Abstract
Glycoprotein (GP) VI is a critical platelet collagen receptor. Phosphoinositide 3-kinase (PI3K) plays an important role in GPVI-mediated platelet activation, yet the major PI3K isoforms involved in this process have not been identified. In addition, stimulation of GPVI results in the activation of Akt, a downstream effector of PI3K. Thus, we investigated the contribution of PI3K isoforms to GPVI-mediated platelet activation and Akt activation. A protein kinase C inhibitor GF 109203X or a P2Y12 receptor antagonist AR-C69931MX partly reduced GPVI-induced Akt phosphorylation. Platelets from mice dosed with clopidogrel also showed partial Akt phosphorylation, indicating that GPVI-mediated Akt phosphorylation is regulated by both secretion-dependent and -independent pathways. In addition, GPVI-induced Akt phosphorylation in the presence of ADP antagonists was completely inhibited by PI3K inhibitor LY294002 and PI3Kβ inhibitor TGX-221 indicating an essential role of PI3Kβ in Akt activation directly downstream of GPVI. Moreover, GPVI-mediated platelet aggregation, secretion, and intracellular Ca2+ mobilization were significantly inhibited by TGX-221, and less strongly inhibited by PI3Kα inhibitor PIK75, but were not affected by PI3Kγ inhibitor AS252424 and PI3Kδ inhibitor IC87114. Consistently, GPVI-induced integrin αIIbβ3 activation of PI3Kγ−/− and PI3Kδ−/− platelets also showed no significant difference compared with wild-type platelets. These results demonstrate that GPVI-induced Akt activation in platelets is dependent in part on Gi stimulation through P2Y12 receptor activation by secreted ADP. In addition, a significant portion of GPVI-dependent, ADP-independent Akt activation also exists, and PI3Kβ plays an essential role in GPVI-mediated platelet aggregation and Akt activation.
Introduction
Glycoprotein VI (GPVI)2 is a platelet collagen receptor that is constitutively associated with Fc receptor-γ chain (1–4). Fc receptor-γ chain is phosphorylated by Src family kinase on tyrosine residue of its immunoreceptor tyrosine-based activation motif upon collagen ligation to GPVI and the tyrosine kinase Syk (spleen tyrosine kinase) binds to the immunoreceptor tyrosine-based activation motif and becomes autophosphorylated (5–10). Tyrosine phosphorylation of Syk leads to phosphorylation of several adaptor proteins such as linker for T-cell activation and Src homology 2-containing leukocyte protein 76, recruitment of Bruton tyrosine kinase, and activation of phosphoinositide 3-kinase (PI3K) (11–15). This phosphorylation process leads to tyrosine phosphorylation and activation of phospholipase Cγ2 (16), which leads to intracellular calcium mobilization and protein kinase C (PKC) activation.
Akt is a 57-kDa serine/threonine kinase that plays an important role in mediating the anti-apoptotic effect of many growth factors (17–19). Akt contains a pleckstrin homology domain adjacent to a centrally located catalytic domain that is connected to a short C-terminal tail (20). Both translocation of Akt to cell membranes and phosphorylation of Thr308/Ser473 are required for full enzyme activity. PI3K is an upstream regulator of Akt (21), and PI3K products phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate trigger the simultaneous phosphorylation of Akt by phosphatidylinositol-dependent kinases 1 and 2 (22). Akt is activated by various agonists, including thrombin, ADP, U46619, and collagen (23–27). We and others have shown that Gi-coupled P2Y12 ADP receptor is responsible for a significant proportion of Akt activation (23, 24). Convulxin (CVX), a snake venom protein belonging to the heterodimeric C-type lectin family, is a selective GPVI agonist that mediates platelet activation by collagen (28). Upon stimulation of platelets with CVX, Akt is translocated to cell membranes via interaction of its pleckstrin homology domain with phosphoinositide products of PI3K and is subsequently phosphorylated at its regulatory threonine and serine phosphorylation sites in association with phosphatidylinositol-dependent kinase 1 and integrin-linked kinase independently of platelet aggregation (25).
PI3K has been shown to play an important role in platelet aggregation (29). Three families of PI3K (classes I, II, and III) are present. The class I PI3K is responsible for agonist-induced phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate production and involved in the activation of integrin αIIbβ3. The class IA (α, β, and δ) isoforms have p55–85 regulatory subunits and are classically regulated by tyrosine kinases, whereas the class IB (γ) isoform has a p101 regulatory subunit and is activated by G protein-coupled receptors (30). Recent studies have reported the selective inhibitors of these PI3K isoforms (31–36). Platelets contain all class I PI3K isoforms with lower levels of p110δ (37). It is shown that PI3Kβ has an important role in ADP-induced platelet aggregation (34). PI3Kγ is also thought to be mediated by the βγ complexes dissociated from Gi proteins upon receptor activation (38) and plays a significant role in ADP-induced platelet aggregation (39). In addition, PI3Kδ plays only a minor role in GPVI-mediated platelet activation (40). Recently, we and others have reported that PI3Kα plays a role in insulin-like growth factor-1-mediated Akt phosphorylation through Gi signaling (41, 42).
Although it has been shown that CVX activates Akt in platelets, the signaling events involved in Akt activation mediated by GPVI signaling are not well established in platelets. Our study reveals that GPVI-mediated Akt phosphorylation is regulated in a dual way: (a) dependent on secreted ADP and Gi signaling and (b) secretion-independent downstream of GPVI. We have found that PI3Kβ isoform plays an essential role in secretion-independent Akt activation as well as aggregation, secretion, and intracellular Ca2+ mobilization.
EXPERIMENTAL PROCEDURES
Materials
Convulxin was purchased from Centerchem, Inc. (Norwalk, CT). Epinephrine, 2-MeSADP, MRS-2179, CDP-Star® chemiluminescent substrates, apyrase (type V), and bovine serum albumin (fraction V) were purchased from Sigma. Anti-phospho-Akt (Ser473 and Thr308) and anti-β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA). Alkaline phosphatase-labeled secondary antibody was from Kirkegaard & Perry Laboratories (Gaithersburg, MD). LY294002 and PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole[3,4-d]pyrimidine) were from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA). TGX-221 was purchased from Cayman Chemical (Ann Arbor, MI). PIK75, AS-252424, and IC87114 were from the laboratory of Shaun Jackson (Monash University, Victoria, Australia). 5,5′-dimethyl-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (dimethyl-BAPTA), Fura 2 acetoxymethyl ester, and Oregon Green 488-labeled fibrinogen (OG488-fibrinogen) were obtained from Molecular Probes (Eugene, OR). AR-C69931MX was a gift from AstraZeneca (Loughborough, UK). Bisindolylmaleimide I (GF 109203X) was from Calbiochem (San Diego, CA). Clopidogrel was from Bristol-Myers Squibb Co. (Princeton, NJ). All other reagents were reagent grade, and deionized water was used throughout.
Animals
P2Y1-deficient mice were generated by subcontract with Lexicon Genetics Inc (Woodlands, TX) as described previously (23). Mice deficient in PI3K p110γ or PI3K p110δ were from sources previously described (34). P2Y12-deficient mice were from Dr. Xiaoping Du (University of Illinois, Chicago, IL).
Preparation of Human Platelets
Human blood was collected from a pool of healthy volunteers in a one-sixth volume of acid-citrate-dextrose (2.5 g of sodium citrate, 1.5 g of citric acid, and 2.0 g of glucose in 100 ml of H2O). Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 230 × g for 20 min at room temperature. Acetylsalicylic acid was added to PRP to a final concentration of 1 mm, and the preparation was incubated for 45 min at 37 °C followed by centrifugation at 980 × g for 10 min at room temperature. The platelet pellet was resuspended in Tyrode buffer (138 mm NaCl, 2.7 mm KCl, 2 mm MgCl2, 0.42 mm NaH2PO4, 5 mm glucose, 10 mm HEPES, pH 7.4, and 0.2% bovine serum albumin) containing 0.1 unit/ml apyrase. This low concentration of apyrase is not enough to block responses to ADP but will prolong the responsiveness of platelets to ADP by preventing desensitization of the P2Y receptors. These conditions have been standardized in the laboratory. The platelet count was adjusted to 2 × 108 cells/ml.
Preparation of Mouse Platelets
Blood was collected from the vena cava of anesthetized mice into syringes containing 1/10th blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100 × g for 10 min at room temperature. PRP was recovered, and platelets were pelleted at 400 × g for 10 min at room temperature. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.05 units/ml apyrase to a density of 2 × 108 cells/ml.
Measurement of Akt Phosphorylation
Platelets were stimulated with agonists under non-stirring condition for the appropriate time, and the Akt phosphorylation was measured as previously described (23). In some experiments, GF 109203X (10 μm), a PKC inhibitor, dimethyl-BAPTA (10 μm), a calcium chelator, and PP2 (10 μm), a Src family kinase inhibitor, were added, and the mixture was incubated for 5 min at 37 °C without stirring before agonist stimulation.
Platelet Aggregation, Secretion, Intracellular Ca2+ Mobilization, and Flow Cytometry
Convulxin-induced platelet aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37 °C with stirring (900 rpm). A 0.5-ml sample of aspirin-treated washed platelets was stimulated with CVX in the presence of ADP receptor antagonists MRS-2179 and AR-C69931MX, and the change in light transmission was measured. Platelets were preincubated with different PI3K inhibitors before agonist stimulation. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s.
Platelet secretion was determined by measuring the release of ATP by adding luciferin-luciferase reagent. Platelet ATP release and aggregation were performed in a lumi-aggregometer at 37 °C with stirring at 900 rpm simultaneously. Platelet secretion was also independently determined by measuring the release of [3H]5-HT. PRP was incubated with 1 μCi/ml [3H]5-HT, and 1 μm imipramine was added to the Tyrode buffer during resuspension of the washed platelets to prevent re-uptake of secreted 5-HT. Secretion experiments were stopped after 2 min with the addition of formaldehyde/EDTA. Samples were then centrifuged at 5000 × g for 1 min, and the radioactivity of the supernatant was measured using a Wallac 1409 liquid scintillation counter (Gaithersburg, MD). Platelet secretion was expressed as the percentage of total [3H]5-HT present in platelets without activation.
Platelet Ca2+ mobilization was also measured. PRP was incubated with 5 μm Fura-2 acetoxymethyl ester and 1 mm aspirin. Fluorescence was measured, and the Ca2+ concentration was calculated as previously described (43).
OG488-fibrinogen binding to washed mouse platelets was quantified by flow cytometry (44). Briefly, washed murine platelets (5 × 107/ml) were stimulated with CRP (10 μg/ml) at room temperature in the presence of Oregon Green-fibrinogen (OG-FGN, 20 μg/ml), mixed and at predetermined time-points fixed with 2% (final) paraformaldehyde for 1 h. Platelets were pelleted by centrifugation at 2,000 × g and resuspended in 500 μl of phosphate-buffered saline. The fluorescent intensity of each platelet sample was analyzed using a FACSCalibur cytometer (BD Biosciences), with OG-FGN absorbing at 498 ± 5 nm and emitting a maximum wavelength of 524 ± 5 nm. The extent of integrin αIIbβ3 activation was represented by the level of OG-FGN binding of 10,000 platelets, expressed numerically as the geometric mean (arbitrary fluorescence units, CellQuest software). Nonspecific fluorescence, as measured in resting platelets, was subtracted from all data points.
Statistical Analysis
Statistical significance was determined by one-way analysis of variance. All statistical tests were carried out using Prism software (Version 3.0). Data are presented as means ± S.E.
RESULTS
Time- and Dose-dependent Phosphorylation of Akt in Response to CVX in Platelets
It has been shown that Akt is phosphorylated in platelets in response to a selective agonist for the platelet collagen receptor GPVI (25). To determine the kinetics of Akt phosphorylation, Ser473 phosphorylation was monitored over a time range of 0.5–20 min. Immunoblot analysis revealed that Akt is phosphorylated on Ser473 (supplemental Fig. S1) in response to CVX in both time-dependent and concentration-dependent manner. An increase in Ser473 phosphorylation was detectable within 30 s of 100 ng/ml CVX stimulation. The level of phosphorylation peaked at around 5 min of stimulation and gradually decreased. These results are consistent with a previous study in which an increase in Ser473 phosphorylation was detectable within 20 s, and the level of phosphorylation peaked at around 5 min (25). We exposed platelets to different concentrations of CVX, and Ser473 phosphorylation was measured at 3 min after the addition of agonist. An increase in Ser473 phosphorylation was detectable at concentrations above 10 ng/ml CVX, and maximal phosphorylation was achieved at concentrations above 50 ng/ml CVX.
Effect of Secretion in Akt Phosphorylation by CVX in Human Platelets
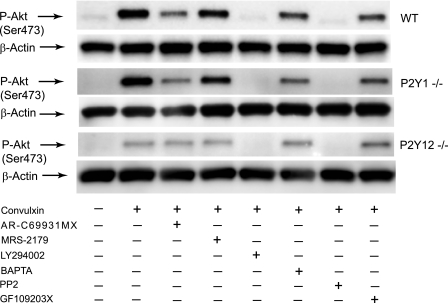
To elucidate downstream pathways involved in GPVI-mediated Akt phosphorylation in platelets, we first focused on the role of secreted ADP. As seen in Fig. 1, treatment of platelets with the PKC inhibitor GF109203X (45) significantly, but not completely, inhibited both Ser473 and Thr308 phosphorylation. We then used the selective antagonists of the platelet ADP receptors, P2Y1 and P2Y12. P2Y12 receptor antagonist AR-C69931MX (46) drastically reduced the Akt phosphorylation to the same extent obtained by GF109203X, but P2Y1 receptor antagonist MRS-2179 (47) had no effect on CVX-stimulated Akt phosphorylation. These results suggest that secreted ADP and subsequent P2Y12 receptor-mediated Gi stimulation plays a major role in GPVI-mediated Akt phosphorylation. However, they also indicate that there is residual Akt phosphorylation induced by CVX in the absence of secretion, which is due to a P2Y12-independent component.
FIGURE 1.
The role of secretion and Gi signaling in Akt phosphorylation by CVX in human platelets. A, platelets preincubated with 100 nm AR-C69931MX, 100 μm MRS2179, 25 μm LY294002, 10 μm dimethyl-BAPTA, 10 μm PP2, or 10 μm GF109203X were stimulated at 37 °C for 3 min with 100 ng/ml CVX. Equal amounts of proteins were separated by SDS-PAGE, Western blotted, and probed for anti-phospho-Akt (Ser473 and Thr308) or anti-β-actin (lane loading control) antibody. B, densitometric measurement of phospho-Akt (Ser473) and phospho-Akt (Thr308), expressed as percentage of agonist control. The blot shown is a representative of three independent experiments. Data are mean ± S.E. (n = 3); *, p < 0.01 compared with agonist.
To further identify the additional signaling molecules involved in P2Y12-independent pathways facilitating GPVI-mediated Akt phosphorylation, we looked at the contribution of GPVI-induced intracellular calcium mobilization in Akt phosphorylation. When we used the high affinity calcium chelator dimethyl-BAPTA, CVX-induced Akt phosphorylation was significantly inhibited, suggesting that intracellular calcium is prerequisite of Akt phosphorylation.
Because of the involvement of Src-family kinases to GPVI signaling in platelets, we used the Src-family kinase inhibitor PP2 (48) to evaluate whether Src-family kinase activation is required for Akt activation mediated by CVX in platelets. Fig. 1 shows that PP2 completely blocked Akt phosphorylation induced by CVX, suggesting the essential role of Src-family kinases in GPVI-mediated Akt activation. PI3K activation through the collagen receptor is downstream of Src-family kinases, and PI3K is the main upstream regulator of Akt (21). The PI3K inhibitor LY294002 (29) completely inhibited CVX-induced Akt phosphorylation confirming the essential role of PI3K in Akt phosphorylation. Thus, these results demonstrate that Src-family kinases and PI3K are the major contributor to the P2Y12-independent pathways in addition to the P2Y12-dependent pathways.
Effect of Secretion on Akt Phosphorylation by CVX in Murine Platelets
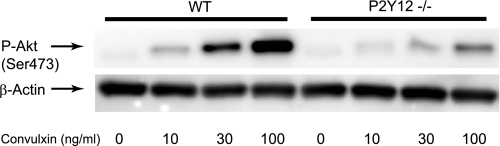
To further confirm the importance of secreted ADP and Gi stimulation in mouse as well as human platelets, we used platelets from P2Y1- and P2Y12-deficient mice or mice dosed with clopidogrel. Clopidogrel blocks activation of platelets by selectively targeting the P2Y12 receptor (49). Consistent with the activation profile seen in human platelets, wild-type murine platelets exhibited an increased Akt phosphorylation in response to CVX, which was significantly inhibited in the presence of AR-C69931MX and GF109203X (Fig. 2). Similar Akt phosphorylation profile in response to CVX was observed in platelets from the P2Y1-deficient mice. However, Akt phosphorylation was significantly diminished in response to CVX in both P2Y12-deficient platelets and clopidogrel-dosed murine platelets further indicating the important role of P2Y12-dependent pathways in CVX-mediated Akt phosphorylation. This residual phosphorylation of Akt was not affected in the presence of AR-C69931MX and GF109203X confirming the contribution of P2Y12-independent pathways to GPVI-mediated Akt phosphorylation. The remaining Akt phosphorylation was eliminated by PP2 and LY294002, demonstrating that Src family kinases and PI3K are required for the P2Y12-independent pathways. In addition, Akt was phosphorylated in response to CVX in a concentration-dependent manner in P2Y12-deficient platelets (Fig. 3). These results further indicate that CVX phosphorylates Akt through P2Y12-dependent and -independent pathways in both human and mouse platelets.
FIGURE 2.
The effect of secretion and Gi signaling in Akt phosphorylation by CVX in mouse platelets. Wild-type, P2Y1-deficient, P2Y12-deficient, and clopidogrel-treated mouse platelets preincubated with 100 nm AR-C69931MX, 100 μm MRS2179, 25 μm LY294002, 10 μm dimethyl-BAPTA, 10 μm PP2, or 10 μm GF109203X were stimulated at 37 °C for 3 min with 100 ng/ml CVX. Samples were analyzed by Western blot analysis with anti-Ser(P)473 antibody. The Western analysis shown is a representative of three independent experiments.
FIGURE 3.
Dose-dependent Akt phosphorylation in response to CVX in P2Y12−/− mice. Platelets isolated from P2Y12−/− mice and their wild-type littermates were stimulated with different concentrations of CVX for 3 min at 37 °C. Equal amounts of proteins were immunoblotted with anti-phospho-Akt (Ser473) and anti-β-actin (lane loading control) antibody. The results shown are representative of three independent experiments.
Restoration of CVX-induced Akt Phosphorylation Inhibited by GF109203X or AR-C69931MX
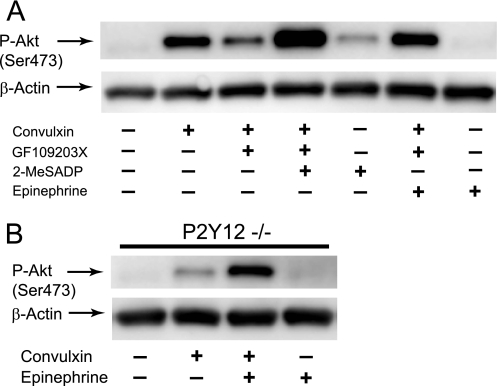
To clarify the role of secreted ADP, we evaluated the effect of selective activation of Gi- or Gz-coupled receptors when secretion was blocked by GF109203X. As shown in Fig. 4A, immunoblot analysis revealed that selective stimulation of Gi signaling through P2Y12 receptor with 2-MeSADP or Gz signaling through α2A adrenergic receptor with epinephrine (50) induced a weak Akt phosphorylation as compared with CVX. However, 2-MeSADP or epinephrine reversed the inhibitory effect of GF109203X on CVX-induced Akt phosphorylation and restored Akt phosphorylation. We also found that CVX-induced Akt phosphorylation was restored by epinephrine in P2Y12-deficient platelets (Fig. 4B) confirming that epinephrine can substitute for the P2Y12 receptor-mediated effect through Gz signaling cascade. These results provide further evidence that Gi stimulation through P2Y12 receptor activation by secreted ADP is partly responsible for Akt phosphorylation induced by CVX.
FIGURE 4.
Selective stimulation of either Gi- or Gz-coupled receptor restores CVX-induced Akt phosphorylation upon blockade of secretion. A, platelets preincubated in the absence and presence of 10 μm GF109203X were stimulated at 37 °C for 3 min with 100 ng/ml CVX. The addition of 100 nm 2-MeSADP or 10 μm epinephrine was made as indicated. B, platelets isolated from P2Y12−/− mice were stimulated at 37 °C for 3 min with 100 ng/ml CVX in the absence and presence of 10 μm epinephrine. Platelet proteins were separated by SDS-PAGE, Western blotted, and probed for anti-phospho-Akt (Ser473) or anti-β-actin (lane loading control) antibody.
Effect of PI3K Inhibitors on Akt Phosphorylation Mediated by CVX
CVX requires PI3K activation to induce Akt phosphorylation (Figs. 1 and 2). To better understand the role of individual PI3K isoforms, we have evaluated the effect of PI3K isoforms on CVX-mediated Akt phosphorylation by using different PI3K-selective inhibitors (31–36). CVX-induced Akt phosphorylation was significantly inhibited in the presence of PI3Kβ inhibitor TGX-221 (p < 0.005), and less strongly inhibited by PI3Kα inhibitor PIK75 (p < 0.01), but was not affected by PI3Kγ inhibitor AS252424 and PI3Kδ inhibitor IC87114 (Fig. 5, A and C). The inhibitory effects of TGX-221 and PIK75 indicate an essential role of PI3Kβ and a less critical role of PI3Kα in CVX-induced Akt phosphorylation in platelets.
FIGURE 5.
The effect of PI3K inhibitors on Akt phosphorylation induced by CVX in platelets. Washed platelets preincubated with 25 μm LY294002, 100 nm PIK75, 500 nm TGX-221, 2 μm AS-252424, or 1 μm IC87114 were stimulated at 37 °C for 3 min with CVX in the absence (A) and presence (B) of 100 nm AR-C69931MX. The reaction was stopped by the addition of 3× SDS sample buffer. Platelet proteins were analyzed by Western blot analysis with anti-phospho-Akt (Ser473) or anti-β-actin (lane loading control) antibody. C, densitometric measurement of phospho-Akt, expressed as percentage of agonist control. The Western analysis shown is a representative of three experiments. Data are mean ± S.E. (n = 3); *, p < 0.005; **, p < 0.01 compared with agonist.
To determine the role of PI3K isoforms on CVX-induced Akt phosphorylation independent of Gi signaling by secreted ADP, we measured the effect of PI3K-selective inhibitors on Akt phosphorylation induced by CVX in the presence of AR-C69931MX. As shown in Fig. 5 (B and C), the remaining Akt phosphorylation in the presence of AR-C69931MX was completely abolished by PI3Kβ inhibitor TGX-221 (p < 0.005), indicating that PI3Kβ is an essential PI3K isoform in the secretion-independent component of CVX-mediated Akt phosphorylation.
We have repeated the same experiment using CRP, GPVI-specific agonist, and have observed the similar Akt phosphorylation profile in the presence of PI3K inhibitors compared with CVX (supplemental Fig. S2), further confirming the essential role of PI3Kβ in GPVI-mediated Akt phosphorylation.
Effect of PI3K Inhibitors on GPVI-induced Platelet Aggregation, Secretion, and Intracellular Ca2+ Mobilization
It has been shown that PI3K and Akt play an important role in collagen-induced platelet aggregation. To identify the role of individual PI3K isoforms on CVX-induced platelet activation, we measured the effect of PI3K-selective inhibitors on platelet aggregation, secretion, and intracellular Ca2+ mobilization induced by CVX in the presence of ADP antagonists AR-C69931MX and MRS-2179. CVX-induced platelet aggregation was markedly reduced by PI3K inhibitor LY294002 and PI3Kβ inhibitor TGX-221 and partially inhibited by PI3Kα inhibitor PIK75 (p < 0.05), whereas PI3Kγ inhibitor AS252424 and PI3Kδ inhibitor IC87114 showed little or no effect on CVX-induced platelet aggregation (Fig. 6A). The dense granule secretion was measured by ATP release (Fig. 6B) and [3H]5-HT release (Fig. 6C). Consistent with the effect of TGX-221 on platelet aggregation, TGX-221 significantly (p < 0.05) inhibited platelet dense granule release consistent with the inhibition of PI3K with LY294002. CVX-induced calcium mobilization was also significantly (p < 0.05) inhibited in the presence of TGX-221 to the same extent with the LY294002-treated platelets (Fig. 6D). In addition, a partial inhibition of platelet secretion and calcium mobilization was observed in the presence of PIK75 (p < 0.05), suggesting the contribution of PI3Kα in GPVI stimulation. These studies confirm the critical role of PI3Kβ and a less important role of PI3Kα in GPVI-mediated platelet responses, including platelet aggregation, secretion, and Ca2+ mobilization.
FIGURE 6.
The effect of PI3K inhibitors on CVX-induced platelet aggregation, secretion, and Ca2+ mobilization. Washed platelets preincubated with 25 μm LY294002, 100 nm PIK75, 500 nm TGX-221, 2 μm AS-252424, or 1 μm IC87114 were stimulated at 37 °C with 100 ng/ml CVX in the presence of 100 nm AR-C69931MX and 10 μm MRS-2179, and platelet aggregation (A), ATP secretion (picomoles/108 platelets) (B), 5-HT secretion (C), and Ca2+ mobilization (D) were measured as noted. Tracings (A) are representative of results obtained from three different donors. The results (B–D) show composite data from three experiments, and values are mean ± S.E. (n = 3); *, p < 0.05 compared with CVX.
GPVI-induced Platelet Aggregation and Integrin αIIbβ3 Activation in PI3K p110γ−/− and PI3K p110δ−/− Platelets
Platelets from PI3K p110γ−/− and PI3K p110δ−/− mice were stimulated with CRP in the presence of a cyclooxygenase inhibitor indomethacin and ADP antagonists AR-C69931MX and MRS-2179, referred to as autocrine stimulation inhibitors. GPVI-induced platelet aggregation of ASI-treated PI3K p110γ−/− and PI3K p110δ−/− platelets revealed, respectively, negligible inhibition and a slight increase in the rate and extent of aggregation at 3 min as compared with control, suggesting that neither of these PI3K isoforms plays a role in GPVI-mediated platelet aggregation (Fig. 7, A–D). Similarly, autocrine stimulation inhibitor-treated platelets from PI3K p110γ−/− and PI3K p110δ−/− mice demonstrated no significant difference in OG-FGN binding compared with control following a 5- and 10-min CRP stimulation (p > 0.05) (Fig. 7, E and F). The response in PI3K p110γ−/− platelets was comparable to that of PI3K p110γ+/+ platelets treated with PI3Kγ inhibitor AS252424 (Fig. 7E), confirming that neither PI3K p110γ nor PI3K p110δ contribute significantly to GPVI-induced activation of integrin αIIbβ3. These findings support a minor role for PI3K p110γ and PI3K p110δ in platelet activation downstream of GPVI.
FIGURE 7.
GPVI-induced platelet aggregation and integrin αIIbβ3 activation in p110γ−/− and p110δ−/− mice. Washed platelets from p110γ+/+ and p110γ−/− (A and B) or p110δ+/+ and p110δ−/− (C and D) mice (2 × 108/ml) were preincubated with autocrine stimulation inhibitors (10 μm AR-C69931MX, 100 μm MRS-2179, and 10 μm indomethacin). Platelets were stimulated with 10 μg/ml CRP in the presence of fibrinogen (0.5 mg/ml), and aggregation was monitored as change in light transmission. A and C, representative aggregation tracings. B and D, quantification of aggregation, expressed as percentage of light transmission at 3 min after CRP addition. Washed platelets from p110γ+/+ and p110γ−/− (E) or p110δ+/+ and p110δ−/− (F) mice (5 × 107/ml), preincubated with autocrine stimulation inhibitors, were stimulated with 10 μg/ml CRP in the presence of OG488-fibrinogen (OG-FGN), and used for flow cytometry. Fibrinogen binding is expressed as percentages relative to the control condition. Data are means ± S.E. (n = 3–5); *, p < 0.05 compared with wild-type mice.
DISCUSSION
PI3K plays an important role in platelet collagen receptor GPVI-mediated platelet activation, but the contribution of the specific PI3K isoforms involved in this process have not been identified. In addition, Akt is the downstream effectors of PI3K, and stimulation of GPVI with CRP or CVX activates Akt (25), which plays an important role in collagen-induced platelet aggregation (51). However, little is known in platelets with regard to the signaling of GPVI-mediated Akt activation. Thus, to determine the role of PI3K isoforms in GPVI-mediated platelet activation, we first have investigated the regulation of Akt downstream of GPVI in platelets. We have shown that Akt phosphorylation in response to thrombin depends on Gi stimulation through P2Y12 receptor activation by secreted ADP (23). Because we have also shown that secreted ADP has a potentiating effect for CVX-induced platelet aggregation (52), we have focused on the contribution of secretion on the Akt phosphorylation following activation of GPVI.
To determine whether CVX can activate Akt independently of secretion and Gi signaling pathways, we have used PKC inhibitor GF109203X and Gi-coupled P2Y12 receptor antagonist AR-C69931MX. Akt phosphorylation in response to CVX was dramatically blocked by GF109203X and AR-C69931MX, suggesting that CVX depends on secretion and Gi signaling to activate Akt. Akt phosphorylation is significantly diminished in response to CVX in clopidogrel-dosed mouse platelets confirming the important role of secreted ADP and Gi stimulation in Akt phosphorylation. Furthermore, CVX induces Akt phosphorylation in P2Y1-deficient platelets indicating that Gαq is not required for GPVI-mediated Akt phosphorylation. Because intracellular calcium has a role in platelet secretion (53), the decreased Akt phosphorylation in the presence of dimethyl-BAPTA might be due to the fact that dimethyl-BAPTA interferes with platelet secretion and subsequent inhibition of Gi stimulation resulting in the inhibition of Akt phosphorylation.
To confirm the contribution of secreted ADP to GPVI-mediated Akt phosphorylation, we have selectively supplemented Gi or Gz signaling with 2-MeSADP and epinephrine under the condition of secretion blockade or P2Y12 receptor blockade and evaluated CVX-mediated Akt phosphorylation. Inhibition of Akt phosphorylation by secretion blockade was overcome to the levels achieved by CVX stimulation by supplemental Gi/Gz signaling, further confirming the essential role of secretion and Gi-dependent signaling pathways in GPVI-mediated Akt phosphorylation.
We have shown that Src family kinases play a role in Akt phosphorylation mediated by Gi signaling (54). Although secreted ADP and Gi signaling contribute to GPVI-induced Akt phosphorylation in platelets, direct GPVI-mediated Akt phosphorylation exists without the contribution of secretion. The CVX-induced Akt phosphorylation was completely inhibited in the presence of Src inhibitor, indicating the involvement of Src family kinases in both secretion-dependent and -independent component of GPVI signaling. It has also been shown that the Src family kinases Fyn and Lyn play an important role in GPVI-mediated platelet activation (8). We have found that Fyn, not Lyn, regulates GPVI-mediated Akt phosphorylation. Fyn-deficient platelets have reduced Akt phosphorylation induced by CVX, whereas Akt phosphorylation does not appear to be affected in Lyn-deficient platelets (not shown). It is consistent with the previous findings showing that GPVI-mediated signaling is inhibited in Fyn-deficient platelets, whereas Lyn-deficient platelets have the initial delay in platelet activation followed by the potentiation of response (8). We have also found that CVX-induced Akt phosphorylation was not affected by Src deficiency (not shown), and others have reported that the Src-family kinase, Fgr, does not affect GPVI signaling (8). However, residual phosphorylation of Akt in Fyn-deficient platelets suggests the contribution of other Src family kinases to GPVI-mediated Akt phosphorylation. Taken together, Src family kinases, especially Fyn, play a major role in Akt phosphorylation by GPVI stimulation.
PI3K also plays a role in the secretion-dependent and -independent GPVI-mediated Akt phosphorylation. Consistent with previous report, our results using the PI3K inhibitor LY294002 show that PI3K is an important downstream molecule of GPVI signaling that leads to Akt phosphorylation (25). However, it was not clear which subtype of PI3K (α, β, δ, or γ) is involved in GPVI-mediated Akt phosphorylation and platelet activation, and we have used several PI3K inhibitors to selectively inhibit different isoforms of PI3K to define the role of individual PI3K isoforms in regulating GPVI-mediated Akt phosphorylation and platelet responses. Our results show that CVX-mediated Akt phosphorylation in the presence of P2Y12 receptor antagonist AR-C69931MX is completely abolished by PI3K p110β inhibitor TGX-221 or PI3K inhibitor LY294002 and a lesser extent by PI3K p110α inhibitor PIK75, whereas PI3Kγ inhibitor AS252424 or PI3Kδ inhibitor IC87114 have little or no effect on Akt phosphorylation, indicating the essential role of PI3K p110β in the GPVI-dependent, secretion-independent Akt phosphorylation. It has also been shown that PI3K activity is required for P2Y12 receptor-mediated platelet aggregation (55), and PI3K activity is necessary for ADP-induced Akt phosphorylation that depends on the Gi-coupled P2Y12 receptor (23, 24). We and others have reported that insulin-like growth factor-1 potentiates platelet activation by supplementing Gi signaling through PI3K p110α/Akt pathways (41, 42). It is also shown that the contribution of PI3Kγ to Gi-mediated Akt and Rap1B activation is minimal (39, 56). In addition, another study has shown that Gi-dependent Rap1B activation is inhibited by >70% by TGX-221 indicating that PI3K p110β has an important role in P2Y12/Gi signaling in platelets (34). Consistent with these findings, our result shows that CVX-mediated Akt phosphorylation is significantly inhibited in the presence of TGX-221, suggesting that PI3K p110β represents the main PI3K isoform in Gi signaling linked to GPVI-mediated Akt phosphorylation. However, some degree of Akt phosphorylation occurs in the presence of TGX-221. There are two possibilities. First, platelet granules contain several other agonists, such as chemokines, that could activate Gi pathways (57). Thus, these other Gi-activating agonists use different signaling pathways to activate Akt through different isoforms of PI3K. The other possibility is that more than one isoform of PI3K is involved in the secretion-dependent Akt phosphorylation.
It has been shown that collagen-induced platelet aggregation is blocked by inhibition of PI3K with wortmannin (11). Because Akt is one of the central downstream effectors of PI3K, Akt may have a role in GPVI-mediated platelet aggregation. It has been shown that Akt1-deficient platelets exhibit a markedly delayed aggregation and spreading in response to collagen (51), but others have reported that both Akt1- and Akt2-deficient platelets aggregate normally in response to collagen (24). We have found that platelet aggregation induced by CVX became reversible and mimicked the effect of LY294002 in the presence of 1L-6-hydroxymethyl-chiro-inositol2-[(R)-2-O-methyl-3-O-octadecylcarbonate] (58) or 1-(5-chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride (59) (data not shown) confirming the importance of Akt ac tivity in GPVI-mediated platelet aggregation. Consistent with the effect of isoform-specific PI3K inhibitors on Akt phosphorylation, PI3K p110β inhibitor TGX-221 and PI3K inhibitor LY294002 blocked CVX-mediated platelet aggregation, secretion, and calcium mobilization, confirming an important role of PI3K p110β in GPVI-mediated platelet responses. PI3K p110γ−/− and PI3K p110δ−/− platelets showed no effect on GPVI-induced platelet aggregation and integrin αIIbβ3 activation, confirming that neither PI3K p110γ nor PI3K p110δ contribute to platelet activation downstream of GPVI. However, a partial inhibition of platelet aggregation and Akt phosphorylation was observed in the presence of PI3Kα inhibitor PIK75 suggesting the contribution of PI3Kα. This finding is consistent with the observation that, in addition to PI3K p110β, the PI3K p110α isoform contributes to GPVI-mediated Rap1B and platelet procoagulant activity (K. Gilio, et al., accompanying paper (61)). Our results further show that TGX-221 has the same extent of inhibition on platelet aggregation, secretion, calcium mobilization, and Akt phosphorylation compared with pan-PI3K inhibitor LY294002. Although both PI3Kα and PI3Kβ seem to be important for GPVI-induced platelet activation, the function of PI3Kβ is less redundant than PI3Kα. Hence, we attribute a major role of PI3K p110β in these events. During the preparation of our manuscript, Canobbio et al. have reported the crucial role of PI3Kβ in GPVI signaling by using the PI3Kβ kinase-dead mice (60), further confirming our results.
In conclusion, we demonstrate that GPVI-mediated Akt phosphorylation depends in part on Gi stimulation through P2Y12 receptor activation by secreted ADP. The GPVI-dependent, ADP-independent portion of Akt phosphorylation also exists, and PI3K p110β isoform plays an essential role in GPVI-induced platelet aggregation and Akt phosphorylation.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL60683 and HL80444 (to S. P. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- GPVI
- glycoprotein VI
- Akt
- protein kinase B
- PI3K
- phosphoinositide 3-kinase
- CVX
- convulxin
- CRP
- collagen-related peptide
- Gi
- heterotrimeric GTP-binding protein, which inhibits adenylyl cyclase
- P2Y12
- platelet ADP receptor coupled to inhibition of adenylyl cyclase
- PKC
- protein kinase C
- 2-MeSADP
- 2-methylthio-ADP
- PRP
- platelet-rich plasma
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole[3,4-d]pyrimidine
- dimethyl-BAPTA
- 5,5′-dimethyl-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- 5-HT
- serotonin
- OG-FGN
- Oregon Green-fibrinogen.
REFERENCES
- 1.Gibbins J., Asselin J., Farndale R., Barnes M., Law C. L., Watson S. P. (1996) J. Biol. Chem. 271, 18095–18099 [DOI] [PubMed] [Google Scholar]
- 2.Gibbins J. M., Okuma M., Farndale R., Barnes M., Watson S. P. (1997) FEBS Lett. 413, 255–259 [DOI] [PubMed] [Google Scholar]
- 3.Nieswandt B., Bergmeier W., Schulte V., Rackebrandt K., Gessner J. E., Zirngibl H. (2000) J. Biol. Chem. 275, 23998–24002 [DOI] [PubMed] [Google Scholar]
- 4.Tsuji M., Ezumi Y., Arai M., Takayama H. (1997) J. Biol. Chem. 272, 23528–23531 [DOI] [PubMed] [Google Scholar]
- 5.Ezumi Y., Shindoh K., Tsuji M., Takayama H. (1998) J. Exp. Med. 188, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichinohe T., Takayama H., Ezumi Y., Arai M., Yamamoto N., Takahashi H., Okuma M. (1997) J. Biol. Chem. 272, 63–68 [DOI] [PubMed] [Google Scholar]
- 7.Ichinohe T., Takayama H., Ezumi Y., Yanagi S., Yamamura H., Okuma M. (1995) J. Biol. Chem. 270, 28029–28036 [DOI] [PubMed] [Google Scholar]
- 8.Quek L. S., Pasquet J. M., Hers I., Cornall R., Knight G., Barnes M., Hibbs M. L., Dunn A. R., Lowell C. A., Watson S. P. (2000) Blood 96, 4246–4253 [PubMed] [Google Scholar]
- 9.Watson S. P., Gibbins J. (1998) Immunol. Today 19, 260–264 [DOI] [PubMed] [Google Scholar]
- 10.Poole A., Gibbins J. M., Turner M., van Vugt M. J., van de Winkel J. G., Saito T., Tybulewicz V. L., Watson S. P. (1997) EMBO J. 16, 2333–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagrue A. H., Francischetti I. M., Guimarães J. A., Jandrot-Perrus M. (1999) FEBS Lett. 448, 95–100 [DOI] [PubMed] [Google Scholar]
- 12.Quek L. S., Bolen J., Watson S. P. (1998) Curr. Biol. 8, 1137–1140 [DOI] [PubMed] [Google Scholar]
- 13.Watson S. P., Asazuma N., Atkinson B., Berlanga O., Best D., Bobe R., Jarvis G., Marshall S., Snell D., Stafford M., Tulasne D., Wilde J., Wonerow P., Frampton J. (2001) Thromb. Haemost. 86, 276–288 [PubMed] [Google Scholar]
- 14.Gibbins J. M., Briddon S., Shutes A., van Vugt M. J., van de Winkel J. G., Saito T., Watson S. P. (1998) J. Biol. Chem. 273, 34437–34443 [DOI] [PubMed] [Google Scholar]
- 15.Asazuma N., Wilde J. I., Berlanga O., Leduc M., Leo A., Schweighoffer E., Tybulewicz V., Bon C., Liu S. K., McGlade C. J., Schraven B., Watson S. P. (2000) J. Biol. Chem. 275, 33427–33434 [DOI] [PubMed] [Google Scholar]
- 16.Daniel J. L., Dangelmaier C., Smith J. B. (1994) Biochem. J. 302, 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellacosa A., Testa J. R., Staal S. P., Tsichlis P. N. (1991) Science 254, 274–277 [DOI] [PubMed] [Google Scholar]
- 18.Cheng J. Q., Godwin A. K., Bellacosa A., Taguchi T., Franke T. F., Hamilton T. C., Tsichlis P. N., Testa J. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 9267–9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakatani K., Thompson D. A., Barthel A., Sakaue H., Liu W., Weigel R. J., Roth R. A. (1999) J. Biol. Chem. 274, 21528–21532 [DOI] [PubMed] [Google Scholar]
- 20.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) The EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 21.Burgering B. M., Coffer P. J. (1995) Nature 376, 599–602 [DOI] [PubMed] [Google Scholar]
- 22.Stokoe D., Stephens L. R., Copeland T., Gaffney P. R., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. (1997) Science 277, 567–570 [DOI] [PubMed] [Google Scholar]
- 23.Kim S., Jin J., Kunapuli S. P. (2004) J. Biol. Chem. 279, 4186–4195 [DOI] [PubMed] [Google Scholar]
- 24.Woulfe D., Jiang H., Morgans A., Monks R., Birnbaum M., Brass L. F. (2004) J. Clin. Invest. 113, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry F. A., Gibbins J. M. (2002) J. Biol. Chem. 277, 12874–12878 [DOI] [PubMed] [Google Scholar]
- 26.Cho M. J., Pestina T. I., Steward S. A., Lowell C. A., Jackson C. W., Gartner T. K. (2002) Blood 99, 2442–2447 [DOI] [PubMed] [Google Scholar]
- 27.Kroner C., Eybrechts K., Akkerman J. W. (2000) J. Biol. Chem. 275, 27790–27798 [DOI] [PubMed] [Google Scholar]
- 28.Polgár J., Clemetson J. M., Kehrel B. E., Wiedemann M., Magnenat E. M., Wells T. N., Clemetson K. J. (1997) J. Biol. Chem. 272, 13576–13583 [DOI] [PubMed] [Google Scholar]
- 29.Trumel C., Payrastre B., Plantavid M., Hechler B., Viala C., Presek P., Martinson E. A., Cazenave J. P., Chap H., Gachet C. (1999) Blood 94, 4156–4165 [PubMed] [Google Scholar]
- 30.Vanhaesebroeck B., Waterfield M. D. (1999) Exp. Cell Res. 253, 239–254 [DOI] [PubMed] [Google Scholar]
- 31.Hayakawa M., Kaizawa H., Moritomo H., Koizumi T., Ohishi T., Okada M., Ohta M., Tsukamoto S., Parker P., Workman P., Waterfield M. (2006) Bioorg. Med. Chem. 14, 6847–6858 [DOI] [PubMed] [Google Scholar]
- 32.Camps M., Rückle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Françon B., Martin T., Gretener D., Perrin D., Leroy D., Vitte P. A., Hirsch E., Wymann M. P., Cirillo R., Schwarz M. K., Rommel C. (2005) Nat. Med. 11, 936–943 [DOI] [PubMed] [Google Scholar]
- 33.Condliffe A. M., Davidson K., Anderson K. E., Ellson C. D., Crabbe T., Okkenhaug K., Vanhaesebroeck B., Turner M., Webb L., Wymann M. P., Hirsch E., Ruckle T., Camps M., Rommel C., Jackson S. P., Chilvers E. R., Stephens L. R., Hawkins P. T. (2005) Blood 106, 1432–1440 [DOI] [PubMed] [Google Scholar]
- 34.Jackson S. P., Schoenwaelder S. M., Goncalves I., Nesbitt W. S., Yap C. L., Wright C. E., Kenche V., Anderson K. E., Dopheide S. M., Yuan Y., Sturgeon S. A., Prabaharan H., Thompson P. E., Smith G. D., Shepherd P. R., Daniele N., Kulkarni S., Abbott B., Saylik D., Jones C., Lu L., Giuliano S., Hughan S. C., Angus J. A., Robertson A. D., Salem H. H. (2005) Nat. Med. 11, 507–514 [DOI] [PubMed] [Google Scholar]
- 35.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadhu C., Masinovsky B., Dick K., Sowell C. G., Staunton D. E. (2003) J. Immunol. 170, 2647–2654 [DOI] [PubMed] [Google Scholar]
- 37.Vanhaesebroeck B., Welham M. J., Kotani K., Stein R., Warne P. H., Zvelebil M. J., Higashi K., Volinia S., Downward J., Waterfield M. D. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4330–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clapham D. E., Neer E. J. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 167–203 [DOI] [PubMed] [Google Scholar]
- 39.Hirsch E., Bosco O., Tropel P., Laffargue M., Calvez R., Altruda F., Wymann M., Montrucchio G. (2001) FASEB. J. 15, 2019–2021 [DOI] [PubMed] [Google Scholar]
- 40.Senis Y. A., Atkinson B. T., Pearce A. C., Wonerow P., Auger J. M., Okkenhaug K., Pearce W., Vigorito E., Vanhaesebroeck B., Turner M., Watson S. P. (2005) Platelets 16, 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hers I. (2007) Blood 110, 4243–4252 [DOI] [PubMed] [Google Scholar]
- 42.Kim S., Garcia A., Jackson S. P., Kunapuli S. P. (2007) Blood 110, 4206–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinter P. G., Dangelmaier C. A., Quinton T. M., Kunapuli S. P., Daniel J. L. (2007) J. Thromb. Haemost 5, 362–368 [DOI] [PubMed] [Google Scholar]
- 44.Maxwell M. J., Yuan Y., Anderson K. E., Hibbs M. L., Salem H. H., Jackson S. P. (2004) J. Biol. Chem. 279, 32196–32204 [DOI] [PubMed] [Google Scholar]
- 45.Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F., et al. (1991) J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- 46.Ingall A. H., Dixon J., Bailey A., Coombs M. E., Cox D., McInally J. I., Hunt S. F., Kindon N. D., Teobald B. J., Willis P. A., Humphries R. G., Leff P., Clegg J. A., Smith J. A., Tomlinson W. (1999) J. Med. Chem. 42, 213–220 [DOI] [PubMed] [Google Scholar]
- 47.Baurand A., Raboisson P., Freund M., Léon C., Cazenave J. P., Bourguignon J. J., Gachet C. (2001) Eur. J. Pharmacol. 412, 213–221 [DOI] [PubMed] [Google Scholar]
- 48.Hanke J. H., Gardner J. P., Dow R. L., Changelian P. S., Brissette W. H., Weringer E. J., Pollok B. A., Connelly P. A. (1996) J. Biol. Chem. 271, 695–701 [DOI] [PubMed] [Google Scholar]
- 49.Geiger J., Brich J., Hönig-Liedl P., Eigenthaler M., Schanzenbächer P., Herbert J. M., Walter U. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 2007–2011 [DOI] [PubMed] [Google Scholar]
- 50.Yang J., Wu J., Kowalska M. A., Dalvi A., Prevost N., O'Brien P. J., Manning D., Poncz M., Lucki I., Blendy J. A., Brass L. F. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9984–9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J., De S., Damron D. S., Chen W. S., Hay N., Byzova T. V. (2004) Blood 104, 1703–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinton T. M., Ozdener F., Dangelmaier C., Daniel J. L., Kunapuli S. P. (2002) Blood 99, 3228–3234 [DOI] [PubMed] [Google Scholar]
- 53.Quinton T. M., Kim S., Dangelmaier C., Dorsam R. T., Jin J., Daniel J. L., Kunapuli S. P. (2002) Biochem. J. 368, 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S., Jin J., Kunapuli S. P. (2006) Blood 107, 947–954 [DOI] [PubMed] [Google Scholar]
- 55.Kauffenstein G., Bergmeier W., Eckly A., Ohlmann P., Léon C., Cazenave J. P., Nieswandt B., Gachet C. (2001) FEBS Lett. 505, 281–290 [DOI] [PubMed] [Google Scholar]
- 56.Lova P., Paganini S., Hirsch E., Barberis L., Wymann M., Sinigaglia F., Balduini C., Torti M. (2003) J. Biol. Chem. 278, 131–138 [DOI] [PubMed] [Google Scholar]
- 57.Kowalska M. A., Ratajczak M. Z., Majka M., Jin J., Kunapuli S., Brass L., Poncz M. (2000) Blood 96, 50–57 [PubMed] [Google Scholar]
- 58.Hu Y., Qiao L., Wang S., Rong S. B., Meuillet E. J., Berggren M., Gallegos A., Powis G., Kozikowski A. P. (2000) J. Med. Chem. 43, 3045–3051 [DOI] [PubMed] [Google Scholar]
- 59.Smith U., Carvalho E., Mosialou E., Beguinot F., Formisano P., Rondinone C. (2000) Biochem. Biophys. Res. Commun. 268, 315–320 [DOI] [PubMed] [Google Scholar]
- 60.Canobbio I., Stefanini L., Cipolla L., Ciraolo E., Gruppi C., Balduini C., Hirsch E., Torti M. (2009) Blood, in press [DOI] [PubMed] [Google Scholar]
- 61.Gilio K., Munnix I. C., Mangin P., Cosemans J. M., Feijge M. A., van der Meijden P. E., Olieslagers S., Chrzanowska-Wodnicka M. B., Lillian R., Schoenwaelder S., Koyasu S., Sage S. O., Jackson S. P., Heemskerk J. W. (2009) J. Biol. Chem. 284, 33750–33762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.