Abstract
Both vascular endothelial growth factor receptors (VEGFR) and integrins are major regulators of VEGF-induced angiogenesis. Previous work has shown that β3 integrin can regulate negatively VEGFR2 expression. Here we show that β3 integrin can regulate negatively VEGF-mediated angiogenesis by limiting the interaction of the co-receptor NRP1 (neuropilin-1) with VEGFR2. In the presence of αvβ3 integrin, NRP1 contributed minimally to VEGF-induced angiogenic processes in vivo, ex vivo, and in vitro. Conversely, when β3 integrin expression is absent or low or its function is blocked with RGD-mimetic inhibitors, VEGF-mediated responses became NRP1-dependent. Indeed, combined inhibition of β3 integrin and NRP1 decreased VEGF-mediated angiogenic responses further than individual inhibition of these receptors. We also show that αvβ3 integrin can associate with NRP1 in a VEGF-dependent fashion. Our data suggest that β3 integrin may, in part, negatively regulate VEGF signaling by sequestering NRP1 and preventing it from interacting with VEGFR2.
Introduction
Vascular endothelial growth factor (VEGF)5 is a key regulator of physiological and pathological angiogenesis. The biological activities of VEGF are primarily mediated by two receptor tyrosine kinases, VEGF-receptor-1 (VEGFR1) and VEGF-receptor-2 (VEGFR2), although substantial evidence suggests that VEGFR2 is the major mediator of VEGF-induced endothelial cell (EC) responses (1). For these reasons, several drugs have been designed to inhibit VEGF binding to and/or signaling through VEGFR2 with the aim of inhibiting tumor angiogenesis (see Ref. 2). So far, these monotherapies have proven less successful than anticipated in a number of clinical trials. Some tumors, for example, become refractory to anti-VEGF therapies (3). This has led to the recent idea of simultaneously targeting convergent proangiogenic pathways.
VEGF-mediated signaling together with integrin-mediated signaling coordinates EC proliferation, migration, invasion, and tube formation (4). In particular, αvβ3 integrin is up-regulated in proliferating ECs during angiogenesis. Disrupting the interactions of this molecule with the ECM prevents blood vessel formation in a number of models in vivo (5). In apparent contrast, we have shown that genetic ablation of β3 integrin enhances VEGFR2-dependent pathological angiogenesis (6). The likely focal point of the coordination between the VEGF- and integrin-mediated angiogenesis pathways is VEGFR2, whose signaling is thought to be, at least in part, modulated by interactions with αvβ3 integrin (7, 8). Recent data have shown that blockade of both αvβ3 integrin and VEGFR2 increases the efficacy of antiangiogenic therapy in vivo (9). Our data suggest that the clinical efficacy of any therapeutic approach that targets these two interacting proteins also is likely to be dependent on other molecules that affect VEGFR2 or αvβ3 integrin activity. For example, VEGFR2 signaling is influenced additionally by associations with NRP1 (neuropilin-1), a co-receptor for some VEGF isoforms (10, 11). Both peptides and antibodies that block the binding of VEGF to NRP1 can enhance anti-VEGF therapy and inhibit tumor growth (12–14). As such, NRP1 is an obvious point of possible convergence of two pathways (VEGF and β3 integrin) that control VEGFR2-dependent EC responses. We thus set out to determine what role NRP1 plays in β3 integrin-dependent, VEGF-induced angiogenesis.
MATERIALS AND METHODS
Reagents and Antibodies
VEGF-A164 was isolated in house according to the method of Krilleke et al. (15). VEGF-A165 and FGF2 were purchased from Peprotech Ltd. (London, UK) and VEGF-A120 from R&D Systems (Abingdon, UK). Antibodies for immunostaining, Western blotting, and immunoprecipitation were as follows: endomucin (clone V.7C7, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), VEGFR2 (clone 55B11, Cell Signaling (Knowl Piece, UK)), phospho-VEGFR2 (clone 19A10, Cell Signaling), NRP1 (catalog number AF566 for mouse, catalog number AF3870 for human, R&D Systems), phospho-ERK (clone 197G2, Cell Signaling), ERK (clone 137F5, Cell Signaling), phospho-p38 MAPK (clone 3D7, Cell Signaling), p38 MAPK (catalog number 9212, Cell Signaling), HSC70 (clone B6, Santa Cruz Biotechnology, Inc.), β3 integrin (catalog number 4702, Cell Signaling), αvβ3 integrin (clone LM609, Millipore (Watford, UK)), transferrin receptor (catalog number ab65831, Abcam (Cambridge, UK)). Antibodies for the DuolinkTM in situ proximity ligation assay were as follows: β3 integrin (rabbit polyclonal clone H-96, Santa Cruz Biotechnology, Inc.), NRP1 (goat polyclonal clone C-19, Santa Cruz Biotechnology, Inc.).
Cell Cultures
Mouse lung ECs were isolated and cultured as described previously (16) from either WT (primary), β3-null (primary), WT temperature-sensitive SV40-large T-antigen immortalized mice (H2kb-tsA58, Charles River, Wilmington, MA), and β3-null/H2kb-tsA58 mice. SV40 ECs were cultured under non-permissive culture conditions for at least 48 h prior to any analysis, thus providing a primary cell equivalent. Polyoma-middle T-antigen immortalized lung endothelial cells (pMT) ECs were generated and cultured as described by May et al. (17). Human umbilical vein EC (HUVEC) were purchased from Lonza Biologics (Woking, UK) and cultured according to the manufacturer's instructions. Tissue culture plates for all experiments were coated with a mixture of collagen (30 μg/ml), gelatin (0.1%), and fibronectin (10 μg/ml).
Transfection of Endothelial Cells
Smart pool duplexes targeting Vegfr2, Nrp1, or β3 integrin were purchased from Dharmacon (Chicago, IL). A non-silencing “control” pool (cp) was used as a control. Mouse ECs at 40–50% confluence were transfected in Opti-MEMTM medium (Invitrogen) using OligofectamineTM (Invitrogen) and a final concentration of siRNA duplexes of 100 nm. After 4 h, Opti-MEMTM was replaced with EC medium, and cultures were maintained for up to 72 h before performing experiments. HUVEC were transiently transfected with β3Δ722 (18) using the appropriate NucleofectorTM kit (Amaxa Biosystems, Cologne, Germany).
Wound Closure Assay in Vitro
Due to their relatively slow growth in culture, we were unable to maintain siRNA knockdown in primary ECs long enough to achieve the confluent monolayer necessary to efficiently perform these studies. Therefore, these studies were performed in SV40 large T-antigen, temperature-sensitive ECs grown under primary-equivalent, non-immortalized conditions. Cells were grown to 50% confluence in 6-well plates, followed by transfection with siRNA duplexes for 72 h, after which time a confluent cell monolayer was achieved. The cells were serum-starved for 3 h in Opti-MEMTM medium, and then a wound was created using a pipette tip. Cultures were then stimulated with VEGF-A164 (20 ng/ml). To determine the rate of cell motility, the scratch width was measured at 0 and 8 h, using a grid inserted in the eyepiece of a Zeiss TelavalTM microscope. For each well at 8 h, the average distance between leading cells was measured at three points along the scratch (left edge, middle, right edge).
125I-VEGF Binding Assays
pMT ECs were grown to subconfluence in 24-well dishes (1 × 105 cells/well). Cells were transferred to 4 °C, and all subsequent steps were performed on ice. Cells were washed twice with PBS and once with binding buffer (Opti-MEMTM, 2 mm HEPES pH 7.5, 0.2% gelatin, 1 μg/ml heparin). Increasing concentrations of 125I-VEGF-A165 (PerkinElmer Life Sciences) ranging from 2 to 40 ng/ml were bound to the cells for 2 h at 4 °C. At the end of the binding reaction, cells were washed three times with PBS and solubilized with 1% Triton X-100, and bound counts were determined. Nonspecific binding was determined in the presence of 1 μg of unlabeled VEGF.
RNA Preparation and Reverse Transcription-PCR
Total RNA was extracted from rings using the TRIzolTM method (Invitrogen). Reverse transcription was performed using oligo(dT) primer and Superscript IIITM reverse transcriptase (Invitrogen). PCR was performed using TaKaRa Ex-TaqTM polymerase (Lonza, Wokingham, UK). Primer sequences for Nrp1 were as follows: forward primer, 5′-GGCTGCCGTTGCTGTGCGCCA-3′; reverse primer, 5′-ATAGCGGATGGAAAACCCTGC-3′.
Primers for Vegfr2 were purchased from R&D Systems, and primers for Acta1 were from Invitrogen. Cycle conditions for all primers (Vegfr2 and Nrp1, 35 cycles; Acta1, 25 cycles) were those set out by R&D Systems for Vegfr2.
Western Blot Analysis
Cells were lysed in 1% Nonidet P-40-buffer (see “Cell Signaling Assays”) and processed as described (19). Densitometric readings of band intensities were obtained using the Image JTM software available at the National Institutes of Health web site.
Ex Vivo Aortic Ring Assays
Thoracic aortas were isolated and prepared for culture as described previously (6). One-mm-thick rings were then transfected in 1 ml of Opti-MEMTM medium with the indicated siRNA “smart” pool duplexes (at a final concentration of 100 nm) using OligofectamineTM. Transfections were performed in 24-well plates with ≤24 rings/well. After 18 h at 37 °C, aortic rings were embedded in growth factor-reduced MatrigelTM (BD Biosciences) and cultured as described (6). Where indicated, culture medium was supplemented with VEGF-A164 at 25 ng/ml. Rings were fed every 2 days with fresh medium with or without VEGF. Sprouting microvessels were counted after 5 days. For RNA or protein isolation, a length of thoracic aorta equivalent to 24 rings was sliced down the midline with a scalpel and transfected as described above. 24–48 h later, two treated aortas were pooled for subsequent RNA or protein extraction. Peptide inhibitors directed against NRP1 or β3 integrin were administered to embedded rings as described by Reynolds et al. (20).
Lentivirus Production and Transduction of Aortic Rings
The pSico Cre-inducible shRNA expression construct was a kind gift from Tyler Jacks (Center for Cancer Research, MIT, Cambridge, MA) (21). The following oligonucleotides were used to insert the appropriate shRNA sequences: scr, sense (5′-TGAACGGACATTTCGAAGTATTCAAGAGATACTTCGAAATGTCCGTTCTTTTTTC-3′) and antisense (5′-TCGAGAAAAAAGAACGGACATTTCGAAGTATCTCTTGAATACTTCGAAATGTCCGTTCA-3′); β3 integrin, sense (5′-TGCAAACAACCCGCTGTATATTCAAGAGATATACAGCGGGTTGTTTGCTTTTTTC-3′) and antisense (5′-TCGAGAAAAAAGCAAACAACCCGCTGTATATCTCTTGAATATACAGCGGGTTGTTTGCA-3′); Vegfr2, sense (5′-TGCGCTCACCTCCTGTTTAATTCAAGAGATTAAACAGGAGGTGAGCGCTTTTTTC-3′) and antisense (5′-TCGAGAAAAAAGCGCTCACCTCCTGTTTAATCTCTTGAATTAAACAGGAGGTGAGCGCA-3′); Nrp1, sense (5′-TGCACAAATCTCTGAAACTATTCAAGAGATAGTTTCAGAGATTTGTGCTTTTTTC-3′) and antisense (5′-TCGAGAAAAAAGCACAAATCTCTGAAACTATCTCTTGAATAGTTTCAGAGATTTGTGCA-3′).
Lentiviruses were generated using the ViraPower Lentiviral Expression SystemTM (Invitrogen). Supernatants were collected 72 h after transfection and filtered through a 0.75-μm filter. The remaining supernatant was centrifuged at 25,000 rpm in a Beckman SW55Ti rotor for 1.5 h. Viral pellets were resuspended in 1 ml of OPTIMEMTM and used directly to infect aortic rings.
Cell Signaling Assays
ECs were transfected with siRNA 48 h prior to analysis. Cells were starved for 3 h in Opti-MEMTM medium and then stimulated with VEGF (20 ng/ml) for 7 min (except where noted). Cells were lysed in 150 mm NaCl, 20 mm Hepes, 2.5 mm EDTA, 10% glycerol, 1% Nonidet P-40 plus freshly added Protease Inhibitor Mixture Set IIITM and Phosphatase Inhibitor Mixture Set ITM (Merck). Western blot analyses were performed as described above.
Immunoprecipitation
Mouse ECs were transfected with siRNA 48 h prior to analysis. Cells were starved for 3 h in Opti-MEMTM medium and then stimulated with VEGF (20 ng/ml) for 7 min and processed as for cell signaling assays. 700 μg of total protein was immunoprecipitated with either 5 μl of the appropriate antibody or IgG control using the Catch and Release SystemTM (Millipore) overnight at 4 °C. Column elution was in the supplied sample buffers. For β3Δ722 studies, transiently transfected HUVEC or pMT ECs were starved for 3 h in Opti-MEMTM medium and then transferred to 4 °C for 30 min. VEGF (20 ng/ml) was added to the appropriate plates, and cells were incubated for an additional 30 min at 4 °C. Cells were then transferred to 37 °C for 7 min and processed as follows. For αvβ3 integrin immunoprecipitations, cells were lysed in 25 mm Tris-HCl, pH 7.6, 0.15% Tween 20, 5% glycerol, 0.5 mm EGTA, and protease/phosphatase inhibitors. For VEGFR2 immunoprecipitations, cells were lysed in 50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 1% Triton X-100, and protease/phosphatase inhibitors. Immunoprecipitations were performed using the DynabeadsTM protein-G immunoprecipitation kit (Invitrogen).
Subcutaneous Growth Factor-induced Angiogenesis Assay
Briefly, two autoclaved sponges (a kind gift from Daryl Harmon, Caligen Foam Ltd., Accrington, UK) of ∼1 cm3 in size were implanted subcutaneously in the flanks of anesthetized WT or β3-null mice. Every 2 days, sponges were injected in situ with 100 μl of PBS containing 10 ng/ml VEGF-A164 and 100 μm scr (LWRPTPA) or nrp1 (ATWLPPR) peptide (see Ref. 13). PBS alone was used as a negative control. After 14 days, sponges were excised and fixed for paraffin embedding. Vessel number density was measured (as described by Thurston et al. (22)) after immunostaining.
Histological Analysis of Sponge Sections
Infiltrating blood vessels were detected by immunohistochemical analysis using anti-endomucin antibody and the Vectastain EliteTM ABC staining kit from Vector Laboratories (Orton Southgate, UK). Images were acquired on a Zeiss AxiophotTM microscope equipped with a ×40/0.75 numerical aperture objective and a Nikon digital DXM1200TM camera.
DuolinkTM in Situ Proximity Ligation Assay (PLA)
Assay components were purchased from Cambridge Bioscience (Cambridge, UK) and were as follows: anti-rabbit PLA plus probe, anti-goat PLA minus probe, and detection kit-563. Cells were grown on 13-mm coverslips and treated as described below for confocal microscopy. The assay was performed following the manufacturer's protocol. DuolinkTM controls were performed with anti-αvβ3 integrin (clone LM609) and anti-transferrin receptor (see above). Results were quantified using BlobFinder, available on the World Wide Web.
Confocal Fluorescence Microscopy
Cells were grown on 13-mm glass coverslips and treated as described above. Cells were washed in PBS, fixed in 4% paraformaldehyde, permeabilized in 0.01% saponin for 10 min on ice, and then incubated with primary antibodies overnight at 4 °C. Cells were washed with PBS and incubated with Alexa-FluorTM-conjugated secondary antibodies (Invitrogen) for 1 h at 37 °C. Cells were analyzed by using a Zeiss LSM 710 ConfocalTM microscope equipped with a ×63/1.4 numerical aperture oil immersion objective. Linear processing of images was performed with Adobe Photoshop CS3TM software (Adobe, San Jose, CA).
Statistical Analysis
Except where noted, data are presented as means ± S.E. Significant differences between means for ex vivo and in vivo studies were evaluated by the Mann-Whitney non-parametric μ-test. All other significant differences were evaluated by Student's t test. p ≤ 0.05 was considered statistically significant.
RESULTS
NRP1 Inhibition Reduces VEGF-induced Angiogenesis in β3-null Mice to a Greater Extent than in WT Mice
We were interested in the contribution that NRP1 makes, in vivo, to pathological angiogenesis. Most tumor cells, however, express NRP1, which itself can promote angiogenesis (23), making it difficult to discriminate between the contribution of endothelial cell-derived and tumor cell-derived-NRP1 to any given result. Therefore, to determine whether NRP1 contributed differently to VEGF-mediated angiogenesis in wild-type (WT) versus β3 integrin-deficient (β3-null) mice, we performed growth factor-dependent subcutaneous sponge implant angiogenesis assays (24). Briefly, synthetic sponges were implanted subcutaneously into WT or β3-null mice and then injected either with VEGF-A164 or VEGF in combination with an NRP1-directed peptide (ATWLPPR, nrp1) that was designed to inhibit VEGF-NRP1 binding (13, 25). A scrambled peptide (LWRPTPA, scr) was used as a control. Vessel infiltration in sponges was assessed after 14 days by immunohistochemical detection of endomucin in blood vessels (Fig. 1A).
FIGURE 1.
NRP1 antagonist treatment inhibits VEGF-mediated angiogenesis more substantially in β3-null mice than WT mice. A, immunohistochemistry of endomucin to detect infiltrating blood vessels in VEGF-induced angiogenesis in subcutaneously implanted sponges in the flanks of WT or β3-null animals. Sponges were injected every other day with either PBS (−VEGF) or VEGF-A164 (+VEGF) and either no peptide (none), scrambled peptide (LWRPTPA; scr), or neuropilin-1-inhibiting peptide (ATWLPPR; nrp1) and harvested after a total of 14 days. The arrows point to examples of endomucin-positive blood vessels. Scale bar, 50 μm. B, quantification of VEGF-induced angiogenesis in vivo. The bar graph shows the relative number of blood vessels/mm2 (means ± S.E.) infiltrating subcutaneously implanted sponges after 14 days of treatment (+) (or not (−)) with VEGF-A164 plus the indicated peptides. n = 3–5 individual animals/genotype/treatment. *, p ≤ 0.03; **, p ≤ 0.01. C, representative Western blot analysis of phosphorylated ERK (pERK) levels in β3-null pMT ECs in the presence of scr or nrp1 peptide. Cells were treated with the indicated peptides for 30 min and then stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were Western blotted for pERK. Blots were stripped and reprobed for total ERK. The accompanying bar graph represents densitometric results (means ± S.E.) showing relative VEGF-A164-induced pERK/total ERK levels from three independent experiments. *, p ≤ 0.02.
Quantification of blood vessel infiltration into the sponges showed that blood vessel infiltration in WT mice was induced by VEGF. This VEGF-induced response was enhanced in β3-null mice (Fig. 1B). Although treatment with the nrp1 peptide reduced vessel density in VEGF-treated sponges in both WT and β3-null mice, this effect was exacerbated in β3-null animals (60% in β3-null compared with 30% in WT), reducing their response to approximately WT levels (Fig. 1B).
Although we cannot rule out off-target effects of the nrp1 peptide, its efficacy in a VEGF-dependent pathway was illustrated by inhibiting VEGF-induced phosphorylation of the mitogen-activated protein kinases Erk1/2 (ERK), known downstream targets of VEGF stimulation (26, 27) (Fig. 1C). Furthermore, treatment of both WT and β3-null endothelial cells (ECs) with nrp1 peptide inhibited long term ERK phosphorylation when compared with scr peptide-treated cells (supplemental Fig. S1). These results imply that the enhanced β3-null response to VEGF in vivo is dependent on NRP1.
Ex Vivo Microvessel Sprouting of β3-null Aortic Rings, but Not WT Aortic Rings, Is Dependent on NRP1
Microvessel sprouting from aortic ring segments is a good model for examining neovascularization ex vivo. When grown in a three-dimensional matrix, aortic rings produce vessels with lumens and many of the salient features associated with angiogenesis, including tip cells and stalk cells involving endothelial and supporting cells (28). Aortic ring assays, therefore, provided a system for continuing our analyses of the functions of VEGFR2, NRP1, and β3 integrin during VEGF-A164-induced angiogenesis. We took, initially, an siRNA approach to knock down the expression of these proteins in WT and β3-null rings (Figs. 2, A and B). Reverse transcription-PCR (supplemental Fig. S2, upper panels) and Western blot (supplemental Fig. S2, lower panels) analyses confirmed that the expression of VEGFR2, NRP1, and β3 integrin were reduced substantially by siRNA treatment of aortic rings from either genotype.
FIGURE 2.
NRP1 plays a significant role in β3-null but not WT VEGF-mediated aortic ring sprouting. A, quantification of VEGF-mediated microvessel sprouting displayed by aortic rings after depletion of Vegfr2 (r2), Nrp1, or β3 integrin (β3) using siRNA duplexes. cp duplexes acted as a negative control. Bars show number of microvessel sprouts per aortic ring (means ± S.E.) after 5 days of stimulation (+) (or not (−)) with VEGF-A164. n > 25 individual rings per genotype per treatment. *, p ≤ 0.01; **, p ≤ 0.0004. nsd, no significant difference. B, representative phase-contrast micrographs of VEGF-A164 induced endothelial sprouts treated with the indicated siRNA duplexes. Scale bar, 0.3 mm. C, quantification of VEGF-mediated microvessel sprouting displayed by aortic rings in the presence of S 36578, an αvβ3/αvβ5 integrin mimetic inhibitor. Bars show the number of microvessel sprouts per aortic ring (means ± S.E.) after 5 days of stimulation (+) (or not (−)) with VEGF-A164. The left bar graph shows rings that were treated with the indicated concentrations of S 36578 (β3-inhib.) plus Nrp1 siRNA duplexes. cp duplexes acted as a negative control. veh, vehicle only control for S 36578 (DMSO). The right bar graph shows rings that were treated with the indicated concentrations of S 36578 plus nrp1 peptide (50 μm). scr peptide at the same concentration served as a negative control. *, p ≤ 0.05. nsd, no significant difference.
As expected, the cp of siRNA had no affect on microvessel sprouting induced by VEGF, but Vegfr2 depletion inhibited sprouting to background levels in both genotypes when compared with either VEGF-only-treated or cp siRNA-transfected controls. In contrast, Nrp1 knockdown did not alter sprouting from WT rings but reduced significantly the degree of microvessel sprouting in β3-null rings, suggesting that β3 integrin limits the participation of NRP1 in VEGF-driven microvessel sprouting (Fig. 2A). Importantly, β3 integrin depletion in WT rings increased VEGF-induced microvessel sprouting to levels similar in β3-null rings (Figs. 2, A and B). This indicates that the enhanced angiogenic response observed in β3-null aortic rings does not occur simply as a result of the long term absence of β3 integrin expression (through developmental compensation) because it can be replicated by β3 integrin knockdown. Moreover, a combination of nrp1 and β3 integrin siRNA treatment inhibited sprouting from WT aortic rings (Fig. 2A).
To further extend the relevance of these observations beyond that of β3 integrin deficiency, we studied the effects of blocking β3 integrin function using αvβ3/αvβ5 RGD-mimetic inhibitors in WT aortic rings. As expected, VEGF-stimulated sprouting from WT aortic rings in the presence of either cp siRNA alone or with scr peptide alone (Fig. 2C). The degree of this response was not altered by treatment with Nrp1 siRNA or nrp1 peptide. In the presence of cp siRNA or scr peptide, sprouting was inhibited, however, in a dose-dependent manner with the αvβ3/αvβ5 RGD-mimetic inhibitor S 36578 (20, 29, 30). This inhibition was augmented by the addition of Nrp1 siRNA or nrp1 peptide (Fig. 2C). These studies provide evidence that our findings are not restricted to the β3-knock-out model but relevant to wild-type systems.
Taken together, our findings indicate that in the presence of β3 integrin, NRP1 normally plays a small role in VEGF-driven angiogenesis both in vivo and ex vivo but that when β3 integrin expression is reduced, angiogenic processes are more NRP1-dependent.
In addition to VEGF, a number of proangiogenic heparin-binding growth factors interact with NRPs (31–34). The interaction between FGF2 and NRP1 in particular is thought to be of physiological relevance because NRP1 was found to enhance the growth-stimulatory activity of FGF2 on endothelial cells (33). We showed that WT aortic rings exhibited a significant increase in fibroblast growth factor-stimulated microvessel sprouting. β3-null aortic rings, on the other hand, failed to respond to fibroblast growth factor stimulation (supplemental Fig. S3). This lack of response in β3-null microvessel sprouting is probably due to the dependence of fibroblast growth factor responses on αvβ3 integrin (35, 36). Thus, αvβ3 integrin is unlikely to regulate negatively the signaling between FGF2 and NRP1 as it appears to do between VEGF and NRP1.
The aortic ring ex vivo angiogenic assay preserves many of the complex interactions between various cell types that might influence VEGF-A164 responses. Although VEGFR2 expression largely is EC-specific, the expression of NRP1 and β3 integrin is more widespread. Therefore, the observed effects in β3-null rings might be attributable to cell types other than ECs. Thus, we directed β3 integrin deficiency to endothelial cells in this system by infecting WT rings from EC-specific Tie1-Cre-positive transgenic animals (37) with a lentivirus carrying a Cre-inducible shRNA construct (21) directed against β3 integrin. Infection of β3 integrin-specific shRNA constructs into Tie1-Cre-positive, but not Tie1-Cre-negative, rings showed a significant increase in VEGF-A164-induced microvessel sprouting (Fig. 3A). This indicated that the reduction of β3 integrin specifically in ECs is sufficient to enhance VEGF-induced angiogenic responses.
FIGURE 3.
The effects of β3 integrin and Nrp1 knockdown on VEGF-induced microvessel sprouting are endothelium-specific. A, quantification of VEGF-mediated microvessel sprouting displayed by endothelium-specific Tie1-Cre-negative (Tie1-Cre−) and Tie1-Cre-positive (Tie1-Cre+) WT aortic rings infected with a lentivirus carrying a Cre-inducible shRNA construct directed against β3 integrin (β3). Bars show total number of microvessel sprouts per aortic ring (means ± S.E.) after 5 days of stimulation (+) (or not (−)) with VEGF-A164. B, quantification of VEGF-mediated microvessel sprouting displayed by WT/Tie1-Cre+ and β3-null/Tie1-Cre+ aortic rings infected with lentiviruses carrying Cre-inducible shRNA constructs directed against Vegfr2 (r2), Nrp1, or β3 integrin (β3). A construct directed against firefly luciferase (scr) acted as a negative control. Bars show total number of microvessel sprouts/aortic ring (means ± S.E.) after 5 days of stimulation (+) (or not (−)) with VEGF-A164. For both A and B, n > 12 individual rings/genotype/treatment. *, p ≤ 0.04; **, p ≤ 0.0001. nsd, no statistical difference.
We then infected both WT/Tie1-Cre-positive and β3-null/Tie1-Cre-positive rings with Cre-inducible shRNA constructs against Vegfr2 and Nrp1 (Fig. 3B). Infection with scr shRNA constructs did not affect the VEGF-induced microvessel sprouting in WT or β3-null rings. Microvessel sprouting in the presence of Vegfr2 shRNA was inhibited in both genotypes. However, microvessel sprouting in β3-null, but not WT, rings was inhibited by Nrp1 shRNA. In addition, infection with β3-specific shRNA enhanced VEGF-mediated sprouting of WT rings to levels similar to those found in VEGF-stimulated β3-null rings. Together, these results establish that knocking down Nrp1 specifically in Tie1-Cre-expressing endothelial cells is sufficient to demonstrate that VEGF-mediated angiogenic responses are more dependent on NRP1 when β3 integrin expression is reduced in endothelial cells.
VEGF-induced Motility of β3-Null ECs in Vitro Is Dependent on NRP1
Confident that we were studying an EC-specific process, we next investigated the contributions made by VEGFR2, NRP1, and β3 integrin to VEGF-A164-mediated events in vitro. In particular, we tested the effect of NRP1 depletion on EC migration, an essential feature of EC behavior during angiogenesis. Due to their relatively slow growth in culture, we were unable to maintain siRNA knockdown long enough in primary ECs to achieve the confluent monolayer necessary to efficiently perform migration studies in primary ECs. Therefore, these studies were performed in SV40 large T-antigen, temperature-sensitive ECs grown under primary-equivalent, non-immortalized conditions. We examined the effects of Vegfr2, Nrp1, and β3 integrin knockdown on EC motility in in vitro wound closure assays. Our results showed that transfection of endothelial cells with cp siRNA did not affect the expected increase in VEGF-mediated β3-null wound closure when compared with WT controls (19) but that knocking down Vegfr2 in both WT and β3-null ECs suppressed significantly EC motility (Fig. 4A). Although Nrp1 knockdown in WT ECs did not affect VEGF-stimulated motility, it did suppress the otherwise enhanced motility of β3-null ECs. Once again, β3 integrin knockdown in WT ECs gave rise to an enhancement in VEGF-stimulated wound closure similar to that observed for β3-null ECs (when compared with WT controls) (Fig. 4A). Western blot analyses confirmed the specificity of the vegfr2 and nrp1 siRNA smart pools for their respective targets (Fig. 4B). Our data indicate, therefore, that VEGF-driven EC motility is dependent on NRP1 when β3 integrin expression is absent.
FIGURE 4.
NRP1 is essential for VEGF-mediated endothelial cell motility in the absence but not the presence of β3 integrin. A, quantification of VEGF-A164-mediated endothelial cell wound closure displayed by SV40-large-T-antigen-immortalized lung WT and β3-null ECs after incubation at 37 °C and knockdown of Vegfr2 (r2), Nrp1, or β3 integrin (β3) using siRNA duplexes. cp duplexes acted as a negative control. Bars show the percentage of wound closure at 8 h relative to 0 h (means ± S.E.). n > 9 individual wells/genotype/treatment. *, p ≤ 0.04; **, p ≤ 0.0003. nsd, no significant difference. B, representative Western blots of VEGFR2 and NRP1 levels in WT SV40-immortalized ECs 72 h after transfection with the indicated siRNA duplexes. Blotting for HSC70 provides a loading control. The underlying bar graphs represent densitometry results (means ± S.E.) from three or four independent experiments.
Enhanced VEGFR2-mediated Signaling in β3-Null Endothelial Cells Is NRP1-dependent
To understand better the molecular mechanisms underlying our results so far, we next investigated the levels of VEGFR2 and NRP1 expressed by WT and β3-null primary lung ECs. As we have shown previously (6), VEGFR2 levels were elevated ∼2-fold in β3-null ECs when compared with WT controls. Moreover, NRP1 expression levels were increased ∼3-fold in β3-null cell lysates (Fig. 5A). Similar increases in VEGFR2 and NRP1 expression were observed in SV40 immortalized ECs. We recognized, therefore, that using these cells, we would not be able to distinguish between increased VEGF-induced responses due simply to the elevated levels of VEGFR2 and NRP1 expression or altered interactions between the two molecules.
FIGURE 5.
Enhanced VEGF-mediated endothelial cell signaling in β3-null ECs is highly dependent on NRP1. A, representative Western blot analyses of VEGFR2 and NRP1 levels in primary lung WT and β3-null ECs. Blotting for HSC70 provides a loading control. The bar graphs below represent densitometry results (means ± S.E.) from three or four independent experiments. B, representative Western blot analyses of total VEGFR2 and NRP1 levels in pMT WT, β3-null, and β3-null-expressing human β3 integrin (+hβ3) ECs show that in these cells, VEGFR2 and NRP1 levels remain unchanged between genotypes. Relative β3 integrin levels are also shown. Blotting for HSC70 provides a loading control. The underlying bar graph represents relative levels of the indicated proteins compared with HSC70 for the shown blots. C, representative Western blot analysis of phosphorylated VEGFR2 levels in pMT ECs. Cells were treated for 48 h with the indicated siRNA duplexes and then stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were Western blotted for phosphorylated VEGFR2 (pVEGFR2). These blots were stripped and reprobed for total VEGFR2 and NRP1 (to assess its knockdown). HSC70 was used as a loading control. The accompanying bar graph represents densitometric results (means ± S.E.) showing relative VEGF-A164-induced phosphorylated VEGFR2/total VEGFR2 levels from three independent experiments. *, p ≤ 0.05; **, p ≤ 0.005. D, representative analysis of pERK levels in pMT ECs. Cells were treated for 48 h with the indicated siRNA duplexes and then stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were Western blotted for pERK. These blots were stripped and reprobed for total ERK and NRP1 (to assess its knockdown). HSC70 was used as a loading control. The accompanying bar graph represents densitometric results (means ± S.E.) showing relative VEGF-A164-induced pERK/total ERK levels from three or four independent experiments. *, p ≤ 0.01; **, p ≤ 0.005.
To overcome this problem, we isolated populations of pMT lung ECs from both WT and β3-null mice that expressed roughly equivalent levels of VEGFR2 and NRP1. We also showed that transduction of human β3 integrin into β3-null pMT ECs did not affect VEGFR2 or NRP1 levels (Fig. 5B). In addition, the saturation kinetics of 125I-VEGF binding to each of the cell lines was indistinguishable (supplemental Fig. S4A). Furthermore, although β3-null pMT ECs showed an enhanced response to VEGF-A164 stimulation as measured by ERK-phosphorylation, all three cell lines maintained a dose responsiveness to increasing concentrations of VEGF-A164 (supplemental Fig. S4B).
The results from these immortalized ECs indicate, therefore, that the affinity of VEGF binding to ECs is unchanged in the absence of β3 integrin and that the enhanced response of ECs to VEGF-A164 in the absence of β3 integrin (as seen in primary ECs) is not due solely to increased expression of VEGFR2 and/or NRP1. Thus, these pMT EC lines provided a good model system for studying the effects of β3 integrin expression on the functions of and interactions between VEGFR2 and NRP1 independent of changes in their expression levels.
We first examined the effect of Nrp1 depletion on VEGF-induced autophosphorylation of VEGFR2 (38) in WT versus β3-null pMT ECs (Fig. 5C). Nrp1 knockdown reduced VEGF-induced VEGFR2 phosphorylation in ECs of both genotypes, showing that NRP1 contributes to VEGFR2 phosphorylation in both genotypes.
We then tested, in these pMT ECs, the influence of NRP1 expression on the activation of ERK after VEGF-A164 stimulation (26, 27). In WT cells, VEGF induced a significant increase in ERK phosphorylation; Nrp1 depletion had a small, but significant, effect on this response (Fig. 5D). The enhanced level of VEGF-stimulated ERK phosphorylation in β3-null cells was reduced to approximate WT levels by Nrp1 depletion, suggesting that the enhanced VEGF response in β3-null cells is dependent on NRP1. Human β3 integrin expression in β3-null ECs restored a WT-like response (Fig. 5D). Taken together, our data demonstrate that β3 integrin expression limits the contribution of NRP1 to VEGF-A164-dependent signaling even when the expression levels of VEGFR2 and NRP1 are unaltered after β3 integrin deletion.
Enhanced VEGF Responses in the Absence of β3 Integrin Are Dependent on Elevated Interactions between VEGFR2 and NRP1
We next tested the influence of β3 integrin expression on VEGFR2/NRP1 association in pMT ECs. We demonstrated that the co-immunoprecipitation of VEGFR2 and NRP1 was elevated in β3-null pMT EC lysates when compared with WT controls, even in the absence of VEGF stimulation and that VEGF-A164 stimulation further enhanced this association (Fig. 6A). Nrp1 knockdown in replicate samples served as a specificity control for the co-immunoprecipitation reactions.
FIGURE 6.
VEGFR2/NRP1 associations are enhanced in β3-null pMT ECs. A, representative analysis of the co-immunoprecipitation of NRP1 with VEGFR2 in WT and β3-null pMT ECs. Cells were stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were immunoprecipitated (IP) with anti-VEGFR2 and Western blotted (WB) for NRP1. Blots were stripped and reprobed for VEGFR2. 48 h pretreatment of replicate samples with Nrp1 siRNA duplexes (as indicated) served as a control for NRP1 blotting. The bar graph below represents densitometric results showing the relative VEGF-A164-induced levels of NRP1/VEGFR2 co-association (means ± S.E.) from four independent experiments. *, p ≤ 0.05. B, quantification of VEGF-A120 mediated microvessel sprouting displayed by WT or β3-null aortic rings after depletion of Vegfr2 (r2) or Nrp1 using siRNA duplexes. Bars show total number of microvessel sprouts/aortic ring (means ± S.E.) after 5 days of stimulation (+) (or not (−)) with VEGF-A120. n > 25 individual rings/genotype/treatment. *, p ≤ 0.01; *, p ≤ 0.001. nsd, no significant difference. C, representative analysis of pERK levels in pMT ECs. Cells were treated for 48 h with the indicated siRNA duplexes and then stimulated (+) (or not (−)) with VEGF-A120 for 7 min. Protein lysates were Western blotted for pERK. Blots were stripped and reprobed for total ERK. The accompanying bar graph represents densitometric results (means ± S.E.) showing relative VEGF-A120-induced pERK/total ERK levels from four independent experiments. *, p ≤ 0.01; **, p ≤ 0.005. nsd, no statistical difference.
These studies suggest that in the absence of β3 integrin, VEGFR2/NRP1 associations are elevated. We wished to test, however, whether a VEGF-induced coupling between VEGFR2 and NRP1 was required for elevated angiogenic responses in the absence of β3 integrin. One way to do this is to use the differential ability of VEGF-A164 and VEGF-A120 (the mouse isoform of human VEGF-A121) to couple VEGFR2 and NRP1. VEGF-A164 is known to interact with VEGFR2 and NRP1 and act as a bridge between these two molecules (10). In contrast, VEGF-A120 can bind to either VEGFR2 or NRP1, but no evidence for a trimeric complex of VEGF-A120·VEGFR2·NRP1 has been demonstrated (1, 11, 39). Therefore, by testing the effects of VEGF-A120 on aortic ring sprouting and ERK phosphorylation, we tested VEGF responses independent of VEGFR2/NRP1 coupling. As expected, VEGF-A120 stimulation did not increase the association between VEGFR2 and NRP1 in either WT or β3-null ECs (supplemental Fig. S5). Furthermore, WT and β3-null aortic rings exhibited similar angiogenic responses to VEGF-A120 that were inhibited by Vegfr2 knockdown but not by Nrp1 knockdown (Fig. 6B). In addition, VEGF-A120-induced ERK phosphorylation was not affected by Nrp1 knockdown in either genotype (Fig. 6C). Thus, in contrast to the enhanced β3-null angiogenic responses to VEGF-A164, where VEGFR2 and NRP1 are coupled together, the lack of VEGFR2/NRP1 coupling after stimulation with VEGF-A120 did not increase angiogenic responses in the β3-nulls. This suggests that the enhanced VEGFR2/NRP1 association in β3-null ECs is functional and required for the elevated angiogenic responses to VEGF-A164.
p38 MAPK is another downstream target of VEGF-A164 whose state of activation is indicative of a functional coupling between VEGFR2 and NRP1 (40). Our conclusions are supported further, therefore, by the observation that VEGFA-164-induced p38 MAPK phosphorylation is more sensitive to Nrp1 depletion in β3-null pMT ECs when compared with either WT ECs or β3-null ECs that are expressing human β3 integrin (supplemental Fig. S6).
The Cytoplasmic Tail of β3 Integrin Regulates Interactions between VEGFR2 and NRP1
Point mutations in the cytoplasmic tail of β3 integrin (where tyrosines 747 and 759 have been replaced with phenylalanines (DiYF) (41)) have indicated that this motif of β3 integrin is required for angiogenesis and that interactions between β3 integrin and VEGFR2 can positively regulate VEGFR2 signaling (8). We therefore wished to determine whether the cytoplasmic tail of β3 integrin regulates the interaction between VEGFR2 and NRP1. We took advantage of a human β3 integrin mutant (β3Δ722), which lacks most of its cytoplasmic tail but is still able to dimerize with endogenous αv integrin and engage ligand (18, 42).
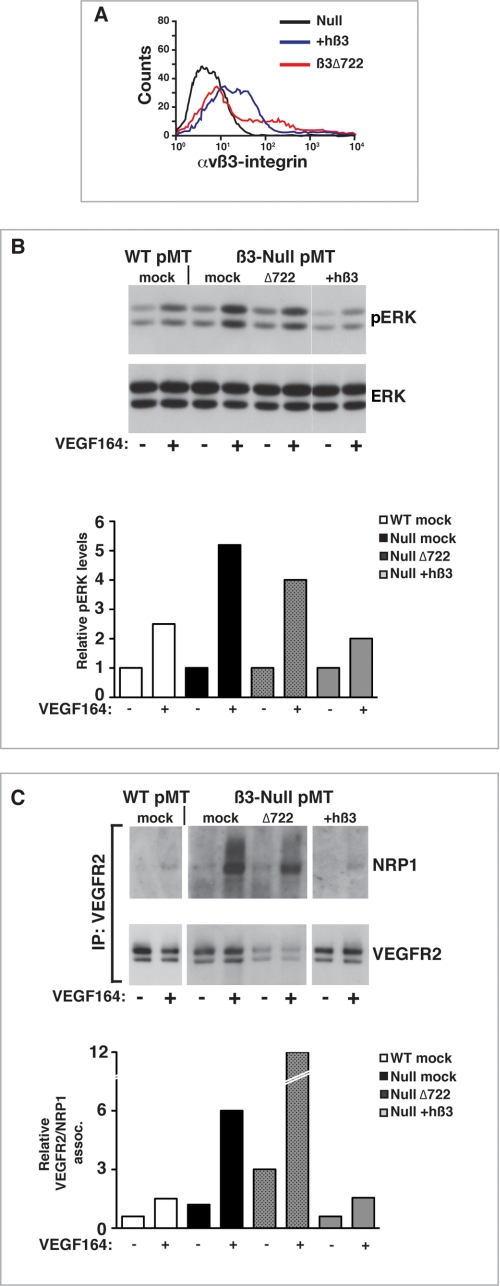
Using an antibody directed specifically against αvβ3 integrin, we confirmed the expression of the β3Δ722 mutant in transiently transfected β3-null pMT ECs by flow cytometry. Full-length human β3 integrin was used as a control; both isoforms of β3 integrin coupled with αv integrin and were expressed on the surface of β3-null pMT ECs (Fig. 7A).
FIGURE 7.
The cytoplasmic tail of β3 integrin regulates interactions between VEGFR2 and NRP1. A, representative flow cytometric analysis showing increased surface expression of αvβ3 integrin in transfected pMT ECs. Black line, β3-null; blue line, β3-null plus full-length human β3 integrin; red line, β3-null plus β3Δ722. B, representative analysis of ERK phosphorylation in WT pMT ECs, mock-transfected β3-null pMT ECs, or β3-null pMT ECs transfected with either β3Δ722 or full-length human β3 integrin (+hβ3). Cells were transfected with the indicated constructs. 48 h later, cells were stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were Western blotted for pERK. These blots were stripped and reprobed for total ERK. The accompanying bar graph represents densitometric results indicating relative VEGF-A164-induced pERK/total ERK levels from the shown blot. C, representative analysis of the co-immunoprecipitation of NRP1 with VEGFR2 in mock-transfected WT pMT ECs, mock-transfected β3-null pMT ECs, or β3-null pMT ECs transfected with either β3Δ722 or full-length human β3 integrin (+hβ3). 48 h after transfection, cells were stimulated (+) (or not (−)) with VEGF-A164 for 7 min. Protein lysates were immunoprecipitated (IP) with anti-VEGFR2 and Western blotted for NRP1. Blots were stripped and reblotted for VEGFR2. The accompanying bar graph represents relative VEGFR2/NRP1 associations for the shown blot; the base-line association between VEGFR2 and NRP1 in WT non-stimulated cells has been arbitrarily set to 1.
We examined then the ability of the two different β3 integrin isoforms to regulate VEGF-induced ERK phosphorylation. As expected, VEGF-induced ERK phosphorylation in β3-null pMT ECs was elevated compared with WT pMT ECs. As shown previously, full-length human β3 integrin restored VEGF-induced ERK phosphorylation to WT-like levels. The β3Δ722 mutant, however, was not able to “rescue” the β3-null response to the same degree as full-length β3 integrin (Fig. 7B).
More importantly, β3Δ722-transfected cells maintained the enhanced VEGF-induced association between VEGFR2 and NRP1 that was observed in mock-transfected β3-null pMT ECs; indeed, as a result of a decreased expression of VEGFR2 in the presence of the β3Δ722, the relative association of VEGFR2 with NRP1 was more dramatic. Transfection with full-length human β3 integrin led to a reduction in the association of these two molecules to WT-like levels (Fig. 7C). These studies highlight two important points: 1) the association between VEGFR2 and NRP1 is regulated by the expression of β3 integrin, and 2) the absence of the β3 integrin cytoplasmic tail is sufficient to increase the association of VEGFR2 and NRP1.
VEGF-A164 Induces an Interaction between αvβ3 Integrin and NRP1 That Can Negatively Regulate VEGFR2 Signaling
It has been demonstrated that NRP1 can associate with β1 integrin (43). Furthermore, Veldembri et al. (44) have shown that, via interactions with α5β1 integrin, NRP1 regulates adhesion to fibronectin. This raised the possibility that interactions between αvβ3 integrin and NRP1 may regulate the processes we have been examining.
Unfortunately, transfection of pMT ECs with either full-length human β3 integrin or the β3Δ722 mutant did not produce high enough levels of αvβ3 integrin to efficiently detect its expression by immunoprecipitation. Therefore, to study the interactions between β3 integrin, NRP1, and VEGFR2, we chose to use HUVEC, which are routinely used as a model system for studying both VEGF- and integrin-mediated angiogenic events.
We showed that αvβ3 integrin and NRP1 associate in a co-immunoprecipitable complex (Fig. 8A, left). This association could be demonstrated by immunoprecipitating αvβ3 integrin and Western blotting for NRP1 or vice versa. IgG isotype controls and total cell lysates were used to demonstrate the specificity of the immunoprecipitations. As a further control, we immunoprecipitated αvβ3 integrin and Western blotted for the transferrin receptor (which is expressed in HUVEC at levels comparable with NRP1) and showed no co-association between the two molecules. Stripping the same blot and reprobing for NRP1 recapitulated the co-immunoprecipitation of αvβ3 integrin with NRP1. In addition, fluorescent confocal mircroscopy confirmed close spatial association of αvβ3 integrin and NRP1 in whole cells (Fig. 8A, right).
FIGURE 8.
VEGF-A induces an interaction between αvβ3 integrin and NRP1. A, Western blots. Representative analysis of the co-immunoprecipitation of NRP1 with β3 integrin in VEGF-stimulated human ECs. 1, protein lysates were immunoprecipitated (IP) with anti-αvβ3 integrin (or an isotype IgG control) and Western blotted for NRP1. Blots were stripped and reprobed for β3 integrin. 2, protein lysates were immunoprecipitated with anti-NRP1 (or an isotype IgG control) and Western blotted for β3 integrin. Blots were stripped and reprobed for NRP1. 3, blotting in total cell lysate (TCL) served as a control for levels of NRP1 and β3 integrin in the starting sample. As a further control for the specificity of the immunoprecipitation (4), protein lysates were immunoprecipitated with anti-αvβ3 integrin and Western blotted for transferrin receptor. The same blot was stripped and reprobed for NRP1 and for β3 integrin. Blotting in total cell lysate served as a control for levels of NRP1, TFR, and β3 integrin in the starting sample. Micrographs, fluorescent confocal microscopy analysis of αvβ3 integrin (red) and NRP1 (green) shows that the two molecules can be spatially co-localized in VEGF-stimulated human ECs (white arrows). Scale bar, 2 μm. B, representative analysis of the co-immunoprecipitation of NRP1 with αvβ3 integrin in mock-transfected and β3Δ722 (Δ722)-transfected HUVEC. Cells were stimulated (+) (or not (−)) with VEGF-A165 for 7 min. Protein lysates were immunoprecipitated with anti-αvβ3 integrin and Western blotted for NRP1. Blots were stripped and reprobed for β3 integrin. Blotting in total cell lysates served as a control for starting levels of β3 integrin and NRP1. HSC70 served as a loading control in total cell lysates. The accompanying bar graph represents densitometric results showing the relative VEGF-A165-induced levels of β3 integrin/NRP1 co-association (means ± S.E.) from three independent experiments. *, p ≤ 0.05. The inset is a Western blot showing that both endogenous and truncated β3 integrin are expressed in β3Δ722-transfected HUVEC (C). Upper micrographs, representative staining of closely associated endogenous β3 integrin and NRP1 (red staining) in mock-transfected and β3Δ722-transfected HUVEC as detected by the DuolinkTM in situ PLA. The system elicits a visible signal only when the two antibodies are in close proximity. Examples of intense staining in VEGF-A165-treated, mock-transfected cells are demarcated by white arrows. Nuclei are stained blue. Scale bar, 10 μm. Lower micrographs, representative controls for the PLA in VEGF-A165-stimulated HUVEC demonstrating the signals generated between β3 integrin and an IgG-matched control for NRP1 (β3/IgG), between β3 integrin and NRP1 (β3/NRP), or between β3 integrin and transferrin receptor (β3/TFR). Examples of intense staining between β3 integrin and NRP1 are demarcated by white arrows. Scale bar, 10 μm. The bar graph below represents quantification of the signal between β3 integrin and NRP1 in VEGF-stimulated (+) and -unstimulated (−) mock-transfected or β3Δ722-transfected HUVEC. Also shown, as a negative control, is the quantification of the signal between β3 integrin and the transferrin receptor in untransfected HUVEC cells. Bars, the average number of signals/cell taken through a midcell confocal z-section and measured using BlobFinderTM. *, p ≤ 0.05. nsd, no statistical difference. D, representative analysis of pERK levels in mock-transfected and β3Δ722-transfected HUVEC. Cells were stimulated (+) (or not (−)) with VEGF-A165 for 7 min. Protein lysates were Western blotted for pERK. Blots were stripped and reblotted for total ERK. The accompanying bar graph represents densitometric results (means ± S.E.) showing relative VEGF-A165-induced pERK/total ERK levels from four independent experiments. *, p ≤ 0.05; **, p ≤ 0.01. E, representative analysis of the co-immunoprecipitation of NRP1 with VEGFR2 in mock-transfected and β3Δ722-transfected HUVEC. Cells were stimulated (+) (or not (−)) with VEGF-A165 for 7 min. Protein lysates were immunoprecipitated with anti-VEGFR2 and Western blotted for NRP1. Blots were stripped and reblotted for VEGFR2.
In order to analyze in more detail the interaction between αvβ3 integrin and NRP1, we made further use of the β3Δ722 mutant; there is evidence to suggest that this mutation has an impact on lateral associations with other transmembrane proteins (18). We postulated that by transfecting HUVEC with the β3Δ722 mutant, we might, therefore, be able to alter the interactions of β3 integrin with other transmembrane proteins, such as NRP1.
We transiently transfected HUVEC with the β3Δ722 mutant and demonstrated, by Western blot analysis, expression of the β3Δ722 mutant (albeit at lower levels when compared with endogenous β3 integrin; Fig. 8B, inset). Stimulation with VEGF-A165 (the human equivalent of mouse VEGF-A164) induced a significant increase in the co-association between αvβ3 integrin and NRP1 in mock-transfected cells (Fig. 8B). In contrast, the association of αvβ3 integrin with NRP1 was reduced significantly by expression of the β3Δ722 mutant. Total cell lysates acted as loading and specificity controls.
The reduced co-immunoprecipitation and relative association of αvβ3 integrin and NRP1 in the presence of the β3Δ722 mutant was enticing. Although total β3 integrin levels did not change in the presence of the mutant, because the total level of immunoprecipitated αvβ3 integrin appeared slightly reduced in the presence of the mutant, the interactions between αvβ3 integrin and NRP1 were substantiated using the DuolinkTM in situ PLA, which measures “direct” interactions between molecules in whole cells by producing a fluorescent amplification product between two primer-ligated secondary antibodies. We showed that treatment with VEGF-A165 induced co-association of αvβ3 integrin and NRP1 in mock-transfected cells but not VEGF-treated β3Δ722-transfected cells (Fig. 8C, upper micrographs). As controls for the specificity of the PLA reaction, we saw no VEGF-induced interaction between αvβ3 integrin and an IgG control antibody or between β3 integrin and the transferrin receptor, which is expressed in HUVEC at levels comparable with NRP1 (Fig. 8C, lower micrographs).
Finally, we showed that even low level expression of the β3Δ722 mutant was sufficient to increase significantly VEGF-A165-stimulated ERK phosphorylation when compared with mock-transfected controls (Fig. 8D). Furthermore, β3Δ722 expression also induced a significant increase in VEGF-A165-stimulated VEGFR2/NRP1 association when compared with similarly treated mock controls (Fig. 8E).
Overall, our data establish not only that the presence of the β3 integrin cytoplasmic tail can limit the interaction of VEGFR2 and NRP1 but that αvβ3 integrin can associate with NRP1 in a VEGF-dependent manner. Our data suggest a model whereby αvβ3 integrin may sequester at least a proportion of NRP1, thus limiting its interaction with VEGFR2. In contrast, in the absence of αvβ3 integrin, both VEGFR2 and NRP1 levels are elevated (at least in primary ECs; see Fig. 5), but, more importantly, base-line associations between the two are enhanced. This leads to a more readily formed close association between VEGFR2 and NRP1 that, upon VEGF stimulation, equates with an enhanced response to VEGF stimulation.
DISCUSSION
Both αvβ3 integrin and NRP1 can be expressed by endothelial cells and tumor cells (10, 45, 46). As such, a precise dissection of their individual roles in tumor angiogenesis is complicated. Nevertheless, it is generally accepted that both are up-regulated by ECs during neovascularization.
Complete ablation of NRP1 is lethal and leads to vascular remodeling and branching defects (47). These defects are further enhanced by the additional loss of NRP2 (48). Although the two proteins may have overlapping functions early in vascular development, the expression of each becomes restricted later with NRP1 expressed in arteries and NRP2 in veins and lymphatic vessels (49, 50). Furthermore, Nrp1 deletion specifically in ECs leads to vascular defects similar to those seen in the total knock-out (51), and defects have been observed in the guidance of endothelial tip cells in the developing hindbrain in Nrp1 KO embryos (52).
αvβ3 integrin is up-regulated in proliferating ECs during angiogenesis. Inhibitors of αvβ3 integrin-mediated adhesion or function-blocking mutations in the β3 integrin cytoplasmic domain suppress pathological angiogenesis in mice. In contrast, genetic ablation of β3 integrin enhances pathological angiogenesis and increases VEGFR2 expression, suggesting multiple roles for β3 integrin in angiogenesis, supporting a role for this molecule in neovascularization (6, 8, 9, 35, 53, 54).
We demonstrate that EC-specific expression of β3 integrin limits VEGF-induced angiogenesis by serving as a negative regulator of VEGFR2/NRP1 co-association in ECs. VEGF-A164-mediated events in vivo, ex vivo, and in vitro were enhanced and dependent on NRP1 expression when β3 integrin was absent. In striking contrast, NRP1 contributed less substantially to these processes when β3 integrin was expressed.
We have shown that when β3 integrin is absent or depleted, blocking NRP1 expression significantly reduces VEGF-mediated aortic ring microvessel sprouting, EC wound closure in vitro, and VEGFR2-mediated signaling. These results correlate with an enhanced association between VEGFR2 and NRP1 in β3-null ECs, as detected by co-immunoprecipitation, even under non-VEGF-stimulated conditions. Although two groups have shown that the association between NRP1 and VEGFR2 is dependent on VEGF treatment (55, 56), Whitaker et al. (38) have shown that co-immunoprecipitation of VEGFR2 and NRP1 in COS-1 cells co-expressing the two receptors and in HUVEC can occur independently of VEGF-A stimulation. Our data suggest that the interaction between VEGFR2 and NRP1 is more readily achieved in the absence of β3 integrin.
Mac Gabhann and Popel (57, 58) have comprehensively modeled the interactions between VEGFR2, NRP1, and VEGFs, and their predictions are in agreement with in vitro studies. Their models demonstrate that the coupling of NRP1-bound VEGF-A165 drives the formation of VEGFR2·NRP1 complexes. Although the influence of the ECM on sequestering VEGF has been considered, what is missing from the computational models is the role of other surface receptors that may positively or negatively regulate this association.
By expressing a β3 integrin cytoplasmic tail mutant in β3-null pMT ECs, we show that the β3 integrin cytoplasmic domain is involved in the regulation of VEGFR2/NRP1 associations and downstream signaling. In HUVEC, expression of the β3Δ722 mutant altered interactions between αvβ3 integrin and NRP1 and between VEGFR2 and NRP1. At present, we cannot distinguish between the following two possibilities of how the β3Δ722 mutant elicits these changes in HUVEC: 1) the presence of β3Δ722 cytoplasmic tail mutation by itself affects co-association between β3 integrin and NRP1, or 2) the presence of the β3Δ722 mutant also changes the confirmation of endogenous β3 integrin, and this alters associations with NRP1. Nevertheless, our studies indicate that the presence of other surface molecules needs to be factored into both mathematical and biological studies that examine how VEGFR2 and NRP1 interact.
It is apparent that β3 integrin regulates VEGFR2 function, and therefore VEGF-induced angiogenesis, in both positive and negative ways and that the two are not mutually exclusive. For example, β3 integrin deficiency can enhance VEGFR2 expression and function (6, 19). In addition, we demonstrate here that β3 integrin (and importantly the cytoplasmic domain of β3 integrin) negatively regulates VEGFR2 signaling by inhibiting the interaction between VEGFR2 and NRP1. However, studies with animals carrying β3 integrin with cytoplasmic tail point mutations (where tyrosines 747 and 759 have been replaced with phenylalanines (DiYF) (41)) have implied that this motif of β3 integrin is required for angiogenesis and that interactions between β3 integrin and VEGFR2 can positively regulate VEGFR2 signaling (8). Although beyond the scope of this study, it is tempting to speculate that the DiYF cytoplasmic tail mutant form of β3 integrin might inhibit VEGFR2/NRP1 interactions more than wild-type β3 integrin and thus reduce angiogenic responses.
Classically, anti-αvβ3 integrin therapy failure is attributed to there being multiple proangiogenic pathways within ECs that compensate for the loss of αvβ3 integrin function. Recent data have shown that low doses of some of these drugs can enhance VEGF-mediated angiogenesis by affecting VEGFR2 recycling (20). Our data strongly imply that this therapy might be unsuccessful as a consequence of removing an inhibitory angiogenic regulator. This raises the possibility that the efficacy of antiangiogenic therapy depends on integrin expression and function. Since the absence of β3 integrin expression in ECs enhances significantly the role played by NRP1 in VEGF-mediated events, targeting both VEGFR2 and NRP1, when β3 integrin expression is low, may prove highly effective. A related observation is that in some tumors, such as angiosarcomas, expression of β3 integrin subunits on ECs decreases during the course of malignant transformation (59). In such situations, targeting NRP1 may prove more effective in preventing disease progression.
If αvβ3 integrin expression is up-regulated on proliferating ECs during angiogenesis and during vascular remodeling, our data would suggest that targeting NRP1 expressed by ECs to control angiogenesis may be of limited use. However, our data indicate that simultaneously targeting β3 integrin and NRP1 may significantly increase the efficacy of antiangiogenic therapy, a hypothesis we are currently testing.
Supplementary Material
Acknowledgments
We thank Nelly Kieffer for the gift of the β3Δ722 mutant construct. We also thank Garry Saunders, Colin Wren, Colin Pegrum, and Claire Darnborough for technical assistance and Ian Hart, Gabriela D'amico, Mitchel Germain, and the other members of the Tumor Biology Centre for support and criticism during this study. We also thank Dr. Mark Karaczun for critical reading of the manuscript and for help with figure design.
This work was supported by a grant from Cancer Research UK.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- VEGF
- vascular endothelial growth factor
- EC
- endothelial cell(s)
- ERK
- extracellular signal-regulated kinase
- pERK
- phospho-ERK
- MAPK
- mitogen-activated protein kinase
- HUVEC
- human umbilical vein endothelial cell(s)
- siRNA
- small interfering RNA
- PBS
- phosphate-buffered saline
- WT
- wild-type
- shRNA
- short hairpin RNA
- pMT
- polyoma-middle T-antigen immortalized lung endothelial cells
- PLA
- proximity ligation assay
- cp
- control pool.
REFERENCES
- 1.Cébe-Suarez S., Zehnder-Fjällman A., Ballmer-Hofer K. (2006) Cell Mol. Life Sci. 63, 601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veeravagu A., Hsu A. R., Cai W., Hou L. C., Tse V. C., Chen X. (2007) Recent Pat. Anticancer Drug Discov. 2, 59–71 [DOI] [PubMed] [Google Scholar]
- 3.Jain R. K., Duda D. G., Clark J. W., Loeffler J. S. (2006) Nat. Clin. Pract. Oncol. 3, 24–40 [DOI] [PubMed] [Google Scholar]
- 4.Eliceiri B. P. (2001) Circ. Res. 89, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 5.Alghisi G. C., Rüegg C. (2006) Endothelium 13, 113–135 [DOI] [PubMed] [Google Scholar]
- 6.Reynolds L. E., Wyder L., Lively J. C., Taverna D., Robinson S. D., Huang X., Sheppard D., Hynes R. O., Hodivala-Dilke K. M. (2002) Nat. Med. 8, 27–34 [DOI] [PubMed] [Google Scholar]
- 7.Soldi R., Mitola S., Strasly M., Defilippi P., Tarone G., Bussolino F. (1999) EMBO J. 18, 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabeleshwar G. H., Feng W., Phillips D. R., Byzova T. V. (2006) J. Exp. Med. 203, 2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strieth S., Eichhorn M. E., Sutter A., Jonczyk A., Berghaus A., Dellian M. (2006) Int. J. Cancer 119, 423–431 [DOI] [PubMed] [Google Scholar]
- 10.Soker S., Takashima S., Miao H. Q., Neufeld G., Klagsbrun M. (1998) Cell 92, 735–745 [DOI] [PubMed] [Google Scholar]
- 11.Pan Q., Chathery Y., Wu Y., Rathore N., Tong R. K., Peale F., Bagri A., Tessier-Lavigne M., Koch A. W., Watts R. J. (2007) J. Biol. Chem. 282, 24049–24056 [DOI] [PubMed] [Google Scholar]
- 12.Jia H., Bagherzadeh A., Hartzoulakis B., Jarvis A., Löhr M., Shaikh S., Aqil R., Cheng L., Tickner M., Esposito D., Harris R., Driscoll P. C., Selwood D. L., Zachary I. C. (2006) J. Biol. Chem. 281, 13493–13502 [DOI] [PubMed] [Google Scholar]
- 13.Starzec A., Vassy R., Martin A., Lecouvey M., Di Benedetto M., Crépin M., Perret G. Y. (2006) Life Sci. 79, 2370–2381 [DOI] [PubMed] [Google Scholar]
- 14.Pan Q., Chanthery Y., Liang W. C., Stawicki S., Mak J., Rathore N., Tong R. K., Kowalski J., Yee S. F., Pacheco G., Ross S., Cheng Z., Le Couter J., Plowman G., Peale F., Koch A. W., Wu Y., Bagri A., Tessier-Lavigne M., Watts R. J. (2007) Cancer Cell 11, 53–67 [DOI] [PubMed] [Google Scholar]
- 15.Krilleke D., DeErkenez A., Schubert W., Giri I., Robinson G. S., Ng Y. S., Shima D. T. (2007) J. Biol. Chem. 282, 28045–28056 [DOI] [PubMed] [Google Scholar]
- 16.Reynolds L. E., Hodivala-Dilke K. M. (2006) Methods Mol. Med. 120, 503–509 [DOI] [PubMed] [Google Scholar]
- 17.May T., Mueller P. P., Weich H., Froese N., Deutsch U., Wirth D., Kröger A., Hauser H. (2005) J. Biotechnol. 120, 99–110 [DOI] [PubMed] [Google Scholar]
- 18.Schaffner-Reckinger E., Gouon V., Melchior C., Plançon S., Kieffer N. (1998) J. Biol. Chem. 273, 12623–12632 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds A. R., Reynolds L. E., Nagel T. E., Lively J. C., Robinson S. D., Hicklin D. J., Bodary S. C., Hodivala-Dilke K. M. (2004) Cancer Res. 64, 8643–8650 [DOI] [PubMed] [Google Scholar]
- 20.Reynolds A. R., Hart I. R., Watson A. R., Welti J. C., Silva R. G., Robinson S. D., Da Violante G., Gourlaouen M., Salih M., Jones M. C., Jones D. T., Saunders G., Kostourou V., Perron-Sierra F., Norman J. C., Tucker G. C., Hodivala-Dilke K. M. (2009) Nat. Med. 15, 392–400 [DOI] [PubMed] [Google Scholar]
- 21.Ventura A., Meissner A., Dillon C. P., McManus M., Sharp P. A., Van Parijs L., Jaenisch R., Jacks T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10380–10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston G., Murphy T. J., Baluk P., Lindsey J. R., McDonald D. M. (1998) Am. J. Pathol. 153, 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttmann-Raviv N., Kessler O., Shraga-Heled N., Lange T., Herzog Y., Neufeld G. (2006) Cancer Lett. 231, 1–11 [DOI] [PubMed] [Google Scholar]
- 24.Andrade S. P., Fan T. P., Lewis G. P. (1987) Br. J. Exp. Pathol. 68, 755–766 [PMC free article] [PubMed] [Google Scholar]
- 25.Starzec A., Ladam P., Vassy R., Badache S., Bouchemal N., Navaza A., du Penhoat C. H., Perret G. Y. (2007) Peptides 28, 2397–2402 [DOI] [PubMed] [Google Scholar]
- 26.Rousseau S., Houle F., Landry J., Huot J. (1997) Oncogene 15, 2169–2177 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T., Ueno H., Shibuya M. (1999) Oncogene 18, 2221–2230 [DOI] [PubMed] [Google Scholar]
- 28.Nicosia R. F., Lin Y. J., Hazelton D., Qian X. (1997) Am. J. Pathol. 151, 1379–1386 [PMC free article] [PubMed] [Google Scholar]
- 29.Perron-Sierra F., Saint Dizier D., Bertrand M., Genton A., Tucker G. C., Casara P. (2002) Bioorg. Med. Chem. Lett. 12, 3291–3296 [DOI] [PubMed] [Google Scholar]
- 30.Maubant S., Saint-Dizier D., Boutillon M., Perron-Sierra F., Casara P. J., Hickman J. A., Tucker G. C., Van Obberghen-Schilling E. (2006) Blood 108, 3035–3044 [DOI] [PubMed] [Google Scholar]
- 31.Rosen E. M., Lamszus K., Laterra J., Polverini P. J., Rubin J. S., Goldberg I. D. (1997) Ciba Found. Symp. 212, 215–226; discussion 227–219 [DOI] [PubMed] [Google Scholar]
- 32.Presta M., Oreste P., Zoppetti G., Belleri M., Tanghetti E., Leali D., Urbinati C., Bugatti A., Ronca R., Nicoli S., Moroni E., Stabile H., Camozzi M., Hernandez G. A., Mitola S., Dell'Era P., Rusnati M., Ribatti D. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 71–76 [DOI] [PubMed] [Google Scholar]
- 33.West H., Richardson W. D., Fruttiger M. (2005) Development 132, 1855–1862 [DOI] [PubMed] [Google Scholar]
- 34.Sulpice E., Plouët J., Bergé M., Allanic D., Tobelem G., Merkulova-Rainon T. (2008) Blood 111, 2036–2045 [DOI] [PubMed] [Google Scholar]
- 35.Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. (1994) Cell 79, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 36.Eliceiri B. P., Klemke R., Strömblad S., Cheresh D. A. (1998) J. Cell Biol. 140, 1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustafsson E., Brakebusch C., Hietanen K., Fässler R. (2001) J. Cell Sci. 114, 671–676 [DOI] [PubMed] [Google Scholar]
- 38.Whitaker G. B., Limberg B. J., Rosenbaum J. S. (2001) J. Biol. Chem. 276, 25520–25531 [DOI] [PubMed] [Google Scholar]
- 39.Shraga-Heled N., Kessler O., Prahst C., Kroll J., Augustin H., Neufeld G. (2007) FASEB J. 21, 915–926 [DOI] [PubMed] [Google Scholar]
- 40.Kawamura H., Li X., Goishi K., van Meeteren L. A., Jakobsson L., Cébe-Suarez S., Shimizu A., Edholm D., Ballmer-Hofer K., Kjellén L., Klagsbrun M., Claesson-Welsh L. (2008) Blood 112, 3638–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips D. R., Nannizzi-Alaimo L., Prasad K. S. (2001) Thromb. Haemost. 86, 246–258 [PubMed] [Google Scholar]
- 42.Schaffner-Reckinger E., Brons N. H., Kieffer N. (2001) Thromb. Haemost. 85, 716–723 [PubMed] [Google Scholar]
- 43.Fukasawa M., Matsushita A., Korc M. (2007) Cancer Biol. Ther. 6, 1173–1180 [DOI] [PubMed] [Google Scholar]
- 44.Valdembri D., Caswell P. T., Anderson K. I., Schwarz J. P., König I., Astanina E., Caccavari F., Norman J. C., Humphries M. J., Bussolino F., Serini G. (2009) PLoS Biol. 7, e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varner J. A., Cheresh D. A. (1996) Important Adv. Oncol. 69–87 [PubMed] [Google Scholar]
- 46.Wey J. S., Stoeltzing O., Ellis L. M. (2004) Clin. Adv. Hematol. Oncol. 2, 37–45 [PubMed] [Google Scholar]
- 47.Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. (1999) Development 126, 4895–4902 [DOI] [PubMed] [Google Scholar]
- 48.Takashima S., Kitakaze M., Asakura M., Asanuma H., Sanada S., Tashiro F., Niwa H., Miyazaki Ji J., Hirota S., Kitamura Y., Kitsukawa T., Fujisawa H., Klagsbrun M., Hori M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3657–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herzog Y., Kalcheim C., Kahane N., Reshef R., Neufeld G. (2001) Mech. Dev. 109, 115–119 [DOI] [PubMed] [Google Scholar]
- 50.Yuan L., Moyon D., Pardanaud L., Bréant C., Karkkainen M. J., Alitalo K., Eichmann A. (2002) Development 129, 4797–4806 [DOI] [PubMed] [Google Scholar]
- 51.Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L., Ginty D. D. (2003) Dev. Cell 5, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerhardt H., Ruhrberg C., Abramsson A., Fujisawa H., Shima D., Betsholtz C. (2004) Dev. Dyn. 231, 503–509 [DOI] [PubMed] [Google Scholar]
- 53.Brooks P. C., Clark R. A., Cheresh D. A. (1994) Science 264, 569–571 [DOI] [PubMed] [Google Scholar]
- 54.Friedlander M., Brooks P. C., Shaffer R. W., Kincaid C. M., Varner J. A., Cheresh D. A. (1995) Science 270, 1500–1502 [DOI] [PubMed] [Google Scholar]
- 55.Soker S., Miao H. Q., Nomi M., Takashima S., Klagsbrun M. (2002) J. Cell. Biochem. 85, 357–368 [DOI] [PubMed] [Google Scholar]
- 56.Barr M. P., Bouchier-Hayes D. J., Harmey J. J. (2008) Int. J. Oncol. 32, 41–48 [PubMed] [Google Scholar]
- 57.Mac Gabhann F., Popel A. S. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H2851–H2860 [DOI] [PubMed] [Google Scholar]
- 58.Mac Gabhann F., Popel A. S. (2007) Am. J. Physiol. Heart Circ Physiol. 292, H459–H474 [DOI] [PubMed] [Google Scholar]
- 59.Mechtersheimer G., Barth T., Hartschuh W., Lehnert T., Möller P. (1994) Virchows Arch. 425, 375–384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.