Abstract
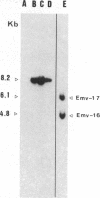
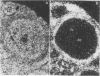
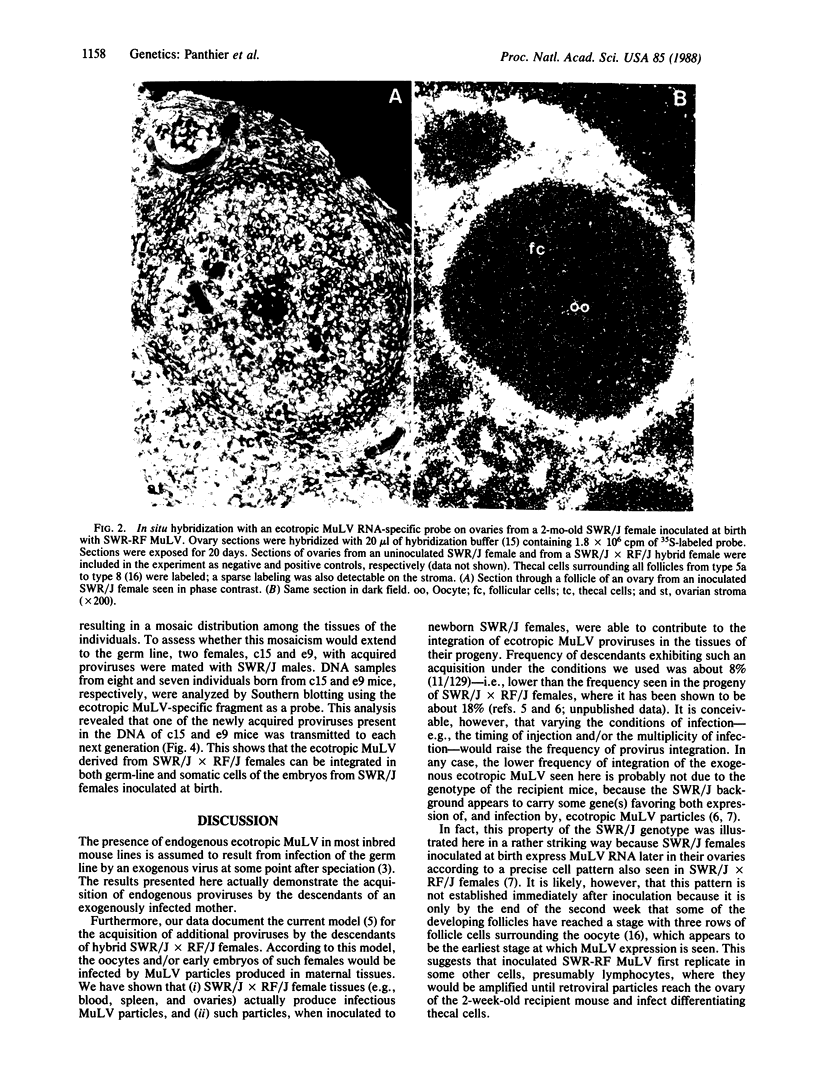
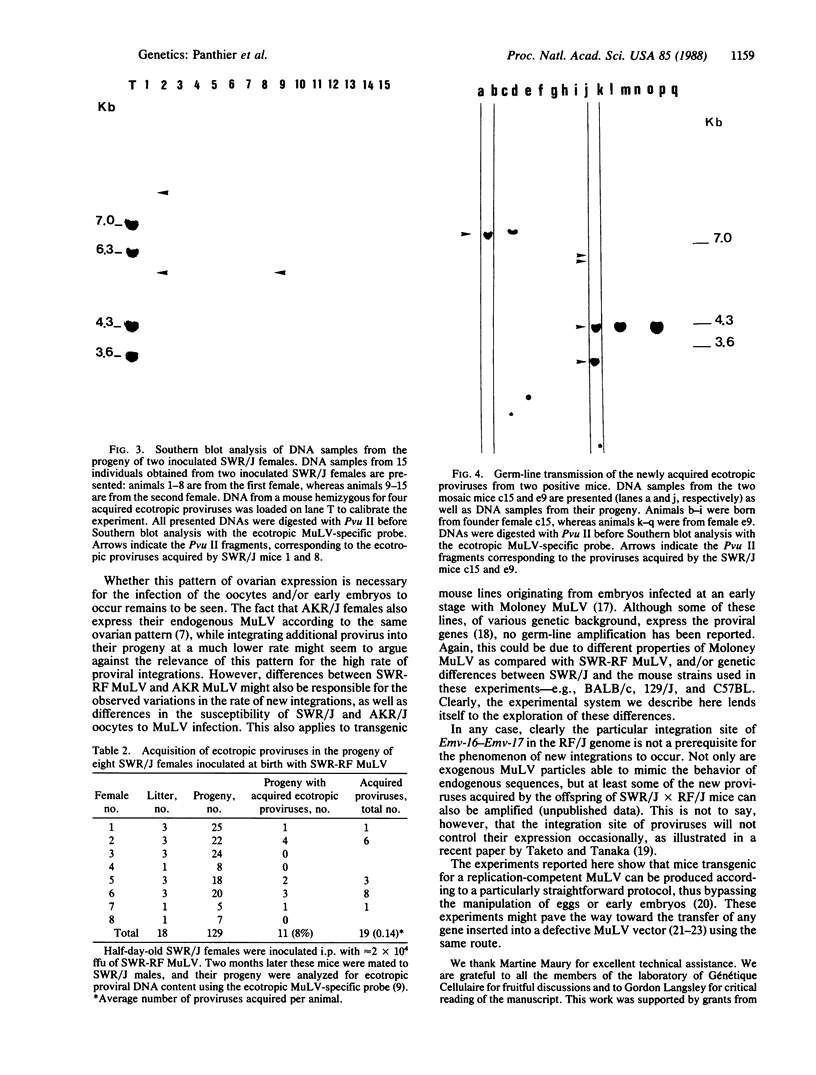
Endogenous ecotropic murine leukemia proviruses that were not present in the parental stock are acquired by the progeny of some SWR/J X RF/J hybrid females. We have made a stock of an ecotropic murine leukemia virus produced by such a hybrid female and inoculated newborn SWR/J females with it. We show that upon crossing of the inoculated females to SWR/J males, some of their progeny acquire ecotropic proviruses. Although most of these proviruses appear to be distributed in somatic tissues in a mosaic way, some are transmitted through the germ line. Thus an exogenous infection is able to mimic the phenomenon observed in SWR/J X RF/J hybrid mice. Available evidence suggests that this infection occurs during oogenesis in the recipient female. Our results document the conversion of an exogenous infectious ecotropic murine leukemia virus to an endogenous provirus without any manipulation of either eggs or embryos.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bautch V. L. Genetic background affects integration frequency of ecotropic proviral sequences into the mouse germ line. J Virol. 1986 Nov;60(2):693–701. doi: 10.1128/jvi.60.2.693-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler C. E., Staal S. P., Rowe W. P., Martin M. A. Variation in the number of copies and in the genomic organization of ecotropic murine leukemia virus proviral sequences in sublines of AKR mice. J Virol. 1982 Aug;43(2):629–640. doi: 10.1128/jvi.43.2.629-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Tuttle-Fuller N., Dunn K. J. Revertants of mouse cells transformed by murine sarcoma virus. 3. Metastable expression of virus functions in revertants retransformed by murine sarcoma virus. Virology. 1974 May;59(1):217–229. [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G. High frequency germline acquisition of ecotropic MuLV proviruses in SWR/J-RF/J hybrid mice. Cell. 1985 Dec;43(3 Pt 2):811–819. doi: 10.1016/0092-8674(85)90254-5. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F., Guenet J. L., Condamine H. Karyologically identified homozygous tw18 embryos: extrauterine growth properties and transformation by SV40. Cell. 1979 Apr;16(4):919–927. doi: 10.1016/0092-8674(79)90107-7. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Horowitz J. M., Risser R. Structure and expression of endogenous ecotropic murine leukemia viruses in RF/J mice. J Exp Med. 1982 Nov 1;156(5):1461–1474. doi: 10.1084/jem.156.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh) 1969 Sep;62(1):98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Introduction of genes into preimplantation mouse embryos by use of a defective recombinant retrovirus. Proc Natl Acad Sci U S A. 1986 Jan;83(2):366–368. doi: 10.1073/pnas.83.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D. L., Bird S., Weinberg R. A. Evidence for the Asiatic origin of endogenous AKR-type murine leukemia proviruses. J Virol. 1980 Sep;35(3):824–835. doi: 10.1128/jvi.35.3.824-835.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Jähner D., Jaenisch R. Infectivity and methylation of retroviral genomes is correlated with expression in the animal. Cell. 1981 Oct;26(2 Pt 2):221–232. doi: 10.1016/0092-8674(81)90305-6. [DOI] [PubMed] [Google Scholar]
- Taketo M., Tanaka M. A cellular enhancer of retrovirus gene expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3748–3752. doi: 10.1073/pnas.84.11.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Botteri F. M., Miller A. D., Rosenfeld M. G., Fan H., Evans R. M., Verma I. M. Efficient insertion of genes into the mouse germ line via retroviral vectors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6148–6152. doi: 10.1073/pnas.82.18.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]