Abstract
Galactofuranose (Galf) containing molecules have been described at the cell surface of several eukaryotes and shown to contribute to the virulence of the parasite Leishmania major and the fungus Aspergillus fumigatus. It is anticipated that a number of the surface glycoconjugates such as N-glycans or glycolipids are galactofuranosylated in the Golgi apparatus. This raises the question of how the substrate for galactofuranosylation reactions, UDP-Galf, which is synthesized in the cytosol, translocates into the organelles of the secretory pathway. Here we report the first identification of a Golgi-localized nucleotide sugar transporter, named GlfB, with specificity for a UDP-Galf. In vitro transport assays established binding of UDP-Galf to GlfB and excluded transport of several other nucleotide sugars. Furthermore, the implication of glfB in the galactofuranosylation of A. fumigatus glycoconjugates and galactomannan was demonstrated by a targeted gene deletion approach. Our data reveal a direct connection between galactomannan and the organelles of the secretory pathway that strongly suggests that the cell wall-bound polysaccharide originates from its glycosylphosphatidylinositol-anchored form.
Introduction
Galactofuranose (Galf)3 is an important constituent of the microbial cell surface (1). It occurs in structures essential for bacterial virulence or growth such as the O-antigen of the outer membrane lipopolysaccharide or the mycobacterial arabinogalactan (2). In eukaryotes, Galf has principally been reported in glycoconjugates and polysaccharides of fungi and protozoan parasites (3), although distribution of the glf gene encoding the UDP-Galf biosynthetic enzyme, UDP-galactopyranose mutase (UGM), suggests its presence in many lower eukaryotes (4, 5). Among fungi, the cell wall of the opportunistic pathogen Aspergillus fumigatus is one of the best studied. In this organism, Galf has been found in the polysaccharides galactomannan (6), on glycoinositolphosphoceramides (7, 8) and in N- and O-linked glycans of glycoproteins (9–11). In A. fumigatus, as in the parasite Leishmania major, generation of a mutant devoid of Galf resulted in attenuated virulence that highlights an important role of Galf for eukaryotic pathogens (12, 13).
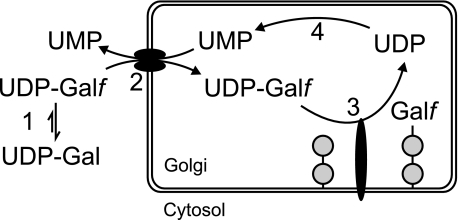
Little is known about the Galf biosynthetic pathways in eukaryotes, although several enzymes involved in the synthesis of Galf containing polysaccharide have been described in prokaryotes (see Ref. 14 for a recent review). Genetic and biochemical studies have shown that UDP-Galf arising from the action of UGM is essential for galactofuranosylation in both prokaryotes and eukaryotes (2, 12, 13, 15, 16). This nucleotide sugar is the established or presumed donor substrate of specific galactofuranosyltransferases (GlfTs) comprising characterized bacterial β-GlfTs (14) and a family of putative β-GlfTs identified in the protozoan parasite L. major (17, 18). In addition, many eukaryotic and prokaryotic GlfTs such as the mycobacterial α-GlfTs are still unidentified. One of the putative eukaryotic transferases, known as LPG1, is involved in the biosynthesis of the lipophosphoglycan (LPG) of L. major and is localized in the Golgi apparatus (19). Similarly, enzymes involved in addition of the terminal Galf to N-glycans, O-glycans, or glycolipids are assumed to be localized in this organelle. UDP-Galf biosynthesis, however, occurs in the cytosol (12), which makes translocation of UDP-Galf across the Golgi membrane necessary (Fig. 1).
FIGURE 1.
Schematic model of galactofuranosylation. UDP-Galf is synthesized from UDP-Galactopyranose (UDP-Gal) (1) and translocated via an antiporter into the Golgi in exchange for UMP (2). Galactofuranosyltransferases transfer Galf moieties from UDP-Galf on various glycoconjugates (3) and UDP is converted to UMP by a nucleoside diphosphatase (4).
Nucleotide sugar transporters (NSTs) are multitransmembrane proteins present in all kinds of eukaryotic organisms. They consist typically of 8–10 transmembrane α-helices linked by short loops. Further structural information is very limited because efforts to obtain crystals for x-ray structure determination have been hindered by the high hydrophobicity of NSTs. Many NSTs have been functionally characterized in biochemical assays measuring incorporation of radioactive nucleotide sugars into membrane vesicles. These experiments led to the development of a mechanistic model, in which NSTs work as antiporters that export a nucleotide sugar molecule in exchange for an equally charged nucleoside monophosphate molecule (20).
Because of their structural conservation, putative NSTs can be readily found by data base mining. In humans, they belong to the SLC35 (solute carrier 35) family (21), which comprise 10 characterized members and 13 proteins whose function is currently unknown. Phylogenetic classification identified subfamilies that contain all of the characterized SLC35 proteins, but do not allow classification of most of the SLC35 proteins with unknown function (22). Furthermore, substrate specificity is hardly conserved within NST subfamilies, thus it is generally not possible to infer substrate specificity from the level of sequence identity or phylogeny.
NSTs are closely related to plastidic phosphate translocators that include translocators for triose phosphate, phosphoenolpyruvate, glucose 6-phosphate, and xylulose phosphate. Additionally, a variety of uncharacterized phosphate translocator-homologous proteins are found in plant and other organisms, including human (23). In this study, we describe the characterization of an A. fumigatus phosphate translocator-homologous protein with NST function and demonstrate its specificity for UDP-Galf. The importance for in vivo galactofuranosylation is shown by a targeted gene deletion approach.
EXPERIMENTAL PROCEDURES
Materials
Radiolabeled nucleotide sugars were purchased from PerkinElmer Life Sciences (UDP-[3H]Gal, UDP-[3H]GlcNAc, and UDP-[14C]GlcA), American Radiolabeled Chemicals (UDP-[3H]GalNAc and [3H]UMP), and GE Healthcare (UDP-[3H]Glc). UDP-Galf was chemically synthesized (24).
Strains, Medium, and Growth Conditions
For protein expression, Saccharomyces cerevisiae strain BY4741 in which the gene for the endoplasmic reticulum UDP-GlcNAc transporter, YEA4, had been deleted (MATa; his3D1; leu2D0; met15D0; ura3D0; YEL004w::kanMX4; EUROSCARF, Frankfurt, Germany) was cultivated in SC minimal media (2% glucose, 1.7 g/liter DifcoTM Yeast Nitrogen Base without amino acids and ammonium sulfate (BD Biosciences), 5 g/liter of ammonium sulfate) supplemented with l-histidine (50 mg/liter), l-methionine (50 mg/liter), and l-leucine (100 mg/liter).
A. fumigatus clinical isolate D141 was used in this study. For gene deletion purposes, a D141 strain deficient in non-homologous end-joining (AfS35) was used (25). Strains were grown at 37 °C on Aspergillus minimal medium containing 1% d-glucose as carbon source and 70 mm NaNO3 as nitrogen source. Phleomycin was added for selection purposes at 30 mg/liter.
Bioinformatic Analyses
Transmembrane helix prediction was carried out using the ConPred II program (26). For plant proteins, predicted transmembrane domains were obtained from the ARAMEMNON data base (27). BLAST searches were performed using default parameter values with the low complexity filter switched off.
Cloning of the glfB Gene
Total RNA was isolated from A. fumigatus mycelium and glfB mRNA was reverse transcribed into single-stranded cDNA using the JE28 primer. All primer sequences are provided in supplemental Table S1. The glfB coding sequence was then amplified by PCR (JE26/JE28) and cloned via BamHI/XbaI into plasmid vector pYEScupFLAGK (28).
A. fumigatus Mutant Generation
Cassettes constructed for targeted gene replacement are described in the supplementary data. Mutants were generated by polyethylene glycol-mediated fusion of protoplasts as described in Ref. 29. Transformants were grown on Aspergillus minimal medium plates containing 1.2 m sorbitol as osmotic stabilizer under appropriate selection conditions and singled out twice before further analysis. Accurate gene deletion and reconstitution were confirmed by Southern hybridization.
Protein Expression in Yeast, Subcellular Fractionation, and in Vitro Transport Assay
The glfB cDNA as well as the human UDP-Gal transporter cDNA (SLC35A2, isoform a) (30) were cloned into the plasmid vector pYEScupFLAGK (complementing uracil auxotrophy) for copper-inducible expression of N-terminal FLAG-tagged proteins in yeast. S. cerevisiae spheroplasts were transformed using the lithium-acetate method described by Invitrogen. Transformants were selected on SC minimal media without uracil. For protein expression, transformants were grown in 1-liter cultures until A600 reached 0.8–0.9, expression was then induced by addition of 0.5 mm CuSO4 (final concentration) and culture was continued for 2 h at 30 °C.
Subcellular fractionation of yeast cells and in vitro transport assay were performed as previously described (28). Briefly, 50 μl of Golgi vesicle preparations (containing typically 80 μg of total protein) and 50 μl of 2 μm 3H-labeled nucleotide sugar or [3H]UMP (0.37 kBq/μl) in assay buffer (10 mm Tris-HCl, pH 7.0, 0.8 m sorbitol, 2 mm MgCl2) were incubated for 30 s at 30 °C. For competition assays, the assay buffer contained in addition 100 μm unlabeled nucleotide sugar (31). Reactions were stopped by dilution with 1 ml of ice-cold assay buffer. The vesicle suspension was then filtered through a mixed cellulose ester membrane (MFTM membrane filters, 0.45 μm, Millipore, Bedford, MA). Vesicles adhering to the filters were washed three times with 2 ml of ice-cold assay buffer and the radioactivity retained on the membrane was measured by liquid scintillation.
Subcellular Fractionation of A. fumigatus
Subcellular fractionation of A. fumigatus largely followed a protocol described for Aspergillus oryzae (32). Briefly, mycelium (approximately 30 g, squeeze-dried) from 1 liter of an A. fumigatus FLAG-glfB overnight culture (inoculum 106 ml−1 conidia) was ground in a mortar with 1–1.5 volumes of sterile sand and 50 ml of lysis buffer (15% (w/w) sucrose, 10 mm HEPES/Tris, pH 7.4, 1 mm EDTA, supplemented with Complete EDTA-free Protease Inhibitors (Roche)). Debris was removed by filtration through Miracloth (Calbiochem) and rinsed further in 45 ml of lysis buffer. Large particles (whole cells, nuclei, and mitochondria) were removed by two sequential centrifugation steps (30 min at 10,000 × g and 20 min at 27,000 × g) and microsomes were pelleted at 110,000 × g (45 min) at 4 °C. The microsomal pellet was suspended in 800 μl of lysis buffer, applied on a multistep sucrose gradient (1 ml of each 60, 50, 40, 38, 36, 34, 32, 30, 28, 26, 24, 20, 18, and 15% (w/w) sucrose in 10 mm HEPES/Tris, pH 7.4, and 1 mm EDTA) and centrifuged at 110,000 × g for 16 h at 4 °C. Fractions (approximately 0.5 ml) were collected from the bottom of the tube and kept at 4 °C until analyzed. Density was calculated from the sucrose concentration determined by a standard optical refractometer and the protein concentration was determined with the Protein BCA Assay (Pierce).
Organelle Marker Enzyme Assays
Cytochrome c oxidoreductase was used as endoplasmic reticulum marker (33) and assayed by adding 20 μl of each fraction to 80 μl of a solution containing 80 mm potassium phosphate, pH 7.5, 150 μm cytochrome c (oxidized, Sigma), 1.5 mm β-NADH, and 7.2 mm NaCN. A550 was recorded over 5 min in a microplate reader, and substrate turnover was calculated from the slope according to Lambert-Beer's law (path length, 0.30 cm; molar absorptivity, 21 mm−1 cm−1). Three days after fractionation, the Golgi marker inosine diphosphatase was assayed as described (34), and phosphate content was determined with the malachite green method (35).
Western Blots
Cell wall glycoproteins and soluble polysaccharides were extracted from 30 mg of ground A. fumigatus mycelium by incubation in 1 ml of sample buffer (15% glycerol, 100 mm Tris-HCl, pH 6.8, 1.5% SDS, 0.25% β-mercaptoethanol, 0.025% bromophenol blue) for 12 min at 95 °C. 20 μl of the supernatant was separated on a 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The monoclonal antibody EB-A2 (39) conjugated to horseradish peroxidase from the Platelia Aspergillus Test (Bio-Rad) or horseradish peroxidase-coupled lectin concanavalin A (Sigma) was used in a 1:50 dilution or at 0.2 μg/ml with or without 200 mm α-methyl-d-mannopyranoside, respectively. Horseradish peroxidase activity was visualized by an enhanced chemiluminescence system (Pierce). For sucrose gradient analysis, 7.5 μl of each fraction were incubated for 10 min at 50 °C with 7.5 μl of sample buffer. Both sample buffer and the polyacrylamide gel contained 4 m urea. For FLAG tag detection, mouse anti-FLAG monoclonal antibody M5 (Sigma) was used in a 1:1000 dilution. IRDye 800CW-coupled secondary antibody enabled band visualization and intensity measurement by infrared scanning on an Odyssey system (Li-Cor, Lincoln, NE).
Purification and Analysis of Glycosylinositolphosphoceramides (GIPCs)
GIPCs were extracted from 0.5 g of mycelium and purified as previously described (13). Purified GIPCs were redissolved in 20 μl of MeOH. High performance thin layer chromatography and immunostaining with the monoclonal antibody MEST-1 were carried out as described (7) using 2 μl for immunostaining and 18 μl for orcinol/H2SO4 staining.
N-Glycan Analysis
N-Glycan preparation and separation were carried out as described previously (36). Glycoproteins from 12 ml (40 × 300 μl) of A. fumigatus culture supernatant were transferred to Immobilon P Multiwell plates (Millipore). After peptide:N-glycanase-mediated N-glycan release and 8- amino-1,3,6-pyrene-trisulfonic acid labeling N-glycans were separated on a capillary electrophoresis DNA Sequencer (ABI PRISM® 3100-Avant Genetic Analyzer, Applied Biosystems, Foster City, CA). Reference glycans were purchased from Dextra Laboratories (Reading, UK).
Growth Assay
For radial growth measurement, a 5-μl drop containing 10,000 A. fumigatus conidia in phosphate-buffered saline was placed in the center of an Aspergillus minimal medium agar plate. Plates were incubated at various temperatures and colony diameters were measured twice daily.
RESULTS
Selection of a UDP-Galf Transporter Candidate Gene
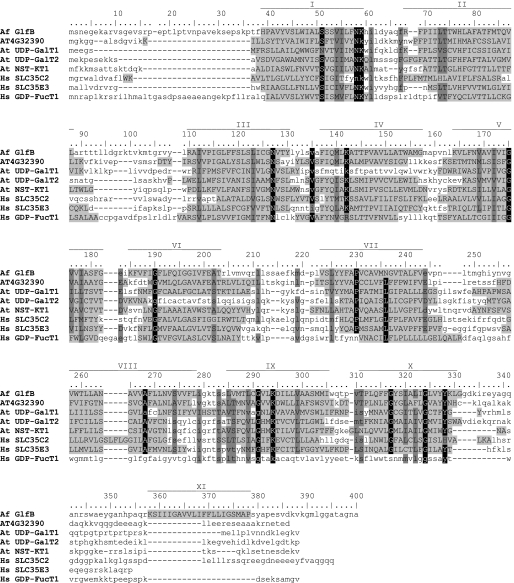
BLAST searches of the A. fumigatus genome (37) with various characterized NST protein sequences identified 16 putative NST genes (supplemental Table S2). One of them (AFUA_3G12700) is adjacent to the recently identified glfA gene (AFUA_3G12690) encoding the UDP-Galf biosynthetic enzyme UGM, and was thus considered a reasonable candidate because clustering of functionally related genes is sometimes observed in A. fumigatus (e.g. the siderophore genes sidF, sidD, and mirB) (38). This gene will be referred to as glfB because of its implication in galactofuranosylation, as demonstrated below. Interestingly, all fungi from the subphylum Pezizomycotina whose genome has been fully sequenced display a clear homolog of glfB clustered with glfA. When present in basiodiomycota (e.g. Cryptococcus neoformans), these two genes are more distant and may be found on different chromosomes. In A. fumigatus, the predicted GlfB protein comprises 400 amino acids and shares up to 40% amino acid identity with uncharacterized Arabidopsis phosphate translocator homologs. The most similar protein with known function is the plant UDP-Gal transporter AtUDP-GalT1 (At1g77610) (39) that displays 21% identity with GlfB. The UDP-Gal transporters AtUDP-GalT2 (At1g76670) and AtNST-KT1 (At4g39390) (39, 40), and the uncharacterized SLC35C2 and SLC35E3 are more distantly related and show 14 to 19% identity with GlfB. Finally, the human GDP-Fuc transporter is the closest characterized transporter of the SLC35 family (12% identity). An alignment of GlfB with these sequences is presented in Fig. 2. This multiple sequence alignment underlines the conservation of two lysine residues (GlfB Lys-59 and Lys-294) that have been proposed to be involved in substrate binding (23, 41). GlfB was predicted to contain 11 transmembrane helices of which the first 10 aligned well with the predicted transmembrane helices of its homologs and other NSTs (fig. 2). We thus hypothesized that glfB encoded a NST and in line with its location in the genome speculated about a specificity for UDP-Galf.
FIGURE 2.
Multiple sequence alignment of A. fumigatus (Af) GlfB and related proteins from Arabidopsis thaliana (At) and humans (Hs) with prediction of transmembrane helices. The Arabidopsis uncharacterized protein encoded by the gene AT4G32390 and UDP-Gal transporter AtUDP-GalT1 are the closest GlfB homologs, whereas the plant UDP-Gal transporters AtUDP-GalT2 and AtNST-KT1, the human proteins SLC35C2 and SLC35E3, and the characterized GDP-fucose transporter (SLC35C1) are more distantly related. Conserved residues, black shading; similar residues, dark-gray shading; predicted transmembrane domains, capital letters and light-gray shading; predicted transmembrane domains for GlfB, roman numbers.
In Vitro Transport and Binding Assays
Uptake of radioactive nucleotide sugars by Golgi-enriched vesicles isolated from yeast expressing a putative NST is a method of choice to determine substrate specificity. Synthesis of radioactive UDP-Galf has been reported (42) but is not commercially available and could thus not be directly tested in this in vitro assay system. Nevertheless, the transport of UDP-Gal, UDP-GlcNAc, UDP-GalNAc, or UDP-GlcA could be excluded. Indeed, the uptake of these nucleotide sugars by Golgi vesicles isolated from yeast cells expressing GlfB or mock transformed was virtually absent (background levels of 1.0 to 1.7 pmol mg−1 min−1), whereas an endogenous UDP-Glc transport of ∼7 pmol mg−1 min−1 verified the quality of the Golgi vesicle preparation. Additionally, transfection of CHO-Lec8 cells with GlfB resulted in Golgi expression of the protein but did not restore galactosylation of the surface glycoconjugates confirming absence of the UDP-Gal transport (data not shown).
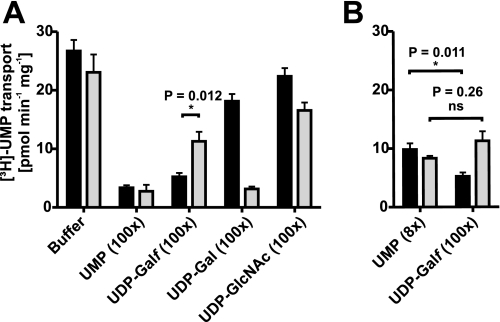
NSTs are likely simple carrier proteins with a binding site alternating between both sides of the membrane (43). Presence of the antiport molecule (in this case UMP) at the trans (inside) or cis (outside) side of the membrane thus leads to stimulation or inhibition of the nucleotide sugar transport, respectively (43, 44). To assess UDP-Galf binding, we thus evaluated the ability of unlabeled UDP-Galf (45) to inhibit the uptake of radioactive UMP, the postulated counter substrate of GlfB (Fig. 3A). For this purpose, Golgi vesicles obtained from cells expressing either GlfB or the human UDP-Gal transporter were incubated with 1 μm [3H]UMP and 100 μm unlabeled UMP, UDP-Galf, UDP-Gal, or UDP-GlcNAc. In GlfB containing vesicles, the addition of UDP-Galf resulted in an 80% inhibition of the [3H]UMP transport and was thus comparable with addition of unlabeled UMP (88% inhibition). In contrast, UDP-GlcNAc and UDP-Gal slightly affected the UMP uptake indicating a limited binding of these nucleotide sugars to GlfB. Unfortunately, because UDP-Galf is rather unstable, contamination of a UDP-Galf solution by UMP is difficult to avoid (46) and was in this case estimated to 6–7% by high pressure liquid chromatography (47) (supplemental Fig. S1). This contaminating UMP explains the 50% decrease of [3H]UMP uptake observed with vesicles expressing the UDP-Gal transporter because a comparable inhibition is observed with 8 μm unlabeled UMP (Fig. 3B). In contrast, inhibition of GlfB-mediated [3H]UMP uptake by the contaminated UDP-Galf solution was significantly higher than observed with 8 μm UMP (p = 0.011, t test), indicating that part of the inhibition observed is actually due to UDP-Galf binding.
FIGURE 3.
UDP-Galf binds to GlfB. A, [3H]UMP uptake by Golgi vesicles isolated from yeast expressing GlfB (black bars) or the human UDP-Gal transporter (gray bars) in the absence or presence of a 100-fold molar excess of unlabeled UMP, UDP-Galf (containing 7% UMP), UDP-Gal, or UDP-GlcNAc. B, comparison of [3H]UMP uptake in the presence of a 100 m excess UDP-Galf containing 7% UMP or an 8 m excess UMP. Each value represents the average of three independent experiments with duplicate measurements. ns, not significant.
Deletion of glfB in A. fumigatus
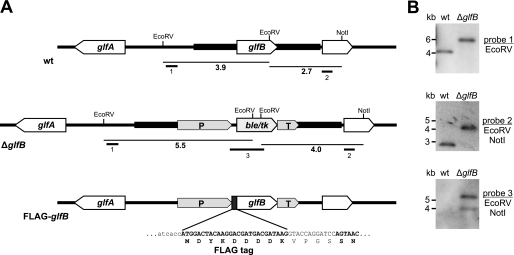
To prove UDP-Galf transport activity in vivo, evaluation of the effect of glfB loss on galactofuranosylation of glycoconjugates in A. fumigatus was undertaken. For this purpose a glfB deletion cassette was constructed by double-joint PCR (48) containing the selectable phleomycin resistance gene ble (49) flanked by up- and downstream regions of the glfB coding sequence. A. fumigatus wild type protoplasts were transformed with the linear cassette for exchange of the genomic glfB coding sequence for the phleomycin resistance gene by homologous recombination (Fig. 4A). Transformants were selected for phleomycin resistance and gene replacement was confirmed by Southern blot analysis (Fig. 4B). A single strain was chosen for further analysis and named ΔglfB.
FIGURE 4.
Gene replacement of glfB in A. fumigatus. A, strategy for the targeted replacement of glfB by the ble/tk selection marker cassette mediated by homologous recombination and subsequent re-introduction of the FLAG-tagged glfB coding sequence. The positions of probes (1–3) used for Southern blot hybridization along with the respective restriction fragments (size in kb) are indicated. B, Southern blots of genomic DNA digested with the indicated restriction enzymes and hybridized to three different digoxigenin-labeled probes. wt, wild type; ble/tk, phleomycin resistance/thymidine kinase fusion gene; P, A. nidulans gpdA promoter; T, A. nidulans trpC terminator; glfA, UDP-galactopyranose mutase open reading frame.
Analysis of Galactofuranosylation in ΔglfB
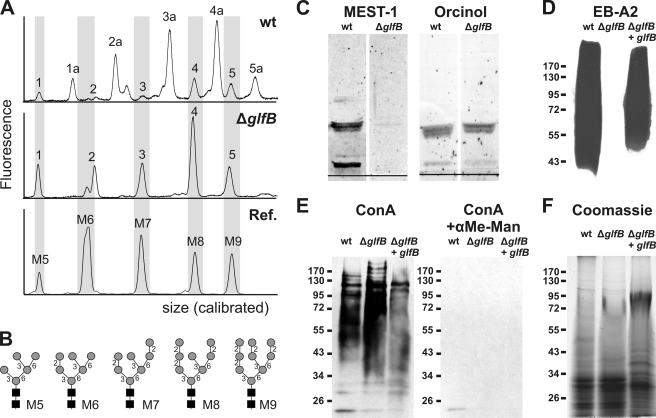
Because N-glycosylation of proteins is known to take place along the secretory pathway and requires various NSTs, we first concentrated on this modification. Proteins from A. fumigatus culture supernatants were immobilized on polyvinylidene difluoride membranes and N-glycans were released by peptide:N-glycanase treatment. After labeling with the negatively charged fluorescent dye 8-amino-1,3,6-pyrene-trisulfonic acid, N-glycans were separated by capillary electrophoresis on a DNA sequencer (Fig. 5A). In the wild type electropherogram (top panel), the peaks labeled 1a to 5a can be assigned to high-mannose type N-glycans bearing a single Galf residue (GalfMan5–9GlcNAc2) as shown previously (10, 13). These Galf-containing glycans were completely absent from the ΔglfB N-glycan electropherogram (middle panel) that exclusively displays nongalactofuranosylated N-glycans (peaks 1–5) co-migrating with Man5–9GlcNAc2 standards (lower panel, Fig. 5B). This finding demonstrates the requirement of GlfB for galactofuranosylation of N-glycans.
FIGURE 5.
The A. fumigatus ΔglfB mutant lacks Galf. A, electropherograms of fluorescently labeled N-glycans enzymatically released from secreted glycoproteins of A. fumigatus wild type (wt) and the ΔglfB mutant. Commercial oligosaccharides (Dextra Laboratories) served as reference (Ref.). The x axis was calibrated to the fragment sizes of the GeneScan-500 ROX standard (Applied Biosystems). B, schematic structures of reference oligosaccharides. Black squares, N-acetylglucosamine; gray circles, mannose. C, GIPCs extracted from A. fumigatus mycelium, separated by high performance thin layer chromatography, and stained with either the Galf(β1–6/β1–3)-specific antibody MEST-1 (left) or with orcinol/H2SO4 (right). The black line indicates the loading spot. D–F, water-soluble extracts of A. fumigatus mycelium separated by SDS-PAGE, transferred onto nitrocellulose membrane, and stained with the Galf-specific monoclonal antibody EB-A2 (D), mannose-specific lectin concanavalin A (ConA) in the presence or absence of 200 mm α-methyl-d-mannopyranoside (E), or Coomassie G-250 as a loading control (F).
The contribution of GlfB to glycolipid biosynthesis was also analyzed by testing their reactivity to monoclonal antibody MEST-1 (50). This antibody reacts specifically with β1–6-Galf found on several glycosphingolipids of A. fumigatus (Fig. 5C, left) (7). However, glycosphingolipids extracted from A. fumigatus ΔglfB mycelium and separated by high performance thin layer chromatography were not stained with MEST-1 indicating the absence of β1–6-linked galactofuranose in these molecules. As loading control, carbohydrates were stained with orcinol/sulfuric acid (Fig. 5C, right).
Finally we tested for reactivity of cell wall components toward the Galf-specific monoclonal antibody EB-A2 (51). A tetrasaccharide of β1–5-linked Galf has been described as the main epitope of EB-A2 (51). This structure is part of the cell wall polysaccharide galactomannan, which can be either linked to the cell wall β1–3/6-glucan (6) or to a GPI anchor (52). Also N-glycans with a single terminal Galf have been reported to be recognized by EB-A2 (10). Aqueous extracts of wild type A. fumigatus mycelium separated on a polyacrylamide gel and transferred to a nitrocellulose membrane bound EB-A2 strongly, whereas in the case of the ΔglfB mutant, binding was totally absent (Fig. 5D). Importantly, episomal expression of the gene in the ΔglfB mutant restores EB-A2 binding excluding any influence of the deletion cassette on the adjacent glfA locus (Fig. 5D). In contrast, staining with the mannose-specific lectin concanavalin A appeared slightly stronger for ΔglfB than for wild type or the episomally complemented mutant, suggesting a higher exposure of cell surface mannan structures as previously observed with the ΔglfA mutant (Fig. 5E, left) (13). Specificity of this staining was demonstrated by α-methyl mannoside inhibition (Fig. 5E, right). Equal loading for EB-A2 and concanavalin A blots was verified by Coomassie staining of a gel run in parallel (Fig. 5F). Thus, the surface glycoconjugates of ΔglfB closely resemble those of the Galf-deficient ΔglfA mutant (13), which suggests a complete loss of galactofuranosylation capacity in the ΔglfB mutant.
Growth and Thermotolerance of the ΔglfB Mutant
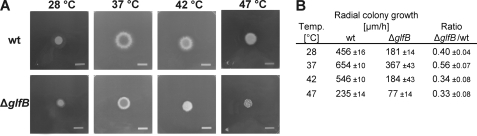
Galf deficiency has been shown to induce an altered culture morphology accompanied by a substantial growth defect in A. fumigatus (13). This effect was more pronounced at higher temperatures than the standard growth temperature of 37 °C indicating a decreased resistance to temperature stress. Indeed, radial colony growth of the ΔglfB mutant was found to be 45% slower than wild type at 37 °C and 66–67% slower at 42 or 47 °C (Fig. 6) in agreement with the observations for the ΔglfA mutant (13).
FIGURE 6.
The morphology and growth of A. fumigatus ΔglfB is altered. A, colony morphology of A. fumigatus wild type (wt) and the ΔglfB mutant after 2 days of growth on minimal agar at various temperatures. B, absolute and relative growth rates obtained from colony diameter measurements (mean ± S.E, n = 3).
Localization of GlfB in A. fumigatus
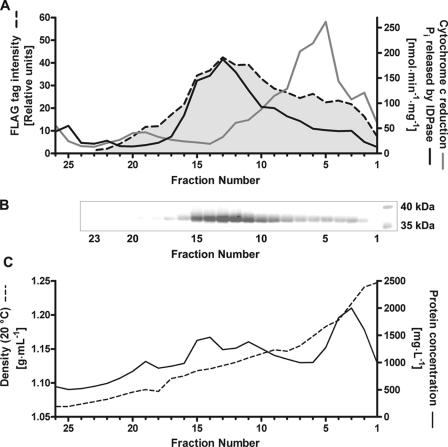
Because of its involvement in protein and lipid glycosylation, GlfB was presumed to be localized in the Golgi apparatus. This localization was observed in transfected mammalian cells by immunofluorescence (supplemental Fig. S2). Moreover, we investigated localization of the GlfB transporter in A. fumigatus by isopycnic ultracentrifugation (Fig. 7). For this purpose, a cassette coding for FLAG-tagged GlfB placed under the gpdA promoter was inserted into the glfB locus (Fig. 4A). Presence of GlfB in fractions collected after ultracentrifugation of microsomes obtained from this transformant on a multistep sucrose gradient was then analyzed by Western blot using an anti-FLAG antibody (Fig. 7B). GlfB was found mainly in fractions 11 to 15 having the same buoyant density (1.12–1.14 g/ml) as Golgi vesicles detected by inosine diphosphatase activity (Fig. 7). In contrast, fractions displaying a high NADPH-cytochrome c oxidoreductase activity (endoplasmic reticulum marker) only contained low amounts of GlfB protein. These data strongly support Golgi localization and are in perfect agreement with a role of GlfB in protein and lipid glycosylation.
FIGURE 7.
Subcellular localization of GlfB in A. fumigatus. A. fumigatus FLAG-glfB microsomes were separated by isopycnic centrifugation on a multistep sucrose gradient. Fractions were analyzed by Western blot and immunostained with an anti-FLAG antibody (A and B) and compared with marker enzyme activities for endoplasmic reticulum (cytochrome c oxidoreductase, gray line) and Golgi (inosine diphosphatase (IDPase), black line) (A). Protein concentration and density were determined (C).
DISCUSSION
This report describes the first identification of a nucleotide sugar transporter with specificity for UDP-Galf. The protein was called GlfB because of its implication in galactofuranose metabolism selected from its homology to other members of the NST family, its phylogenetic classification, as well as the location on chromosome 3 directly downstream of the glfA gene, which encodes the UDP-Galf biosynthesis enzyme UGM (13). It is a 400-amino acid protein with 11 predicted transmembrane helices. This represents a distinctiveness of the GlfB protein because NSTs classically exhibit 8–10 predicted hydrophobic domains and follow an experimentally determined model in which both the N and C terminus are situated on the cytoplasmic side of the organelle (53).
Remarkably, filamentous fungi of the subphylum Pezizomycotina, including many human or plant pathogens, all seem to exhibit adjacent glfA and glfB genes and the presence of Galf has been reported in many species of this subphylum. These genes are also found in a few basidiomycota such as the human pathogen C. neoformans but are absent from other fungi, notably yeasts. Thus, Galf seems particularly important for filamentous fungi. Indeed several studies have already shown the role of this monosaccharide form for growth, hyphal morphology, and sporulation of the Aspergillus species (13, 54, 55). Hence we speculate that Galf plays major roles in hyphal development and/or reproduction of all filamentous fungi of Pezizomycotina and thus has been maintained during evolution.
To address the function of a nucleotide sugar transporter, two approaches are commonly used. Complementation of a mutant strain or cell line not only provides a way to determine NST function in vivo, but also allows identification of the underlying gene(s) by expression of a cDNA library combined with sibling selection (39). Alternatively, NST substrates can be identified by measuring transport of radiolabeled nucleotide sugars into vesicles, either prepared from cell lysates or artificially reconstituted proteoliposomes (56, 57). Because of the unavailability of a UDP-Galf transporter-deficient mutant or cell line and radioactive UDP-Galf, we opted for targeted gene deletion of candidate genes in the opportunistic fungus A. fumigatus. This approach was enabled by the restricted number of candidate genes, the existence of a haploid stage that facilitates the isolation of clones by molecular techniques, and a comprehensive knowledge of the galactofuranosylated structures in this organism (11). Targeted replacement of the most promising candidate gene, glfB, led to the absence of Galf bringing direct evidence of its involvement in Galf metabolism.
The specificity of GlfB for UDP-Galf was established by an indirect assay showing competitive inhibition of [3H]UMP transport by GlfB with unlabeled UDP-Galf. In addition, the transport of several other nucleotide sugars was excluded using a direct in vitro transport assay suggesting that GlfB is highly specific. In particular, absence of the UDP-Gal transport, which was confirmed by the inability of glfB to complement the CHO cell line Lec8, indicates that the transporter is able to discern the ring conformation of the monosaccharide. Vice versa, our data show that the human UDP-Gal transporter used as control in this study does not recognize UDP-Galf. Similarly L. major UDP-Gal transporters LPG5A and LPG5B appear to be specific for the pyranic form of galactose because their concomitant deletion results in the synthesis of glycoconjugates containing Galf but devoid of galactopyranose (58). The obvious difference in the three-dimensional structure of the furanic and pyranic rings certainly plays a role in the ability of these NSTs to differentiate the two cyclic forms. Initially NSTs were thought to be monospecific. However, several NSTs have now been shown to be multifunctional in vitro and usually recognize sugars activated with the same nucleotide that led to the assumption that the nucleotide part is a major player in recognition. With the exception of the Fringe Connection (59), which is thought to be a general UDP-sugar transporter, the specificity of NSTs is generally restricted to a few related nucleotide sugars demonstrating that the sugar part also plays a significant role in the recognition.
Detailed analyses of A. fumigatus ΔglfB total extract and purified glycoconjugates revealed the complete absence of galactofuranose in N-glycans, glycolipids as well as galactomannan. It can be inferred from this result that GlfB is the only NST in A. fumigatus capable of UDP-Galf transport. The Golgi localization of this transporter is moreover in perfect agreement with its implication in galactofuranosylation of N-glycans and glycolipids. The lack of Galf in galactomannan was, however, less predictable because its biosynthesis is currently unknown. Recently, we demonstrated that the terminal sugar of this polysaccharide arises from UDP-Galf synthesized in the cytoplasm by UGM (13). Galactomannan is either linked to the membrane by a GPI anchor, covalently bound to the cell wall β1,3/1,6-glucan, or secreted in the environment (6, 52). Because they present the same carbohydrate structure, it has been postulated that these three forms of galactomannan share a common biosynthetic pathway. By analogy to the biosynthesis of the Leishmania GPI-anchored polysaccharide LPG (19), we assumed that biosynthesis of the GPI-linked galactomannan takes place in the Golgi apparatus. The total absence of EB-A2 staining in the ΔglfB mutant, indicative of the absence of all forms of galactomannan, supports this location. Galactomannan would then be transferred to the β1,3/1,6-glucan from the GPI-anchored polymer as it has been proposed for some GPI-anchored proteins in ascomycetous yeasts (60). The secreted form would arise from enzymatic cleavage of surface galactomannan. Our data demonstrating the absence of galactofuran in the ΔglfB mutant establish a clear link between galactomannan including the cell wall bound form and the secretory pathway and thus strongly support this model. In contrast, the synthesis of α1–3-glucan, β1–3-glucan, and chitin seem to occur at the plasma membrane (61).
In agreement with the complete absence of Galf in glycoconjugates and galactomannan, the growth phenotype of the glfB gene deletion mutant closely resembles the previously described Galf-deficient ΔglfA (13, 16). In the case of ΔglfA, the growth defect was correlated with a reduction of virulence (13). It can thus be extrapolated that glfB is most probably required for full virulence of the fungus. Another example of nucleotide sugar transporter implicated in pathogenicity and restricted to certain organisms is the Golgi GDP-Man transporter. Deletion of this NST led to avirulence of the parasite L. major (62) and is lethal in S. cerevisiae, Candida albicans, and Candida glabrata (63–65). In the latter organisms, Golgi mannosylation comprises N-glycan outer chain elongation, O-mannosylation of proteins, and GIPC biosynthesis. The A. fumigatus genome contains a clear GDP-Man transporter homolog (AFUA_ 5G05740) whose importance is difficult to predict. It has recently been shown that protein O-mannosylation is dispensable in this fungus but is required for cell wall stability and full virulence (66, 67). Yet the importance of N-glycan branching and elongation, and mannosylation of GIPCs, is currently undetermined. The absence of Galf is, however, not sufficient to abolish growth of A. fumigatus as shown previously (13, 16) and confirmed in this study. Even chitin, a polysaccharide that is believed to contribute to the rigidness of the fungal cell wall is not strictly essential for S. cerevisiae (68, 69). Therefore, therapeutic strategies directed against cell wall biosynthesis will likely have to address several targets to be successful.
Supplementary Material
Acknowledgments
We thank Dr. Sven Krappmann for support and A. fumigatus strain AfS35, Dr. Anita Straus and Dr. Helio Takahashi for the monoclonal antibody MEST-1, Dr. C. Nugier-Chauvin, Dr. R. Daniellou, and Dr. P. Peltier for involvement in the synthesis of UDP-Galf, and Dr. Rita Gerardy-Schahn and Dr. Hans Bakker for helpful discussion and critical reading of the manuscript.
This work was supported by Graduate School 745 of the German Research Foundation (Deutsche Forschungsgemeinschaft), French Research Agency Grant ANR JCJC06_140075, and the Région Bretagne.

The on-line version of this article (available at http://www.jbc.org) contains supplemental data, Tables S1 and S2, and Figs. S1 and S2.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) FJ746723.
- Galf
- galactofuranose
- UGM
- UDP-galactopyranose mutase
- NST
- nucleotide sugar transporter
- GlcA
- glucuronic acid
- GalNAc
- N-acetylgalactosamine
- SLC
- solute carrier
- GIPC
- glycoinositolphosphoceramides
- LPG
- lipophosphoglycan
- GlfT
- galactofuranosyltransferase
- GPI
- glycosylphosphatidylinositol.
REFERENCES
- 1.Peltier P., Euzen R., Daniellou R., Nugier-Chauvin C., Ferrières V. (2008) Carbohydr. Res. 343, 1897–1923 [DOI] [PubMed] [Google Scholar]
- 2.Pan F., Jackson M., Ma Y., McNeil M. (2001) J. Bacteriol. 183, 3991–3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lederkremer R. M., Colli W. (1995) Glycobiology 5, 547–552 [DOI] [PubMed] [Google Scholar]
- 4.Beverley S. M., Owens K. L., Showalter M., Griffith C. L., Doering T. L., Jones V. C., McNeil M. R. (2005) Eukaryot. Cell 4, 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakker H., Kleczka B., Gerardy-Schahn R., Routier F. H. (2005) Biol. Chem. 386, 657–661 [DOI] [PubMed] [Google Scholar]
- 6.Latgé J. P., Kobayashi H., Debeaupuis J. P., Diaquin M., Sarfati J., Wieruszeski J. M., Parra E., Bouchara J. P., Fournet B. (1994) Infect. Immun. 62, 5424–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo M. S., Levery S. B., Bennion B., Guimaraes L. L., Castle S. A., Lindsey R., Momany M., Park C., Straus A. H., Takahashi H. K. (2007) J. Lipid Res. 48, 1801–1824 [DOI] [PubMed] [Google Scholar]
- 8.Simenel C., Coddeville B., Delepierre M., Latgé J. P., Fontaine T. (2008) Glycobiology 18, 84–96 [DOI] [PubMed] [Google Scholar]
- 9.Leitao E. A., Bittencourt V. C., Haido R. M., Valente A. P., Peter-Katalinic J., Letzel M., de Souza L. M., Barreto-Bergter E. (2003) Glycobiology 13, 681–692 [DOI] [PubMed] [Google Scholar]
- 10.Morelle W., Bernard M., Debeaupuis J. P., Buitrago M., Tabouret M., Latgé J. P. (2005) Eukaryot. Cell 4, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latge J. P. (2009) Med. Mycol. 47, Suppl. 1,S104– S109 [DOI] [PubMed] [Google Scholar]
- 12.Kleczka B., Lamerz A. C., van Zandbergen G., Wenzel A., Gerardy-Schahn R., Wiese M., Routier F. H. (2007) J. Biol. Chem. 282, 10498–10505 [DOI] [PubMed] [Google Scholar]
- 13.Schmalhorst P. S., Krappmann S., Vervecken W., Rohde M., Müller M., Braus G. H., Contreras R., Braun A., Bakker H., Routier F. H. (2008) Eukaryot. Cell 7, 1268–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards M. R., Lowary T. L. (2009) Chembiochem. 10, 1920–1938 [DOI] [PubMed] [Google Scholar]
- 15.Novelli J. F., Chaudhary K., Canovas J., Benner J., Medinger C., Kelly P., Hodgkin J., Carlow C. K. (2009) Dev. Biol. 335, 340–355 [DOI] [PubMed] [Google Scholar]
- 16.Lamarre C., Beau R., Balloy V., Fontaine T., Hoi J. W., Guadagnini S., Berkova N., Chignard M., Beauvais A., Latgé J. P. (2009) Cell Microbiol. 11, 1612–1623 [DOI] [PubMed] [Google Scholar]
- 17.Zhang K., Barron T., Turco S. J., Beverley S. M. (2004) Mol. Biochem. Parasitol. 136, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Späth G. F., Epstein L., Leader B., Singer S. M., Avila H. A., Turco S. J., Beverley S. M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9258–9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha D. S., Schwarz J. K., Turco S. J., Beverley S. M. (1996) Mol. Biochem. Parasitol. 77, 57–64 [DOI] [PubMed] [Google Scholar]
- 20.Gerardy-Schahn R., Oelmann S., Bakker H. (2001) Biochimie 83, 775–782 [DOI] [PubMed] [Google Scholar]
- 21.Ishida N., Kawakita M. (2004) Pflugers Arch. Eur. J. Physiol. 447, 768–775 [DOI] [PubMed] [Google Scholar]
- 22.Jack D. L., Yang N. M., Saier M. H., Jr. (2001) Eur. J. Biochem. 268, 3620–3639 [DOI] [PubMed] [Google Scholar]
- 23.Knappe S., Flügge U. I., Fischer K. (2003) Plant Physiol. 131, 1178–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltier P., Guégan J. P., Daniellou R., Nugier-Chauvin C., Ferrières V. (2008) Eur. J. Org. Chem. 5988– 5994 [Google Scholar]
- 25.Krappmann S., Sasse C., Braus G. H. ( 2006) Eukaryot. Cell 5, 212– 215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai M., Mitsuke H., Ikeda M., Xia J. X., Kikuchi T., Satake M., Shimizu T. (2004) Nucleic Acids Res. 32, W390–W393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwacke R., Schneider A., van der Graaff E., Fischer K., Catoni E., Desimone M., Frommer W. B., Flügge U. I., Kunze R. (2003) Plant Physiol. 131, 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashikov A., Routier F., Fuhlrott J., Helmus Y., Wild M., Gerardy-Schahn R., Bakker H. (2005) J. Biol. Chem. 280, 27230–27235 [DOI] [PubMed] [Google Scholar]
- 29.Punt P. J., van den Hondel C. A. (1992) Methods Enzymol. 216, 447–457 [DOI] [PubMed] [Google Scholar]
- 30.Kabuss R., Ashikov A., Oelmann S., Gerardy-Schahn R., Bakker H. (2005) Glycobiology 15, 905–911 [DOI] [PubMed] [Google Scholar]
- 31.Muraoka M., Miki T., Ishida N., Hara T., Kawakita M. (2007) J. Biol. Chem. 282, 24615–24622 [DOI] [PubMed] [Google Scholar]
- 32.Record E., Asther M., Moukha S., Marion D., Burlat V., Ruel K., Asther M. (1998) Can. J. Microbiol. 44, 945–953 [PubMed] [Google Scholar]
- 33.Lord J. M., Kagawa T., Moore T. S., Beevers H. (1973) J. Cell Biol. 57, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green J. R. (1983) in Isolation of Membranes and Organelles from Plant Cells ( Hall J. L., Moore A. L. eds) pp.135– 152, Academic Press, London [Google Scholar]
- 35.Baykov A. A., Evtushenko O. A., Avaeva S. M. (1988) Anal. Biochem. 171, 266–270 [DOI] [PubMed] [Google Scholar]
- 36.Laroy W., Contreras R., Callewaert N. (2006) Nat. Protoc. 1, 397–405 [DOI] [PubMed] [Google Scholar]
- 37.Nierman W. C., Pain A., Anderson M. J., Wortman J. R., Kim H. S., Arroyo J., Berriman M., Abe K., Archer D. B., Bermejo C., Bennett J., Bowyer P., Chen D., Collins M., Coulsen R., Davies R., Dyer P. S., Farman M., Fedorova N., Fedorova N., Feldblyum T. V., Fischer R., Fosker N., Fraser A., García J. L., García M. J., Goble A., Goldman G. H., Gomi K., Griffith-Jones S., Gwilliam R., Haas B., Haas H., Harris D., Horiuchi H., Huang J., Humphray S., Jiménez J., Keller N., Khouri H., Kitamoto K., Kobayashi T., Konzack S., Kulkarni R., Kumagai T., Lafon A., Lafton A., Latgé J. P., Li W., Lord A., Lu C., Majoros W. H., May G. S., Miller B. L., Mohamoud Y., Molina M., Monod M., Mouyna I., Mulligan S., Murphy L., O'Neil S., Paulsen I., Peñalva M. A., Pertea M., Price C., Pritchard B. L., Quail M. A., Rabbinowitsch E., Rawlins N., Rajandream M. A., Reichard U., Renauld H., Robson G. D., Rodriguez de Córdoba S., Rodríguez-Peña J. M., Ronning C. M., Rutter S., Salzberg S. L., Sanchez M., Sánchez-Ferrero J. C., Saunders D., Seeger K., Squares R., Squares S., Takeuchi M., Tekaia F., Turner G., Vazquez de Aldana C. R., Weidman J., White O., Woodward J., Yu J. H., Fraser C., Galagan J. E., Asai K., Machida M., Hall N., Barrell B., Denning D. W. (2005) Nature 438, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 38.Schrettl M., Bignell E., Kragl C., Sabiha Y., Loss O., Eisendle M., Wallner A., Arst H. N., Jr., Haynes K., Haas H. (2007) PLoS Pathog. 3, 1195–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakker H., Routier F., Oelmann S., Jordi W., Lommen A., Gerardy-Schahn R., Bosch D. (2005) Glycobiology 15, 193–201 [DOI] [PubMed] [Google Scholar]
- 40.Rollwitz I., Santaella M., Hille D., Flügge U. I., Fischer K. (2006) FEBS Lett. 580, 4246–4251 [DOI] [PubMed] [Google Scholar]
- 41.Gao X. D., Nishikawa A., Dean N. (2001) J. Biol. Chem. 276, 4424–4432 [DOI] [PubMed] [Google Scholar]
- 42.Mariño K., Marino C., Lima C., Baldoni L., de Lederkremer R. M. (2005) Eur. J. Org. Chem. 2958– 2964 [Google Scholar]
- 43.Tiralongo J., Ashikov A., Routier F., Eckhardt M., Bakker H., Gerardy-Schahn R., von Itzstein M. ( 2006) Glycobiology 16, 73– 81 [DOI] [PubMed] [Google Scholar]
- 44.Capasso J. M., Hirschberg C. B. (1984) Biochim. Biophys. Acta 777, 133–139 [DOI] [PubMed] [Google Scholar]
- 45.Peltier P., Daniellou R., Nugier-Chauvin C., Ferrières V. (2007) Organic Lett. 9, 5227–5230 [DOI] [PubMed] [Google Scholar]
- 46.Marlow A. L., Kiessling L. L. (2001) Organic Lett. 3, 2517–2519 [DOI] [PubMed] [Google Scholar]
- 47.Lee R., Monsey D., Weston A., Duncan K., Rithner C., McNeil M. (1996) Anal. Biochem. 242, 1–7 [DOI] [PubMed] [Google Scholar]
- 48.Yu J. H., Hamari Z., Han K. H., Seo J. A., Reyes-Domínguez Y., Scazzocchio C. (2004) Fungal. Genet. Biol. 41, 973–981 [DOI] [PubMed] [Google Scholar]
- 49.Krappmann S., Bayram O., Braus G. H. (2005) Eukaryot. Cell 4, 1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki E., Toledo M. S., Takahashi H. K., Straus A. H. (1997) Glycobiology 7, 463–468 [DOI] [PubMed] [Google Scholar]
- 51.Stynen D., Sarfati J., Goris A., Prévost M. C., Lesourd M., Kamphuis H., Darras V., Latgé J. P. (1992) Infect. Immun. 60, 2237–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costachel C., Coddeville B., Latgé J. P., Fontaine T. (2005) J. Biol. Chem. 280, 39835–39842 [DOI] [PubMed] [Google Scholar]
- 53.Eckhardt M., Gotza B., Gerardy-Schahn R. (1999) J. Biol. Chem. 274, 8779–8787 [DOI] [PubMed] [Google Scholar]
- 54.Damveld R. A., Franken A., Arentshorst M., Punt P. J., Klis F. M., van den Hondel C. A., Ram A. F. (2008) Genetics 178, 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Ganiny A. M., Sanders D. A., Kaminskyj S. G. (2008) Fungal. Genet. Biol. 45, 1533–1542 [DOI] [PubMed] [Google Scholar]
- 56.Puglielli L., Hirschberg C. B. (1999) J. Biol. Chem. 274, 35596–35600 [DOI] [PubMed] [Google Scholar]
- 57.Segawa H., Soares R. P., Kawakita M., Beverley S. M., Turco S. J. (2005) J. Biol. Chem. 280, 2028–2035 [DOI] [PubMed] [Google Scholar]
- 58.Capul A. A., Barron T., Dobson D. E., Turco S. J., Beverley S. M. (2007) J. Biol. Chem. 282, 14006–14017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto S., Taniguchi M., Muraoka M., Toyoda H., Sado Y., Kawakita M., Hayashi S. (2001) Nat. Cell Biol. 3, 816–822 [DOI] [PubMed] [Google Scholar]
- 60.Kollár R., Reinhold B. B., Petráková E., Yeh H. J., Ashwell G., Drgonová J., Kapteyn J. C., Klis F. M., Cabib E. (1997) J. Biol. Chem. 272, 17762–17775 [DOI] [PubMed] [Google Scholar]
- 61.Klis F. M., Ram A. F. J., De Groot P. W. J. (2007) in Biology of the Fungal Cell ( Howard R. J., Gow N. A. R., eds) pp.97– 120, 2nd Ed., Springer, Berlin [Google Scholar]
- 62.Späth G. F., Lye L. F., Segawa H., Sacks D. L., Turco S. J., Beverley S. M. (2003) Science 301, 1241–1243 [DOI] [PubMed] [Google Scholar]
- 63.Dean N., Zhang Y. B., Poster J. B. (1997) J. Biol. Chem. 272, 31908–31914 [DOI] [PubMed] [Google Scholar]
- 64.Nishikawa A., Mendez B., Jigami Y., Dean N. (2002) Yeast 19, 691–698 [DOI] [PubMed] [Google Scholar]
- 65.Nishikawa A., Poster J. B., Jigami Y., Dean N. (2002) J. Bacteriol. 184, 29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou H., Hu H., Zhang L., Li R., Ouyang H., Ming J., Jin C. (2007) Eukaryot. Cell 6, 2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagener J., Echtenacher B., Rohde M., Kotz A., Krappmann S., Heesemann J., Ebel F. (2008) Eukaryot. Cell 7, 1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roy S. K., Chiba Y., Takeuchi M., Jigami Y. (2000) J. Biol. Chem. 275, 13580–13587 [DOI] [PubMed] [Google Scholar]
- 69.Schmidt M. (2004) Microbiology 150, 3253–3260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.