Abstract
Chk1, one of the critical transducers in DNA damage/replication checkpoints, prevents entry into mitosis through inhibition of Cdk1 activity. However, it has remained unclear how this inhibition is cancelled at the G2/M transition. We reported recently that Chk1 is phosphorylated at Ser286 and Ser301 by Cdk1 during mitosis. Here, we show that mitotic Chk1 phosphorylation is accompanied by Chk1 translocation from the nucleus to the cytoplasm in prophase. This translocation advanced in accordance with prophase progression and was regulated by Crm-1-dependent nuclear export. Exogenous Chk1 mutated at Ser286 and Ser301 to Ala (S286A/S301A) was observed mainly in the nuclei of prophase cells, although such nuclear accumulation was hardly observed in wild-type Chk1. Induction of S286A/S301A resulted in the delay of mitotic entry. Biochemical analyses using immunoprecipitated cyclin B1-Cdk1 complexes revealed S286A/S301A expression to block the adequate activation of Cdk1. In support of this, S286A/S301A expression retained Wee1 at higher levels and Cdk1-induced phosphorylation of cyclin B1 and vimentin at lower levels. A kinase-dead version of S286A/S301A also localized predominantly in the nucleus but lost the ability to delay mitotic entry. These results indicate that Chk1 phosphorylation by Cdk1 participates in cytoplasmic sequestration of Chk1 activity, which releases Cdk1 inhibition in the nucleus and promotes mitotic entry.
Introduction
DNA damage stresses and stalled DNA replication forks activate evolutionarily conserved checkpoint pathways, which arrest the cell cycle and allow repair of the damaged DNA (1, 2). In the center of these pathways, there exists a protein kinase cascade from ATR (ataxia telangiectasia mutated- and Rad3-related) to Chk1, which is activated by a broad spectrum of genotoxic stimuli such as ultraviolet light and DNA replication inhibitors (3, 4). ATR induces Chk1 phosphorylation at Ser317 and Ser345, which facilitates Chk1 functions (5). Chk1 itself phosphorylates and inhibits Cdc25 family phosphatases, thus blocking the activation of Cdk1 (cyclin-dependent kinase 1; also called Cdc2) and preventing premature mitotic entry (3, 6). Recent reports suggested that Chk1 has a basal kinase activity (7–9) and acts as a negative regulator of cell cycle progression even in unperturbed cells (7). Thus, Chk1 function is likely to be inhibited at the G2/M transition, but its precise mechanism remains largely unknown.
We reported previously that Cdk1 phosphorylates Chk1 at Ser286 and Ser301 during mitosis (10). In this work, we found that this phosphorylation regulates not only Chk1 transport from the nucleus to the cytoplasm at the G2/M transition but also the adequate activation of Cdk1 in the nucleus. The disturbance of this process results in a delay in mitotic entry.
EXPERIMENTAL PROCEDURES
Antibodies
We produced site- and phosphorylation state-specific antibodies for Ser55 in vimentin (mouse monoclonal antibody) (11); Ser28 in histone H3 (12); and Ser286, Ser301, Ser317, and Ser345 in Chk1 (rat monoclonal antibodies) (13) as described previously (14). Antibodies from commercial sources were as follows: mouse Chk1 (G4) and cyclin B (GNS-1) (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit lamin B1 (ab16048) and cyclin B1 phospho-Ser126 (ab55184) (Abcam, Cambridge, UK); rabbit Cdc25C phospho-Ser216 (63F9), Wee1 (4936), Chk1 phospho-Ser345 (133D3), and mouse Chk1 (2G1D5) (Cell Signaling Technology, Beverly, MA); mouse Chk1 (DCS310), α-tubulin (DM1a), γ-tubulin (GTU-88), and vimentin (V9) (Sigma); mouse Crm-1/exportin-1 (clone 44) and Cdk1 (clone 1), (BD Transduction Laboratories); and mouse Myc (4A6, 05-724; Millipore, Bedford, MA).
Small Interfering RNA Transfection
Five 21-nucleotide double-stranded RNAs were purchased from Qiagen (Valencia, CA): Chk1 target sequence 1 (Chk1 S1), (AA)CTGAAGAAGCAGTCGCAGT; Chk1 target sequence 2 (Chk1 S2), (AA)CCAGATGCTCAGAGATTCT; Crm-1 target sequence 1 (Crm-1 S1), (TA)CATGTTACTCCCTAATCAA; Crm-1 target sequence 2 (Crm-1 S2), (TT)CTCAGAATATGAATACGAA; and non- silencing sequence (control), (AA)TTCTCCGAACGTGTCACGT. Transfection was performed with a mixture of each siRNA3 (final concentration, 10 nm) and LipofectamineTM RNAiMAX reagent (Invitrogen) according to the manufacturer's protocol.
Immunocytochemistry
Cells growing on coverslips were fixed with 3.7% formaldehyde in phosphate-buffered saline at room temperature for 10 min and treated with −20 °C methanol for 10 min. Cells were incubated with primary antibodies for 1 h and then with appropriate Alexa Fluor-conjugated secondary antibodies (Invitrogen) for 30 min at room temperature. DNA was also stained with 0.5 μg/ml DAPI for 5 min. Each fluorescence image was captured as a single optical section using a Zeiss LSM 510 confocal laser-scanning microscope. We calculated nuclear or cytoplasmic proportions of antibody signals in each digital image using the public domain ImageJ program (Version 1.38x for Macintosh OS X, developed at the National Institutes of Health and available at rsb.info.nih.gov/ij/).
Evaluation of Cell Cycle Progression Including Mitotic Entry
We established HeLa cells in which each type of Myc-tagged Chk1 was expressed in a tetracycline/doxycycline-dependent manner as described previously (13). Before Myc-Chk1 induction, each HeLa Tet-On cell line was synchronized at the G1/S boundary by the method of double thymidine block: we used 2 mm thymidine for each block. At the release of the second thymidine block, we added 1 μg/ml doxycycline to the growth medium. In most experiments (except for those in Fig. 3, A and B), we simultaneously added 50 ng/ml nocodazole to prevent passage through mitosis (see supplemental Fig. S2D). For the calculation of mitotic indices, cells were fixed at each time point and then stained with anti-Myc (to detect exogenous Myc-Chk1 expression) and anti-histone H3 phospho-Ser28 (as a mitotic marker) antibodies (12). We judged mitotic cells not only by morphological features of nuclei or chromosomes (DAPI staining patterns) but also by the detection of mitotic histone H3 Ser28 phosphorylation in the chromosomes (12).
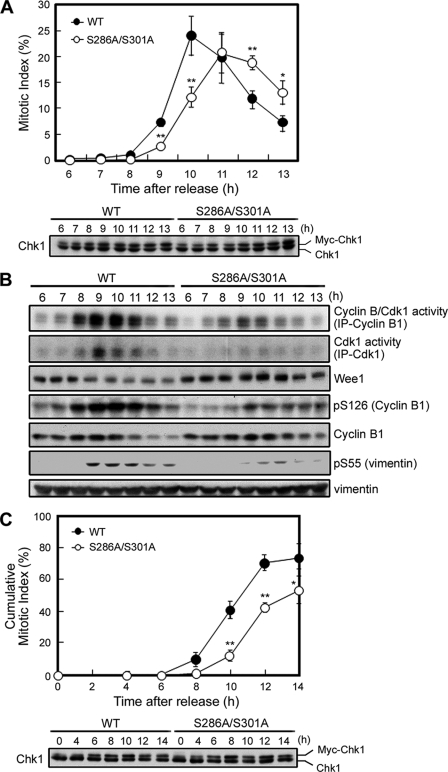
FIGURE 3.
Phosphorylation at Ser286 and Ser301 amplifies the Cdk1 activation loop and then promotes mitotic entry. After the release of the second thymidine block, experiments were performed in the presence (C) or absence (A and B) of nocodazole as described under “Experimental Procedures” (see supplemental Fig. S2D). Graph data of the (cumulative) mitotic index at each time point indicate the mean ± S.E. for 200 cells from three independent experiments (A and C). *, 0.01 < p < 0.05; **, p < 0.01 (A and C). To evaluate the expression level of each exogenous Myc-Chk1 protein, samples were immunoblotted with anti-Chk1 antibody (A and C). Cyclin B1-Cdk1 complexes were purified from cell extracts as anti-cyclin B1 or anti-Cdk1 immunocomplexes, and in vitro kinase assays of activity toward histone H1 were performed. Activity was visualized by autoradiography of H1 (B, first and second rows). To further evaluate Cdk1 kinase activity, each sample was also subjected to immunoblotting with the indicated antibodies (B, lower rows). IP, immunoprecipitation.
Immunoprecipitation and Immunoblotting
We performed the immunoprecipitation as described previously (13). In some immunoblot experiments, we used immunoreaction enhancer solutions (Can Get Signal®, Toyobo, Osaka, Japan) for dilution of primary and secondary antibodies.
RESULTS AND DISCUSSION
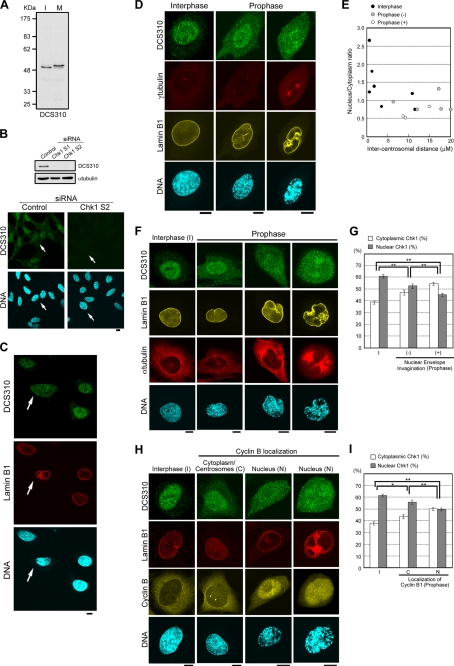
We first examined the change in Chk1 localization by using three independent mouse anti-Chk1 monoclonal antibodies (DCS310 (7), G4, and 2G1D5). Immunoblot analysis revealed that each anti-Chk1 antibody immunoreacts specifically with a band corresponding to the position of Chk1 in HeLa cell lysates; all antibodies detected a mitotic mobility shift due to Chk1 phosphorylation (Fig. 1A and supplemental Fig. S1A) (10). RNA interference-mediated Chk1 depletion diminished anti-Chk1 signals not only upon immunoblotting but also upon immunocytochemistry (Fig. 1B and supplemental Fig. S1B). Using these antibodies, we found the changes in Chk1 localization at the G2/M transition. In interphase cells, anti-Chk1 signals were observed mainly in the nucleus (Fig. 1C and supplemental Fig. S1C). However, Chk1 was likely to move from the nucleus to the cytoplasm at prophase, the first mitotic phase morphologically characterized by chromosome condensation without NE breakdown (Fig. 1C and supplemental Fig. S1C).
FIGURE 1.
Chk1 is translocated from the nucleus to the cytoplasm in prophase cells. A, interphase (I) and mitotic (M) HeLa cell lysates were subjected to immunoblotting with mouse anti-Chk1 monoclonal antibody (DCS310). B, 48 h after transfection of a siRNA, HeLa cells were subjected to immunoblotting or immunocytochemistry. Arrows indicate prophase cells. Similar diminishment of DCS310 signals upon immunocytochemistry was observed in cells transfected with Chk1 S1 siRNA (data not shown). C–I, shown are confocal microscopic images of HeLa cells stained with DCS310 (Alexa 488-conjugated anti-mouse IgG2b, green). Nuclear membranes (C and H, red; D and F, yellow), mitotic spindles (F, red), cyclin B1 (H, yellow), centrosomes (D, red), and DNA (blue) were simultaneously stained with anti-lamin B1 (Alexa 546- or Alexa 680-conjugated anti-rabbit), anti-α-tubulin (Alexa 680-conjugated anti-mouse IgG1), anti-cyclin B1 (Alexa 546-conjugated anti-mouse IgG1), anti-γ-tubulin (Alexa 680-conjugated anti-mouse IgG1) antibodies and DAPI, respectively. The arrows (C) and arrowheads (H) indicate a prophase cell (C) and centrosomal signals of anti-cyclin B1 antibody (H), respectively. Scale bars = 10 μm. Profiles for intercentrosomal distance (μm) and nucleus/cytoplasm ratios of DCS310 signals in each cell are shown in E. The bar graphs show cytoplasmic or nuclear proportions of DCS310 signals in each cell group (G and I). Prophase cells were divided into two subgroups based on the presence (+)/absence (−) of nuclear membrane invagination (E and G) or the subcellular localization of cyclin B1 (I). Interphase cells are indicated as I (G and I). Each proportion was calculated as described under “Experimental Procedures.” Data represent means ± S.E. for at least 20 cells in each cell group (G and I). *, 0.01 < p < 0.05; **, p < 0.01 (G and I).
In prophase, several changes are known to occur together with chromosome condensation. Centrosomes move apart along the NE at the G2/M transition (Fig. 1D), and microtubules are nucleated at centrosomes and attached to the outer face of the NE (Fig. 1F). These morphological changes exert pulling forces that cause NE invaginations around centrosomes (Fig. 1, D and F) (15). Cyclin B localizes in the cytoplasm in interphase, accumulates at centrosomes at the G2/M transition, and then moves into the nucleus in later prophase (Fig. 1H) (16). As judged by the degree of chromosome condensation (Fig. 1, D, F, and H; and supplemental Fig. S1, D and E), NE invagination (Fig. 1, D and F–H; and supplemental Fig. S1, D and E), centrosome separation (Fig. 1, D–F), microtubule nucleation (Fig. 1, D and F), and the change in cyclin B localization (Fig. 1, H and I), Chk1 translocation from the nucleus to the cytoplasm appeared to correlate with prophase progression.
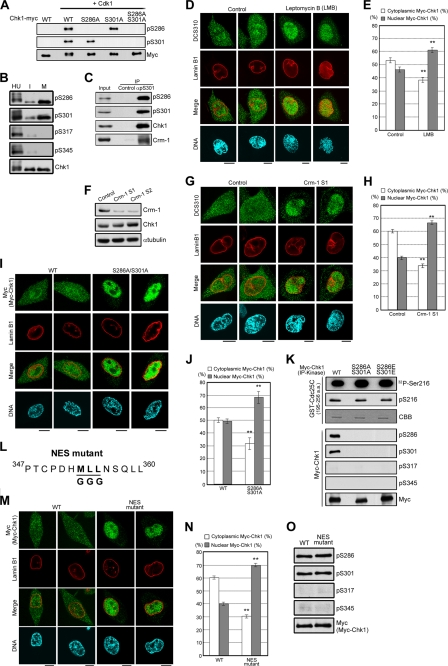
We produced each site- and phosphorylation state-specific antibody (14) for Ser286 and Ser301 (referred to as pS286 and pS301, respectively) (13). Immunoblot analysis confirmed that pS286 and pS301 recognize Chk1 in a Cdk1 phosphorylation-dependent manner (Fig. 2A). Chk1 mutation at Ser286 or Ser301 to Ala diminished the immunoreactivity of pS286 or pS301 (Fig. 2A), respectively. Using these antibodies, we demonstrated that Chk1 Ser286 and Chk1 Ser301 were highly phosphorylated in mitosis compared with interphase (Fig. 2B) (13). However, Chk1 phosphorylation at Ser317 and Ser345 was hardly detected in mitosis (Fig. 2B), consistent with our previous observation that mitotic Chk1 phosphorylation occurs predominantly at Ser286 and Ser301 (10).
FIGURE 2.
Chk1 transport to the cytoplasm is regulated not only by Crm-1-dependent nuclear export but also by Cdk1-induced Chk1 phosphorylation. A, each glutathione S-transferase-Chk1-Myc construct incubated with or without cyclin B1-Cdk1 (10, 13) was subjected to the immunoblotting. B and C, interphase (I), mitotic (M), or hydroxyurea (HU)-treated HeLa cells were prepared as described (10, 13). Immunoprecipitation (IP) of Chk1 from cell extracts (B) or of Ser301-phosphorylated Chk1 from mitotic cell extracts (C) was performed as described (13). d--J, M, and N, HeLa cells were treated with 2 ng/ml leptomycin B or an equal volume of 70% ethanol (control) for 1 h (D and E). HeLa cells were transfected with the indicated siRNAs for 48 h (F–H); there was only a marginal difference in Chk1 localization between Crm-1 S1 and S2 (data not shown). Tet-On HeLa cell lines were treated with doxycycline to introduce exogenous Myc-tagged WT Chk1, S286A/S301A, or the NES mutant (I, J, M, and N). Cells were stained with DCS310 (D and G) or anti-Myc antibody (I and M, green). Nuclear membranes and DNA were simultaneously stained with anti-lamin B1 antibody (red) and DAPI (blue), respectively (D, G, I, and M). Scale bars = 10 μm. The bar graphs show the cytoplasmic or nuclear proportion of DCS310 (E and H) or anti-Myc (J and N) signals. Data represent means ± S.E. for at least 20 cells in cell group. **, p < 0.01 compared with control cells (E and H) or WT-expressing cells (J and N). K, the kinase activity of each Myc-Chk1 construct in mitosis was measured. Myc-Chk1 was purified from each mitotic cell extract as an anti-Myc immunocomplex. The phosphorylation reaction was performed as described previously (5). For the detection of Chk1 kinase activity, each sample was subjected to autoradiography (32P-Ser216, first row) or immunoblotting with anti-phospho-Ser216 antibody in Cdc25C (pS216, second row). As a loading control for each substrate, Coomassie Brilliant Blue staining was performed. For assessment of the Chk1 phosphorylation status in anti-Myc immunoprecipitates, immunoblots were made with anti-phospho-Chk1 and anti-Myc antibodies (lower rows). a.a., amino acids. L, the schema shows the Chk1 mutant (NES mutant) in which the underlined amino acids were changed to Gly. O, each immunoprecipitated Myc-Chk1 construct was subjected to immunoblotting.
Using pS301, we performed the immunoprecipitation of Chk1 phosphorylated at Ser301 from mitotic cell extracts. As shown in Fig. 2C, we detected Crm-1 in the precipitate of pS301 but not of control rat IgG. Crm-1 is known to bind to NES sequences on target proteins and ensure their transport to the cytoplasm (17). Therefore, we synchronized cells at the G2/M transition and treated cells with leptomycin B, a potent inhibitor of Crm-1-mediated nuclear export (18). This treatment induced nuclear retention of Chk1 in prophase (Fig. 2, D and E). Similar results were obtained when cells were treated with Crm-1-specific siRNAs (Fig. 2, F–H). These observation suggest that nuclear exclusion of Chk1 at the G2/M transition is regulated by Crm-1-dependent nuclear export.
We examined whether Chk1 translocation is regulated by Chk1 phosphorylation at Ser286 and Ser301 by Cdk1 using Myc-tagged Chk1 mutated to Ala (non-phosphorylatable mutant) or to Asp or Glu (phosphomimetic mutants). However, transient transfection induced Chk1 Ser345 phosphorylation even in the absence of genotoxic stimuli (supplemental Fig. S2A). Thus, we established HeLa cells in which Myc-Chk1 was expressed in a tetracycline-dependent manner (supplemental Fig. S2, B and C). Induced expression did not elevate the phosphorylation level of Chk1 Ser345 (supplemental Fig. S2A). Because Chk1 Ser345 phosphorylation was reported to change the subcellular localization of Chk1 (19, 20) and was hardly observed in mitosis (Fig. 2B), we chose the inducible expression system.
Chk1 mutated at Ser286 and Ser301 to Ala (S286A/S301A) was localized predominantly in the nuclei of prophase cells (Fig. 2, I and J). Such nuclear accumulation was hardly observed in prophase cells when Myc-tagged WT Chk1 was introduced (Fig. 2, I and J). Only marginal differences were observed in the catalytic activity of Myc-Chk1 mutants (Fig. 2K), suggesting that Chk1 phosphorylation at Ser286 and Ser301 is required for Chk1 translocation from the end of G2 to prophase.
How does Ser286 and Ser301 phosphorylation control Chk1 transport from the nucleus to the cytoplasm in prophase? Because Ser286 and Ser301 are separate from a putative nuclear localization signal of Chk1 (20), this phosphorylation may not inhibit nuclear import of Chk1 directly. Because Chk1 export in prophase is regulated by Crm-1 (Fig. 2, D–H), we considered the possible existence of a NES sequence in Chk1. Because a putative NES sequence was reported to be located around Met353 in Chk1 (20), we produced a Chk1 mutant (NES mutant) in which three key (hydrophobic) amino acids (Met353, Leu354, and Leu355) were changed to Gly (Fig. 2L and supplemental Fig. S2C). This NES mutation abolished Chk1 transport from the nucleus to the cytoplasm in prophase (Fig. 2, M and N), although Ser286 and Ser301 phosphorylation was observed in this mutant in mitosis (Fig. 2O). Thus, Ser286 and Ser301 phosphorylation is likely to promote the accessibility of Crm-1 to a known NES sequence in Chk1 rather than to create a new NES sequence.
To analyze the effect of mitotic Chk1 phosphorylation on the timing of mitotic entry, we performed the following experiments (supplemental Fig. S2D). Before Myc-Chk1 induction, each HeLa Tet-On cell line was synchronized at the G1/S boundary by the method of double thymidine block. At the release of the second thymidine block, we added doxycycline to the growth medium for Myc-Chk1 induction. Compared with WT Chk1, the S286A/S301A mutant was delayed in mitotic entry (Fig. 3A). S286A/S301A expression also weakened the in vitro kinase activity of the cyclin B-Cdk1 immunocomplex toward histone H1 (Fig. 3B, first and second rows). In S286A/S301A-expressing cells, Wee1 degradation (21), which participates in the process of Cdk1 activation, was also abrogated (Fig. 3B). In support of the reduction of Cdk1 activity, Cdk1 phosphorylated cyclin B1 Ser126 (16) and vimentin Ser55 (11) to a lesser degree in S286A/S301A-expressing cells (Fig. 3B). Similar results were obtained when we added the microtubule-depolymerizing reagent nocodazole (together with doxycycline) to evaluate cumulative mitotic proportions at each time point (Fig. 3C and supplemental Fig. S2E). Fluorescence-activated cell sorter analysis revealed that there were limited differences in S phase progression between the two cell lines (data not shown). Without the induction of Myc-Chk1, there was no significant difference in mitotic entry between the two cell lines (data not shown; supplemental Fig. S2F). These results suggest that S286A/S301A expression inhibits the adequate activation of Cdk1 in the nucleus and thus induces a delay in mitotic entry.
Expression of the phosphomimetic mutant S286E/S301E appeared to induce the acceleration of mitotic entry compared with WT expression (Fig. 4A). However, there was only a marginal change in mitotic entry between Tet-On cells without protein induction and S286E/S301E-expressing cells (supplemental Fig. S2F). Because S286E/S301E was localized predominantly in the cytoplasm in prophase cells (Fig. 4, B and C), S286E/S301E expression may have only a marginal effect on mitotic entry. These observations also support the hypothesis that Chk1 phosphorylation by Cdk1 is required for Chk1 export to the cytoplasm and the timing of mitotic entry.
FIGURE 4.
Appropriate mitotic entry is regulated by the elimination of Chk1 kinase from the nucleus. Experiments were performed as described for Figs. 3C (A and D), 2I (B and E), and 2J (C and F). Scale bars = 10 μm (B and E). G, model of the feedback loop of Cdk1 through down-regulation of Chk1 at the G2/M transition.
To examine the importance of Chk1 catalytic activity in the unperturbed cell cycle, we analyzed a kinase-dead plus double-Ala mutant (K38M/S286A/S301A). Like S286A/S301A (Fig. 2, I and J), K38M/S286A/S301A was localized predominantly in the nucleus in prophase cells (Fig. 4, E and F), but expression had no significant effect on mitotic entry (Fig. 4D). Together with the observation that treatment with the potent Chk1 inhibitor UCN-01 (22) accelerated mitotic entry in HeLa cells (supplemental Fig. S2G), these results suggest that Chk1 negatively regulates mitotic entry through its catalytic activity.
These observations led us to propose the following model of a feedback loop between Cdk1 and Chk1 at the G2/M transition (Fig. 4G). Before mitosis (at S or G2 phase), Chk1 has a basal activity in unperturbed cells (7–9). Because Chk1 is localized predominantly in the nuclei of interphase cells (Fig. 1C and supplemental Fig. S1C), it would be expected to inhibit Cdc25 phosphatase activity in the nucleus and thus block premature Cdk1 activation in the nuclei of interphase cells. Once the initial activation of Cdk1 occurs (likely in late G2 phase), Cdk1 starts to phosphorylate Chk1 at Ser286 and Ser301. This phosphorylation induces Chk1 translocation from the nucleus to the cytoplasm. The elimination of Chk1 kinase activity from the nucleus triggers a positive feedback loop of Cdk1 activation in the nucleus, which promotes mitosis. Because Chk1 export from the nucleus to the cytoplasm occurred in concert with centrosome separation before chromosome condensation (Fig. 1, D and E), the above feedback loop between Chk1 and Cdk1 works from the end of G2 and thus affects the timing of mitotic entry.
Cyclin B-Cdk1 activation was considered to initiate at centrosomes and then occur in the nucleus (16): the active cyclin B-Cdk1 complex may be transported from centrosomes to the nucleus. In this study, we demonstrated that Chk1 moves from the nucleus to the cytoplasm through mitotic phosphorylation at Ser286 and Ser301. Other groups reported that Chk1, which localizes at centrosomes in interphase cells, dissociates at centrosomes in prophase (7, 23). Thus, such movements of cyclin B-Cdk1 and Chk1 may enable the ordered activation of cyclin B-Cdk1, which is likely to play critical roles in proper mitotic progression. Interestingly, after the nucleation of microtubules at centrosomes, microtubules are attached to the outer face of the NE (15). Because many molecules are transported through the microtubule-based transport system, the dynamics of mitotic microtubules may be correlated with the accumulation of cyclin B-Cdk1 and the exclusion of Chk1 at proper cyclin B-Cdk1 activation site(s). These issues will be addressed in the future.
Here, we have described a novel regulatory pathway between Cdk1 and Chk1 for maintaining the proper order at the G2/M transition. Our study should facilitate a better understanding of the relationship between the progression (acceleration) and arrest (braking) of the cell cycle.
Supplementary Material
Acknowledgments
We thank W. C. Earnshaw (University of Edinburgh) for helpful discussion; T. Urano (Shimane University) for providing an anti-INCENP antibody; M. Matsuyama for preparation of Chk1 constructs; Y. Ikegami (Nagoya City University), Y. Hayashi, K. Kobori, and C. Yuhara for technical assistance; Y. Takada for secretary service; and M. Moore for critical comments on the manuscript.
This work was supported in part by grants-in-aid for scientific research from the Japan Society for the Promotion of Science and from the Ministry of Education, Science, Technology, Sports, and Culture of Japan, a grant-in-aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan, the Uehara Memorial Foundation, the Naito Foundation, the Takeda Science Foundation, and a research grant from the Princess Takamatsu Cancer Research Fund.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- siRNA
- small interfering RNA
- DAPI
- 4′,6-diamidino-2-phenylindole
- NE
- nuclear envelope
- NES
- nuclear export signal
- WT
- wild-type.
REFERENCES
- 1.Hartwell L. H., Weinert T. A. (1989) Science 246, 629–634 [DOI] [PubMed] [Google Scholar]
- 2.Elledge S. J. (1996) Science 274, 1664–1672 [DOI] [PubMed] [Google Scholar]
- 3.Bartek J., Lukas J. (2003) Cancer Cell 3, 421–429 [DOI] [PubMed] [Google Scholar]
- 4.Rhind N., Russell P. (2000) J. Cell Sci. 113, 3889–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H., Piwnica-Worms H. (2001) Mol. Cell. Biol. 21, 4129–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B. B., Elledge S. J. (2000) Nature 408, 433–439 [DOI] [PubMed] [Google Scholar]
- 7.Krämer A., Mailand N., Lukas C., Syljuåsen R. G., Wilkinson C. J., Nigg E. A., Bartek J., Lukas J. (2004) Nat. Cell Biol. 6, 884–891 [DOI] [PubMed] [Google Scholar]
- 8.Shimada M., Niida H., Zineldeen D. H., Tagami H., Tanaka M., Saito H., Nakanishi M. (2008) Cell 132, 221–232 [DOI] [PubMed] [Google Scholar]
- 9.Sørensen C. S., Syljuåsen R. G., Falck J., Schroeder T., Rönnstrand L., Khanna K. K., Zhou B. B., Bartek J., Lukas J. (2003) Cancer Cell 3, 247–258 [DOI] [PubMed] [Google Scholar]
- 10.Shiromizu T., Goto H., Tomono Y., Bartek J., Totsukawa G., Inoko A., Nakanishi M., Matsumura F., Inagaki M. (2006) Genes Cells 11, 477–485 [DOI] [PubMed] [Google Scholar]
- 11.Tsujimura K., Ogawara M., Takeuchi Y., Imajoh-Ohmi S., Ha M. H., Inagaki M. (1994) J. Biol. Chem. 269, 31097–31106 [PubMed] [Google Scholar]
- 12.Goto H., Tomono Y., Ajiro K., Kosako H., Fujita M., Sakurai M., Okawa K., Iwamatsu A., Okigaki T., Takahashi T., Inagaki M. (1999) J. Biol. Chem. 274, 25543–25549 [DOI] [PubMed] [Google Scholar]
- 13.Ikegami Y., Goto H., Kiyono T., Enomoto M., Kasahara K., Tomono Y., Tozawa K., Morita A., Kohri K., Inagaki M. (2008) Biochem. Biophys. Res. Commun. 377, 1227–1231 [DOI] [PubMed] [Google Scholar]
- 14.Goto H., Inagaki M. (2007) Nat. Protoc. 2, 2574–2581 [DOI] [PubMed] [Google Scholar]
- 15.Güttinger S., Laurell E., Kutay U. (2009) Nat. Rev. Mol. Cell Biol. 10, 178–191 [DOI] [PubMed] [Google Scholar]
- 16.Jackman M., Lindon C., Nigg E. A., Pines J. (2003) Nat. Cell Biol. 5, 143–148 [DOI] [PubMed] [Google Scholar]
- 17.Mosammaparast N., Pemberton L. F. (2004) Trends Cell Biol. 14, 547–556 [DOI] [PubMed] [Google Scholar]
- 18.Kudo N., Wolff B., Sekimoto T., Schreiner E. P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. (1998) Exp. Cell Res. 242, 540–547 [DOI] [PubMed] [Google Scholar]
- 19.Niida H., Katsuno Y., Banerjee B., Hande M. P., Nakanishi M. (2007) Mol. Cell. Biol. 27, 2572–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang K., Pereira E., Maxfield M., Russell B., Goudelock D. M., Sanchez Y. (2003) J. Biol. Chem. 278, 25207–25217 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe N., Arai H., Nishihara Y., Taniguchi M., Watanabe N., Hunter T., Osada H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4419–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graves P. R., Yu L., Schwarz J. K., Gales J., Sausville E. A., O'Connor P. M., Piwnica-Worms H. (2000) J. Biol. Chem. 275, 5600–5605 [DOI] [PubMed] [Google Scholar]
- 23.Tibelius A., Marhold J., Zentgraf H., Heilig C. E., Neitzel H., Ducommun B., Rauch A., Ho A. D., Bartek J., Krämer A. (2009) J. Cell Biol. 185, 1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.