Abstract
Isothermal calorimetry (ITC) measurements yielded the binding constants during complex formation of light-inducible histidine kinases (HK) and their cognate CheY-type response regulators (RR). HK-RR interactions represent the core function of the bacterial two-component system, which is also present in many bacterial phytochromes. Here, we have studied the recombinant forms of phytochromes CphA and CphB from the cyanobacterium Tolypothrix PCC7601 and their cognate RRs RcpA and RcpB. The interaction between the two reaction partners (HK and RR) was studied in the presence and absence of ATP. A complex formation was observable in the presence of ATP, but specific interactions were only found when a non-hydrolyzable ATP derivative was added to the mixture. Also, the incubation of the HK domain alone (expressed as a recombinant protein) with the RR did not yield specific interactions, indicating that the HK domain is only active as a component of the full-length phytochrome. Considering also previous studies on the same proteins (Hübschmann, T., Jorissen, H. J. M. M., Börner, T., Gärtner, W., and de Marsac, N. (2001) Eur. J. Biochem. 268, 3383–3389) we now conclude that the HK domains of these phytochromes are active only when the chromophore domain is in its Pr form. The formerly documented phosphate transfer between the HK domain and the RR takes place via a transiently formed protein-protein complex, which becomes detectable by ITC in the presence of a non-hydrolyzable ATP derivative. This finding is of interest also in relation to the function of some (blue light-sensitive) photoreceptors that carry the HK domain and the RR fused together in one single protein.
Introduction
The detection of external stimuli is essential for uni- and multicellular organisms to adapt their metabolism to changing environmental conditions. The process to transmit a stimulus-generated biological signal into the cell interior comprises interactions of proteins or protein domains, during which conformational changes and/or transient modifications take place, e.g. (trans) phosphorylation of amino acid side chains, generation or hydrolysis of cyclic nucleotides, or the release of low molecular weight second messenger molecules (Ca2+, IP3 etc.).
Light is one of the most important stimuli that has evoked the development of numerous photoreceptor types for nearly every organism. Among the best studied photoreceptors are the phytochromes, which are bilin-binding red/far red light-sensitive chromoproteins. Phytochromes are ubiquitous in higher and lower plants and have also been detected in a large number of prokaryotes (1, 2). Many prokaryotic phytochromes perform the signaling via a light-regulated histidine kinase (HK)3 activity, located in the C-terminal part of the photoreceptor, whereas the N-terminal portion binds the chromophore and is responsible for light detection (3) and spectroscopic changes (4). HKs are constituents of the so-called two-component system, which has been identified in many prokaryotic signal-transducing processes (5, 6). A large number of HK domains has been characterized, and in most cases, a homodimeric arrangement has been found. Their function is based on an ATP binding capacity. Upon (light-induced) activation, HKs phosphorylate a conserved histidine residue in the HK domain. Only after such protein modification, a specific protein-protein interaction can be accomplished between the HK domain and a so-called response regulator (RR) protein, during which the phosphate group is transferred from the histidine residue to an aspartate of the RR protein (Scheme 1). This activated RR can subsequently initiate downstream physiological processes that allow the organism to respond to the external stimulus.
SCHEME 1.
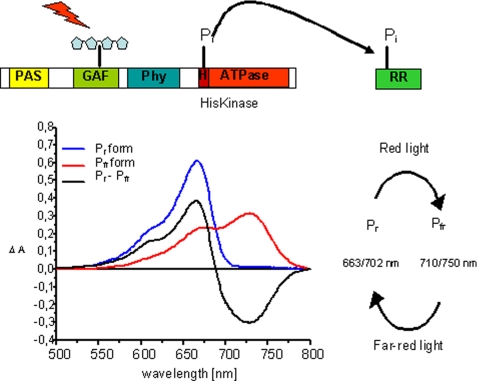
Schematic presentation of the prokaryotic phytochrome domain structure and model for a HK activity. Phytochromes bind their chromophore in the N-terminal domain; shown here is the canonical situation for phytochromes where the chromophore is bound to a cysteine in the GAF domain (realized in this study with CphA). Some bacterial phytochromes (realized here with CphB) bind biliverdin as chromophore at a cysteine in the PAS domain. The histidine kinase activity is located in both types of phytochromes in the C-terminal domain. Upon excitation, HK utilizes a bound ATP molecule and phosphorylates a conserved histidine residue. Upon protein-protein contact between the HK domain and its response regulator, the phosphate group is transferred to a conserved aspartate residue in the RR protein. Also shown (bottom left) are the absorption spectra of the two forms of phytochrome, Pr and Pfr, the difference spectrum Pr − Pfr, and (bottom right) a scheme indicating the red and far-red light-induced Pr and Pfr formation, together with the λmax values of the respective states (Pr/Pfr). Wavelengths for PCB-assembled phytochromes are given first, followed by those for BV-assembled phytochromes.
Besides the inherent fascinating properties of biological photoreceptors, light-induced signal transduction can serve as a model to study signaling processes in detail, allowing for an almost instantaneous switch between the active and inactive states. Light induction of HKs has been reported for several systems, amongst which the first identified cyanobacterial phytochrome, Cph1 (3), for bacteriophytochromes (7, 8), and even for blue light-sensitive photoreceptors, where a flavin chromophore-binding domain activates an HK just in the same way as in the red light-sensing phytochromes (9). Recently, we have demonstrated light-inducible kinase activity for two cyanobacterial phytochromes, CphA and CphB from Tolypothrix PCC7601, and the transfer of the phosphate group from the histidine in the HK domain to an added response regulator (10, 11). These two proteins show a very similar overall architecture (as deduced from sequence comparison, and secondary and tertiary structure prediction); however, they show a different chromophore preference. CphA binds covalently phycocyanobilin as chromophore and shows all features of typical plant-related phytochromes. CphB, instead, binds biliverdin covalently in its N-terminally located PAS domain, as shown previously by us by homologous expression in Tolypothrix, affinity purification of the recombinant protein, and spectroscopic and kinase activity analysis (12). Irrespective of the differences in their chromophore binding properties, both phytochromes exhibit light-regulated HK activity and a highly specific phosphate transfer upon incubation with their cognate response regulators, RcpA and RcpB (11). Interestingly, both phytochromes showed a high kinase activity and transphosphorylation to the response regulators in their dark-adapted (Pr) states. When converted into the Pfr forms, a reduced activity of only 30% of the Pr form was determined. Considering the photochemical properties of phytochromes, one has to keep in mind that, because of the spectral overlap of the Pr and the Pfr forms, only 70% of the Pfr form can be generated in a photoequilibrium (see also Scheme 1). Clearly, the residual HK activity observed in the Pfr state has to be ascribed to the residual amount of Pr present in the sample. Phosphate transfer to the response regulators that was demonstrated by the use of radioactive ATP, could independently also be demonstrated by the crystal structures that were obtained for the phosphate-loaded and -unloaded states of the RRs (11, 13).
Although the function of these light-regulated signal transduction systems has been demonstrated, a quantitative analysis and a detailed characterization of the complex formation process between CphA/ B and their respective partners, RcpA/B, has not been performed so far. Here, we present quantitative measurements of these protein-protein interactions by applying isothermal titration calorimetry (ITC), and demonstrate that only very specific experimental conditions allow the detection of a stable complex between cyanobacterial phytochromes and their cognate response regulators.
EXPERIMENTAL PROCEDURES
Sample Generation
Recombinant CphA and CphB from the cyanobacterium Tolypothrix PCC7601 and their cognate response regulators RcpA and RcpB were heterologously expressed as previously described (10, 12, 14). The HK domain of CphA was generated by introducing a start codon at positions 1375–1377 of cphA (originally coding for Glu-459) and subsequent cloning of this new open reading frame into pET28a between restriction sites for NcoI and XhoI. HK from CphB was generated similarly, inserting a start codon at positions 1408–1410 (originally coding for Trp-469). Cloning was at the same sites of pET28 as for the HK of CphA. Both HK proteins from CphA and CphB were modified to include a His6 tag at their C-terminal end by inserting an octadecanucleotide at the 3′-end of the gene. All recombinant proteins were purified via Ni-NTA columns, eluting with increasing concentrations of imidazole. After elution, samples were washed extensively to remove imidazole using Amicon concentration cartridges to ensure that no residual imidazole remained. The non-hydrolyzable ATP analogue, adenosine 5′(β-γ-imido)triphosphate tetralithium salt, was purchased from Sigma-Aldrich and was used without further purification.
Sample Preparation for Microcalorimetric Measurements
Isothermal titration calorimetry measurements were performed at 25 °C, using a VP-ITC MicroCalorimeter (MicroCal, Northampton, MA). Experiments were performed with three separately prepared protein samples. Each preparation was used for two parallel sets of experiments. Data acquisition and subsequent nonlinear regression analysis were performed using ORIGIN software package for a simple binding model. All samples for the microcalorimetry measurements were prepared in 50 mm NaCl, 50 mm Tris-Cl buffer, pH 8.0. Protein samples and buffer solutions were gently degassed prior to each experiment. Control experiments for all titrations were performed by titrating either the response regulator (RcpA/B) to buffer solution or buffer solution to the phytochrome (CphA/B) protein in the presence or absence of the non-hydrolyzable ATP analogue. An automatic injection syringe was used with injection intervals of 4 min. The sample cuvette was stirred at 300 rpm. In all experiments, the response regulator (RcpA/B), typically at a concentration of 200 μm, was injected in 15 μm increments into the calorimetry cell, until complete saturation. Initially, the calorimetry cell contained the phytochrome protein (as either the full-length CphA/B protein or the truncated HK domain) in 2 ml of buffer at a concentration of 15 μm and ATP or the non-hydrolyzable ATP analogue at a concentration of 1 mm. For comparison, experiments were also performed in the absence of the non-hydrolyzable ATP analogue.
RESULTS
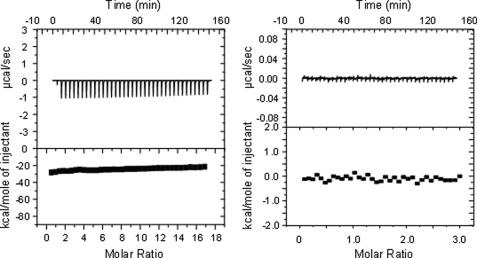
Microcalorimetric experiments were performed under precisely thermostatted conditions at 25 °C, allowing an exact determination of the heat released or taken up. Because the Pr form of cyanobacterial phytochromes have been previously demonstrated to be the active state (11, 12), the dark-adapted Pr form was used in all experiments. Protein interaction measurements were always performed such that the RR proteins (in high concentration) were titrated into the calorimetric cell, containing the chromophore-assembled phytochromes. For control measurements, a blank buffer solution was titrated stepwise into the reservoir of the cell, which contained a solution of BV-reconstituted CphB, and the heat development was registered. Alternatively, RcpA was titrated with the blank buffer solution (Fig. 1). Similar shapes of the curves were found when PCB-reconstituted CphA or RcpB were titrated with a buffer solution (data not shown).
FIGURE 1.
Control experiments of isothermal titration calorimetry measurements. Titration of the buffer into a solution of BV-reconstituted CphB (left) and of buffer into a solution of RcpA (right).
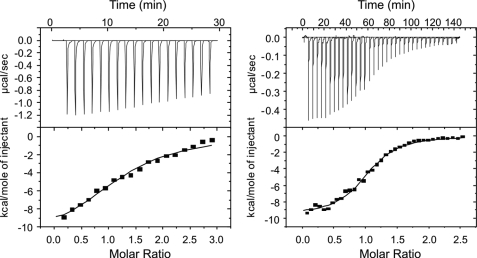
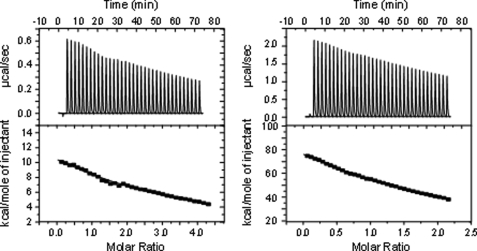
Interestingly, no meaningful binding constants could be determined, when CphB was titrated with its cognate RR protein RcpB, even when the experiments were repeated in the presence of ATP (Fig. 2, left). In the case where ATP was added, the titration of RcpB to CphB resulted in a titration curve without a clearly pronounced inflection point, indicating a complex reaction process. Formally, thermodynamic values could be determined similar to those obtained from titrations using the non-hydrolyzable ATP analog (see below). However, it was not possible to fit the data satisfactorily, because of the fact that this titration mirrors three different reactions: Binding of RcpB to CphB(ATP) and the hydrolysis of ATP to ADP while phosphorylating RcpB. A significant binding curve was only obtained when a non-hydrolyzable ATP analogue was present during the experiment (Fig. 2, right). Under these conditions, clear evidence for a complex formation between CphB and its respective partner protein, RcpB, was found. Data analysis revealed a binding constant of 1.1 μm for the CphB/RcpB couple. The stoichiometry was determined as close to 1 (1.05). A nearly identical behavior was found for the CphA/RcpA couple. Also here, no distinct binding was found, when only the two components (even with addition of ATP) were mixed (control experiment, data not shown). Distinct binding was only detected when the non-hydrolyzable ATP derivative was present (Fig. 3). Under these conditions, a binding constant of 1.2 μm and a stoichiometry of 0.9 were calculated (Table 1). Evidently, the addition of the non-hydrolyzable ATP sufficiently stabilizes the complexes between the cyanobacterial phytochromes and their cognate response regulators, allowing them to be detectable by ITC.
FIGURE 2.
Incubation of BV-reconstituted CphB with RcpB. Left, titration of both components in the presence of ATP; right, titration of both components in the presence of the non-hydrolyzable ATP derivative.
FIGURE 3.
Interactions between CphA and RcpA in the presence of the non-hydrolyzable ATP derivative.
TABLE 1.
Thermodynamic parameters of CphA-RcpA and CphB-RcpB binding reactions
| ΔH° | K | ΔG | TΔS | KD | n | |
|---|---|---|---|---|---|---|
| kJ mol−1 | m−1105 | kJ mol−1 | kJ mol−1K−1 | μm | ||
| CphB/RcpB (ATP analog) | −9.6 | 9.19 | −8.3 | −1.47 | 1.08 | 1.05 |
| CphB/RcpB | No binding | |||||
| CphA/RcpA (ATP analog) | −19.4 | 8.05 | −8.2 | −11.2 | 1.2 | 0.9 |
| HK domain/RcpA | No binding | |||||
| HK domain/RcpA (ATP analog) | No binding | |||||
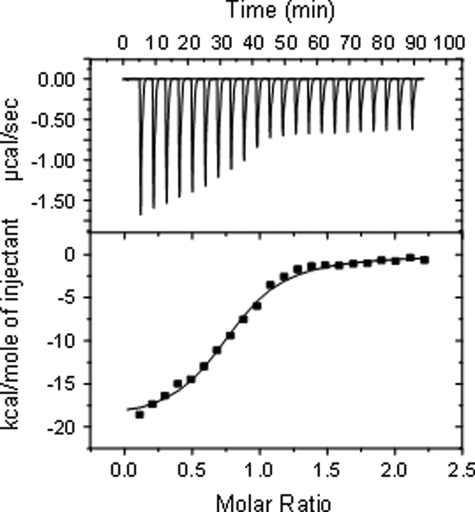
Because all the experiments thus far were performed on full-length photoreceptors, it was of interest to learn whether the HK domain alone, expressed separately as a recombinant protein, would also be able to form a stable complex with the RR protein. Expression yielded a fully soluble protein of expected size. However, no complex formation between the HK domain and its cognate RR protein was observed either in the absence or in the presence of the non-hydrolyzable ATP analogue (Fig. 4). For this experiment, even the course of the titration curve has changed, indicating a completely different behavior of the two proteins.
FIGURE 4.
Incubation of heterologously expressed HK domain from CphA with RcpA in the (left) presence or (right) absence of the non-hydrolyzable ATP derivative.
DISCUSSION
Two-component signal transduction systems are well characterized for many different stimuli in numerous microorganisms. The light-inducible components reported here, the cyanobacterial phytochromes CphA and CphB, together with their cognate response regulators, RcpA and RcpB, are well suited for protein-protein interaction studies, because the active and inactive state can readily be interconverted upon light irradiation. Moreover, once formed, both Pr and Pfr remain stable in the dark for several hours under the experimental conditions applied here. The principal interaction between these phytochromes and their response regulators and the transfer of a phosphate group had already been demonstrated formerly (11). Even for the case of two phytochromes being present in Tolypothrix PCC7601, the functionality of both and the strict selection with respect to interaction with their cognate RRs was shown (11). Even more, irrespective of CphA binding PCB and CphB binding BV (in both cases the chromophores are bound covalently) the signaling process works the same. Moreover, the crystallization of both RR forms, unphosphorylated and phosphate-loaded, revealed the structure of the final products of this signaling pathway (13). It is important to note here, that our crystal structures for RcpA and RcpB showed an upside-down arrangement of two RR molecules in the unit cell, which, on the basis of the commonly accepted reaction mechanisms, makes an interaction with HKs difficult to imagine. Histidine phosphorylation in HKs is based on a parallel arrangement of both protein domains, as also had been shown for the ortholog protein Cph1 from Synechocystis PCC6803 (15, 16). In addition, a bacteriophytochrome has recently been characterized as a homodimer via the small-angle scattering technique, acknowledging the commonly proposed dimeric arrangement also for this protein class (17). This assumption would also be in accordance with a parallel arrangement of the two chromophore-binding domains in a phytochrome homodimer. Phosphate transfer from the HK to the RR was shown by us for both systems, CphA and CphB, utilizing radioactive ATP under very similar concentration conditions. Thus, the dimer formation that we obtained in the crystallization experiments is most probably caused by the high concentrations (>10 mg/ml) required for protein crystallization.
On the basis of the formerly presented data, it is most interesting that under the conditions of ITC experiments, a stable complex between the photoreceptor and its RR protein can only be detected in the presence of a non-hydrolyzable ATP analogue. Clearly, the above described data on the auto- and transphosphorylation that prove functionality can only be explained such that the phosphate transfer takes place during a transient complex formation between both reaction partners. The observed molar ratio is determined as 1:1, preferring a 2:2 complex as the more reasonable form on the basis of above cited literature reports. In fact, most HK proteins are reported to assemble as homodimers (making the phosphorylation reaction a transphosphorylation) (6).
Previous phosphorylation assays using radioactive ATP (11) showed a preferred enzymatic activity when the photoreceptor was in its Pr state. Upon conversion into the Pfr form, only ∼30% of the Pr-related enzymatic activity was observed, which have to be ascribed to the remaining portion of Pr molecules in the photoequilibrium (the partially overlapping Pr and Pfr absorption spectra do not allow a complete conversion into the Pfr form, see absorption spectra in Scheme 1). Interestingly, former experiments also showed an autophosphorylation capability for the apo-phytochrome, i.e. the chromophore-free protein (11). This observation is in agreement with the Pr-ascribed phosphorylation activity, because phytochromes are known to adopt the Pr state during biosynthesis or upon assembly with the chromophore in the dark. Thus, it was of interest to learn whether the HK domain, when expressed on its own, is in its active or inactive form. The results reported here clearly demonstrate that the HK is expressed in an inactive form, being unable to undergo autophosphorylation; also the interaction with added RR does not establish the enzyme activity, even when all the appropriate conditions for complex formation (as established between the full-length phytochromes and the RR proteins) are fulfilled.
It can thus be concluded for the full-length, chromophore-reconstituted phytochromes from Tolypothrix PCC7601 and their cognate RR proteins that the dark-adapted Pr form of these photoreceptors is the active state (with respect to kinase activity and transphosphorylation). Only under these conditions the HK domain is able to autophosphorylate the histidine residue and to transfer the phosphate group to the added RR protein. Photoconversion of the chromophore-bearing N-terminal domain into the Pfr form causes an inactivation of the HK domain. Apparently, this is also the state which the HK adopts when expressed independently.
The observation that a stable complex is formed only after addition of a non-hydrolyzable ATP derivative is quite indicative for the mechanism and function of this particular protein-protein interaction. Further kinetic experiments may help to clarify the complex reactions that were observed in the combination CphB/RcpB/ATP. Apparently, the use of the non-hydrolyzable ATP analog ADPNHP decouples the kinase reaction from the binding process and allows determining the binding parameters. The transient nature of complex formation enabling phosphate transfer is clearly an advantage, because it enables one phytochrome molecule (in its Pr state) to activate several response regulator molecules; thus, causing an amplification of the light-generated signal. Such amplification is consistent with the observation that only very few molecules of cyanobacterial phytochrome molecules are present in a single cell (18). The transient complex formation as an argument for an amplification mechanism has to be seen in relation to a recently characterized, highly homologous HK domain in a blue light-sensitive photoreceptor (9). This photoreceptor from the plant pathogen Pseudomonas syringae shows a similar domain architecture, consisting of a chromophore-binding domain, which is followed by a light-regulated HK. In addition, this photoreceptor has also an RR domain fused to the HK domain. Thus, irrespective of the similar structure and function of these domains, the process of signal propagation is apparently different for the various members of the two-component signaling family. The key issue in the cyanobacterial phytochrome signaling process is to generate the active state of the HK domain, which is accomplished only when the chromophore domain is in its Pr state, then allowing a stable and physiologically functional interaction between the HK and the RR protein.
Although no physiological response induced by these cyanobacterial phytochromes has been reported up to now, their light-regulated enzymatic activity has been clearly documented. Interestingly, in the case of the cyanobacterial phytochromes studied here, irradiation with red light (i.e. conversion into the Pfr form) causes an inactivation of the HK domain, whereas in many two-component systems a phosphorylation of the response regulator is considered as the signal transducing step. In consequence, if the light-generated Pfr state is assumed the active conformation, it has to be proposed that the response regulator is active in its non-phosphorylated form, i.e. the phosphorylation is assumed to halt the RR molecules in an inactive state.
This study adds to recent reports on light-inducible HK/RR two-component systems and extends the number and variability of these signaling devices. The similar function for either BV- or PCB-binding prokaryotic phytochromes allows direct comparison with Cph1, which had already been shown by radioactivity-based experiments to function as a light-driven HK (3). The extension of this signal transduction system into the domain of blue light-sensing photoreceptors, either from cultivated organisms (9) or even from enriched metagenomes (19), demonstrates the prominent role of these light-detecting signaling mechanism for many microbes.
Acknowledgments
We thank Daniel Estevez Prado and Christina Reetz for help during the protein preparations.
This work was supported in part by the Helmholtz-Society (VIBS VH-VI-157, to M. E. and W. G.) and the International Max Planck Research School Chemical Biology (to Y. J. K.).
- HK
- histidine kinase
- BV
- biliverdin (IXα)
- CphA
- PCB-binding cyanobacterial phytochrome
- CphB
- BV-binding cyanobacterial phytochrome
- ITC
- isothermal titration calorimetry
- PCB
- phycocyanobilin
- RR
- response regulator.
REFERENCES
- 1.Hughes J., Lamparter T., Mittmann F., Hartmann E., Gärtner W., Wilde A., Börner T. (1997) Nature 386, 663. [DOI] [PubMed] [Google Scholar]
- 2.Karniol B., Wagner J. R., Walker J. M., Vierstra R. D. (2005) Biochem. J. 392, 103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh K. C., Wu S. H., Murphy J. T., Lagarias J. C. (1997) Science 277, 1505–1508 [DOI] [PubMed] [Google Scholar]
- 4.Rockwell N. C., Su Y. S., Lagarias J. C. (2006) Annu. Rev. Plant Biol. 57, 837–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stock A. M., Robinson V. L., Goudreau P. N. (2000) Annu. Rev. Biochem. 69, 183–215 [DOI] [PubMed] [Google Scholar]
- 6.Szurmant H., White R. A., Hoch J. A. (2007) Curr. Opin. Struct. Biol. 17, 706–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giraud E., Fardoux L., Fourrier N., Hannibal L., Genty B., Bouyer P., Dreyfus B., Verméglio A. (2002) Nature 417, 202–205 [DOI] [PubMed] [Google Scholar]
- 8.Bhoo S. H., Davis S. J., Walker J., Karniol B., Vierstra R. D. (2001) Nature 414, 776–779 [DOI] [PubMed] [Google Scholar]
- 9.Cao Z., Buttani V., Losi A., Gärtner W. (2008) Biophys. J. 94, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorissen H. J. M. M., Quest B., Remberg A., Coursin T., Braslavsky S. E., Schaffner K., Tandeau de Marsac N., Gärtner W. (2002) Eur. J. Biochem. 269, 2662–2671 [DOI] [PubMed] [Google Scholar]
- 11.Hübschmann T., Jorissen H. J. M. M., Börner T., Gärtner W., Tandeau, de Marsac N. (2001) Eur. J. Biochem. 268, 3383–3389 [DOI] [PubMed] [Google Scholar]
- 12.Quest B., Hübschmann T., Sharda S., Tandeau de Marsac N., Gärtner W. (2007) FEBS Journal 274, 2088–2098 [DOI] [PubMed] [Google Scholar]
- 13.Benda C., Scheufler C., Tandeau de Marsac N., Gärtner W. (2004) Biophys. J. 87, 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quest B., Gärtner W. (2004) Eur. J. Biochem. 271, 1117–1126 [DOI] [PubMed] [Google Scholar]
- 15.Otto H., Lamparter T., Borucki B., Hughes J., Heyn M. (2003) Biophys. J. 84, 291A [Google Scholar]
- 16.Esteban B., Carrascal M., Abian J., Lamparter T. (2005) Biochemistry 44, 450–461 [DOI] [PubMed] [Google Scholar]
- 17.Evans K., Grossmann J. G., Fordham-Skelton A. P., Papiz M. Z. (2006) J. Mol. Biol. 364, 655–666 [DOI] [PubMed] [Google Scholar]
- 18.Hübschmann T., Börner T., Hartmann E., Lamparter T. (2001) Eur. J. Biochem. 268, 2055–2063 [DOI] [PubMed] [Google Scholar]
- 19.Pathak G. P., Ehrenreich A., Losi A., Streit W. R., Gärtner W. (2009) Environ. Microbiol. 11, 2388–2399 [DOI] [PubMed] [Google Scholar]