Abstract
Background
Depression is highly prevalent among HIV-infected injection drug users (IDUs) and has been associated with poor adherence to antiretroviral therapy and increased morbidity and mortality. Factors associated with changes in depressive symptoms among this group receiving antiretroviral treatment that have not been extensively evaluated.
Methods
This post-hoc analysis of prospective clinical trial analyzes the factors associated with changes in depressive symptomatology using the Center for Epidemiologic Studies of Depression Scale (CES-D) among HIV-infected IDUs enrolled in a prospective, six months randomized controlled trial of directly administered antiretroviral therapy (DAART) versus self-administered therapy.
Results
Of the 127 evaluable IDUs enrolled in the study, 89 subjects (70%) had complete six-month follow-up data. Of these, 58 (63%) met baseline criteria for severe or major depressive disorder (MDD) using the CES-D. CES-D scores improved significantly from baseline to six months overall for the 89 subjects (p=0.01) and for the 58 who had MDD with six-month data (p=0.001). Using multiple regression, an improvement in CES-D score was independently associated with: (1) increase in CD4 count; (2) increase in adherence; (3) non-Caucasian race; and (4) older age. Worsening in CES-D score was associated with: (1) increase in HIV-1 RNA levels; (2) homelessness; (3) poor self-efficacy; (4) active drug use; and (5) male gender. Factors not correlated with changes in CES-D were receipt of DAART, engagement in drug treatment, use of antidepressant medication, and employment. Using generalized estimating equation modeling, factors that remained positively associated with improvements in CES-D score were absence of drug use at six months, having housing, higher self-efficacy, increase in CD4 count and increases in adherence.
Conclusion
Improvements in depressive symptoms could occur with improvement of alterable factors that are associated with strengthening adherence such as linkages to case management, mental health and substance abuse treatment services as well as through enhancement of social stabilization factors through social support and supportive housing.
Keywords: HIV/AIDS, depression, antiretroviral therapy, HIV-1 RNA, CD4 lymphocyte count, directly administered antiretroviral therapy, CES-D, substance abuse, injection drug users, DAART
Introduction
Depression is two fold greater in HIV-infected persons than among demographically matched uninfected persons (Ciesla & Roberts, 2001). Not only is major depression highly prevalent among drug users in general and ranging from 38 to 47% (Johnson, Rabkin, Lipsitz, Williams, & Remien, 1999; Knowlton et al., 2001; Perdue, Hagan, Thiede, & Valleroy, 2003), but has been reported to approach 70% among drug users with HIV infection (Berger-Greenstein et al., 2007). Co-morbid depression among those who are HIV-infected has been associated with an accelerated CD4 count decline (Burack et al., 1993; Ickovics et al., 2001), and increased mortality when compared to non-depressed HIV-infected persons (Bouhnik et al., 2005; Cook et al., 2004).
Depression has been associated with poor adherence to antiretroviral therapy (ART) in HIV-infected substance misusers (Berger-Greenstein et al., 2007), which may result in decreased clinical effectiveness and potential development of drug resistance (Bangsberg, Moss, & Deeks, 2004; Kozal et al., 2005). Little is known, however, about the changes in depressive symptoms over time among HIV-infected drug users who are receiving treatment. This post-hoc analysis evaluates the longitudinal factors associated with changes in depressive symptoms among HIV-infected injection drug users (IDUs) receiving highly active antiretroviral therapy (HAART) as part of a randomized controlled adherence trial of Directly Administered Antiretroviral Therapy (DAART) versus self-administered therapy (SAT).
Methods
Patient population and study design
The parent study design and DAART intervention have been previously described (Altice et al., 2004; Altice, Maru, Bruce, Springer, & Friedland, 2007; Maru et al., 2008; Smith-Rohrberg & Altice, 2006; Smith-Rohrberg, Mezger, Walton, Bruce, & Altice, 2006). Briefly, DAART was provided as modified directly observed therapy five days per week with one dose per day observed in a community setting through a mobile outreach program. The DAART intervention was provided for six months to 88 subjects and compared to a control group of 53 who self-administered their therapy. Data analysis for this paper was restricted to recruited subjects who took at least one dose of medication (N=127), 14 of the original subjects who were assigned to DAART refused DAART upon learning of their randomization. Subjects were recruited from all of the HIV clinics in New Haven, Connecticut. Entry criteria included: (1) being HIV seropositive; (2) being eligible for and/or being prescribed antiretroviral medications; (3) residing within the city of New Haven; (4) reporting heroin and/or cocaine use in the previous six months; and (5) receiving no more than a twice-daily regimen. The study was approved by the Yale University Institutional Review Board and had a Certificate of Confidentiality, and is registered at www.clinicaltrials.gov (NCT00367172). All subjects received monetary compensation only for interviews and phlebotomy.
HIV-1 RNA levels (Amplicor 1.5; Roche) and CD4+ T lymphocyte counts (FACS; Quest); and standardized questionnaires were obtained at baseline, one, three, and six months. Baseline interviews assessed psychosocial and demographic characteristics, as well as adherence strategies, personal priorities and attitudes toward DAART. Standardized scales included the Center for Epidemiologic Studies-Depression (CES-D for depression) (Radloff, 1977), self-efficacy scale (Huba & Melchior), Drug Abuse Symptoms Test (DAST-10 for addiction severity) (Skinner, 1982), and AIDS Clinical Trial Group (ACTG) three-day recall for adherence (Chesney et al., 2000) that were also assessed at baseline, one, three, and six months. The primary outcome of the original trial was virological success, defined as an HIV-RNA level reduction of ≥1.0 log10 or an HIV-1 RNA <400 copies/mL at the end of the six-month intervention (Altice et al., 2007; Smith-Rohrberg et al., 2006). This outcome was achieved in the original trial (Altice et al., 2007; Maru et al., 2008).
Definitions and measurements
Major Depressive Disorder (MDD) was defined as having a CES-D score ≥16, while self-efficacy, or the ability to have control over their own life was based on Module 64: Self-efficacy Form (Huba & Melchior). Adherence was measured using the three-day recall from the ACTG and is reported as a mean change in percentage adherence between baseline and six-month. Drug use was defined responding yes to using either heroin and/or cocaine during any standardized questionnaire. Being in drug treatment was defined broadly as anyone who was enrolled in formal drug treatment programs or attending substance abuse support or self-help groups. Addiction severity was measured using the 10-item Drug Abuse Screening Test (DAST-10).
Statistical analysis
For demographics and baseline information, means and standard deviations were provided for continuous variables, while number and proportions were provided for categorical variables. A paired t-test was used to test the mean changes of CES-D scores from baseline to six months. Generalized Estimating Equation (GEE) multivariate regression models assessed longitudinal association of risk factors with CES-D score. Proc Mixed procedure in SAS V9.13 (Cary, NC), without Bonferoni correction, was used to build GEE multivariate regression models. Statistical significance was considered to be two-sided P<0.05. Backward selection method was used to keep the risk factors with P<0.05 in the final model.
Results
Demographic characteristics
Table 1 compares the baseline characteristics of the 127 subjects with only baseline data to the 89 subjects for whom there was complete six-month data. The 89 subjects with complete data did not differ statistically from the 38 subjects without complete data enrolled in the parent study. Among 127 subjects enrolled, the mean age was 43.4 years; most were male (70.3%; N=89); and most were of non-Caucasian race (80%; N=92). Thirty five percent (N=45) did not finish high school; 91% were unemployed and 40% (N=51) were homeless. All subjects used illegal drugs and 64% stated they were involved in drug treatment at baseline. Approximately, one-third of subjects, irrespective of assignment, were being prescribed antidepressants at enrollment. Mean CD4 count of the cohort was 389 cells/μL and mean viral load was 3.54 log10 at enrollment. All subjects were receiving ART.
Table 1.
Demographics characteristics of baseline subjects (N=127); subjects with six-month data (N=89); and subjects who had missing six month data (N=38)
| Characteristic | N=127 | N=89 | N=38 | P-value | |
|---|---|---|---|---|---|
| Mean age in years (standard deviation) | 43.4 (7.49) | 43.6 (7.37) | 43.2 (7.73) | 0.81 | |
| Gender | Male N (%) | 89 (70.1) | 61 (68.5) | 28 (73.7) | 0.56 |
| Female N (%) | 38 (29.7) | 28 (31.5) | 10 (26.3) | ||
| Race | Black N (%) | 74 (58.3) | 56 (62.9) | 18 (47.4) | 0.15 |
| White N (%) | 24 (18.9) | 17 (19.1) | 7 (18.4) | ||
| Hispanic N (%) | 28 (22.1) | 15 (16.9) | 13 (34.2) | ||
| Other N (%) | 1 (0.8) | 1 (1.1) | 0 | ||
| Arm | DAART N (%) | 74 (58.3) | 54 (60.7) | 20 (52.6) | 0.40 |
| SAT N (%) | 53 (41.7) | 35 (39.3) | 18 (47.4) | ||
| Education | Did not complete High School N (%) | 45 (35.4) | 30 (33.7) | 15 (39.5) | 0.64 |
| High School graduate N (%) | 52 (40.9) | 36 (40.5) | 16 (42.1) | ||
| Beyond High School N (%) | 30 (23.6) | 23 (25.8) | 7 (18.4) | ||
| Monthly income | <$500 | 56 (44.8) | 37 (42.1) | 19 (51.4) | 0.45 |
| $500-$1000 | 58 (46.4) | 44 (50.0) | 14 (37.8) | ||
| >$1000 | 11 (8.8) | 7 (7.9) | 4 (10.8) | ||
| Homelessness N (%) | 51 (40.1) | 11 (29.0) | 40 (44.9) | 0.09 | |
| Social support score mean (standard deviation) | 68.1 (17.98) | 67.5 (17.31) | 69.8 (19.43) | 0.51 | |
| Self-efficacy mean (standard deviation) | 14.0 (4.04) | 22.5 (5.07) | 23.8 (6.22) | 0.19 | |
| DAST score mean (standard deviation) | 4.2 (3.27) | 4.2 (3.27) | 4.4 (3.26) | 0.72 | |
| Receiving antidepressant treatment N (%) | 40 (31.8) | 28 (32.2) | 12 (31.5) | 0.95 | |
| In drug treatment program N (%) | 80 (64.0) | 55 (62.5) | 26 (68.4) | 0.52 | |
| CD4 count cells/mL mean (standard deviation) | 389.6 (341.8) | 359.2 (325.6) | 461.9 (379.0) | 0.12 | |
| Viral load (Log10) mean (standard deviation) | 3.54 (1.53) | 3.56 (1.57) | 3.57 (1.47) | 0.96 | |
Note: P-value is for comparing the group of subjects with six-month data to the group ofsubjects without six-month data.
Major depressive symptoms
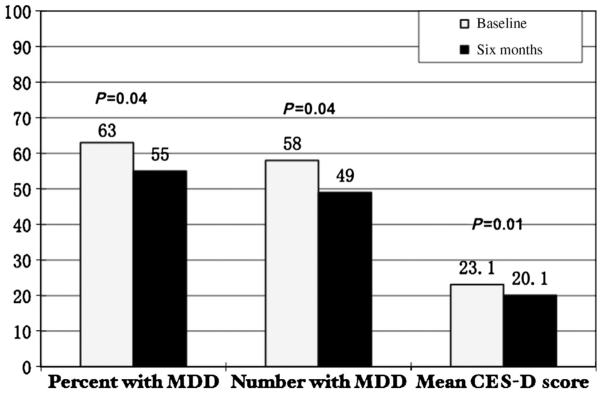
The CES-D was used to assess depressive symptomatology in subjects. Among 127 subjects at baseline, six-month CES-D scores were available for 89 (70%) of the subjects. Mean CES-D score of the cohort significantly improved from 23.1 to 20.1 during the study period (P=0.01) (see Table 2 and Figure 1). Of the 58 subjects (63%) who had major depression symptomatology (CES-D score of ≥16) at baseline, the mean CES-D significantly improved from 30.8 to 25.7 in six months (P=0.001) for this group (see Table 3).
Table 2.
CES-D scores and proportions of Major Depressive Disorder (MDD). Among 127 subjects, 89 (70%) subjects had CES-D score at baseline and six months, the following table shows their CES-D changes and N (%) with MDDa
|
N (%) with MDD |
Mean CES-D (standard deviation) N=89 |
|
|---|---|---|
| Baseline | 58 (63) | 23.1 (13.45) |
| Six months | 49 (55) | 20.1 (13.46) |
| P-valueb | 0.04 | 0.01 |
No statistical difference was found between baseline demographic characteristics of the 89 subjects who had six-month data compared to the 38 subjects who had missing six-month data (data not shown).
Paired t-test.
Figure 1.
First bar graphs depicts a statistically significant decrease in percentage of the 89 study subjects with Major Depressive Disorder (MDD) symptoms at baseline as compared to six months; middle bar graph depicts the absolute number of subjects who had MDD at baseline compared to six months; and the last bar graph depicts the mean decrease in CES-D scores over a six-month time period.
Table 3.
Among 78 subjects who had major depression at baseline, 58 subjects had CES-D score at six months; the following table shows their CES-D changes
| Overall N=58 | |
|---|---|
| Baseline mean (standard deviation) | 30.8 (9.67) |
| six months mean (standard deviation) | 25.7 (12.91) |
| P-valuea | 0.001 |
Paired t-test.
GEE multivariate regression models were utilized to access the longitudinal association of risk factors with changes in CES-D score. Using univariate model results, improvements in depressive symptoms (decreases in CES-D score) were significantly associated with older age (Δ=-0.26), non-Caucasian race (Δ=-5.3), increase in CD4 count (Δ=-0.009) and for each 1% self-reported increase in adherence (Δ=-5.0). Worsening of depressive symptoms (increases in CES-D score) were associated with female gender (Δ=+4.32), homelessness (Δ=+6.4), poor self-efficacy (Δ=+1.0), increase in HIV-1 RNA level (Δ=+1.5), and use of illegal drugs (Δ=+3.25). Factors not significantly associated with improvements in CES-D were randomization to DAART, engagement in drug treatment, use of antidepressant medication, and employment. The multivariate model, however, demonstrated that only use of illegal drugs, homelessness, and having poor self-efficacy was associated with an increase in depressive symptomatology; while an increase in CD4 count and adherence to HAART was associated with improved depressive symptomatology. After controlling for illegal drug use (β=2.57, P=0.03), homelessness (β=4.95, P=0.03), self-efficacy (β=0.86, P<.0001), and CD4 count (β= (0.005, P=0.03), increased adherence was significantly associated to CES-D score (e.g. ifadherence increased by 1%, CES-D score decreased by 3.79.) changes. See Table 4 for details.
Table 4.
Risk factors associated with CES-D score changes. MIXED model (N=127)
| Univariate model |
Multivariate modela |
|||
|---|---|---|---|---|
| Risk factors | β | P-value | β | P-value |
| Age (continuous) | -0.26 | 0.07 | - | - |
| Gender (Female vs. Male) | 4.32 | 0.06 | - | - |
| Race (non-Caucasian vs. Caucasian) | -5.26 | 0.05 | - | - |
| Random (DARRT vs. SAT) | 2.51 | 0.24 | - | - |
| Baseline DAST score | 0.96 | 0.03 | - | - |
| Any drug use | 3.25 | 0.01 | 2.57 | 0.03 |
| Drug treatmentb | -1.26 | 0.36 | - | - |
| Using anti-depressive medication | 1.82 | 0.19 | - | - |
| Unemployed | -3.4 | 0.14 | - | - |
| Homelessness | 6.44 | 0.003 | 4.95 | 0.01 |
| Self-efficacy | 0.99 | <0.0001 | 0.86 | <0.0001 |
| CD4 lymphocyte count | -0.009 | 0.002 | -0.005 | 0.03 |
| Viral load (log10) | 1.47 | 0.003 | - | - |
| Adherence | -5.01 | 0.001 | -3.79 | 0.01 |
Backward selection, and the final model only include those variables with P<0.05.
“Drug treatment”=self-report at baseline for “Are you currently in drug treatment, or going to substance abuse support groups? (yes/no).”
Discussion
The majority of document studies that depression is highly associated with non-adherence to ART. (Bouhnik et al., 2005; Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000; Olatunji, Mimiaga, O’Cleirigh, & Safren, 2006). It stands to reason then that factors that influence depression will likely result in improved clinical outcomes. Multiple factors have been associated with worsening depression and some of these may be alterable, potentially through intervention. This study prospectively examines social, behavioral, and biological factors associated with changes in depressive symptoms among HIV-infected drug users enrolled in a clinical trial. Identifying the possible alterable factors that are associated with depression in this marginalized group could lead to better and more comprehensive adherence interventions that will likely improve clinical outcomes and ultimately reduce development of drug resistance and transmission of HIV.
This study demonstrates that severe depressive symptoms were highly prevalent in this cohort of HIV-infected drug users. Identification and treatment of depression in this population is therefore critical. Overall, depressive symptoms improved significantly over a six-month period in this study. Associations with improvement in depressive symptomatology were found to include higher self-efficacy, having housing, remaining abstinent from illicit drugs, and improvements in CD4 count and adherence to prescribed ART.
While DAART itself was not associated with improvements in depressive symptomatology, it may serve as the pathway by which other identified factors influenced improvements in depressive symptoms (Altice et al., 2007; Maru et al., 2008; Maru et al., 2007; Smith-Rohrberg et al., 2006). For instance, in the parent trial, DAART was associated with improvements in HIV-1 RNA levels and CD4 lymphocyte counts (Altice et al., 2007; Smith-Rohrberg et al., 2006), both of which were independently associated with improved depressive symptomatology in this post-hoc study. DAART was also associated with improvements in adherence itself (Maru et al., 2008). The DAART intervention, in addition to observing an individual take medication doses, provides social support with identification and linkage to needed “enhanced” services such as medical care, drug treatment, and social services. Provision of these “enhanced” services among our DAART subjects was correlated with markedly improved virological outcomes compared to those who did not utilize such services (Smith-Rohrberg et al., 2006). Thus, the complexity of the DAART intervention itself may be the indirect pathway by which depressive symptoms improve.
Other studies have similarly demonstrated the importance of case management services and the improvement with adherence to ART and HIV clinical outcomes (Hollon et al., 2005; Kushel et al., 2006). There are several possible explanations for why improvements in depressive symptomatology occurred in this study population. Increased social support provided by the DAART program itselfmay decrease social isolation and improve symptoms of depression and helplessness. Previous studies have found that improvements in coping techniques have improved depressive symptomatology in HIV-infected persons (Cruess et al., 2002). Improvements in coping patterns may have been available to all subjects through the availability of case management services either provided through the DAART program or through other community resources. The provision of case management services of ten provides individual-based advice that improves self-efficacy and resulted in a more optimistic outlook toward their lives and overall well-being.
Paradoxically, the use of antidepressant treatment was not associated with improvement in CES-D for this cohort. Several reasons may explain this finding. First, nearly all subjects who were prescribed antidepressant medications were receiving it at study entry and therefore already achieved maximum antidepresssive benefit. Additionally, the small number of subjects who received antidepressant therapy, despite the tremendous need, could have caused this lack of association. There is some evidence, however, that antidepressant therapy alone may not be as effective as one would think. One recent meta-analysis of randomized controlled trials evaluating efficacy of antidepressant treatment among HIV-infected depressed persons found that placebo response explained 60% of the variance across the studies (Himelhoch & Medoff, 2005). Psychosocial treatment alone, similar to what is provided when the DAART-team encounters the subject on a daily basis, may improve depressive symptoms through a number of mechanisms, including addressing adverse drug reactions, drug interactions and ongoing counseling and referral to drug treatment (Hollon et al., 2005).
Although, this particular study did not find that active drug treatment was associated with improvements in depressive symptomatology, very few participants enrolled in active drug treatment with opiate substitution therapy (e.g. methadone or buprenorphine) in this study, and buprenorphine was not readily available while this study was on-going. We also defined drug treatment broadly and being enrolled in more evidence-based treatment may have portended an improved outcome. The majority who identified themselves as enrolled in drug treatment were attending 12-step meetings or individual/group drug counseling. This study did, however, find a significant association with “not actively using drugs” and improvement in CES-D score perhaps suggesting that becoming abstinent from illicit drugs improves adherence. Medication assisted therapy for opioid dependence has been associated with improving adherence to ART among HIV-infected persons (Moatti et al., 2000). This suggests that opiate substitution therapy may be beneficial in reducing depressive symptomatology as well as improving adherence to ART.
Furthermore, active drug use and HIV itselfare associated with decreased neurocognitive function. Though not discernable in this study, research has shown that both HAART and cessation of illicit drugs improves neurocognitive function in HIV-1 infected persons (Cohen et al., 2001; Sacktor et al., 2006; Suarez et al, 2001). Hence, it is possible that subjects in this group who had improvements in adherence to HAART could have improved psychomotor function and therefore improved depressive symptoms by virtue of their ART alone.
There are some limitations to this study. First, though validated in several studies as being a sensitive marker for depression, the CES-D is not specific for depression and is perhaps a better surrogate of psychological distress (Fechner-Bates, Coyne, & Schwenk, 1994). False positives using this measure has been associated with co-occurring drug dependence, alcohol dependence, and anxiety disorder (Boyd, Weissman, Thompson, & Myers, 1982). It is possible that more significant changes of depressive symptoms could have been seen using a different measure. Nonetheless, the prevalence of MDD within this cohort is similar to that reported in other cohorts of HIV-infected drug users using other measures.
Second, 30% of the original cohort did not have six-month interview follow-up and resulted in missing data and potential decreased power to test some associations. Nonetheless, the final assessment group did not differ statistically from those with missing data at six months making our findings generalizable to the entire cohort. Last, the parent study was powered to determine differences in virological outcomes within a randomized controlled trial, not to evaluate differences in depressive symptomatology. Future prospective studies utilizing standardized tests of depression that can be used as repeated measures (e.g. Beck’s Depression Inventory, the Hamilton-Anxiety Depression Scale or the Quick Inventory of Depressive Symptomatology) would be better suited in this study population to assess associations of depression and adherence. More studies of this nature are necessary to better understand associations with improvements in depression and adherence to ART in this group of challenging patients.
Conclusions
Future programs that are designed to improve adherence to ART for drug users should incorporate factors associated with improving depression symptomatology. These improvements include linkages with case management, mental health, and substance abuse treatment provided as a comprehensive array of services potentially within a DAART or clinic-based program. Overall it is important to note that factors associated with improvement in depression can be alterable by improving social support in conjunction, or potentially without, explicit psychological counseling, and pharmacotherapy. Future adherence programs for HIV-infected persons should incorporate all of these services to improve not only adherence to medical treatment but also to improve psychological and social well-being of our patients.
Acknowledgements
This research was funded by National Institute on Drug Abuse for the parent study (R01-DA013805) as well as career development awards for Drs. Springer (K23-DA019381) and Altice (K24-DA017072). The funding source played no role in the study design or interpretation of the data.
References
- Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: A prospective, randomized, controlled trial. Clinical Infectious Disease. 2007;45(6):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: Implications for program replication. Clinical Infectious Disease. 2004;38(Suppl 5):376–387. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Moss A, Deeks S. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. Journal of Antimicrobial Chemotherapy. 2004;53:696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- Berger-Greenstein JA, Cuevas CA, Brady SM, Trezza G, Richardson MA, Keane TM. Major depression in patients with HIV/AIDS and substance abuse. AIDS Patient Care STDS. 2007;21(12):942–955. doi: 10.1089/apc.2006.0153. [DOI] [PubMed] [Google Scholar]
- Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antiviral Therapy. 2005;10(1):53–61. [PubMed] [Google Scholar]
- Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Archives of General Psychiatry. 1982;39(10):1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270(21):2568–2573. [PubMed] [Google Scholar]
- Catz S, Kelly JA, Bogart L, Benotsch E, McAuliffe T. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19:124–133. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Boland R, Paul R, Tashima KT, Schoenbaum EE, Celentano DD, et al. Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS. 2001;15(3):341–345. doi: 10.1097/00002030-200102160-00007. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. American Journal of Public Health. 2004;94(7):1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruess S, Antoni MH, Hayes A, Penedo F, Ironson G, Fletcher MA, et al. Changes in mood and depressive symptoms and related change processes during cognitive-behavioral stress management in HIV infected men. Cognitive Therapy Research. 2002;26:373–392. [Google Scholar]
- Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to depressive disorders and other psychopathology. Journal of Consulting and Clinical Psychology. 1994;62(3):550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: A systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19(12):813–822. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Archives of General Psychiatry. 2005;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Huba GJ, Melchior LA, Staff of the Measurement Group. HRSA/HAB’s SPNS Cooperative Agreement Steering Committee Module 64: Self-Efficacy Form. 1996 Retrieved April 10, 2008, from www.The MeasurementGroup.com.
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Rabkin JG, Lipsitz JD, Williams JB, Remien RH. Recurrent major depressive disorder among human immunodeficiency virus (HIV)-positive and HIV-negative intravenous drug users: Findings of a 3-year longitudinal study. Comparitive Psychiatry. 1999;40(1):31–34. doi: 10.1016/s0010-440x(99)90073-1. [DOI] [PubMed] [Google Scholar]
- Knowlton AR, Latkin CA, Schroeder JR, Hoover DR, Ensminger M, Celentano DD. Longitudinal predictors of depressive symptoms among low income injection drug users. AIDS Care. 2001;13(5):549–559. doi: 10.1080/09540120120063197. [DOI] [PubMed] [Google Scholar]
- Kozal MJ, Amico KR, Chiarella J, Cornman D, Fisher W, Fisher J, et al. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. Journal of Acquired Immuno Deficiency Syndrome. 2005;40(1):106–109. doi: 10.1097/01.qai.0000159666.95455.d2. [DOI] [PubMed] [Google Scholar]
- Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clinical Infectious Disease. 2006;43(2):234–242. doi: 10.1086/505212. [DOI] [PubMed] [Google Scholar]
- Maru DS, Bruce RD, Walton M, Mezger JA, Springer SA, Shield D, et al. Initiation, adherence, and retention in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Behaviour. 2008;12(2):284–293. doi: 10.1007/s10461-007-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru DS, Kozal MJ, Bruce RD, Springer SA, Altice FL. Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: Results from a randomized controlled trial. Journal of Acquired Immune Deficiency Syndrome. 2007;46(5):555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti JP, Carrieri MP, Spire B, Gastault JA, Cassuto JP, Moreau J. Adherence to HAART in French HIV-infected injecting drug users: The contribution of buprenorphine drug maintenance treatment. AIDS and Behavior. 2000;14:151–155. doi: 10.1097/00002030-200001280-00010. [DOI] [PubMed] [Google Scholar]
- Olatunji B, Mimiaga M, O’Cleirigh C, Safren S. A review of treatment studies of depression in HIV. Topics in HIV Medicine. 2006;14(3):112–124. [PubMed] [Google Scholar]
- Perdue T, Hagan H, Thiede H, Valleroy L. Depression and HIV risk behavior among Seattle-area injection drug users and young men who have sex with men. AIDS Education Preview. 2003;15(1):81–92. doi: 10.1521/aeap.15.1.81.23842. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky R, Robertson K, Wong M, Musisi S, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67(2):311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behaviour. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: Questions about study population and analytical approach. Clinical Infectious Diseases. 2006;43(9):1221–1222. doi: 10.1086/508357. author reply 1222-1223. [DOI] [PubMed] [Google Scholar]
- Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. Journal of Acquired Immuno Deficiency Syndrome Human Reterovirology. 2006;43(Suppl 1):48–53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- Suarez S, Baril L, Stankoff B, Khellaf M, Dubois B, Lubetzki C, et al. Outcome of patients with HIV-1-related cognitive impairment on highly active antiretroviral therapy. AIDS. 2001;15(2):195–200. doi: 10.1097/00002030-200101260-00008. [DOI] [PubMed] [Google Scholar]