Abstract
Factor VIII-von Willebrand factor (FVIII·vWF) complex, a molecule involved in coagulation, can be physically associated with osteoprotegerin (OPG). OPG is an anti-osteoclastic protein and a soluble receptor for the proapoptotic protein TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), suggesting a potential role of FVIII·vWF complex in bone and cancer biology. We, thus, assessed the effects of FVIII·vWF complex on osteoclastogenesis and cell survival. We first evidenced that FVIII·vWF complex inhibited RANKL-induced osteoclastogenesis and enhanced the inhibitory effect of OPG. Interestingly, we revealed by surface plasmon resonance that FVIII·vWF complex bound to RANKL, whereas recombinant FVIII and vWF did not. By modeling, we showed that the OPG binding domain to the A1 domain of vWF was closely located and partially overlapped to its binding site to RANKL. Then, we demonstrated that FVIII·vWF complex cancelled the inhibitory activity of OPG on TRAIL-induced apoptosis and characterized interactions between these molecules. The present work evidenced a direct activity of FVIII·vWF complex on osteoclasts and on induced cell apoptosis, pointing out its potential involvement in physiological bone remodeling or in bone damages associated with severe hemophilia and cancer development.
Introduction
The molecular triad osteoprotegerin (OPG)3/RANK/RANKL is a crucial parameter of bone biology. Receptor activator of nuclear factor κB ligand (RANKL), a member of the tumor necrosis factor family, is mainly expressed by osteoblasts in the bone microenvironment and acts as a pro-resorption factor (1, 2); RANKL binds to its receptor RANK expressed at the cell surface of osteoclast precursors and induces osteoclastic differentiation and maturation, leading to bone resorption (3, 4). OPG, also mainly produced by osteoblasts, is a soluble decoy receptor for RANKL, preventing the binding of RANKL to RANK and, thus, inhibiting osteoclastogenesis (5–7). Bone turnover is tightly controlled by the OPG/RANK/RANKL triad, and any change in the balance OPG/RANKL leads to pathological conditions (7). OPG is also a receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (8, 9), a cytokine that is able to induce a rapid cancer cell death by apoptosis (9–11). Interestingly, the binding of OPG to TRAIL completely inhibits TRAIL-induced cytotoxicity (8). OPG possesses anti-apoptotic properties and, therefore, could be considered as a pro-tumoral agent.
Factor VIII is a plasma glycoprotein mainly synthesized by hepatocytes but also by kidney, sinusoidal endothelial cells, and in small amounts by lymphatic tissues (12). Factor VIII is one of the main coagulation factors and allows the completion of the coagulation process. Factor VIII circulates in plasma in a non-covalent complex with the von Willebrand factor (FVIII·vWF complex). The most well known genetic disease associated with Factor VIII is hemophilia A, which shows an X-linked inheritance (13). A second important disease associated with low Factor VIII levels is von Willebrand disease, a bleeding disorder (14, 15). Patients suffering from von Willebrand disease have primary hemostasis defects leading to mucocutaneous bleeding or spontaneous deep tissue bleeding, such as in hemophilia A, or both (14). Bleeding diseases could be associated with different bone phenotypes. For instance, in a murine model of platelet-type von Willebrand disease, a significant increase of bone mass and cortical thickness due to a reduction of the number of osteoclasts is observed (16). In contrast, various case reports suggest that children suffering from severe hemophilia have more risks for low bone density and osteopenia/osteoporosis, preferentially caused by physical inactivity and leading to loss of joint function, shorter height, lower weight, and muscle atrophy (17, 18).
von Willebrand factor is a multimeric protein containing many binding domains for various proteins such as the D′-D3 domain, which binds Factor VIII (FVIII) (19), and the A1 domain, which can bind different proteins such as the platelet glycoprotein Ib (20), heparin (21), and snake venom toxins (bitiscetin (22) and botrocetin (23)). Recently, it has been shown that the vWF is physically complexed to OPG (through the A1 domain) within the Weibel-Palade bodies and also in plasma, revealing a possible modulatory role of OPG in hemostasis (24, 25). The aim of the present study was to characterize the effects of FVIII·vWF complex on osteoclastogenesis and cancer cell survival and then interactions between complex FVIII·vWF complex and three members of tumor necrosis factor cytokine/cytokine receptor family: OPG, RANKL, and TRAIL. The data obtained demonstrated that FVIII·vWF complex binds to OPG and RANKL and, thus, indirectly participates in bone biology. Indeed, we first demonstrated that the FVIII·vWF complex inhibits RANKL-induced osteoclastogenesis. Second, we demonstrated that the FVIII·vWF complex abolished the inhibitory effect of OPG on TRAIL-induced apoptosis, revealing a key role of FVIII·vWF complex in cancer development.
EXPERIMENTAL PROCEDURES
Osteoclast Differentiation Assay
Generation of osteoclasts from murine RAW 264.7 cells was performed as previously described (26) in the presence of recombinant human RANKL (100 ng/ml) (kindly provided by Amgen Inc.), human OPG (100 ng/ml) (R&D systems), FVIII·vWF complex purified from plasma (ProSpec), or recombinant FVIII (Octocog α, kindly provided by CSL Behring) (1 or 2 units/ml). Generation of osteoclasts from human CD14+ monocytes was described previously (26). Briefly, purified CD14+ cells were cultured in α-minimum essential medium with 10% fetal calf serum and 25 ng/ml human macrophage colony-stimulating factor (R&D systems). After 3 days of culture, 100 ng/ml RANKL, 100 ng/ml OPG, and 1 unit/ml FVIII or FVIII·vWF were added. Multinucleated cells formed with three nuclei, and more were counted after TRAP staining (Sigma).
Cell Proliferation
Human osteosarcoma cell lines MG63 and SaOS2 as well as the Ewing's sarcoma cell line TC71 were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. MG63 and SaOS2 cells were seeded at 500 cells/well in 96-well plates, and TC71 cells were seeded at 1500 cells/well. Cells were treated with 50–100 ng/ml TRAIL (R&D Systems), 50–100 ng/ml OPG, and 1 unit/ml FVIII·vWF complex for 72 h. After the culture period, cell viability was determined by a cell proliferation reagent assay kit using sodium 3′-(1-(phenylaminocarbonyl)-3,4-tetrazolium)-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate (XTT Roche Applied Science).
Hoechst Staining and Caspase-3 Activity
Cell death was monitored microscopically after Hoechst 33258 (Sigma) staining. MG63, SaOS2, and TC71 cells were seeded in a 96-multiwell plate and treated or not with TRAIL (50 ng/ml), OPG (50 ng/ml), and FVIII·vWF complex (1 unit/ml) for 16 h, stained with 10 μg/ml Hoechst reagent for 20 min at 37 °C, and then observed under UV microscopy (DMRXA; Leica, Germany). Caspase-3 activity was assessed on 10 μl of total treated cell lysates using the kit CaspACE assay system, fluorometric (Promega) following the manufacturer's recommendations. Results are expressed in arbitrary units and are corrected for protein content.
Surface Plasmon Resonance Binding Assays
Experiments were carried out on a BIAcore 3000 instrument (BIAcore). OPG (1 μg/ml in 5 mm maleate, pH 6.0), RANKL (2 μg/ml in 5 mm maleate, pH 5.75), and TRAIL (10 μg/ml in 10 mm acetate, pH 5.5) were covalently immobilized to the dextran matrix of a CM5 sensor chip (BIAcore) at a flow rate of 5 μl/min. Immobilization levels ranging from 300 RU for OPG and 400 RU for RANKL to 700 RU for TRAIL were obtained. vWF (Hematologic Technologies) was immobilized on a C1 sensor chip at 2000 RU. Binding assays were performed at 25 °C in 10 mm Hepes buffer, pH 7.4, containing 0.15 m NaCl and 0.005% P20 surfactant (HBS-P buffer, BIAcore) or in a pH 6.5 buffer containing 20 mm Bis-Tris and 10 mm CaCl2 at a flow rate of 30 μl/min for immobilized-OPG and 20 μl/min for immobilized RANKL and immobilized-TRAIL. OPG Kd values for vWF and FVIII·vWF were determined using single cycle kinetics, starting with 25 nm OPG or with 300 nm FVIII·vWF, then ½ dilutions. For binding analysis over the immobilized RANKL or immobilized-TRAIL chip, binding of OPG alone or preincubated for 120 min with different concentrations of FVIII·vWF complex was performed for 4 min at a flow rate of 20 μl/min followed by dissociation for 2.5 min. The resulting sensorgrams were fitted using BiaEval 4.1 software (BIAcore). For Kd calculations, the following molecular masses were used: recombinant FVIII, 330 kDa; FVIII·vWF complex, 540 kDa.
ELISA Assay
FVIII·vWF complex was coated at 1 unit/ml on a 96-well plate overnight at room temperature. OPG or RANKL (both were tested at 500, 100, and 10 ng/ml) were incubated for 2 h at room temperature. After 2 washes with PBS, Tween 0.05%, the revelation of the binding of OPG or RANKL to FVIII·vWF complex was performed using a specific biotinylated antibody against each molecule (anti-OPG was from R&D systems and anti-RANKL from Peprotech). Streptavidin conjugated to horseradish peroxidase (R&D systems) was incubated for 20 min, then the revelation solution (Promega) was added for 20 min, and the reaction was stopped with sulfuric acid solution. The absorbance at 450 nm was measured using a microplate reader (Victor II, PerkinElmer Life Sciences).
Modeling Analysis
To design OPG and RANKL, sequences were retrieved from the Universal Protein resource. Each protein was subjected to BLAST searches on the organism species (Homo sapiens) and on the organism classification (Mammalia, Vertebrata) (27). These sequences were further analyzed using multiple sequence alignments to extract the most conserved residues (28). The multiple alignments were manually adjusted using Jalview (29). Human OPG and human RANKL models were built using Modeler 9v5 (30) from these refined alignments, respectively, using substructure of DR5 (PDB code 1D4V) (31) and mouse RANKL (PDB code 1IQA) (32). All resulting models were assessed using the Protein Health module of Discovery Studio 2.1 (Accelrys Inc.). Alignment of the A1 domain of vWF on OPG has been realized as described below. Structural figures were produced with VMD (33) and rendered using Pov-Ray.
OPG, RANKL, and TRAIL Effect on the Coagulation Cascade
Plasma of a healthy donor was drawn on 0.109 m buffered citrate. OPG, TRAIL, and RANKL were added to the plasma at 100 ng/ml. Primary hemostasis was tested using PFA 100 automate (Siemens). The Quick time was determined using the reagent RecombiPlasTin (Instrumentation Laboratory) on the ACL TOP automate (Instrumentation Laboratory). The activated partial thromboplastin time (aPTT) was tested using TriniCLOT Thrombin Time reagent (Trinity Biotech) on ACL TOP. The thrombin time was tested using thrombin (Siemens) on ACL TOP. The FVIII/C method was based on the aPTT. This assay was performed using FVIII-deficient plasma (Biopep), an aPTT reagent with kaolin as contact phase activator (CK Prest, Diagnostica Stago, France), and an ACL TOP coagulometer.
Statistical Analysis
The mean ± S.D. was calculated for all conditions and compared by analysis of variance with Bonferroni multiple comparisons test as the post hoc test. Differences relative to a probability of two-tailed p < 0.05 were considered significant.
RESULTS
FVIII·vWF Inhibits Murine and Human Osteoclast Differentiation Induced by RANKL
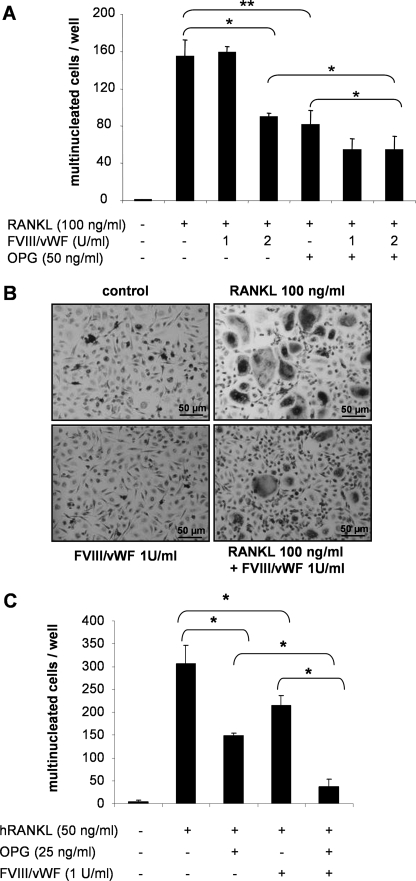
The impact of the FVIII·vWF complex on osteoclastogenesis was first examined using the cellular model RAW 264.7. After 5 days of culture with 100 ng/ml RANKL, RAW 264.7 cells differentiated into multinucleated cells. As expected, 50 ng/ml OPG inhibited the RANKL-induced osteoclastogenesis by 47% (p < 0.01) (Fig. 1A). Surprisingly, 2 units/ml FVIII·vWF complex inhibited RANKL-induced osteoclastogenesis by 42% (p < 0.01), whereas 1 unit/ml FVIII·vWF complex had no effect on RANKL-induced osteoclastogenesis (Fig. 1A). Furthermore, when 2 units/ml FVIII·vWF complex were added to the culture medium in the presence of OPG, the inhibition of osteoclastogenesis was significantly stronger than that observed with OPG alone. Indeed, the inhibition of RANKL-induced osteoclastogenesis reached 65% in the presence of a mixture OPG, FVIII·vWF complex compared with 47% with OPG alone (p < 0.05). The recombinant FVIII alone had no effect on RANKL-induced osteoclastogenesis of RAW 264.7 cells (data not shown).
FIGURE 1.
FVIII·vWF complex inhibits RANKL-induced osteoclastogenesis. A, RAW 264.7 cells were cultured for 5 days in the presence or not of 100 ng/ml human RANKL (hRANKL), 100 ng/ml OPG, and 1 or 2 units/ml FVIII·vWF complex. After May Grünwald/Giemsa staining. B, purified human CD14+ monocytes were cultured for 15 days in the presence of 25 ng/ml human macrophage colony-stimulating factor and 100 ng/ml human RANKL and 1 unit/ml FVIII·vWF. TRAP coloration was performed at the end of the culture period. C, purified human CD14+ monocytes were cultured for 15 days in the presence of 25 ng/ml human macrophage colony-stimulating factor and 100 ng/ml human RANKL with or without 50 ng/ml OPG and 1 unit/ml FVIII·vWF. Multinucleated TRAP-positive cells were counted under a light microscope. A and C, results are expressed as the number of multinucleated cells (more than three nuclei) per well; each value represents the mean ± S.D. All experiments were performed independently three times in triplicate. *, p < 0.05, **, p < 0.01.
To ascertain the effect of FVIII·vWF complex on osteoclastogenesis, we next generated osteoclasts from human CD14+ purified from total peripheral blood mononuclear cells upon macrophage colony-stimulating factor and RANKL activation (26). As shown in Fig. 1, B and C, and similarly to RAW 264.7 cells, 1 unit/ml FVIII·vWF complex significantly inhibited by 30% the RANKL-induced osteoclastogenesis of CD14+ cells (p < 0.05) (Fig. 1C). Furthermore, 1 unit/ml FVIII·vWF complex reinforced the OPG inhibitory activity on RANKL-induced osteoclastogenesis (p > 0.05) (Fig. 1C). In accordance with the RAW 264.7 cells, the recombinant FVIII alone had no effect on RANKL-mediated osteoclastogenesis (data not shown).
RANKL Binds to FVIII·vWF Complex Similarly to OPG
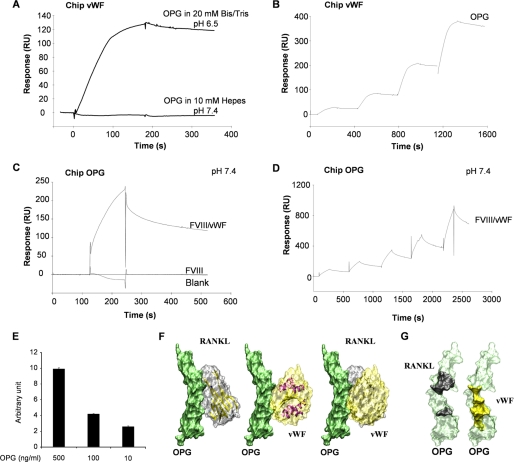
To explore the molecular mechanism underlying the effect of the FVIII·vWF complex on RANKL-induced osteoclastogenesis and the possible synergistic effect of OPG and FVIII·vWF complex, we investigated the molecular interactions between RANKL, OPG, FVIII·vWF complex, recombinant FVIII, and vWF by surface plasmon resonance. It has been determined that OPG and vWF are physically associated in Weibel-Palade bodies of endothelial cells and also in the plasma (24, 25). Thus, we first immobilized vWF and confirmed that the interaction between OPG and vWF depends on the biochemical environment (25). In fact, the binding of OPG to immobilized-vWF occurred only with 20 mm Bis-Tris, pH 6.5, and not with 10 mm Hepes, pH 7.4 (Fig. 2A), and the dissociation constant obtained was Kd = 3.51 10−9 m (Fig. 2B). Then we revealed that in the pH 7.4 buffer FVIII·vWF complex was also able to bind to immobilized-OPG, whereas recombinant FVIII was not (Fig. 2C). Furthermore, using a single cycle kinetic assay, the Kd of OPG for FVIII·vWF complex was 7.19 10−8 m (Fig. 2D). The binding of OPG to the FVIII·vWF complex was also confirmed by ELISA assay. As shown in Fig. 2E, OPG can bind in a dose-dependent manner to the FVIII·vWF complex previously coated. Taken together, these results revealed that the interaction between OPG and the FVIII·vWF complex occurred through the vWF.
FIGURE 2.
The interaction between OPG and the FVIII·vWF complex occurred through the vWF. A, OPG binds to immobilized-vWF chip in specific biochemical conditions. Binding assays were performed using 2 different buffers (pH 7.4 or 6.5) as described under “Experimental Procedures.” B, determination of the Kd of OPG for vWF using a single cycle kinetic assay is shown. OPG was injected over immobilized vWF in pH 6.5 buffer at 25 nm and then ½ dilutions. C, in the pH 7.4 buffer, FVIII·vWF complex, but not recombinant Factor VIII, binds to OPG. FVIII·vWF complex (50 units/ml) or recombinant Factor VIII (50 units/ml) was injected at a flow rate of 30 μl/min over the immobilized-OPG sensor chip for 5 min, and the dissociation was monitored for 10 min. D, determination of the Kd of OPG for FVIII·vWF complex in pH 7.4 buffer using a single cycle kinetic assay is shown. FVIII·vWF complex was injected over immobilized-OPG starting at 300 nm and then ½ dilutions. E, OPG (500, 100, and 10 ng/ml) bound to the coated FVIII·vWF complex (1 unit/ml) using an ELISA assay is shown. Results are expressed using arbitrary units. F, modeling of the interactions between OPG (green), RANKL (gray), and the A1 domain of vWF (yellow) is shown. OPG has the same orientation in the three illustrations. The right illustration is an overlay of left and middle illustrations. G, shown is a representation of the binding domains involved in the interaction OPG-RANKL and OPG-vWF. OPG amino acids involved for the binding with RANKL are schematized in black, and those involved for the binding with vWF are schematized in yellow.
To explore the putative mode of ligand-receptor binding, we modeled the OPG-RANKL interaction using constructs obtained from crystallographic coordinates of homologous proteins TRAIL-DR5 complex as described by Cheng et al. (34). We confirmed that OPG-RANKL binding model is closely related to TRAIL-DR5 binding mode (data not shown). The data obtained clearly showed that the OPG binding domain to A1 domain of vWF is closely located and partly overlaps to its binding site to RANKL (Fig. 2F). Indeed, the interface shape consists of two anchoring points on OPG for RANKL by amino acids 68, 69, 82, and amino acids 88–91, 111, and 116–120, whereas the contact surface is a continuum for A1 domain of vWF to OPG (amino acids 62–69 and 82–89) (Fig. 2G).
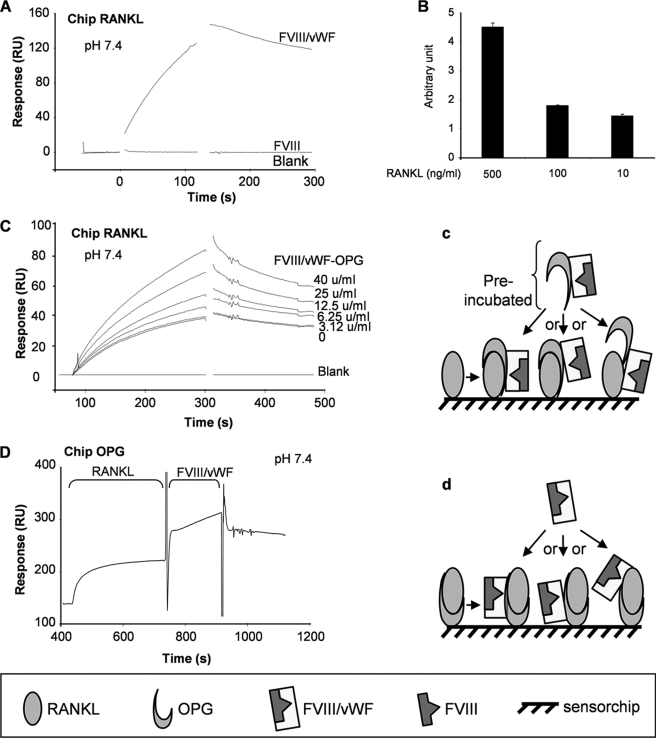
The binding of FVIII·vWF complex to immobilized RANKL was then investigated in the pH 7.4 buffer. Surprisingly, FVIII·vWF complex was able to bind to immobilized RANKL (response of 150 RU), whereas recombinant FVIII was not (Fig. 3A). However, when using an immobilized-vWF sensor chip, no binding was observed whatever the biochemical parameters used (20 mm Bis-Tris, pH 6.5, or 10 mm Hepes, pH 7.4) (data not shown). As for the binding of OPG to the FVIII·vWF complex, we performed an ELISA assay using a coating of FVIII·vWF complex. As shown in Fig. 3B, RANKL was able to bind to FVIII·vWF complex in a dose-dependent manner, confirming the results obtained by surface plasmon resonance experiments. Thus, the present data demonstrated for the first time the capacity of FVIII·vWF complex to bind RANKL. However, in contrast to OPG, for which the interaction with this complex is done via the vWF, our results suggested that the tridimensional structure of the FVIII·vWF complex is mandatory for its interaction with RANKL. To further explore the involvement of the FVIII·vWF complex in the RANKL/OPG interactions, the effect of a preincubation of OPG (100 ng/ml) and increasing concentrations of FVIII·vWF complex for 2 h was assessed. The pre-formed FVIII·vWF complex·OPG complex was then injected over the immobilized RANKL (Fig. 3C). This experiment revealed that the pre-formed complex FVIII·vWF/OPG did not prevent the binding of OPG to RANKL or the binding of FVIII·vWF to RANKL. Furthermore, the binding of OPG was higher in the presence of FVIII·vWF complex than without this complex. These results suggest that the FVIII·vWF complex, by binding to RANKL or OPG, induced some modifications in the three-dimensional structure of OPG, RANKL, or FVIII·vWF, resulting in a higher affinity between OPG and RANKL and then potentially increasing its biological activity. Such hypothesis was supported by the synergistic effect of OPG-FVIII·vWF complex observed on RAW 264.7 cells (Fig. 1A). Similarly, Fig. 3D showed that FVIII·vWF complex was still able to bind RANKL or OPG even if RANKL had already been bound to immobilized-OPG, demonstrating that these three molecules can interact together without interfering the binding of one to another (see Fig. 3, c and d, for schematic explanations). The same results were observed using immobilized vWF; indeed, a pre-formed complex OPG/RANKL was able to bind to immobilized vWF in the same way as OPG alone (see the supplemental figure).
FIGURE 3.
Complex FVIII·vWF can bind to RANKL and OPG prevents its binding. A, in the pH 7.4 buffer, FVIII·vWF complex, but not recombinant Factor VIII, binds to RANKL. FVIII·vWF complex (50 units/ml) or recombinant Factor VIII (50 units/ml) was injected at a flow rate of 10 μl/min over the immobilized RANKL sensor chip for 5 min, and the dissociation was monitored for 10 min. B, shown is RANKL (500, 100, and 10 ng/ml) bound to the coated FVIII·vWF complex (1 unit/ml) using an ELISA assay. Results are expressed in arbitrary units. C, FVIII·vWF complex increases the binding of OPG to RANKL. OPG was incubated with increasing concentrations of FVIII·vWF complex for 2 h before the injection to immobilized RANKL. D, FVIII·vWF complex, OPG, and RANKL can form a tripartite complex. Human RANKL (5 μg/ml) was injected to immobilized OPG with a flow rate of 20 μl/min, then the FVIII·vWF complex was injected. Schematic explanations are represented in c and d.
TRAIL Binds to FVIII without Affecting TRAIL/OPG Interactions
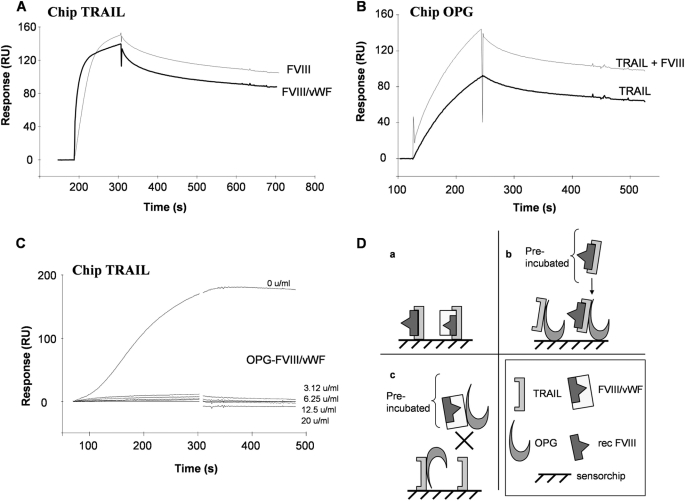
OPG is not only a decoy receptor for RANKL but also acts as soluble receptor for TRAIL and, thus, inhibits its proapoptotic activity (8, 10, 11). To determine whether or not the FVIII·vWF complex could affect the complex OPG/TRAIL, TRAIL has been immobilized on a sensor chip, and the capacity of FVIII·vWF complex to bind to TRAIL was analyzed. In contrast to the previous experiments with OPG and RANKL, both FVIII·vWF complex and recombinant FVIII were able to bind to immobilized TRAIL (Fig. 4A and summarized in Fig. 4D). Furthermore, as for OPG binding to immobilized-vWF, only specific biochemical conditions of 20 mm Bis-Tris, pH 6.5, allowed the binding of TRAIL to immobilized vWF (no binding with 10 mm Hepes, pH 7.4) (data not shown). We confirmed that TRAIL bound to immobilized-OPG and showed that the complex formed by TRAIL and recombinant FVIII was still able to bind similarly to OPG (Fig. 4B). To further explore the involvement of the FVIII·vWF complex in the OPG/TRAIL interactions, the effect of a preincubation of OPG and increasing concentrations of FVIII·vWF has been investigated. Whatever the concentration of FVIII·vWF complex used to form a complex with OPG, all these combinations completely inhibited the binding of OPG to TRAIL (Fig. 4C and summarized in Fig. 4D). These results suggested that the binding domains of OPG to vWF and TRAIL is very closed, and one molecule bound to OPG can then block the binding sites of the second.
FIGURE 4.
TRAIL binds to FVIII without affecting TRAIL/OPG interactions. A, recombinant Factor VIII and FVIII·vWF complex bind to immobilized-TRAIL. Recombinant Factor VIII (50 units/ml) or FVIII·vWF complex (50 units/ml) were injected at a flow rate of 30 μl/min over the immobilized-TRAIL for 2 min association. B, recombinant Factor VIII bound to TRAIL does not interfere the binding of TRAIL to immobilized-OPG. 50 units/ml recombinant Factor VIII was preincubated with TRAIL for 2 h at room temperature. The complex formed was then injected to immobilized-OPG. C, FVIII·vWF complex inhibits the binding of OPG to TRAIL. FVIII·vWF complex was incubated with OPG for 2 h at room temperature; the new complex formed was then injected at a flow rate of 30 μl/min for 2 min over immobilized-TRAIL. D, schematic representation of plasmon resonance experiments a, b, and c summarized respectively A, B, and C.
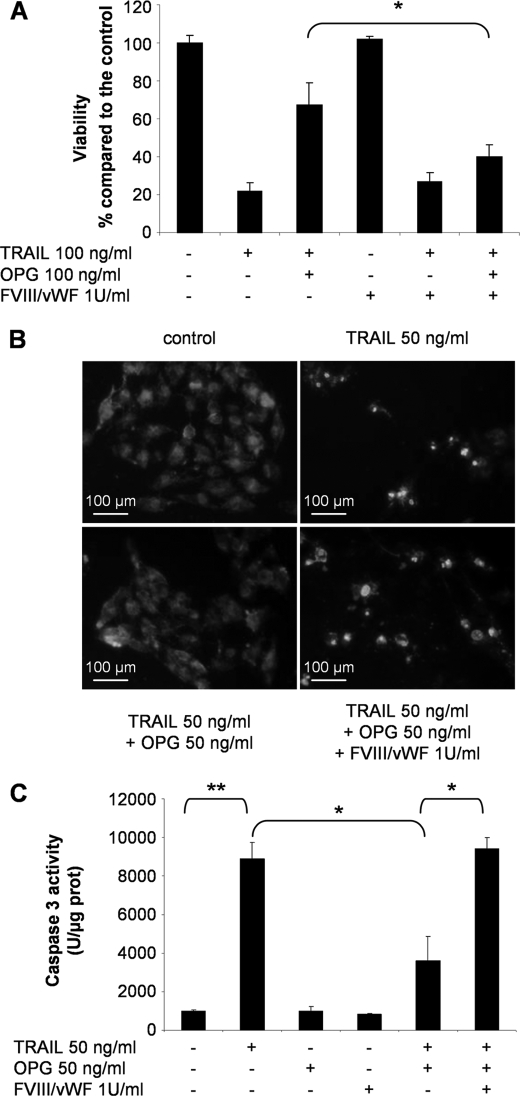
To investigate the relevance of this inhibition in a biological experiment, we performed a viability assay on the human osteosarcoma cell line MG63 sensitive to TRAIL-induced apoptosis. As shown in Fig. 5A, the ability of TRAIL to induce MG63 cell death (∼75%, p < 0.01) was prevented by the addition of OPG. In contrast, when 1 unit/ml FVIII·vWF complex was added to the culture medium, OPG was not able to prevent the capacity of TRAIL to induce MG63 cell death (∼60%, p < 0.05). Furthermore, the apoptotic effect of TRAIL was confirmed even in the presence of FVIII·vWF complex and OPG. Nucleus fragmentation was observed in MG63 (Fig. 5B). In the same manner of 50 ng/ml TRAIL, the combination TRAIL + FVIII·vWF complex + OPG induced nucleus fragmentation, as the cells exhibited a characteristic kidney-like form with condensed chromatin clumps compared with control cells. Moreover, TRAIL induced a significant increase of caspase-3 activity in MG63 cells (p < 0.01) (Fig. 5C) which was significantly reduced in the presence of OPG. But the caspase-3 activity was not decreased by OPG when FVIII·vWF complex was added (p < 0.05). The same results of viability, Hoechst staining, and caspase-3 activation were obtained using other cell lines sensitive to TRAIL-induced apoptosis such as the human osteosarcoma cell line SaOS2 and the human Ewing's sarcoma cell line TC71 (data not shown). Thus, these data revealed that the inhibitory effect of OPG on TRAIL-induced apoptosis can be reversed by FVIII·vWF complex and then evidenced the role of FVIII·vWF complex in the control of cell death.
FIGURE 5.
FVIII·vWF complex blocks the inhibitory effect of OPG on TRAIL-induced apoptosis on MG63 cells. A, osteosarcoma cell line MG63 was cultured with 100 ng/ml TRAIL ± 100 ng/ml OPG ± 1 unit/ml FVIII·vWF complex. After 72 h of culture, cell viability was determined by XTT assay. Results were expressed as percentage of control. Experiments were performed at least three times (*, p < 0.05). B, nuclear morphological changes induced by TRAIL, OPG, and FVIII·vWF complex were analyzed by Hoechst staining on MG63 cells. C, caspase-3 activity was assessed on MG63 cells after 16 h of treatment with TRAIL, OPG, and FVIII·vWF complex (*, p < 0.05; **, p < 0.01).
Recombinant Human OPG, RANKL, and TRAIL Do Not Affect the Coagulation Cascade
Because of the different interactions evidenced in our study between OPG, RANKL, TRAIL, and the FVIII·vWF complex, we evaluated the potential implications of these three molecules on the coagulation cascade. We demonstrated that 100 ng/ml OPG, RANKL, and TRAIL have no effect on the following assays: primary hemostasis, Quick time, aPTT, thrombin time, and the FVIII/C method based on the aPTT (data not shown).
DISCUSSION
FVIII associated with the vWF is a key protagonist of the coagulation process as evidenced in patients suffering from hemophilia A. Recent papers revealed the physical interaction between vWF and OPG (24, 25), a powerful inhibitor of osteoclastogenesis and, therefore, of bone resorption (7). Although severe hemophilia patients have also joint diseases, to our knowledge there is no evidence of the effect of FVIII·vWF complex on bone cells and especially on osteoclasts. The present work demonstrates that FVIII·vWF complex binds to OPG and RANKL and, thus, indirectly participates to bone biology. This paper is, thus, the first evidence that FVIII·vWF complex inhibits RANKL-induced osteoclastogenesis. Furthermore, in a second part of the manuscript, we also demonstrated for the first time that the FVIII·vWF complex abolishes the inhibitory effect of OPG on TRAIL-induced apoptosis, suggesting a potential function of FVIII·vWF complex in cancer development (Fig. 6).
FIGURE 6.
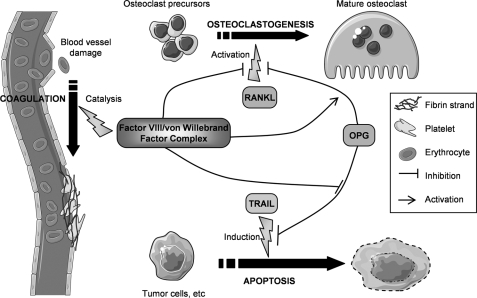
Schematic representation describing the involvement of FVIII·vWF complex in coagulation cascade, bone, and cancer biology. FVIII·vWF complex is one of the main complex involved in coagulation; FVIII is released from vWF by the action of thrombin and becomes a cofactor for Factor IX to stimulate coagulation cascade, whereas vWF is essential in platelet activation. FVIII·vWF complex also plays a major role in other biological processes. Indeed, this complex inhibits RANKL-induced osteoclastogenesis by binding to RANKL and also by increasing the anti-osteoclastic activity of OPG. Furthermore, FVIII·vWF complex may be involved in cell apoptosis (endothelial, bone, and cancer cells); through its binding to OPG, the FVIII·vWF complex inhibits the OPG protective effect on TRAIL-induced apoptosis that occurs in inflammation and cancer disorders.
In two different models FVIII·vWF complex regulates osteoclastogenesis by inhibiting the pro-osteoclastic activity of RANKL. Two different effects can be involved in this inhibition; the first way of inhibition occurs through a physical interaction between FVIII·vWF complex and RANKL, leading to an inactivation of RANKL, and the second potential effect is a synergic effect of the FVIII·vWF complex with OPG. In fact, both molecules inhibit RANKL-induced osteoclastogenesis by themselves, but their association in the culture medium increased this inhibitory effect. However, different mechanisms could be proposed. OPG could bind to the FVIII·vWF complex through the vWF, and this complex could increase the affinity of OPG to RANKL, or the complex FVIII·vWF could bind to both RANKL and OPG, leading to a stronger inhibition of RANKL activity.
These interactions between FVIII·vWF complex, OPG, and RANKL point out their potential involvement in bone and vascular system (7). Indeed, the hallmark of severe hemophilia is repeated bleedings into joints and muscles resulting in a severe and painful inflammation of synovitis named hemophilic synovitis (35, 36). However, the exact mechanism related to blood-induced joint disease is not precisely known even if some mechanisms are now settled. The processes that occur at the early stages of blood-induced joint disease associated with infiltration of inflammatory cells releasing high amounts of inflammatory cytokines, enzymes (36), proteins such as hemoglobin, and an increase of intra-articular pressure and synovial proliferation. The later stages are characterized by a promotion of angiogenesis, cartilage cell apoptosis, and subchondral bone destruction. Thus, hemophilic arthropathy shares several biological features with rheumatoid arthritis (37). Numerous studies in rheumatoid arthritis models have produced evidence for a causal role of excessive RANKL activity in associated-bone loss (38). Indeed, RANKL levels were concomitantly increased in inflamed joint leading to an increase in the RANKL/OPG ratio, which appears positively correlated with bone destruction and osteoclast activity (39). The present data evidenced for the first time that FVIII·vWF complex inhibits RANKL-induced osteoclastogenesis. Moreover FVIII·vWF complex did not abolish OPG activity on osteoclastogenesis but reinforced its activity in murine and human models. In this context hemophilic arthritis may be associated with an intra-articular inflammatory process concomitantly with an increased osteoclastogenesis due to a deficiency of FVIII·vWF complex.
OPG/RANK/RANKL triad constitutes a molecular bridge spanning bone metabolism, vascular biology, and immunity (7). The first evidence linking the OPG/RANK/RANKL system to the vessel biology has been provided by the vascular phenotype of OPG-deficient mice (40). Indeed, OPG-deficient mice exhibited medial calcification of the aorta and renal arteries and not of smaller vessels, suggesting that OPG and its molecular partners may play a role in the long term-observed association between osteoporosis and vascular calcification (40). OPG physically associated with the vWF is localized in the Weibel-Palade bodies of endothelial cells and is rapidly secreted in response to inflammatory stimuli (24). More recently, in a case-control study Bilora et al. (41) assessed the presence of atherosclerosis in 50 patients suffering from hemophilia and in 50 age-matched control individuals. Their results suggest that hemophilia could protect against asymptomatic atherosclerosis. Overall, these observations strongly support that the OPG/RANK/RANKL and FVIII·vWF systems constitute a molecular cascade essential in the development of atherosclerotic lesions. Furthermore, our present work gives a biologically direct relationship between the FVIII·vWF system and osteoclastic cells, strengthening the interests of prophylaxis in young patients suffering from severe hemophilia. Even if prophylaxis seems to be the best therapeutic option for severe hemophilia A to prevent joint damages in evidence-based medicine (42, 43), these results give a basic explanation for the effect of prophylaxis in joint damage and subchondral bone erosion prevention.
The second important result reported in our study is the control of cell apoptosis by the FVIII·vWF complex. We observed in vitro that OPG did not inhibit TRAIL-induced cell apoptosis when FVIII·vWF complex was present in the culture medium. Physical interactions between the FVIII·vWF complex, OPG, and TRAIL were confirmed by surface plasmon resonance experiments. We showed that the FVIII·vWF complex was able to bind to TRAIL, and then we demonstrated that, when associated to OPG, FVIII·vWF complex prevented the binding TRAIL/OPG, correlating the in vitro apoptosis experiment. To our knowledge, the functional relationship between Factor VIII and/or vWF and apoptosis has never been investigated. TRAIL is a cytotoxic ligand that binds to type I transmembrane receptors (DR4 and DR5) possessing death domains and which ultimately activates the caspase cascade, inducing cell death (44). TRAIL also has two decoy receptors (DcR1, DcR2) that lack a functional death domain and explain in part the absence of massive apoptosis in cells that express functional membrane receptor (45). However, normal and cancer cells lacking these decoy receptors can escape to cell death through the expression of OPG, which is able to block TRAIL transduction signaling (8). It is well established that the coagulation cascade contributes to cancer development (46), and a clear correlation between thrombosis and cancer progression has been established. Indeed, tissue factor is up-regulated on both tumor and host cells in cancer patients and initiates protease-activated receptor-mediated cell signaling that leads to the production of soluble cytokines and angiogenic growth factors (47). More recently, Ho-Tin-Noé et al. demonstrated that platelets support tumor vascular homeostasis by regulating the stability of tumor vessels (48). Thus, tumor development appears as the equilibrium between cell proliferation and cell death actively controlled by blood vessels and coagulation cascade. By reversing the inhibitory effect of OPG on TRAIL-induced apoptosis, FVIII·vWF may control tumor growth. Hemophilia A has been recently reported after tumor resection in patients suffering from glioblastoma (49), and it has been suggested that cancer cells could produce factor VIII-like tumor antigens. Such hypothesis has been also strengthened by Franchini et al. (50), who recently reviewed the acquired factor VIII inhibitors in oncohematology. If the origin of such nonclassical antibodies against FVIII is not yet defined, these autoantibodies may complicate the clinical course of the malignancy (51). All these data associated with the present work are in favor of a contribution of FVIII·vWF complex during cancer disorders. Then the interaction between OPG-TRAIL-FVIII·vWF complex may be involved in induced cell apoptosis (endothelial, cartilage, bone, and tumor cells), which is essential during angiogenesis associated with inflammation and cancer disorders.
Supplementary Material
This work was supported in part by the Région des Pays de la Loire (Program “Ciblage Moléculaire et Applications Thérapeutique”) and by the ANR 2007 INSERM Pathophysiology of Human Diseases Project R07196NS.

The on-line version of this article (available at http://www.jbc.org) contains a supplemental figure.
- OPG
- osteoprotegerin
- RANKL
- receptor activator of nuclear factor κB ligand
- FVIII
- Factor VIII
- FVIII·vWF complex
- factor VIII-von Willebrand complex
- TRAIL
- tumor necrosis factor-related apoptosis-inducing ligand
- RU
- response units
- aPTT
- activated partial thromboplastin time
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- ELISA
- enzyme-linked immunosorbent assay.
REFERENCES
- 1.Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 2.Theoleyre S., Wittrant Y., Tat S. K., Fortun Y., Redini F., Heymann D. (2004) Cytokine Growth Factor Rev. 15, 457–475 [DOI] [PubMed] [Google Scholar]
- 3.Burgess T. L., Qian Y., Kaufman S., Ring B. D., Van G., Capparelli C., Kelley M., Hsu H., Boyle W. J., Dunstan C. R., Hu S., Lacey D. L. (1999) J. Cell Biol. 145, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu H., Lacey D. L., Dunstan C. R., Solovyev I., Colombero A., Timms E., Tan H. L., Elliott G., Kelley M. J., Sarosi I., Wang L., Xia X. Z., Elliott R., Chiu L., Black T., Scully S., Capparelli C., Morony S., Shimamoto G., Bass M. B., Boyle W. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda E., Goto M., Mochizuki S., Yano K., Kobayashi F., Morinaga T., Higashio K. (1997) Biochem. Biophys. Res. Commun. 234, 137–142 [DOI] [PubMed] [Google Scholar]
- 6.Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T. M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W. J. (1997) Cell 89, 309–319 [DOI] [PubMed] [Google Scholar]
- 7.Baud'huin M., Lamoureux F., Duplomb L., Rédini F., Heymann D. (2007) Cell. Mol. Life Sci. 64, 2334–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery J. G., McDonnell P., Burke M. B., Deen K. C., Lyn S., Silverman C., Dul E., Appelbaum E. R., Eichman C., DiPrinzio R., Dodds R. A., James I. E., Rosenberg M., Lee J. C., Young P. R. (1998) J. Biol. Chem. 273, 14363–14367 [DOI] [PubMed] [Google Scholar]
- 9.Degli-Esposti M. (1999) J. Leukocyte Biol. 65, 535–542 [DOI] [PubMed] [Google Scholar]
- 10.Wiley S. R., Schooley K., Smolak P. J., Din W. S., Huang C. P., Nicholl J. K., Sutherland G. R., Smith T. D., Rauch C., Smith C. A. (1995) Immunity 3, 673–682 [DOI] [PubMed] [Google Scholar]
- 11.Pitti R. M., Marsters S. A., Ruppert S., Donahue C. J., Moore A., Ashkenazi A. (1996) J. Biol. Chem. 271, 12687–12690 [DOI] [PubMed] [Google Scholar]
- 12.Hollestelle M. J., Thinnes T., Crain K., Stiko A., Kruijt J. K., van Berkel T. J., Loskutoff D. J., van Mourik J. A. (2001) Thromb. Haemost. 86, 855–861 [PubMed] [Google Scholar]
- 13.Bolton-Maggs P. H., Pasi K. J. (2003) Lancet 361, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 14.Sadler J. E. (2005) Annu. Rev. Med. 56, 173–191 [DOI] [PubMed] [Google Scholar]
- 15.Nichols W. L., Hultin M. B., James A. H., Manco-Johnson M. J., Montgomery R. R., Ortel T. L., Rick M. E., Sadler J. E., Weinstein M., Yawn B. P. (2008) Haemophilia 14, 171–232 [DOI] [PubMed] [Google Scholar]
- 16.Suva L. J., Hartman E., Dilley J. D., Russell S., Akel N. S., Skinner R. A., Hogue W. R., Budde U., Varughese K. I., Kanaji T., Ware J. (2008) Am. J. Pathol. 172, 430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs C. S. (2008) Transfus. Apher Sci. 38, 33–40 [DOI] [PubMed] [Google Scholar]
- 18.Wallny T. A., Scholz D. T., Oldenburg J., Nicolay C., Ezziddin S., Pennekamp P. H., Stoffel-Wagner B., Kraft C. N. (2007) Haemophilia 13, 79–84 [DOI] [PubMed] [Google Scholar]
- 19.Lollar P., Hill-Eubanks D. C., Parker C. G. (1988) J. Biol. Chem. 263, 10451–10455 [PubMed] [Google Scholar]
- 20.Dumas J. J., Kumar R., McDonagh T., Sullivan F., Stahl M. L., Somers W. S., Mosyak L. (2004) J. Biol. Chem. 279, 23327–23334 [DOI] [PubMed] [Google Scholar]
- 21.Adachi T., Matsushita T., Dong Z., Katsumi A., Nakayama T., Kojima T., Saito H., Sadler J. E., Naoe T. (2006) Biochem. Biophys. Res. Commun. 339, 1178–1183 [DOI] [PubMed] [Google Scholar]
- 22.Maita N., Nishio K., Nishimoto E., Matsui T., Shikamoto Y., Morita T., Sadler J. E., Mizuno H. (2003) J. Biol. Chem. 278, 37777–37781 [DOI] [PubMed] [Google Scholar]
- 23.Fukuda K., Doggett T. A., Bankston L. A., Cruz M. A., Diacovo T. G., Liddington R. C. (2002) Structure 10, 943–950 [DOI] [PubMed] [Google Scholar]
- 24.Zannettino A. C., Holding C. A., Diamond P., Atkins G. J., Kostakis P., Farrugia A., Gamble J., To L. B., Findlay D. M., Haynes D. R. (2005) J. Cell. Physiol. 204, 714–723 [DOI] [PubMed] [Google Scholar]
- 25.Shahbazi S., Lenting P. J., Fribourg C., Terraube V., Denis C. V., Christophe O. D. (2007) J. Thromb. Haemost. 5, 1956–1962 [DOI] [PubMed] [Google Scholar]
- 26.Duplomb L., Baud'huin M., Charrier C., Berreur M., Trichet V., Blanchard F., Heymann D. (2008) Endocrinology 149, 3688–3697 [DOI] [PubMed] [Google Scholar]
- 27.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990) J. Mol. Biol. 215, 403–410 [DOI] [PubMed] [Google Scholar]
- 28.Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 29.Clamp M., Cuff J., Searle S. M., Barton G. J. (2004) Bioinformatics 20, 426–427 [DOI] [PubMed] [Google Scholar]
- 30.Sali A., Blundell T. L. (1993) J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 31.Mongkolsapaya J., Grimes J. M., Chen N., Xu X. N., Stuart D. I., Jones E. Y., Screaton G. R. (1999) Nat. Struct. Biol. 6, 1048–1053 [DOI] [PubMed] [Google Scholar]
- 32.Ito S., Wakabayashi K., Ubukata O., Hayashi S., Okada F., Hata T. (2002) J. Biol. Chem. 277, 6631–6636 [DOI] [PubMed] [Google Scholar]
- 33.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 34.Cheng X., Kinosaki M., Takami M., Choi Y., Zhang H., Murali R. (2004) J. Biol. Chem. 279, 8269–8277 [DOI] [PubMed] [Google Scholar]
- 35.De Palma A. F., Cotler J. M. (1956) AMA Arch. Surg. 72, 247–250 [DOI] [PubMed] [Google Scholar]
- 36.Valentino L. A., Hakobyan N., Rodriguez N., Hoots W. K. (2007) Haemophilia 13, 10–13 [DOI] [PubMed] [Google Scholar]
- 37.Lafeber F. P., Miossec P., Valentino L. A. (2008) Haemophilia 14, 3–9 [DOI] [PubMed] [Google Scholar]
- 38.Kearns A. E., Khosla S., Kostenuik P. J. (2008) Endocr. Rev. 29, 155–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolon B., Campagnuolo G., Feige U. (2002) Cell. Mol. Life Sci. 59, 1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucay N., Sarosi I., Dunstan C. R., Morony S., Tarpley J., Capparelli C., Scully S., Tan H. L., Xu W., Lacey D. L., Boyle W. J., Simonet W. S. (1998) Genes Dev. 12, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilora F., Zanon E., Petrobelli F., Cavraro M., Prandoni P., Pagnan A., Girolami A. (2006) Clin. Appl. Thromb. Hemost. 12, 193–198 [DOI] [PubMed] [Google Scholar]
- 42.Astermark J., Petrini P., Tengborn L., Schulman S., Ljung R., Berntorp E. (1999) Br. J. Haematol. 105, 1109–1113 [DOI] [PubMed] [Google Scholar]
- 43.Manco-Johnson M. J., Abshire T. C., Shapiro A. D., Riske B., Hacker M. R., Kilcoyne R., Ingram J. D., Manco-Johnson M. L., Funk S., Jacobson L., Valentino L. A., Hoots W. K., Buchanan G. R., DiMichele D., Recht M., Brown D., Leissinger C., Bleak S., Cohen A., Mathew P., Matsunaga A., Medeiros D., Nugent D., Thomas G. A., Thompson A. A., McRedmond K., Soucie J. M., Austin H., Evatt B. L. (2007) N. Engl. J. Med. 357, 535–544 [DOI] [PubMed] [Google Scholar]
- 44.Johnstone R. W., Frew A. J., Smyth M. J. (2008) Nat. Rev. Cancer 8, 782–798 [DOI] [PubMed] [Google Scholar]
- 45.Pan G., Ni J., Wei Y. F., Yu G., Gentz R., Dixit V. M. (1997) Science 277, 815–818 [DOI] [PubMed] [Google Scholar]
- 46.Dogan M., Demirkazik A. (2005) Support Cancer Ther. 3, 28–34 [DOI] [PubMed] [Google Scholar]
- 47.Pawlinski R., Mackman N. (2008) Semin. Thromb. Hemostasis 34, 182–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho-Tin-Noé B., Goerge T., Cifuni S. M., Duerschmied D., Wagner D. D. (2008) Cancer Res. 68, 6851–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Durme C. M., Idema R. N., van Guldener C. (2008) Neth. J. Med. 66, 286–288 [PubMed] [Google Scholar]
- 50.Franchini M., Targher G., Manzato F., Lippi G. (2008) Crit. Rev. Oncol. Hematol. 66, 194–199 [DOI] [PubMed] [Google Scholar]
- 51.Meeks S. L., Healey J. F., Parker E. T., Barrow R. T., Lollar P. (2008) Blood 112, 1151–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.