Abstract
The aim of our study was to examine in detail the impact of NF-E2-related factor (Nrf2) activation on endothelial cell function with focus on redox homeostasis and the endothelial nitric oxide synthase (eNOS) system. We administered 2-cyano-3,12-dioxooleana-1,9-dien-28-oic imidazolide (CDDO-IM), a known activator of Nrf2, to primary human umbilical vein endothelial cells. Activation of Nrf2 by CDDO-IM increased the amount of bioavailable nitric oxide (NO), a major contributor to vascular homeostasis, in naive and stressed cells. Concomitantly, intracellular reactive oxygen species were dose-and time-dependently reduced. In apparent contrast to elevated NO levels, eNOS protein expression was transiently decreased in an Nrf2-dependent manner. Employing pharmacological inhibitors as well as a small interfering RNA approach, we identified de novo protein synthesis of heme oxygenase 1 (HO-1) and its enzymatic activity as cause for the observed reduction of eNOS. We hypothesize that under redox stress, when the availability of tetrahydrobiopterin, a pivotal stoichiometric cofactor for eNOS, is limited, activation of Nrf2 leads (a) to transient reduction of eNOS protein levels and (b) to an antioxidant defense in human umbilical vein endothelial cells. Both activities ensure that a stoichiometric ratio of eNOS and tetrahydrobiopterin is sustained and that the risk of eNOS uncoupling is reduced. Our study is the first to provide a causal link between Nrf2 activation and eNOS expression and NO levels, respectively.
Introduction
NF-E2-related factor (Nrf2)2 is a redox-sensitive transcription factor that normally resides in the cytoplasm bound to Kelch-like ECH-associated protein (Keap)-1. Upon exposure to pro-oxidative or electrophilic stimuli, cysteine residues of Keap are oxidized or covalently modified, and Nrf2 is released to the nucleus. By binding to antioxidant-response element consensus sequences, Nrf2 initiates transcription of antioxidant and phase 2 defense enzymes including γ-glutamyl-cysteine ligase, quinone reductase, and heme oxygenase 1 (HO-1) (1, 2). Moreover, an anti-inflammatory activity was also reported for active Nrf2 (3–5).
In the context of vascular health, the antioxidant and anti-inflammatory features of Nrf2 may be of benefit because the onset of endothelial dysfunction is initiated and/or accompanied by proinflammatory (6, 7) and pro-oxidative conditions (8–10). Interestingly, vessel regions that are exposed to laminar flow are commonly accepted to be atheroprotected (in contrast to atheroprone oscillatory flow regions) and are characterized by activated Nrf2 (11–13). Moreover, regions of laminar flow show elevated levels of bioavailable nitric oxide (NO), the main mediator of vascular homeostasis and product of the enzymatic activity of endothelial NO synthase (14, 15). The enzymatic activity of eNOS is tightly regulated by a plethora of mechanisms ranging from changes in expression and phosphorylation to availability of cofactors such as tetrahydrobiopterin (BH4). Stoichiometric amounts of BH4 are pivotal to keep eNOS in the coupled state, i.e. the electron flow through the enzyme is coupled to NO production. If there is a lack of BH4 (e.g. by oxidation to 7,8-dihydrobiopterin (BH2) under redox stress), the electron flow through the enzyme is uncoupled from NO production. In this case, superoxide is formed, which further aggravates the redox imbalance (16).
There are currently no reports on the immediate impact of Nrf2 activation on the endothelial NO system. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid imidazolide (CDDO-IM, see Fig. 1A), a synthetic triterpenoid derived from oleanolic acid, has been used as a potent activator of Nrf2 in various models mainly related to cancer and inflammation (17, 18). From a mechanistic point of view, CDDO-IM was reported to covalently modify cysteine residues of Keap-1 and hereby lead to stabilization and nuclear translocation of Nrf2 (19). In this study, we employed CDDO-IM as a tool to selectively and strongly activate Nrf2 in primary endothelial cells and to examine subsequent changes in reactive oxygen species (ROS) and BH4 levels, bioavailability of NO, and eNOS expression.
FIGURE 1.
Activation of Nrf2 by CDDO-IM elevates bioavailability of NO. A, chemical structure of CDDO-IM. B, confluent HUVEC were treated with DMSO or 100 nmol/liter CDDO-IM for 24 h, and then a DAF2 assay was performed as described under “Experimental Procedures.” The depicted graph compiles data of three independent experiments (* p < 0.05; n = 3; ANOVA, Dunnett's post test versus DMSO control ;in all panels, error bars indicate S.E.). 2 nmol/liter phorbol-12-myristoyl-13-acetate (PMA) were used as a positive control, and N-nitro-l-arginine-methyl ester (l-NAME, 200 μmol/liter) was used as eNOS inhibitor to assess NO-independent DAF2 fluorescence. C, HUVEC were treated with DMSO or 100 nmol/liter CDDO-IM for 24 h and with N-nitro-l-arginine (l-NNA, irreversible NOS inhibitor) as indicated, loaded with DAF2-DA, and then subjected to a flow cytometric analysis of intracellular NO levels as described under “Experimental Procedures” (* p < 0.05; n = 5; Student's t test). D, cells were transfected with scrambled siRNA (scr) and siRNA targeted against Nrf2 (siNrf2), respectively. After 48 h, a 24-h treatment with CDDO-IM (100 nmol/liter) (+) or DMSO (−) followed, and NO levels were determined with DAF2-DA and subsequent flow analysis. A subsequent lysis of the cells and Western blot (IB) analysis (upper panel) confirmed the successful knockdown of Nrf2. Numbers below the lanes hereby depict arbitrary densitometric values of Nrf2 levels The depicted bar graphs represent the compilation of at least three independent experiments (* p < 0.05, n = 3; Student's t test). E, HUVEC were cultivated in normal (5 mmol/liter; plus 20 mmol/liter mannitol as osmotic control) or high glucose (25 mmol/liter, HG) medium for 24 h in the absence (−) or presence (+) of 100 nmol/liter CDDO-IM before NO levels were determined as described in C. (* p < 0.05; n = 3; ANOVA, Bonferroni's post test.)
EXPERIMENTAL PROCEDURES
Chemicals, Reagents, and Antibodies
CDDO-IM was kindly provided by M. Sporn, Dartmouth Medical School, Hanover, NH. Chemicals were obtained from Sigma-Aldrich unless stated otherwise. Primary antibodies were purchased from Cell Signaling (eNOS), Sigma-Aldrich (HO-1), and Santa Cruz Biotechnology (Nrf2, iNOS, nNOS, actin, and tubulin), respectively.
Isolation and Cultivation of Human Umbilical Vein Endothelial Cells (HUVEC)
HUVECs were obtained from Lonza and cultivated in EBM-1 medium (Lonza) with the supplements provided by the manufacturer. Confluent HUVECs not older than passage 3 were used for experiments. Solvent (DMSO) concentration never exceeded 0.1% during the treatment protocols.
Knockdown of Nrf2 and HO-1
HUVEC in 6-well plates were transfected with 200 pmol of specific siRNA (SMARTpool (mixture of four different target-specific sequences), Thermo Scientific) and scrambled control (Invitrogen), respectively, using the OptiMEM/Oligofectamine system (Invitrogen). 16 h after transfection for HO-1 (to block induction of de novo synthesis of HO-1) and 48 h after transfection for Nrf2 (to allow turnover of residual Nrf2 protein and to block de novo synthesis of Nrf2), cells were used for experiments. Successful knockdown of the target proteins was confirmed by Western blot analysis.
Ectopic Expression of HO-1
HUVEC were grown in 6-well plates and transfected with 1 μg of an expression vector for HO-1 (pcDNA-HO-1, kindly provided by M. Soares, Gulbenkian Institute of Science, Oeiras, Portugal) and empty control vector, respectively, using FuGENE HD (Roche Applied Science) as transfection reagent and following the manufacturers' instructions.
Assessment of ROS
Levels of intracellular ROS were determined using dihydrodichlorofluorescein diacetate (Molecular Probes, Invitrogen)-based flow cytometric analysis as described before (20).
Quantification of NO by Diaminofluorescein-Diacetate (DAF2-DA)
Quantification of intracellular levels of NO or NO released into the supernatant by cultivated HUVEC was performed using DAF2-DA and DAF2, respectively (Molecular Probes, Invitrogen) as reported previously (21, 22) and described in detail in the supplemental materials.
[14C]l-Arginine/[14C]l-Citrulline Conversion Assay
NO is produced via NOS-mediated conversion of [14C]l-arginine to [14C]l-citrulline, which renders [14C]l-citrulline a surrogate marker for NO production. The assay was performed essentially as described previously for an immortalized endothelial cell line (23). For further information, please refer to the supplemental materials.
Gel Electrophoresis and Immunoblot Analysis
Preparation of cell extracts, SDS-PAGE, immunoblot analysis, and densitometric evaluations were performed as described previously (20). For detection of multiple proteins with similar molecular weights in one sample, two or more identical membranes were processed in parallel.
Statistical Methods
Statistical analysis was done using GraphPad Prism software version 4.03 (GraphPad Software Inc., La Jolla, CA). One-way ANOVA was used for comparison of different treatment regimens. If two groups were compared, Student's t test was applied. p values <0.05 were considered significant (*). In figures with bar graphs, these show means ± S.E. of at least three independent experiments unless stated otherwise.
RESULTS
Nrf2 Is Activated by CDDO-IM, and Active Nrf2 Elevates the Bioavailability of NO in Primary Human Endothelial Cells
First we aimed to demonstrate Nrf2 activation by CDDO-IM in primary HUVEC, which has not been reported hitherto. As seen in other cell systems, CDDO-IM (100 nmol/liter; Fig. 1A) potently activated Nrf2 in HUVEC, as shown by nuclear translocation of Nrf2 as well as by the massive Nrf2-dependent expression of HO-1 upon CDDO-IM exposure (supplemental Fig. I). CDDO-IM at 100 nmol/liter did not reduce the viability of HUVEC after 48 h as measured by trypan exclusion (data not shown).
We therefore employed CDDO-IM to decipher the impact of active Nrf2 on endothelial NO production. We determined the amount of bioavailable NO upon CDDO-IM (100 nmol/liter) treatment for 24 h employing a modified DAF2 assay (22) that gives a measure of the amount of NO in the supernatant of cultured cells. We observed a moderate but significant elevation of bioavailable NO by 46% when compared with control cells (Fig. 1B). As a positive control, we used phorbol-12-myristoyl-13-acetate (PMA; 2 nmol/liter) commonly accepted to elevate the amount of NO by up-regulation of eNOS protein levels within 24 h. We obtained consistent results when we assessed intracellular NO levels with the cell-permeable diacetate of DAF2 and flow cytometry (Fig. 1C). Co-incubation with an inhibitor of eNOS enzyme activity (N-nitro-l-arginine or N-nitro-l-arginine-methyl ester) markedly reduced the measured fluorescence and ensured NO specificity of the signal. Up-regulation of NO levels by CDDO-IM was dependent on Nrf2 activation because it was clearly sensitive to knockdown of Nrf2 by siRNA (Fig. 1D). In cells that were additionally (redox-) stressed by cultivation in high glucose medium (24), activation of Nrf2 by CDDO-IM exerted an even stronger positive effect on bioavailable NO. Although bioavailable NO in glucose-stressed cells was decreased when compared with control cells, activation of Nrf2 by CDDO-IM not only completely compensated this stress-mediated down-regulation of NO but also led to NO levels higher than in naive control cells (Fig. 1E).
These findings support a causal link between Nrf2 activation and elevated NO levels in unstressed (apart from the potential stress occurring during in vitro cultivation) and stressed endothelial cells. An increased amount of bioavailable NO after Nrf2 activation can be explained by a reduction of ROS levels because bioavailability of NO is massively impaired by the presence of superoxide and the subsequent formation of mainly peroxynitrite and/or an elevated eNOS expression or activity. We therefore set out to investigate ROS and eNOS levels in HUVEC upon activation of Nrf2.
Active Nrf2 Elicits an Antioxidant Response in Primary Endothelial Cells
Activation of Nrf2 by CDDO-IM led, as expected from the antioxidant profile of Nrf2 target genes, to a dose- and time-dependent reduction of ROS in endothelial cells (Fig. 2, A and B). 100 nmol/liter CDDO-IM led to a reduction of ROS (assessed by dichlorofluorescein (DCF) fluorescence) by ∼35% after 24 h, and the employed positive control diphenyliodonium, a flavoprotein inhibitor, led to a 70% reduction of ROS. Knockdown of Nrf2 by 80% almost completely abrogated the observed CDDO-IM-mediated decrease in ROS (Fig. 2C), indicating that Nrf2 is prominently involved. The antioxidant effect of Nrf2 activation was even more pronounced (up to 53% reduction of ROS) in cells that were cultivated under high concentrations of glucose (25 mmol/liter), a known oxidative stressor in endothelial cells (24) (Fig. 2D). Determination of intracellular ROS via oxidized DCF can be prone to artifacts (25–28). We therefore wanted to corroborate our findings concerning the antioxidant activity of CDDO-IM. We additionally assessed intracellular superoxide using dihydroethidium and extracellular H2O2 using Amplex Red. In both assays, CDDO-IM moderately reduced basal ROS and strongly interfered with high glucose-triggered ROS (see supplemental Fig. II). Thus, activation of Nrf2 not only compensated for the glucose-mediated redox stress but even reduced the redox load below the level of untreated naive cells.
FIGURE 2.
Activation of Nrf2 reduces ROS in HUVEC. A, cells were treated with different concentrations of CDDO-IM as indicated for 24 h and with diphenyliodonium (3 μm) as positive control and subjected to flow cytometric analysis of intracellular ROS levels as described under “Experimental Procedures” (* p < 0.05; n = 3, ANOVA, Dunnett's versus control; in all panels, error bars indicate S.E.). B, HUVEC were treated for the indicated periods of time with 100 nmol/liter CDDO-IM, loaded with DCF-DA, and then subjected to flow cytometric analysis of total ROS levels as in A (* p < 0.05, n = 3, ANOVA, Dunnett's post test versus control). C, cells were transfected with scrambled siRNA (scr) and siRNA targeted against Nrf2 (siNrf2), respectively. After 48 h, a 24-h treatment with CDDO-IM (100 nmol/liter) (+) or DMSO (−) followed, and total ROS levels were determined. A subsequent lysis of the cells and Western blot (IB) analysis (upper panel) confirmed the successful knockdown of Nrf2. Numbers below the lanes hereby depict arbitrary densitometric values of Nrf2 levels. The depicted bar graphs represent the compilation of at least three independent experiments (* p < 0.05, n = 3; Student's t test). D, HUVEC were cultivated in normal (5 mmol/liter; plus 20 mmol/liter mannitol as osmotic control) or high glucose (25 mmol/liter, HG) medium for 24 h in the absence (−) or presence (+) of 100 nmol/liter CDDO-IM before total ROS levels were determined (* p < 0.05; n = 3; ANOVA, Bonferroni's post test).
Activation of Nrf2 Reduces eNOS Protein Expression in HUVEC
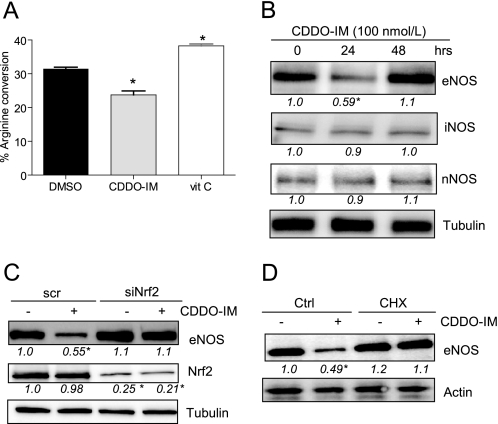
Apart from reduced ROS levels, a rise in eNOS activity and/or protein level could additionally account for the observed increase of NO bioavailability upon Nrf2 activation. Therefore, we investigated the impact of Nrf2 activation on the enzymatic activity and expression of eNOS. Administering radiolabeled arginine as eNOS substrate to confluent HUVEC, we observed a rather unexpected reduced overall conversion of arginine to citrulline in cells that were treated with 100 nmol/liter CDDO-IM (Fig. 3A). Vitamin C was used as a positive control and clearly increased eNOS enzymatic activity, presumably due to stabilization of necessary eNOS cofactors (29). The decreased utilization of eNOS substrate was explained by a reduction of total eNOS protein (preferentially of the dimeric form; supplemental Fig. III) with unaltered levels of inducible NO synthase, a potential alternative source of NO in the endothelium, or neuronal NO synthase (Fig. 3B). 48 h after CDDO-IM administration, eNOS protein levels returned to basal values (Fig. 3B). To ensure that eNOS reduction is causally linked to Nrf2 activation and not a mere off-target effect of CDDO-IM, we transfected cells with siRNA targeted against Nrf2. Knockdown of Nrf2 by 75–80% clearly overcame the observed reduction of eNOS (Fig. 3C). These data indicate that activation of Nrf2 creates a redox milieu that favors freely available NO even if cellular eNOS protein levels are transiently diminished. When we relate the produced NO (from Fig. 1) to the amount of present eNOS dimer (the catalytically active form of eNOS), the positive influence of Nrf2 activation on NO availability becomes even more apparent; CDDO-IM-treated cells release up to 2.6-fold more NO per eNOS than control cells. CDDO-IM had hereby no effect on the degree of phosphorylation at Ser1177 and Thr495, the main regulatory phosphorylation sites for eNOS activity (30), on expression levels of heat shock protein (hsp) 90 and caveolin-1, which are both known to modulate eNOS activity (31), nor on intracellular localization of eNOS (data not shown). Furthermore, levels of the pivotal cofactor BH4, of total biopterin, or of GTP cyclohydrolase 1 (GCH-1), the rate-limiting enzyme for BH4 biosynthesis (32) (supplemental Fig. IV), were not altered by CDDO-IM. DMSO did not change any mentioned parameter over the time of the experiment.
FIGURE 3.
Activation of Nrf2 and de novo protein synthesis reduce eNOS enzymatic activity and eNOS expression. A, confluent HUVEC were treated with DMSO, 100 nmol/liter CDDO-IM, or 100 μmol/liter vitamin C (vit C; positive control) for 24 h and then subjected to an arginine-citrulline conversion assay as described under “Experimental Procedures.” The bar graph depicts the compilation of three independent experiments (* p < 0.05; n = 3; ANOVA, Dunnett's post test versus DMSO control; error bars indicate S.E.). B, HUVEC were treated with 100 nmol/liter CDDO-IM for the indicated periods of time before total cell lysates were subjected to a Western blot analysis for eNOS, iNOS, nNOS, and tubulin. C, HUVEC were transfected with scrambled siRNA (scr) and siRNA targeted against Nrf2 (siNrf2), respectively. 48 h later, they were exposed to DMSO (−) or 100 nmol/liter CDDO-IM (+) for another 24 h before total cell lysates were subjected to Western blot analysis for eNOS, Nrf2, and tubulin. D, HUVEC were pretreated with cycloheximide (CHX; 5 μg/ml) for 30 min and then exposed to DMSO (−) or 100 nmol/liter CDDO-IM (+) for 24 h. Immunoblot analysis for eNOS and tubulin were performed. Blots depict examples from at least three independent experiments showing consistent results. The numbers below the blots depict mean densitometric arbitrary units (ratio protein/loading control as -fold DMSO control) of all performed experiments (* p < 0.05, ANOVA, Dunnett's post test versus control (Ctrl).
Nrf2-mediated Reduction of eNOS Protein Depends on de Novo Synthesis of HO-1
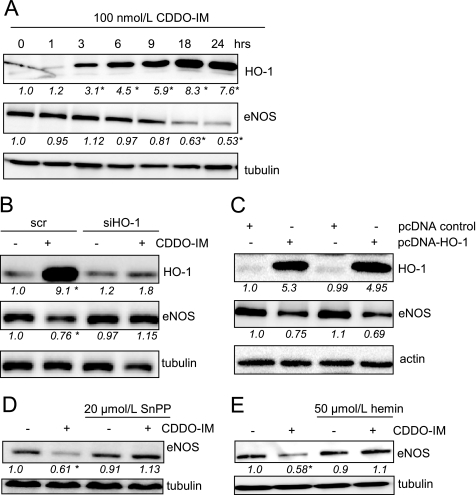
Interestingly, co-incubation with the protein synthesis inhibitor cycloheximide (CHX) overcame the down-regulation of eNOS protein (Fig. 3D), ruling out an immediate transcriptional repression of eNOS promotor activity by Nrf2. Activation of Nrf2 is instead followed by de novo protein synthesis of Nrf2 target genes that elicit down-regulation of eNOS. These findings prompted us to identify the protein(s) accounting for the observed Nrf2-triggered down-regulation of eNOS. HO-1, the enzyme that degrades heme to carbon monoxide (CO), biliverdin, and iron, was seen to be massively up-regulated by CDDO-IM in an Nrf2-dependent manner (supplemental Fig. IB) (17). Up-regulation of HO-1 occurred as early as 3 h (Fig. 4A) and clearly preceded eNOS down-regulation upon CDDO-IM exposure. Transfection with siRNA targeting HO-1 blocked Nrf2-mediated up-regulation of HO-1 by nearly 100% and overcame down-regulation of eNOS by CDDO-IM (Fig. 4B). Thus, induction of HO-1 protein synthesis seems to be involved in the down-regulation of eNOS upon activation of Nrf2. Consistently, ectopic expression of HO-1 led to a reduction of eNOS levels (Fig. 4C). Moreover, co-incubation with 20 μmol/liter tin protoporphyrin IX (SnPP), an inhibitor of heme oxygenase, overcame the effect of CDDO-IM on eNOS protein levels (Fig. 4D), stressing the crucial role of HO-1 enzymatic activity for eNOS down-regulation. Because eNOS is a heme-containing enzyme, one could speculate that increased degradation of heme by elevated HO-1 activity causes heme deficiency and leads to a reduction of eNOS production due to the altered heme pool. To test this hypothesis, we incubated confluent HUVEC with CDDO-IM for 6 h (when no significant effect on eNOS levels could be detected yet) followed by an 18-h supplementation with 50 μmol/liter exogenous heme. Heme was able to prevent CDDO-IM/Nrf2-induced down-regulation of eNOS (Fig. 4E). These findings support the notion that eNOS down-regulation occurs via HO-1 induction and subsequent (local and transient) heme deficiency. The reaction products of heme degradation, CO and biliverdin/bilirubin, have been shown to exert biological activities, such as antioxidative and anti-inflammatory effects (33–36). We therefore examined the impact of biliverdin and CO on eNOS protein levels. Incubation of HUVEC with bilirubin, which is physiologically quickly formed from biliverdin by biliverdin reductase, had no effect on eNOS protein expression (supplemental Fig. VA). Thus, biliverdin/bilirubin can most likely be excluded as the culprit for the observed eNOS reduction. Incubation of cells with a rising concentration of a CO-releasing molecule led to a reduction of eNOS, although only at rather high concentrations (supplemental Fig. VB).
FIGURE 4.
Activation of Nrf2 down-regulates eNOS levels via elevation of HO-1 activity. A, confluent HUVEC were treated with 100 nmol/liter CDDO-IM for the indicated periods of time before total cell lysates were subjected to Western blot analyses for eNOS, HO-1, and tubulin. B, HUVEC were transfected with siRNA targeted against HO-1 (siHO-1) and scrambled siRNA (scr), respectively, and after 16 h, treated with DMSO (−) or CDDO-IM (100 nmol/liter) (+) for another 24 h. Total cell lysates were prepared and subjected to Western blot analysis for HO-1, eNOS, and tubulin. C, HUVEC were transiently transfected with 1 μg of HO-1 expression plasmid (data of two transfections shown). 36 h after transfection, total cell lysates were subjected to Western blot analysis for HO-1, eNOS, and actin. D, HUVEC were pretreated for 30 min with the HO-1 inhibitor tin protoporphyrin IX (SnPP) (20 μmol/liter) before another 24-h incubation with DMSO (−) or CDDO-IM (100 nmol/liter) (+) followed. Total cell lysates were prepared and subjected to Western blot analysis for eNOS and tubulin. E, confluent HUVEC were treated with DMSO (−) or CDDO-IM (100 nmol/liter) (+) for 6 h followed by another 18 h with or without 50 μmol/liter hemin. Representative blots out of at least three independent experiments with consistent results are shown. The numbers below the blots depict mean densitometric arbitrary units (ratio protein/tubulin as -fold DMSO control) of all performed experiments (* p < 0.05, ANOVA, Dunnett's post test versus control).
Nrf2 May Act as a Sensor for BH4 and Maintain eNOS Coupling by Adjustment of eNOS Levels
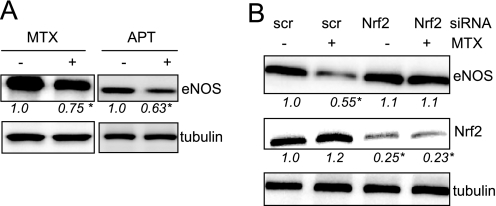
So far we have shown that exogenous strong activation of Nrf2 by CDDO-IM leads to elevated amounts of bioavailable NO in naive and stressed endothelial cells. The observed reduction of ROS could contribute via a reduced inactivation of NO. However, the concomitant reduction of eNOS levels, although mechanistically traced back to increased heme oxygenase activity, is still somewhat puzzling. As already mentioned, stoichiometric BH4 levels are important to keep eNOS in the coupled and NO-producing state. In situations of reduced BH4 levels, Nrf2 activation and subsequent transient eNOS reduction could relieve an eventual imbalance of eNOS and BH4, keep eNOS in the coupled state, and maintain or even enhance NO production (despite reduced eNOS levels). To assess whether reduced BH4 levels themselves could be a trigger for Nrf2 activation and eNOS reduction, we treated HUVEC with methotrexate (MTX) or aminopterin, two inhibitors of dihdrofolatreductase that also interfere with regeneration of BH4 from BH2 (37), to induce BH4 deficiency. MTX- and aminopterin-treated cells consistently showed reduced eNOS levels (Fig. 5A). The reduction of eNOS upon MTX incubation was Nrf2-dependent, as shown by knockdown experiments (Fig. 5B). These findings support the hypothesis of Nrf2 as (direct or indirect) translator of reduced BH4 levels into down-regulation of eNOS to maintain a 1:1 ratio of BH4 and eNOS.
FIGURE 5.
Reduced BH4 levels lead to down-regulated eNOS expression in an Nrf2-dependent manner. A, BH4 levels were reduced by treatment of HUVEC with either MTX (10 μmol/liter) or aminopterin (APT; 100 μmol/liter). Total cell lysates were subjected to Western blot analysis for eNOS and tubulin. B, HUVEC were transfected either with scrambled siRNA (scr) or siRNA targeted against Nrf2 and then treated with MTX as indicated. Total cell lysates were subjected to Western blot analysis for eNOS, Nrf2, and tubulin. Representative blots out of three independent experiments with consistent results are shown. The numbers below the blots depict mean densitometric arbitrary units (ratio protein/tubulin as -fold DMSO control) of all performed experiments (* p < 0.05, ANOVA, Dunnett's post test versus control).
DISCUSSION
We showed in this study that activation of Nrf2 leads to a reduction of reactive oxygen species, to elevated levels of NO, and to a transient reduction of eNOS protein levels in primary human endothelial cells. We provided, furthermore, data indicating that Nrf2 can be activated upon the reduction of BH4 levels and possibly ensures a stoichiometric BH4: eNOS ratio and hereby eNOS coupling. Reduced eNOS levels could be mechanistically traced back to elevated HO-1 activity and heme deficiency.
Activation of Nrf2 in our study was mainly achieved by treatment with the synthetic triterpenoid CDDO-IM, and Nrf2 involvement was confirmed by knockdown of Nrf2 and subsequent abrogation of the observed effects. Other known pharmacological activities of CDDO-IM such as inhibition of IκB kinase-β (38, 39) and peroxisome proliferator activated receptor-γ agonism (40, 41) could be excluded in our setting (supplemental Fig. VI). Key findings such as the reduction of ROS, increase of bioavailable NO, down-regulation of eNOS, and up-regulation of HO-1 by CDDO-IM could also be observed in human and/or porcine aortic endothelial cells (supplemental Fig. VII), demonstrating a general and not vein-specific character of the reported findings.
The increase of bioavailable NO by Nrf2 activation and the concomitant reduction of eNOS levels are at first sight contradictory. However, they can be reconciled in the context of redox stress and its impact on proper eNOS function. Higher eNOS levels do not necessarily mean higher amounts of produced NO. Due to uncoupling of eNOS enzymatic activity under situations of reduced availability of its cofactor BH4, i.e. under oxidative stress, eNOS can turn into a major source of superoxide (9) and trigger a vicious circle of excessive production of ROS and depletion of NO. Activation of Nrf2 may therefore be a safeguard mechanism to reduce the risk of eNOS uncoupling and contribute to vascular homeostasis in the redox-stressed endothelium. Nrf2-mediated down-regulation of eNOS relieves the transient imbalance between eNOS levels and bioavailable BH4 until concomitant up-regulation of the cellular antioxidant defense has restored sufficient BH4 levels (see hypothetical scheme in supplemental Fig. VIII). In Fig. 5, we accordingly demonstrate that reduced BH4 levels can reduce the amount of eNOS protein in an Nrf2-dependent manner. How Nrf2 is exactly activated by reduced BH4 levels and whether depletion of absolute BH4 levels or an increase of the BH2:BH4 ratio is the initial trigger still needs to be deciphered. Preliminary data support a predominant role for the BH2:BH4 ratio because preincubation with sepiapterin, a precursor for total biopterin synthesis, failed to affect the MTX-induced eNOS down-regulation (data not shown). Furthermore the importance of the BH2:BH4 ratio for eNOS coupling has recently been stressed by others (42, 43). Future studies need to define the parameters that favor or disturb Nrf2 activation in the endothelium. We were able to activate Nrf2 strongly by CDDO-IM and moderately by BH4 depletion. However, induced oxidative stress such as cultivation in high glucose apparently failed to trigger the Nrf2 safeguard mechanism and resulted in reduced NO production (as seen in Fig. 1E). In this case, only exogenous activation of Nrf2 by CDDO-IM led to recovery of NO levels. Because most studies dealing with Nrf2 in endothelial cells were performed in the context of shear stress, the impact of laminar or oscillatory flow deserves special attention in future investigations. Nrf2 is selectively activated in atheroprotected vessel regions that are exposed to laminar flow (4, 12, 44, 45). Moreover, recent reports showed synergistic action between Nrf2 and Krüppel-like factor (KLF)-2 (46, 47), another transcription factor that is predominantly activated by atheroprotective blood flow and positively involved in eNOS expression (48–50). One could speculate that under laminar flow conditions, activated KLF-2 and Nrf2 complement each other and secure unhindered NO production by taking care of eNOS expression and eNOS coupling, respectively. Overall, there seems to be a complex and delicate interplay between flow, Nrf2, KLF-2, and levels of ROS that maintains endothelial homeostasis, which prompts further investigations. The beneficial impact of exogenous activation of the Nrf2/HO-1 axis for the endothelium in situations of stress is underlined in Figs. 1E and 2D and supported by Xue et al. (51) and Iori et al. (52) They observed alleviated endothelial dysfunction under hyperglycemic conditions via activation of Nrf2 and induction of HO-1, respectively.
During our work, we further provided evidence that increased activity of HO-1 and presumably heme deficiency are underlying mechanistic triggers for Nrf2-mediated reduction of eNOS protein. Decreased levels of eNOS protein occur as late as 18–20 h after exposure to CDDO-IM and primarily affect the amount of eNOS dimers (supplemental Fig. III). This is in agreement with the half-life of residing eNOS protein and the fact that dimerization of newly synthesized eNOS is dependent on heme binding. Heme deficiency may also negatively feed back to eNOS mRNA stability or transcription. CDDO-IM reproducibly reduced levels of eNOS mRNA, which was overcome by exogenous heme (data not shown). Involvement of CO in the down-regulation of eNOS cannot be completely ruled out at this point because the addition of a CO-releasing molecule (100 μmol/liter) was able to reduce eNOS levels. Moreover, Thorup et al. (53) reported a negative regulation of eNOS by high concentrations of CO, and CO is, like NO, a vasoactive gas (54) with similar functions and targets. Although affinities for soluble GMP cyclase and vasodilatatory activities are much lower than those found for NO (55), high levels of HO-1/CO could negatively feed back to the eNOS system, as already indicated by Batzlsperger et al. (56).
Overall, we provide novel mechanistic insight in the impact of Nrf2 for endothelial homeostasis. Besides its general anti-inflammatory (see also supplemental Fig. IX) and antioxidant effect, activated Nrf2 transiently decreases eNOS levels and can hereby contribute to eNOS coupling by ensuring stoichiometric balance between BH4 and eNOS.
Supplementary Material
Acknowledgments
We express our gratitude to M. B. Sporn, Dartmouth Medical School, Hanover, NH for providing aliquots of CDDO-IM; to P. Soares, Gulbenkian Institute of Science, Oeiras, Portugal, for kindly sending us the HO-1 expression vector; as well as to H. Beres, R. Leitner (University of Vienna), and N. Madl (Innsbruck Medical University) for expert technical assistance.
This work was supported in part by grants from the “Hochschuljubiläumsstiftung der Stadt Wien” (Grant H-01509/2007 to E. H. H.) and the Austrian Research Funds “zur Förderung der wissenschaftlichen Forschung” (Grant P19764 to E. R. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. I–IX.
- Nrf2
- NF-E2-related factor
- HO-1
- heme oxygenase-1
- BH2
- 7,8-dihydrobiopterin
- BH4
- tetrahydrobiopterin
- NO
- nitric oxide
- NOS
- NO synthase
- eNOS
- endothelial NOS
- iNOS
- inducible NOS
- nNOS
- neuronal NOS
- CO
- carbon monoxide
- ROS
- reactive oxygen species
- CDDO-IM
- 2-cyano-3,12-dioxooleana-1,9-dien-28-oic imidazolide
- DAF2-DA
- diaminofluorescein-diacetate
- HUVEC
- human umbilical vein endothelial cells
- MTX
- methotrexat
- siRNA
- small interfering RNA
- DMSO
- dimethyl sulfoxide
- ANOVA
- analysis of variance
- DCF
- dichlorofluorescein
- KLF-2
- Krüppel-like factor 2.
REFERENCES
- 1.Kang K. W., Lee S. J., Kim S. G. (2005) Antioxid. Redox Signal. 7, 1664–1673 [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal A. K. (2004) Free Radic. Biol. Med. 36, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 3.Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. (2004) Mol. Cell. Biol. 24, 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X. L., Dodd G., Thomas S., Zhang X., Wasserman M. A., Rovin B. H., Kunsch C. (2006) Am. J. Physiol. Heart Circ Physiol. 290, H1862–1870 [DOI] [PubMed] [Google Scholar]
- 5.Chen X. L., Varner S. E., Rao A. S., Grey J. Y., Thomas S., Cook C. K., Wasserman M. A., Medford R. M., Jaiswal A. K., Kunsch C. (2003) J. Biol. Chem. 278, 703–711 [DOI] [PubMed] [Google Scholar]
- 6.Zhang C. (2008) Basic Res. Cardiol. 103, 398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zandbergen F., Plutzky J. (2007) Biochim. Biophys. Acta 1771, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hink U., Li H., Mollnau H., Oelze M., Matheis E., Hartmann M., Skatchkov M., Thaiss F., Stahl R. A., Warnholtz A., Meinertz T., Griendling K., Harrison D. G., Forstermann U., Munzel T. (2001) Circ. Res. 88, E14–E22 [DOI] [PubMed] [Google Scholar]
- 9.Förstermann U., Münzel T. (2006) Circulation 113, 1708–1714 [DOI] [PubMed] [Google Scholar]
- 10.Le Brocq M., Leslie S. J., Milliken P., Megson I. L. (2008) Antioxid. Redox Signal. 10, 1631–1674 [DOI] [PubMed] [Google Scholar]
- 11.Dai G., Vaughn S., Zhang Y., Wang E. T., Garcia-Cardena G., Gimbrone M. A., Jr. (2007) Circ. Res. 101, 723–733 [DOI] [PubMed] [Google Scholar]
- 12.Warabi E., Takabe W., Minami T., Inoue K., Itoh K., Yamamoto M., Ishii T., Kodama T., Noguchi N. (2007) Free Radic. Biol. Med. 42, 260–269 [DOI] [PubMed] [Google Scholar]
- 13.Hosoya T., Maruyama A., Kang M. I., Kawatani Y., Shibata T., Uchida K., Warabi E., Noguchi N., Itoh K., Yamamoto M. (2005) J. Biol. Chem. 280, 27244–27250 [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Pan S., Berk B. C. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1283–1288 [DOI] [PubMed] [Google Scholar]
- 15.Gallis B., Corthals G. L., Goodlett D. R., Ueba H., Kim F., Presnell S. R., Figeys D., Harrison D. G., Berk B. C., Aebersold R., Corson M. A. (1999) J. Biol. Chem. 274, 30101–30108 [DOI] [PubMed] [Google Scholar]
- 16.Förstermann U. (2006) Biol. Chem. 387, 1521–1533 [DOI] [PubMed] [Google Scholar]
- 17.Liby K., Hock T., Yore M. M., Suh N., Place A. E., Risingsong R., Williams C. R., Royce D. B., Honda T., Honda Y., Gribble G. W., Hill-Kapturczak N., Agarwal A., Sporn M. B. (2005) Cancer Res. 65, 4789–4798 [DOI] [PubMed] [Google Scholar]
- 18.Sussan T. E., Rangasamy T., Blake D. J., Malhotra D., El-Haddad H., Bedja D., Yates M. S., Kombairaju P., Yamamoto M., Liby K. T., Sporn M. B., Gabrielson K. L., Champion H. C., Tuder R. M., Kensler T. W., Biswal S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samudio I., Konopleva M., Hail N., Jr., Shi Y. X., McQueen T., Hsu T., Evans R., Honda T., Gribble G. W., Sporn M., Gilbert H. F., Safe S., Andreeff M. (2005) J. Biol. Chem. 280, 36273–36282 [DOI] [PubMed] [Google Scholar]
- 20.Heiss E. H., Schilder Y. D., Dirsch V. M. (2007) J. Biol. Chem. 282, 26759–26766 [DOI] [PubMed] [Google Scholar]
- 21.Räthel T. R., Leikert J., Vollmar A. M., Dirsch V. M. (2003) Biol. Proced. Online 5, 136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leikert J. F., Räthel T. R., Müller C., Vollmar A. M., Dirsch V. M. (2001) FEBS Lett. 506, 131–134 [DOI] [PubMed] [Google Scholar]
- 23.Leikert J. F., Räthel T. R., Wohlfart P., Cheynier V., Vollmar A. M., Dirsch V. M. (2002) Circulation 106, 1614–1617 [DOI] [PubMed] [Google Scholar]
- 24.Cosentino F., Eto M., De Paolis P., van der Loo B., Bachschmid M., Ullrich V., Kouroedov A., Delli Gatti C., Joch H., Volpe M., Lüscher T. F. (2003) Circulation 107, 1017–1023 [DOI] [PubMed] [Google Scholar]
- 25.Rota C., Fann Y. C., Mason R. P. (1999) J. Biol. Chem. 274, 28161–28168 [DOI] [PubMed] [Google Scholar]
- 26.Dikalov S., Griendling K. K., Harrison D. G. (2007) Hypertension 49, 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchesi E., Rota C., Fann Y. C., Chignell C. F., Mason R. P. (1999) Free Radic. Biol. Med. 26, 148–161 [DOI] [PubMed] [Google Scholar]
- 28.Rota C., Chignell C. F., Mason R. P. (1999) Free Radic. Biol. Med. 27, 873–881 [DOI] [PubMed] [Google Scholar]
- 29.Heller R., Unbehaun A., Schellenberg B., Mayer B., Werner-Felmayer G., Werner E. R. (2001) J. Biol. Chem. 276, 40–47 [DOI] [PubMed] [Google Scholar]
- 30.Fleming I., Busse R. (2003) Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1–R12 [DOI] [PubMed] [Google Scholar]
- 31.Fleming I., Busse R. (1999) Cardiovasc. Res. 43, 532–541 [DOI] [PubMed] [Google Scholar]
- 32.Cai S., Khoo J., Channon K. M. (2005) Cardiovasc. Res. 65, 823–831 [DOI] [PubMed] [Google Scholar]
- 33.Zabalgoitia M., Colston J. T., Reddy S. V., Holt J. W., Regan R. F., Stec D. E., Rimoldi J. M., Valente A. J., Chandrasekar B. (2008) Free Radic. Biol. Med. 44, 284–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.True A. L., Olive M., Boehm M., San H., Westrick R. J., Raghavachari N., Xu X., Lynn E. G., Sack M. N., Munson P. J., Gladwin M. T., Nabel E. G. (2007) Circ. Res. 101, 893–901 [DOI] [PubMed] [Google Scholar]
- 35.Kirkby K. A., Adin C. A. (2006) Am. J. Physiol. Renal Physiol. 290, F563–571 [DOI] [PubMed] [Google Scholar]
- 36.Noriega G. O., Tomaro M. L., del Batlle A. M. (2003) Biochim. Biophys. Acta 1638, 173–178 [DOI] [PubMed] [Google Scholar]
- 37.Werner E. R., Werner-Felmayer G., Wachter H., Mayer B. (1996) Rev. Physiol. Biochem. Pharmacol. 127, 97–135 [DOI] [PubMed] [Google Scholar]
- 38.Ahmad R., Raina D., Meyer C., Kharbanda S., Kufe D. (2006) J. Biol. Chem. 281, 35764–35769 [DOI] [PubMed] [Google Scholar]
- 39.Yore M. M., Liby K. T., Honda T., Gribble G. W., Sporn M. B. (2006) Mol. Cancer Ther. 5, 3232–3239 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Porter W. W., Suh N., Honda T., Gribble G. W., Leesnitzer L. M., Plunket K. D., Mangelsdorf D. J., Blanchard S. G., Willson T. M., Sporn M. B. (2000) Mol. Endocrinol. 14, 1550–1556 [DOI] [PubMed] [Google Scholar]
- 41.Tabe Y., Konopleva M., Kondo Y., Contractor R., Tsao T., Konoplev S., Shi Y., Ling X., Watt J. C., Tsutsumi-Ishii Y., Ohsaka A., Nagaoka I., Issa J. P., Kogan S. C., Andreeff M. (2007) Cancer Biol. Ther. 6, 1967–1977 [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama T., Levy B. D., Michel T. (2009) J. Biol. Chem. 284, 12691–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crabtree M. J., Tatham A. L., Al-Wakeel Y., Warrick N., Hale A. B., Cai S., Channon K. M., Alp N. J. (2009) J. Biol. Chem. 284, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 44.Jyrkkänen H. K., Kansanen E., Inkala M., Kivelä A. M., Hurttila H., Heinonen S. E., Goldsteins G., Jauhiainen S., Tiainen S., Makkonen H., Oskolkova O., Afonyushkin T., Koistinaho J., Yamamoto M., Bochkov V. N., Ylä-Herttuala S., Levonen A. L. (2008) Circ. Res. 103, e1–e9 [DOI] [PubMed] [Google Scholar]
- 45.Levonen A. L., Inkala M., Heikura T., Jauhiainen S., Jyrkkänen H. K., Kansanen E., Määttä K., Romppanen E., Turunen P., Rutanen J., Ylä-Herttuala S. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 741–747 [DOI] [PubMed] [Google Scholar]
- 46.Fledderus J. O., Boon R. A., Volger O. L., Hurttila H., Ylä-Herttuala S., Pannekoek H., Levonen A. L., Horrevoets A. J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1339–1346 [DOI] [PubMed] [Google Scholar]
- 47.Ali F., Zakkar M., Karu K., Lidington E. A., Hamdulay S. S., Boyle J. J., Zloh M., Bauer A., Haskard D. O., Evans P. C., Mason J. C. (2009) J. Biol. Chem. 284, 18882–18892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekker R. J., van Thienen J. V., Rohlena J., de Jager S. C., Elderkamp Y. W., Seppen J., de Vries C. J., Biessen E. A., van Berkel T. J., Pannekoek H., Horrevoets A. J. (2005) Am. J. Pathol. 167, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parmar K. M., Larman H. B., Dai G., Zhang Y., Wang E. T., Moorthy S. N., Kratz J. R., Lin Z., Jain M. K., Gimbrone M. A., Jr., García-Cardeña G. (2006) J. Clin. Invest. 116, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinderlerer A. R., Ali F., Johns M., Lidington E. A., Leung V., Boyle J. J., Hamdulay S. S., Evans P. C., Haskard D. O., Mason J. C. (2008) J. Biol. Chem. 283, 14636–14644 [DOI] [PubMed] [Google Scholar]
- 51.Xue M., Qian Q., Adaikalakoteswari A., Rabbani N., Babaei-Jadidi R., Thornalley P. J. (2008) Diabetes 57, 2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iori E., Pagnin E., Gallo A., Calò L., Murphy E., Ostuni F., Fadini G. P., Avogaro A. (2008) Life Sci. 82, 383–392 [DOI] [PubMed] [Google Scholar]
- 53.Thorup C., Jones C. L., Gross S. S., Moore L. C., Goligorsky M. S. (1999) Am. J. Physiol. 277, F882–F889 [DOI] [PubMed] [Google Scholar]
- 54.Durante W., Schafer A. I. (1998) Int. J. Mol. Med. 2, 255–262 [DOI] [PubMed] [Google Scholar]
- 55.Furchgott R. F., Jothianandan D. (1991) Blood Vessels 28, 52–61 [DOI] [PubMed] [Google Scholar]
- 56.Batzlsperger C. A., Achatz S., Spreng J., Riegger G. A., Griese D. P. (2007) Cardiovasc. Drugs Ther. 21, 347–355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.