Abstract
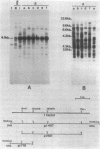
The I factor is a transposable element controlling inducer-reactive (IR) hybrid dysgenesis in Drosophila melanogaster, which occurs when males from the class of inducer strains are crossed with females from the class of reactive strains. Inducer strains contain several copies of the complete 5.4-kilobase (kb) I factor at various sites on the chromosomal arms; reactive strains contain no complete I factor. Incomplete and defective I elements occur at constant locations in pericentromeric heterochromatin of both types of strains. The 5.4-kb I factors transpose, whereas incomplete I elements do not transpose. The constant location of defective I elements in all strains indicates that they were in the genome before the spread of D. melanogaster throughout the world. Sequences homologous to I occur in other Drosophila species, and their distribution correlates with the phylogenetic relationships between species. We have studied the organization of I homologues in Drosophila simulans and Drosophila teissieri. These species seem to contain both transposable I elements, even though their structure may differ from that of the 5.4-kb I factors of the inducer strains of D. melanogaster, and nontransposable I elements, which are always at the same place in the genome when different stocks of the same species are compared. These results suggest that both mobile and nonmobile I elements are very old components of the Drosophilidae genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brookfield J. F., Montgomery E., Langley C. H. Apparent absence of transposable elements related to the P elements of D. melanogaster in other species of Drosophila. 1984 Jul 26-Aug 1Nature. 310(5975):330–332. doi: 10.1038/310330a0. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Lavige J. M., Picard G., L'Heritier P. Non-mendelian female sterility in Drosophila melanogaster: quantitative variations in the efficiency of inducer and reactive strains. Heredity (Edinb) 1976 Jun;36(3):305–314. doi: 10.1038/hdy.1976.38. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Casari G. Related polypeptides are encoded by Drosophila F elements, I factors, and mammalian L1 sequences. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5843–5847. doi: 10.1073/pnas.84.16.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. H., Lister C. K., Kellett E., Finnegan D. J. Transposable elements controlling I-R hybrid dysgenesis in D. melanogaster are similar to mammalian LINEs. Cell. 1986 Dec 26;47(6):1007–1015. doi: 10.1016/0092-8674(86)90815-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Hattori M., Kuhara S., Takenaka O., Sakaki Y. L1 family of repetitive DNA sequences in primates may be derived from a sequence encoding a reverse transcriptase-related protein. Nature. 1986 Jun 5;321(6070):625–628. doi: 10.1038/321625a0. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G. Evolution of hybrid dysgenesis determinants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1655–1659. doi: 10.1073/pnas.80.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., Wiernasz D., Schedl P. Evolution of Drosophila repetitive-dispersed DNA. J Mol Evol. 1983;19(3-4):203–213. doi: 10.1007/BF02099967. [DOI] [PubMed] [Google Scholar]
- Picard G., Bregliano J. C., Bucheton A., Lavige J. M., Pelisson A., Kidwell M. G. Non-mendelian female sterility and hybrid dysgenesis in Drosophila melanogaster. Genet Res. 1978 Nov;32(3):275–287. doi: 10.1017/s0016672300018772. [DOI] [PubMed] [Google Scholar]
- Picard G. Non-mendelian female sterility in Drosophila melanogaster: hereditary transmission of I factor. Genetics. 1976 May;83(1):107–123. doi: 10.1093/genetics/83.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Telford J., Baldari C., Pirrotta V. Isolation of cloned genes differentially expressed at early and late stages of Drosophila embryonic development. Dev Biol. 1981 Sep;86(2):438–447. doi: 10.1016/0012-1606(81)90202-5. [DOI] [PubMed] [Google Scholar]
- Singer M. F. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982 Mar;28(3):433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stacey S. N., Lansman R. A., Brock H. W., Grigliatti T. A. Distribution and conservation of mobile elements in the genus Drosophila. Mol Biol Evol. 1986 Nov;3(6):522–534. doi: 10.1093/oxfordjournals.molbev.a040413. [DOI] [PubMed] [Google Scholar]
- Will B. M., Bayev A. A., Finnegan D. J. Nucleotide sequence of terminal repeats of 412 transposable elements of Drosophila melanogaster. A similarity to proviral long terminal repeats and its implications for the mechanism of transposition. J Mol Biol. 1981 Dec 25;153(4):897–915. doi: 10.1016/0022-2836(81)90458-7. [DOI] [PubMed] [Google Scholar]