan;144(1):32–40. doi: 10.1016/0003-2697(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Spät A., Catt K. J. Intracellular receptors for inositol 1,4,5-trisphosphate in angiotensin II target tissues. J Biol Chem. 1987 Jan 25;262(3):1010–1015. [PubMed] [Google Scholar]
- Iacopetta B., Carpentier J. L., Pozzan T., Lew D. P., Gorden P., Orci L. Role of intracellular calcium and protein kinase C in the endocytosis of transferrin and insulin by HL60 cells. J Cell Biol. 1986 Sep;103(3):851–856. doi: 10.1083/jcb.103.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Keller G. A., Tokuyasu K. T., Dutton A. H., Singer S. J. An improved procedure for immunoelectron microscopy: ultrathin plastic embedding of immunolabeled ultrathin frozen sections. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5744–5747. doi: 10.1073/pnas.81.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Smith M., Macer D., Webster P., Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986 Dec;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Lew P. D. Subcellular distribution of Ca2+ pumping sites in human neutrophils. J Clin Invest. 1987 Jul;80(1):107–116. doi: 10.1172/JCI113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Michalak M., Campbell K. P., MacLennan D. H. Localization of the high affinity calcium binding protein and an intrinsic glycoprotein in sarcoplasmic reticulum membranes. J Biol Chem. 1980 Feb 25;255(4):1317–1326. [PubMed] [Google Scholar]
- Nauseef W. M., Clark R. A. Separation and analysis of subcellular organelles in a human promyelocytic leukemia cell line, HL-60: application to the study of myeloid lysosomal enzyme synthesis and processing. Blood. 1986 Aug;68(2):442–449. [PubMed] [Google Scholar]
- Payne R., Fein A. Inositol 1,4,5 trisphosphate releases calcium from specialized sites within Limulus photoreceptors. J Cell Biol. 1987 Apr;104(4):933–937. doi: 10.1083/jcb.104.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler M., Williams J. A. Localization of ATP-dependent calcium transport activity in mouse pancreatic microsomes. J Membr Biol. 1983;73(2):137–144. doi: 10.1007/BF01870437. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982 Oct;7(10):2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]