Abstract
Hyperhomocysteinemia has been correlated with hepatic steatosis and activation of the unfolded protein response (UPR), yet a causal relationship has not been established. Although methionine and choline are essential components of homocysteine metabolism, the role of homocysteine in the pathogenesis of a methionine- and choline-deficient (MCD) diet remains unknown. We explored the effects of homocysteine supplementation on hepatic steatosis and the UPR in mice fed a control or MCD diet. Mice fed the MCD diet developed severe hyperhomocysteinemia and activation of the hepatic UPR. Supplementing the MCD diet with homocysteine attenuated the MCD diet-induced hepatic UPR activation and other injurious effects of the MCD diet including hepatic cholesterol accumulation, weight loss, and plasma ALT elevation. Homocysteine supplementation replenished the MCD diet-induced depletion of hepatic S-adenosylmethionine (SAM). Depleting SAM in HepG2 cells using MAT1α siRNA or cycloleucine resulted in enhanced activation of the UPR upon exposure to thapsigargin. Mice fed a control diet supplemented with homocysteine had a 3-fold elevation in plasma homocysteine level by 2 weeks and 6-fold elevation by 6 weeks but demonstrated no other pathophysiologic change. In summary, we found that homocysteine attenuates MCD diet-induced hepatic UPR activation, likely via repletion of hepatic SAM. Furthermore, homocysteine supplementation alone does not cause hepatic steatosis or UPR activation despite inducing hyperhomocysteinemia. These studies indicate that although hyperhomocysteinemia is often associated with hepatic steatosis and UPR activation, these effects may be a secondary response rather than a direct effect of homocysteine.
Introduction
Homocysteine has been implicated in the pathogenesis of the metabolic syndrome and non-alcoholic fatty liver disease (NAFLD)2 (1–3). Hepatic steatosis is often a feature of severe hyperhomocysteinemic disease in human and animal models. Patients with genetic diseases that cause severe hyperhomocysteinemia, including cystathionine β-synthase (CBS) deficiency, frequently develop fatty liver disease (4). Likewise, CBS-deficient mice develop severe hyperhomocysteinemia and hepatic steatosis (5, 6). Murine dietary models that induce hyperhomocysteinemia, such as the high methionine, low folate diet or intragastric alcohol feeding have also been shown to induce hepatic steatosis (7, 8).
Hyperhomocysteinemia has been identified as a risk factor for coronary artery disease and has been implicated in the pathogenesis of atherosclerosis (9). Interestingly, however, data have emerged indicating that lowering serum homocysteine levels with pharmacologic therapy does not reduce the risk of cardiac events (10, 11). This has raised the possibility that homocysteine may be a marker, rather than a cause, of cardiovascular disease.
Despite the recognized correlations between severe hyperhomocysteinemia and fatty liver disease, there is no in vivo data demonstrating that homocysteine directly causes hepatic steatosis or injury. As with cardiovascular disease, the possibility exists that homocysteine may be a marker rather than a cause of fatty liver disease. Additionally, although severe hyperhomocysteinemia in the setting of rare genetic diseases may be linked to hepatic steatosis, it is unclear whether the modest elevations in homocysteine, commonly seen in the general population, have a significant impact on the development of fatty liver disease. In fact, recent work in humans has shown that obese females with advanced NAFLD did not have elevated serum homocysteine levels compared with control subjects without NAFLD (12). This further calls into question the role of homocysteine as a causative agent of fatty liver disease.
Homocysteine has also been implicated in activation of the unfolded protein response (UPR) (7). Stimuli that induce endoplasmic reticulum (ER) stress, such as oxidative stress, hypoxia, calcium depletion, and toxin exposure, result in accumulation of unfolded proteins in the ER, leading to activation of an intracellular signaling cascade known as the UPR (13, 14). The UPR promotes cell survival by activating genes and proteins that halt further ER protein accumulation. Three UPR transducers (IRE1, PERK, and ATF6) and one master regulator, BiP (Grp78) are central to this process. In the nonstressed state, IRE1, PERK, and ATF6 are bound by BiP. In response to ER stress, dissociation of BiP initiates activation of these proteins. IRE1 forms a dimer and autophosphorylates, resulting in mRNA splicing of the X-box-binding protein 1 (XBP-1). Activated XBP-1 up-regulates ER chaperone genes such as BiP. Release of BiP from PERK leads to its activation by dimerization and autophosphorylation. Activated PERK phosphorylates the eukaryotic-initiating factor 2α (eIF-2α) resulting in its activation. ER stress leads to release of BiP from ATF6, allowing its cleavage and formation of a cytosolic fragment that migrates to the nucleus and activates UPR-responsive genes. If the survival response is inadequate, a cell signaling cascade to induce apoptosis is initiated, which includes activation of GADD153/CHOP (15, 16). ER stress and activation of the UPR have been implicated in the pathogenesis of diseases such as obesity (17), diabetes (17, 18), Alzheimer disease (14), and numerous liver diseases including α-1-antitrypsin deficiency (19), hepatitis C (20), alcoholic liver disease (8), and NAFLD (21–23).
Several studies have shown an association between hyperhomocysteinemia and activation of the UPR, and, therefore, it has been postulated that homocysteine may be a stimulus that induces ER stress. In vitro studies have demonstrated up-regulation of markers of the UPR, including BiP and CHOP, in HepG2 cells treated with millimolar concentrations of homocysteine, however, in vivo serum levels are typically 1000 times lower (7, 24). Furthermore, it has been shown that activation of the hepatic UPR enhances hepatic cholesterol and triglyceride biosynthesis, and it has been proposed that the accumulation of hepatic lipids seen in association with hyperhomocysteinemia may be mediated by this mechanism (7). However, in vivo data demonstrating a clear association between homocysteine and activation of the hepatic UPR are lacking.
It is well established that methionine metabolism is altered in some patients with liver disease. Hepatic depletion of S-adenosylmethionine (SAM) predisposes to liver injury and may lead to steatohepatitis, hepatocyte apoptosis, fibrosis, and hepatocellular carcinoma (25). Rodents fed a methionine- and choline-deficient (MCD) diet exhibit depletion of hepatic SAM and develop severe steatohepatitis (26). Although methionine, choline, SAM, and SAH are essential components of homocysteine metabolism, the role of homocysteine in the pathogenesis of MCD diet-induced steatohepatitis remains unknown.
The hepatic effects of homocysteine supplementation in vivo have not been explored. We supplemented a control and MCD diet with homocysteine to determine the in vivo effects of homocysteine in isolation and in conjunction with this nutritional model of steatohepatitis. We specifically addressed the effect of homocysteine on hepatic lipid accumulation, hepatic SAM levels, and activation of the hepatic unfolded protein response.
EXPERIMENTAL PROCEDURES
Animals and Diet
Female FVB/NJ mice (8–10 weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were divided into five groups and fed one of five diets for a total of 14 days: (a) control diet identical to the MCD diet except replete with methionine and choline (ICN Biomedicals, Aurora, OH); (b) control diet supplemented with homocysteine; (c) MCD diet (ICN Biomedicals); (d) MCD diet supplemented with homocysteine; or (e) MCD diet supplemented with methionine. dl-homocysteine, and l-methionine (Sigma-Aldrich) were supplemented as 1.8 g/liter and 5 g/liter in drinking water, respectively. An additional two cohorts of mice were fed either a control diet or control diet supplemented with homocysteine for 6 weeks. Mice were housed in colony cages with a 12-h light/dark cycle and were given free access to food and water. Mice were fasted for 4 h prior to sacrifice. Body weight was recorded at the beginning and end of the experimental protocol. Mice were euthanized by CO2 inhalation. Blood was collected via cardiac puncture and centrifuged at 5,000 rpm at 4 °C for 10 min to collect the plasma. Livers were rapidly excised, weighed, and flushed with ice-cold saline. An aliquot was fixed in 10% formalin for histologic analysis and TUNEL staining, and the remainder of the liver was sectioned, snap-frozen in liquid nitrogen, and stored at −80 °C until analyzed. All animal protocols were approved by the Northwestern University Animal Care and Use Committee (ACUC).
Plasma and Liver Chemistries
After collection, plasma was stored for less than 48 h at 4 °C before analysis. Plasma homocysteine and ALT were measured per protocol in the clinical laboratory at Northwestern Memorial Hospital (Chicago, IL). Liver samples were homogenized in Dulbecco's phosphate-buffered saline (DPBS) for hepatic lipid analysis (100 mg liver tissue/1 ml). Triglyceride and cholesterol levels were measured in plasma and liver homogenate using an Infinity spectrophotometric assay per the manufacturer's protocol (Thermo Electron Corp., Melbourne, Australia).
Histology
Formalin-fixed liver was embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin. Sections were scored for hepatic steatosis as follows: grade 0, none present; grade 1 steatosis, <25% of parenchyma; grade 2 steatosis, 26–50%; grade 3 steatosis, 51–75%; grade 4 steatosis, >75% of parenchyma.
Analysis of Gene Expression by Real-time Quantitative PCR
Total RNA from frozen liver samples was isolated using TRIzol reagent (Invitrogen). Two micrograms of total RNA was used for reverse transcription PCR using a SuperScript First-Strand kit (Invitrogen). Real-time quantitative PCR was performed using 2 μl of cDNA from each sample in a 25-μl reaction mixture containing Quantitect SYBR Green PCR Mastermix (Qiagen, Valencia, CA) along with primers specific for the gene of interest. Mouse glyceraldehyde-3-phosphate dehydrogenase and human actin were employed as housekeeping genes. The primer sequences are shown in supplemental Table S1. Human MAT-1α primers were targeted to a silenced region of the MAT-1α gene (Santa Cruz Biotechnology, cat. sc-106202-PR). Amplification was performed on an ABI 7300 sequence detector (Applied Biosystems, Foster City, CA). Relative gene expression was calculated using the comparative threshold cycle method as described in the Applied Biosystems Sequence Detection Systems instruction guide.
Analysis of Protein Expression by Western Blot
Liver samples were homogenized in radioimmune precipitation assay buffer (0.1% SDS, 0.5% sodium deoxycholate, 1% Igepal-630, and phosphate-buffered saline) containing protease mixture inhibitor (Calbiochem, La Jolla, CA). Homogenates were centrifuged at 12,000 rpm for 5 min at 4 °C. Protein was extracted from HepG2 cells using T-Per (Thermo Scientific) containing Halt phosphatase inhibitor (Thermo Scientific) and protease mixture inhibitor. Protein concentrations of homogenates were determined by the Bradford assay using Coomassie Blue reagent (Pierce) and subsequently diluted with Laemmli buffer (Bio-Rad) containing β-mercaptoethanol to a standard concentration of 1 μg/μl and heated at 95 °C for 5 min. Samples containing 25 μg of protein were separated on a 12% SDS-polyacrylamide gel by electrophoresis. Protein was then transferred to a nitrocellulose membrane by electrophoresis. Protein detection was performed using polyclonal rabbit antibodies to total and phosphorylated eukaryotic initiation factor 2 α (Cell Signaling Technology, Danvers, MA), BiP (Santa Cruz Biotechnology), CHOP (Cell Signaling Technology, Danvers, MA), and MAT1α (Santa Cruz Biotechnology), and a monoclonal mouse antibody to β-actin (Sigma-Aldrich). Bound antibody was detected using goat anti-rabbit or goat anti-mouse polyclonal HRP antibody (Santa Cruz Biotechnology) and developed using ECL Western blotting Substrate (Pierce).
HPLC Assay
Hepatic SAM and SAH levels were measured by high performance liquid chromatography (HPLC) using the method of Wang et al. (27). Fresh liver specimens were homogenized in 0.4 m perchloric acid and centrifuged at 10,000 × g for 15 min. The aqueous layer was removed and filtered through a 0.2-μm syringe filter. 20 μl of solution was injected directly onto a Whatman PartiSphere C18 reversed-phase analytical column (250 mm × 4.6 mm I.D., 5-μm particle) (Clifton, NJ). The mobile phase consisted of two solvents: Solvent A, 8 mm octanesulfuronic acid sodium salt and 50 mm NaH2PO4 adjusted to pH 3.0 with H3PO4; Solvent B, 100% methanol. The HPLC column was equilibrated with 80% Solvent A and 20% Solvent B. Separation was obtained using a step gradient, increasing Solvent B to 40% at 10 min and returning to initial conditions at 21 min. The flow rate was 1.0 ml/min, and detection was monitored at 254 nm. SAM and SAH were identified by their characteristic retention times as well as spiking with SAM and SAH standards. The amount of SAM and SAH was calculated from a standard curve. SAM and SAH levels are reported as nmol/gram liver tissue.
Cell Culture
HepG2 cells (ATCC, Manassas, VA) were cultured in DMEM (ATCC) with 10% fetal bovine serum and maintained at 37 °C in 5% CO2. In the first set of experiments, cells were treated with 20 mm cycloleucine (Sigma-Aldrich) in serum-free DMEM for 24 h followed by treatment with 10, 50, or 100 nm thapsigargin (Sigma-Aldrich) in serum-free DMEM for 2, 4, or 6 h. In a second set of experiments, HepG2 cells were treated with siRNA targeted against MAT-1α or control siRNA (Santa Cruz Biotechnology) per protocol. After treatment with siRNA, cells were exposed to 100 nm thapsigargin for 6 h. In a third set of experiments, HepG2 cells were treated with 10 μm, 150 μm, or 5 mm dl-homocysteine in serum-free DMEM for 18 h. RNA isolation was performed using TRIzol reagent per protocol.
Statistical Analysis
Data are presented as mean ± S.D. Comparisons between groups were performed using Student's t test analysis.
RESULTS
The MCD Diet Induces Severe Hyperhomocysteinemia and Activates the Unfolded Protein Response
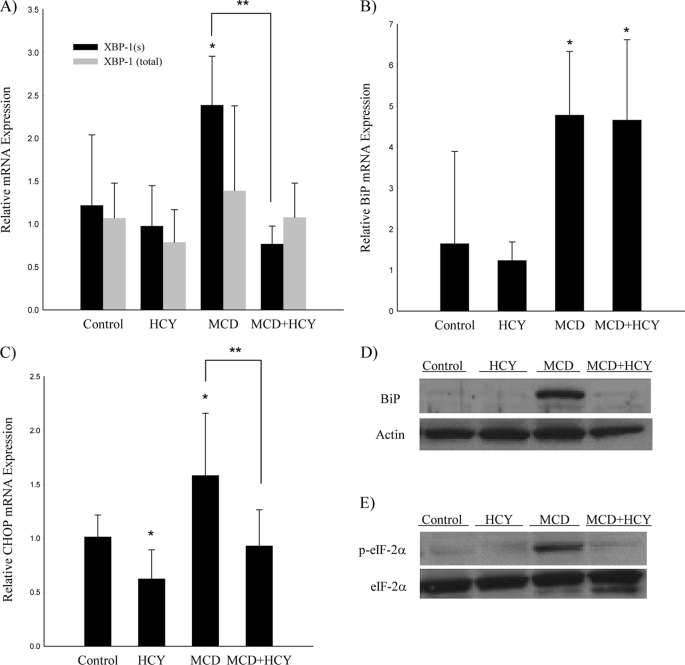
Mice fed the MCD diet for 14 days developed severe hyperhomocysteinemia (102.7 ± 17.2 μm versus 13.6 ± 1.6 μm in control, p < 0.001), which was not previously known to be a feature of this dietary model (Table 1). Activation of the UPR has been demonstrated in other dietary models of hyperhomocysteinemia. Therefore, we measured the expression of UPR markers in mice fed the MCD diet (Fig. 1). We determined that hepatic mRNA levels of CHOP, BiP, and the spliced (active) form of XBP-1 (XBP-1(s)) were 1.6-, 4.8-, and 2.4-fold elevated relative to controls (p < 0.05). Western blot analysis revealed a greater than 10-fold increase in hepatic BiP and p-eIF-2α expression compared with controls. There was no up-regulation of CHOP at the protein level.
TABLE 1.
Weight, plasma, and liver chemistries of mice fed the control, MCD, MCD + homocysteine, or homocysteine diets for 14 days
Values are expressed as mean ± S.D. n = 7 for all parameters except plasma homocysteine for which n = 5 pooled samples with 2–3 mice per sample. The asterisk indicates that in a subgroup of mice in which homocysteine-infused drinking water was replaced with distilled water for the 4-h fast prior to sacrifice, plasma homocysteine was 75.0 ± 3.8 μm in the MCD + homocysteine cohort and 29.0 ± 6.2 μm in the homocysteine cohort.
| Control | MCD | MCD + homocysteine | Homocysteine | |
|---|---|---|---|---|
| Plasma homocysteine (μm) | 13.6 ± 1.6 | 102.7 ± 17.2a | 113.0* ± 22.0a | 43.1* ± 7.8a,b |
| Body weight (g) | 21.0 ± 0.4 | 17.5 ± 1.1a | 21.1 ± 0.6b | 21.3 ± 2.2b |
| Change in body weight (%) | +1.6 ± 1.6 | −14.9 ± 1.4a | +3.5 ± 4.1b | +7.1 ± 7.8b |
| Plasma ALT (IU/L) | 35 ± 7 | 203 ± 57a | 129 ± 30a,b | 44 ± 9b |
| Plasma cholesterol (mg/dL) | 212 ± 17 | 102 ± 9a | 138 ± 8a,b | 192 ± 24b |
| Plasma triglyceride (mg/dL) | 241 ± 10 | 204 ± 28 | 206 ± 22 | 205 ± 27 |
| Plasma glucose (mg/dL) | 143 ± 32 | 79 ± 25a | 181 ± 17b | 109 ± 21b |
| Liver weight (g) | 1.00 ± 0.09 | 1.13 ± 0.16 | 1.42 ± 0.20a,b | 0.99 ± 0.06 |
| Liver wt/body wt (%) | 4.4 ± 0.2 | 6.5 ± 0.7a | 6.7 ± 0.8a | 4.5 ± 0.3b |
| Hepatic triglyceride (mg/mg protein) | 0.22 ± 0.06 | 0.72 ± 0.14a | 0.64 ± 0.12a | 0.23 ± 0.02b |
| Hepatic cholesterol (μg/mg protein) | 25.5 ± 3.2 | 47.6 ± 7.6a | 33.2 ± 8.3a,b | 26.6 ± 2.0b |
a p < 0.05 versus control.
b p < 0.05 versus MCD.
FIGURE 1.
Gene expression of markers of the unfolded protein response in mice fed the control, HCY, MCD, or MCD + HCY diet for 14 days. Real-time quantitative PCR for XBP-1(s) and XBP-1(total) (A); BiP (B); CHOP (C), and Western blot for BiP (D); and p-eIF2α (E). Quantitative PCR values are mean (n = 5–7) relative expression ± S.D. n = 5 for Western blot. *, p < 0.05 versus control; **, p < 0.05 for MCD versus MCD + HCY.
Homocysteine Supplementation Attenuates MCD Diet-induced Activation of the Unfolded Protein Response
Although the MCD diet induced both hyperhomocysteinemia and activation of the UPR, it was unclear whether the MCD diet-induced UPR activation was caused by homocysteine. Therefore, we explored the impact of supplementing homocysteine in conjunction with the MCD diet on activation of the hepatic UPR. As was observed with MCD feeding, mice fed the MCD + homocysteine diet developed severe hyperhomocysteinemia (113.0 ± 22.0 μm), which was not significantly different than the MCD-fed group (Table 1).
Homocysteine supplementation abolished the up-regulation of hepatic CHOP and XBP-1(s) mRNA induced by the MCD diet alone (p < 0.05) (Fig. 1). Additionally, the MCD diet-induced up-regulation of hepatic p-eIF-2α was markedly attenuated with homocysteine supplementation. BiP expression remained similarly up-regulated in MCD and MCD + homocysteine groups at the mRNA level, but was attenuated in the MCD + homocysteine group at the protein level. There was no detectable change in CHOP protein expression.
Given the modest up-regulation of CHOP mRNA induced by the MCD diet, we evaluated for apoptosis using a TUNEL assay. Consistent with previously reported findings (28), there was minimal apoptosis on the MCD diet or MCD + homocysteine diet by TUNEL assay (supplemental Fig. S1).
Homocysteine Supplementation Attenuates MCD Diet-induced Weight Loss, Serum ALT Elevation, and Hepatic Cholesterol Accumulation
In addition to attenuating the hepatic UPR, homocysteine supplementation attenuated numerous other injurious sequelae of the MCD diet (Table 1). After 14 days of MCD feeding, mice had significant weight loss compared with controls (−14.9 ± 1.4% versus +1.6 ± 1.6%, p < 0.001), which is a well established consequence of this dietary model (29). The addition of homocysteine to the MCD diet completely prevented this weight loss (p < 0.001) and attenuated the MCD diet-induced elevation in plasma ALT levels (129 ± 30 international unit/liter versus 203 ± 57 international unit/liter, p < 0.05). Homocysteine also attenuated the suppression of total plasma cholesterol (138 ± 8 mg/dL versus 102 mg/dL ± 9, p < 0.05) and suppression of fasting plasma glucose (181 ± 17 versus 79 ± 25 mg/dL, p < 0.05) induced by the MCD diet. There was no significant change in fasting plasma triglycerides with the addition of homocysteine to the MCD diet.
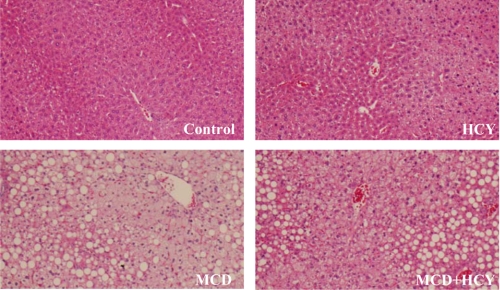
Both the MCD and MCD + homocysteine diets induced hepatomegaly relative to body weight (Table 1). Histologic analysis of liver samples revealed grade 4 steatosis in both of these groups (Fig. 2). Consistent with the histologic findings, both MCD- and MCD + homocysteine-fed mice had markedly elevated hepatic triglyceride content compared with controls (0.72 ± 0.14 and 0.64 ± 0.12 versus 0.22 ± 0.06 mg trig/mg protein, p < 0.001) (Table 1). There was a trend toward a greater increase in triglyceride content in MCD diet-fed mice compared with MCD + homocysteine-fed mice; however, this did not reach statistical significance. Homocysteine supplementation did, however, significantly impact hepatic cholesterol accumulation. The MCD diet induced significant hepatic cholesterol accumulation, which was attenuated with the addition of homocysteine to the MCD diet (47.6 ± 7.6 versus 33.2 ± 8.3 μg chol/mg protein, p < 0.01) (Table 1).
FIGURE 2.
Histology of liver samples from mice fed the control (A), HCY (B), MCD (C), or MCD + HCY (D) diet for 14 days (hematoxylin and eosin stain, 10× original magnification).
Given the alterations observed in hepatic cholesterol content, the hepatic expression of genes regulating lipid metabolism was analyzed by real-time quantitative PCR (Table 2). A ∼60% increase in expression of HMG-CoA-reductase (HMG-CoAR) was observed in mice fed the MCD diet relative to controls (p < 0.05), yet there was no up-regulation of HMG-CoAR in the MCD + homocysteine group. Hepatic mRNA levels of sterol regulatory-binding protein 2 (SREBP-2) and scavenger receptor type B1 (SR-B1) showed a trend toward up-regulation on the MCD diet, which did not reach statistical significance. However, the MCD group had a statistically significant up-regulation of SREBP-2 expression relative to MCD + homocysteine-fed mice (p < 0.05). The mRNA levels of SREBP-1c and LDL receptor (LDL-R) were unchanged among the various cohorts. Consistent with prior studies, we found that MCD-fed mice exhibited profound (86%) suppression of hepatic stearoyl-CoA-desaturase 1 (SCD-1) mRNA (29). MCD + homocysteine-fed mice showed some suppression of SCD-1 mRNA, but the levels remained 2.5 times higher than the MCD-fed mice (p < 0.05).
TABLE 2.
Expression of genes regulating hepatic lipid metabolism in mice fed the control, MCD, MCD + homocysteine, or homocysteine diets for 14 days
Relative expression, mean ± S.D. of n = 5–7, is shown.
| Control | MCD | MCD + homocysteine | Homocysteine | |
|---|---|---|---|---|
| HMG-CoAR | 1.06 ± 0.37 | 1.64 ± 0.48a | 1.04 ± 0.21b | 0.80 ± 0.25b |
| SREBP-2 | 1.15 ± 0.59 | 1.46 ± 0.54 | 0.94 ± 0.25b | 0.88 ± 0.33b |
| SR-B1 | 1.02 ± 0.20 | 1.50 ± 0.92 | 0.88 ± 0.28 | 1.16 ± 0.75 |
| SREBP-1c | 1.05 ± 0.40 | 1.11 ± 0.77 | 0.83 ± 0.38 | 1.30 ± 0.77 |
| LDL-R | 1.07 ± 0.43 | 1.59 ± 0.87 | 1.66 ± 0.54 | 1.00 ± 0.50 |
| SCD-1 | 1.06 ± 0.38 | 0.14 ± 0.07a | 0.37 ± 0.15a,b | 0.84 ± 0.11b |
a p < 0.05 versus Control.
b p < 0.05 versus MCD.
Homocysteine Supplementation Alone Does Not Induce Hepatic Steatosis or Activate the Unfolded Protein Response
Our finding that homocysteine attenuated the UPR in the setting of the MCD diet suggests that homocysteine may not directly induce UPR activation. To explore this hypothesis, we analyzed the effects of homocysteine administration in vitro and in vivo.
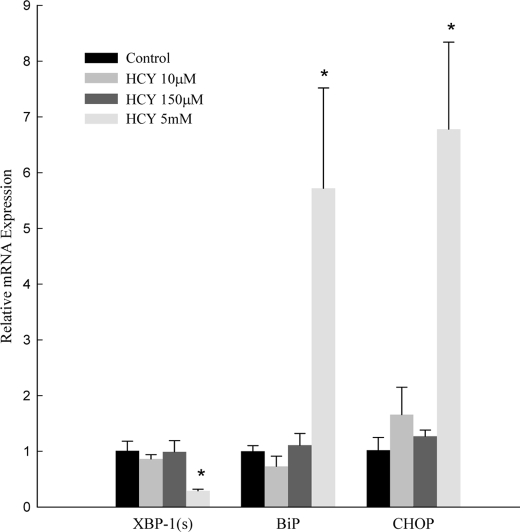
Previous in vitro studies have demonstrated that pharmacologic doses of homocysteine (>1000 times pathophysiologic serum levels) activate the UPR. We treated HepG2 cells with 10 μm, 150 μm, and 5 mm homocysteine and evaluated for activation of the UPR (Fig. 3). Administration of 10 or 150 μm homocysteine (a level consistent with severe hyperhomocysteinemia in humans) resulted in no change in BiP, CHOP, or XBP-1(s). Consistent with prior results, treatment with 5 mm homocysteine for 18 h resulted in up-regulation of BiP and CHOP mRNA (5.7 ± 1.8 and 6.8 ± 1.6). XBP-1(s) was down-regulated by 70%.
FIGURE 3.
Effect of homocysteine supplementation on mRNA levels of XBP-1(s), BiP, and CHOP in HepG2 cells. Values are mean (n = 6) ± S.D. *, p < 0.05 versus control.
To explore these findings in vivo, we determined the effects of dietary homocysteine supplementation in mice fed a control diet. Homocysteine supplementation for 2 weeks resulted in a greater than 3-fold elevation in serum homocysteine (43.1 ± 7.8 μm) compared with controls (p < 0.05) (Table 1). These mice did not demonstrate abnormalities in any other parameters evaluated. Homocysteine supplementation did not induce hepatic steatosis (Fig. 2). Homocysteine-fed mice had normal body and liver weight, normal liver histology, normal plasma ALT, and no significant increase in hepatic triglyceride content compared with controls (Table 1). Accordingly, there was no up-regulation in any of the genes we measured regulating hepatic lipid metabolism including HMG-CoAR, SREBP-2, SREBP-1c, SR-B1, SCD-1, or LDL-R (Table 2). Homocysteine supplementation for 2 weeks did not cause activation of the hepatic UPR genes, BiP and XBP-1(s), or UPR protein, p-eIF-2α. In fact, there was a modest, but statistically significant, down-regulation of CHOP mRNA in this group (Fig. 1).
Although homocysteine supplementation for 2 weeks did not result in hepatic steatosis or activation of the UPR, we examined whether a more prolonged homocysteine supplementation may be necessary to induce phenotypic changes. Therefore, we fed mice a control diet supplemented with homocysteine for 6 weeks. These mice exhibited severe hyperhomocysteinemia (134.7 ± 29.5 μm versus 21.0 ± 3.2 in control, p < 0.001) (Table 3). Homocysteine-supplemented mice had an increased body weight relative to controls but no significant increase in liver weight or plasma ALT. Despite inducing severe hyperhomocysteinemia, 6 weeks of homocysteine supplementation did not result in an increase in hepatic triglycerides relative to control (0.41 ± 0.08 versus 0.33 ± 0.11 mg/mg protein). After 6 weeks of homocysteine supplementation, CHOP mRNA levels did not differ from controls. However, mRNA levels of BiP and XBP-1(s) were unexpectedly down-regulated by 63 and 73%, respectively (Table 3).
TABLE 3.
Phenotype and hepatic gene expression in mice fed the control or homocysteine diets for 6 weeks
Values are expressed as mean ± S.D. n = 6 for all parameters except plasma homocysteine for which n = 3 pooled samples with 2 mice per sample.
| Control | Homocysteine | |
|---|---|---|
| Plasma homocysteine (μm) | 21.0 ± 3.2 | 134.7 ± 29.5a |
| Body weight (g) | 20.5 ± 1.7 | 24.4 ± 1.3a |
| Change in body weight (%) | +1.8 ± 3.3 | +15.1 ± 3.3a |
| Plasma ALT (IU/L) | 27 ± 5 | 42 ± 10 |
| Liver weight (g) | 1.00 ± 0.05 | 1.07 ± 0.11 |
| Liver wt/body wt (%) | 4.9 ± 0.5 | 4.4 ± 0.4 |
| Hepatic triglyceride (mg/mg protein) | 0.33 ± 0.11 | 0.41 ± 0.08 |
| Hepatic SAM (nmol/g liver) | 52.4 ± 6.4 | 63.6 ± 5.8 |
| Hepatic SAH (nmol/g liver) | 69.7 ± 6.0 | 74.9 ± 18.2 |
| SAM/SAH ratio | 0.8 ± 0.1 | 0.9 ± 0.2 |
| Relative mRNA levels | ||
| BiP | 1.05 ± 0.34 | 0.37 ± 0.11a |
| CHOP | 1.03 ± 0.32 | 1.02 ± 0.13 |
| XBP-1(s) | 1.35 ± 1.20 | 0.27 ± 0.15a |
a p < 0.05 versus control.
Homocysteine Replenishes MCD Diet-induced Depletion of Hepatic SAM
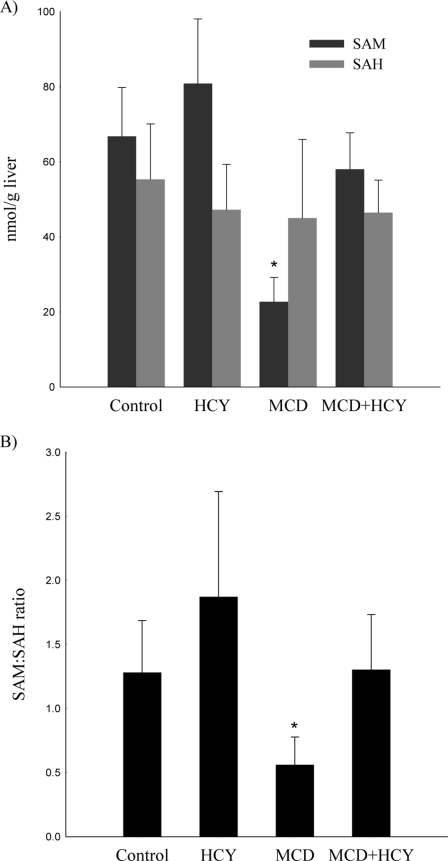
Homocysteine supplementation attenuated numerous deleterious effects of the MCD diet, including weight loss, plasma ALT elevation, hepatic cholesterol accumulation, and activation of the hepatic unfolded protein response. Given that hepatic SAM depletion, an established consequence of the MCD diet, predisposes to liver injury, we considered the possibility that exogenous homocysteine administration may replenish hepatic SAM resulting in less hepatic injury. To explore this hypothesis, we measured hepatic levels of SAM and SAH by HPLC (Fig. 4). The MCD diet caused marked suppression of hepatic SAM (22.7 ± 6.5 versus 66.8 ± 13.0 nmol/g liver, p < 0.001) with no significant change in hepatic SAH compared with controls. The addition of homocysteine to the MCD diet resulted in repletion of hepatic SAM levels (58.0 ± 9.7 nmol/g liver). The hepatic SAM:SAH ratio, which was suppressed by the MCD diet (0.6 ± 0.2), normalized with homocysteine supplementation (1.3 ± 0.4, p < 0.01 versus MCD). Homocysteine supplementation with a control diet for 2 weeks (Fig. 4) or 6 weeks (Table 3) did not cause a significant change in hepatic SAM and SAH levels compared with controls.
FIGURE 4.
A, hepatic levels of SAM and SAH, and B, ratio of hepatic SAM to SAH in mice fed the control, HCY, MCD, or MCD + HCY for 14 days. Values are mean (n = 5) ± S.D. *, p < 0.01 versus control, HCY, and MCD + HCY.
Choline Deficiency Alone Does Not Activate the Unfolded Protein Response
We have shown that homocysteine supplementation attenuates MCD diet-induced UPR activation, which we hypothesize is due to repletion of hepatic SAM. To demonstrate the importance of methionine in regulation of the UPR, we addressed whether a choline-deficient (CD) diet alone activates the UPR by treating mice with the MCD diet supplemented with methionine. As expected, the CD diet resulted in normal levels of hepatic SAM (Table 4). Additionally, the CD group exhibited normal body weight, liver weight, and plasma ALT. Hepatic triglyceride content was modestly increased relative to control (0.36 ± 0.10 versus 0.22 ± 0.06 mg/mg protein, p < 0.05). The CD diet induced marked hyperhomocystemia (75.9 ± 26.1 μm). The plasma homocysteine level in the CD group showed a trend toward a decrease compared with the MCD diet group; however, this did not reach statistical significance. The livers of CD diet-treated mice demonstrated no activation of the hepatic UPR as evidenced by normal mRNA levels of BiP and XBP-1(s).
TABLE 4.
Phenotype and hepatic gene expression in mice fed the control or CD diets for 14 days
Values are expressed as mean ± S.D. n = 6 for all parameters except plasma homocysteine for which n = 3 pooled samples with 2 mice per sample.
| Control | CD | |
|---|---|---|
| Plasma homocysteine (μm) | 13.6 ± 1.6 | 75.9 ± 26.1a |
| Body weight (g) | 21.0 ± 0.4 | 21.3 ± 1.0 |
| Change in body weight (%) | +1.6 ± 1.6 | +2.6 ± 4.4 |
| Plasma ALT (IU/L) | 35 ± 7 | 33 ± 5 |
| Plasma glucose (mg/dL) | 143 ± 32 | 175 ± 22 |
| Liver weight (g) | 1.00 ± 0.09 | 1.00 ± 0.03 |
| Liver wt/body wt (%) | 4.4 ± 0.2 | 4.7 ± 0.2 |
| Hepatic triglyceride (mg/mg protein) | 0.22 ± 0.06 | 0.36 ± 0.10a |
| Hepatic SAM (nmol/g liver) | 66.8 ± 13.0 | 55.3 ± 9.0 |
| Hepatic SAH (nmol/g liver) | 55.3 ± 14.8 | 71.8 ± 15.6 |
| SAM/SAH ratio | 1.3 ± 0.4 | 0.8 ± 0.3 |
| Relative mRNA levels | ||
| BiP | 1.3 ± 1.0 | 1.7 ± 0.8 |
| XBP-1(s) | 1.4 ± 1.3 | 1.4 ± 0.8 |
a p < 0.05 versus Control.
SAM Depletion Sensitizes HepG2 Cells to ER Stress
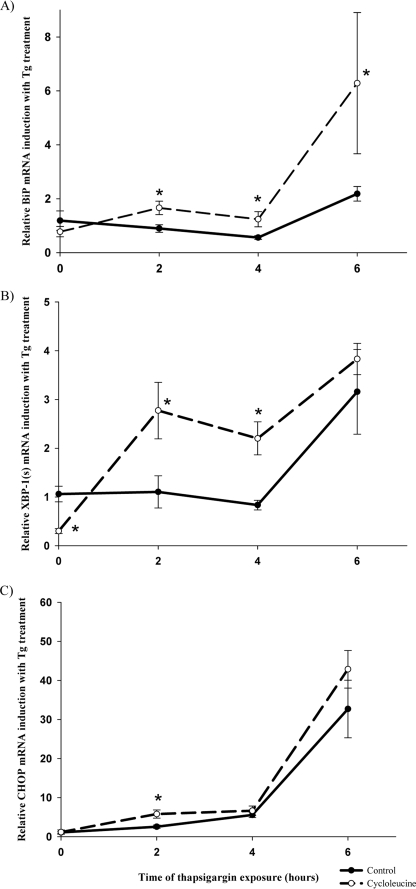
Our in vivo data indicate that SAM depletion may be important in the regulation of the hepatic UPR. To explore this hypothesis more directly, we examined the effects of SAM depletion in vitro on UPR activation. HepG2 cells were treated with cycloleucine (an inhibitor of methionine adenosyltransferase 1α (MAT1α)) for 24 h to deplete intracellular levels of SAM. Consistent with previous findings (30), cycloleucine treatment of HepG2 cells resulted in a 78% reduction in SAM (50.0 ± 40.6 versus 229.9 ± 9.5 nmol/g protein in controls, p < 0.05). After depleting SAM with cycloleucine, the cells were exposed to the ER stress-inducing agent thapsigargin for 2, 4, or 6 h (Fig. 5). Treatment with thapsigargin for 2 h resulted in enhanced up-regulation of BiP, CHOP, and XBP-1(s) mRNA in cycloleucine-treated cells relative to control cells that were not pretreated with cycloleucine. At the 4 h time point, there was a greater up-regulation of BiP and XBP-1(s) in cycloleucine-treated cells. The response to lower doses of thapsigargin (10 and 50 nm) was also examined. At lower doses of thapsigargin, there continued to be enhanced up-regulation of XBP-1(s) in SAM-depleted cells relative to control cells; however, there was no up-regulation of BiP and a similar degree of up-regulation of CHOP (supplemental Fig. S2).
FIGURE 5.
Effect of thapsigargin (Tg) treatment for 2, 4, or 6 h on relative mRNA levels of BiP (A), XBP-1(s) (B), and CHOP (C), in control or cycloleucine-treated HepG2 cells, normalized to non-thapsigargin-treated controls. Error bars are ± S.E. *, p < 0.05, thapsigargin + cycloleucine-treated cells versus thapsigargin-treated control cells.
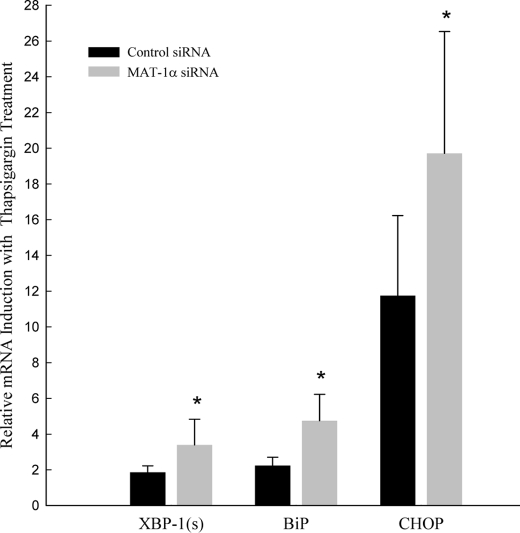
As an alternative means of depleting SAM in vitro, we treated HepG2 cells with siRNA targeted against MAT1α. Treatment with MAT-1α siRNA resulted in a 50% suppression of MAT-1α mRNA and protein (supplemental Fig. S3). After silencing MAT1α, the cells were exposed to 100 nm thapsigargin for 6 h, and gene expression of UPR markers was measured (Fig. 6). MAT1α-silenced cells showed enhanced thapsigargin-induced up-regulation of XBP-1(s), BiP, and CHOP relative to thapsigargin-treated control cells. SAM depletion alone, via treatment with cycloleucine or MAT1α siRNA, did not induce UPR activation.
FIGURE 6.
Effect of thapsigargin treatment for 6 h on relative mRNA levels of XBP-1(s), BiP, and CHOP in HepG2 cells treated with MAT1α siRNA or control siRNA normalized to non-thapsigargin-treated controls. Values are mean (n = 6) ± S.D. *, p < 0.05, thapsigargin-treated MAT1α-silenced cells versus thapsigargin-treated control cells.
DISCUSSION
The MCD diet is a well established nutritional model of steatohepatitis. Methionine and choline are essential components in the metabolism of homocysteine; however, the role of homocysteine in the pathogenesis of MCD diet-induced steatohepatitis remains unclear. A recent study demonstrated that the MCD diet causes up-regulation of the UPR markers CHOP, phosphorylated PERK, and phosphorylated eIF-2α (31). Additionally, recent studies have found a correlation between hyperhomocysteinemia, hepatic steatosis, and activation of the UPR (7, 24). However, the potential role of homocysteine in activation of the UPR in NAFLD remains poorly understood.
We found that the MCD diet induces severe hyperhomocysteinemia, which had not previously been identified as a feature of this dietary model of experimental steatohepatitis. We also found that a choline deficient diet alone causes hyperhomocysteinemia. We therefore hypothesize that the mechanism of hyperhomocysteinemia in the MCD diet is the absence of choline, resulting in impaired conversion of homocysteine to methionine.
Additionally, we demonstrated that the MCD diet induces ER stress, as evidenced by the up-regulation of UPR markers, BiP, XBP-1(s), CHOP, and p-eIF-2α. The degree of up-regulation of the various UPR markers was variable. This variation may be related to the fact that the UPR is a dynamic process. In these experiments, all UPR markers were measured at a single time point, yet it is recognized that there are earlier and later signals in the UPR pathways. The possibility cannot be excluded that other factors such as oxidative stress may also be contributing to the increased phosphorylation of eIF-2α in this model. The significance of the modest up-regulation of CHOP mRNA and absence of protein up-regulation remains unclear, particularly given the minimal degree of apoptosis detected by TUNEL assay. Rahman et al. (31) reported that mice fed the MCD diet for 4 weeks showed up-regulation of CHOP at both the mRNA and protein level. The discrepancy in our findings may relate to our different length of MCD feeding or to the fact that different mouse strains were used.
If the UPR activation induced by the MCD diet were directly due to hyperhomocysteinemia, supplementing the MCD diet with homocysteine should amplify the UPR. However, we found that the addition of homocysteine to the MCD diet attenuated the UPR, as well as numerous other injurious effects of this diet.
The attenuating effect of homocysteine with the MCD diet strongly suggests that despite the correlative data linking hyperhomocysteinemia and ER stress, homocysteine may not directly activate the UPR. Consistent with this hypothesis, we found that in vivo homocysteine supplementation to mice fed a control diet did not cause UPR activation or hepatic steatosis despite inducing a 3-fold elevation in plasma homocysteine by 2 weeks (i.e. a degree of hyperhomocysteinemia correlated with increased cardiovascular risk) and a 6-fold elevation by 6 weeks (9). At the 6-week time point, the plasma homocysteine concentration in control-fed mice was slightly higher than that of standard chow-fed mice. It should be noted that the control diet used for all experiments was not a standard chow diet but rather was identical to the MCD diet except replete with methionine and choline. This may account for the slight elevation in serum homocysteine level and subtle variations in other baseline parameters in our control-fed mice.
Our data challenge some previous data regarding the relationship between homocysteine, the UPR, and hepatic steatosis. In vitro studies have found that hepatocytes treated with millimolar concentrations of exogenous homocysteine exhibit induction of the UPR (7, 24, 32) and increased cholesterol secretion via activation of HMG-CoAR (7, 24, 32, 33). One explanation for these disparate results is that the media concentration of homocysteine used in prior in vitro studies was over 1000-fold greater than pathophysiologic plasma levels. The plasma levels achieved in our homocysteine-feeding study were consistent with levels observed in patients with clinical hyperhomocysteinemia. To examine this further we treated HepG2 cells with varying doses of homocysteine to determine whether UPR activation occurs at physiologic or pathophysiologic concentrations of homocysteine. Consistent with prior studies we demonstrated UPR activation in cells treated with 5 mm homocysteine. However, HepG2 cells treated with homocysteine concentrations as high as 150 μm (a level consistent with severe hyperhomocysteinemia in humans) did not result in UPR activation. Similarly, a recent in vitro study (32) reported that activation of the UPR could not be detected in HepG2 cells and primary mouse hepatocytes treated with less than 2 mm concentrations of homocysteine (about 1000-fold greater than normal serum levels). This raises the possibility that there may be a dose-dependent association between homocysteine and activation of the UPR or hepatic cholesterol production limited only to pharmacologic doses. It is possible that moderate elevations in serum homocysteine, as seen in patients with hyperhomocysteinemia, may have little or no impact on activation of the hepatic UPR. After all, even in the most severe human hyperhomocysteinemic diseases, serum homocysteine concentrations are generally 200–1000-fold lower than the media homocysteine concentrations required to elicit the UPR in vitro.
The high methionine, low folate diet has been used to explore the relationship between hyperhomocysteinemia, the UPR, and hepatic steatosis in vivo (7). This model is inherently limited by the fact that it does not solely induce hyperhomocysteinemia, and, therefore, it is difficult to isolate the effects of homocysteine from those of folate depletion and excess methionine. Our model of homocysteine supplementation is a more direct way to isolate the effects of homocysteine in vivo, which may explain some of the discrepant findings between our data and data using the high methionine, low folate diet. Another potential contributing factor is that the strain of mice used in our experiments was different than the strains used in other studies of diet-induced hyperhomocysteinemia.
We hypothesized that the attenuating effects of homocysteine supplementation in mice fed the MCD diet may relate to changes in hepatic levels of SAM, SAH, and the SAM/SAH ratio. Depletion of hepatic SAM, a well established consequence of the MCD diet, has been associated with increased liver injury (34, 35). We determined that the MCD diet-induced hepatic SAM depletion was nearly normalized by administration of exogenous homocysteine. No significant changes in hepatic SAH levels were observed; as such, homocysteine supplementation corrected the MCD diet-induced suppression of the SAM/SAH ratio.
Similarly, we fed mice the MCD diet supplemented with methionine (a choline-deficient diet) as another means to replenish hepatic SAM. These mice exhibited hyperhomocysteinemia but showed no significant up-regulation in the markers of the UPR. Thus, choline deficiency alone does not activate the UPR but rather UPR activation requires methionine depletion.
We found that supplementing a control diet with homocysteine for 2 or 6 weeks did not cause significant changes in hepatic SAM or SAH. This may explain the absence of pathophysiologic changes observed in this model. The possibility remains that more prolonged hyperhomocysteinemia may cause alterations in hepatic SAM and SAH leading to liver injury.
Mice fed homocysteine with the MCD diet developed marked steatosis and an equal degree of hepatic triglyceride accumulation as mice fed the MCD diet alone. This suggests that, although MCD diet-induced plasma ALT elevation and activation of the hepatic UPR may be caused, in part, by hepatic SAM depletion, the severe steatosis induced by the MCD diet may not be a function of hepatic SAM depletion.
Our in vivo data suggest that depletion of hepatic SAM has an important role in activation of the UPR. To test this hypothesis more directly, in vitro experiments of SAM depletion were performed. HepG2 cells were treated with either the MAT1α inhibitor, cycloleucine, or siRNA-targeted against MAT1α to deplete hepatic SAM. SAM depletion alone did not induce the UPR. However, exposing SAM-depleted cells to the ER stress-inducing agent, thapsigargin, resulted in a greater degree of UPR activation than in control cells replete with SAM. This indicates that SAM-depleted cells are more vulnerable to ER stress and UPR activation. SAM depletion induces hepatic injury and it is thought that this relates to hypomethylation of DNA and proteins. It is conceivable that altered methylation, triggered by SAM depletion, may induce misfolding of proteins leading to activation of the UPR. Further studies will need to be performed to explore this hypothesis.
In summary, we have shown that the MCD diet induces severe hyperhomocysteinemia and activates the hepatic UPR in mice. Supplementing the MCD diet with homocysteine attenuates the hepatic UPR, suggesting that MCD diet-induced UPR activation is not directly due to homocysteine. Homocysteine supplementation in the MCD diet serves as a source of hepatic SAM repletion, which may mediate some of its ameliorating effects. Supporting this hypothesis is the finding that SAM-depleted HepG2 cells are more prone to UPR activation upon exposure to an ER stress inducing agent. Furthermore, dietary homocysteine administration alone increases plasma homocysteine levels 3-fold by 2 weeks and 6-fold by 6 weeks but does not activate the hepatic UPR or cause hepatic steatosis in mice.
These results indicate that homocysteine at physiologic or pathophysiologic levels does not directly cause hepatic steatosis or activation of the UPR. These data raise the possibilities that the moderate degrees of hyperhomocysteinemia seen in the general population may not significantly impact the development of fatty liver disease, and homocysteine may be a marker, rather than the cause, of hepatic steatosis.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3 and Table S1.
- NAFLD
- non-alcoholic fatty liver disease
- UPR
- unfolded protein response
- MCD
- methionine- and choline-deficient
- SAM
- S-adenosylmethionine
- SAH
- S-adenosylhomocysteine
- CBS
- cystathionine β-synthase
- ER
- endoplasmic reticulum
- BiP
- glucose-related protein 78
- XBP-1(s)
- X-box-binding protein 1 spliced
- eIF
- eukaryotic-initiating factor
- CHOP
- C/EBP homologous protein
- HMG-CoAR
- 3-hydroxy-3-methyl-glutaryl-CoA-reductase
- SREBP
- sterol regulatory-binding protein
- SR-B1
- scavenger receptor type B1
- SCD-1
- stearoyl-CoA-desaturase-1
- HCY
- homocysteine
- DMEM
- Dulbecco's modified Eagle's medium
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling
- CD
- choline-deficient
- wt
- wild type.
REFERENCES
- 1.Hajer G. R., van der Graaf Y., Olijhoek J. K., Verhaar M. C., Visseren F. L. (2007) Heart 93, 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulsen M., Yesilova Z., Bagci S., Uygun A., Ozcan A., Ercin C. N., Erdil A., Sanisoglu S. Y., Cakir E., Ates Y., Erbil M. K., Karaeren N., Dagalp K. (2005) J. Gastroenterol. Hepatol. 20, 1448–1455 [DOI] [PubMed] [Google Scholar]
- 3.Björck J., Hellgren M., Råstam L., Lindblad U. (2006) Metabolism 55, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 4.Gaull G., Sturman J. A., Schaffner F. (1974) J. Pediatr. 84, 381–390 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert K., Nehmé J., Bourdon E., Pivert G., Friguet B., Delcayre C., Delabar J. M., Janel N. (2005) Gastroenterology 128, 1405–1415 [DOI] [PubMed] [Google Scholar]
- 7.Werstuck G. H., Lentz S. R., Dayal S., Hossain G. S., Sood S. K., Shi Y. Y., Zhou J., Maeda N., Krisans S. K., Malinow M. R., Austin R. C. (2001) J. Clin. Invest. 107, 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji C., Kaplowitz N. (2003) Gastroenterology 124, 1488–1499 [DOI] [PubMed] [Google Scholar]
- 9.Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. (1991) N. Engl. J. Med. 324, 1149–1155 [DOI] [PubMed] [Google Scholar]
- 10.Lonn E., Yusuf S., Arnold M. J., Sheridan P., Pogue J., Micks M., McQueen M. J., Probstfield J., Fodor G., Held C., Genest J., Jr. (2006) N. Engl. J. Med. 354, 1567–1577 [DOI] [PubMed] [Google Scholar]
- 11.Bønaa K. H., Njølstad I., Ueland P. M., Schirmer H., Tverdal A., Steigen T., Wang H., Nordrehaug J. E., Arnesen E., Rasmussen K. (2006) N. Engl. J. Med. 354, 1578–1588 [DOI] [PubMed] [Google Scholar]
- 12.Hirsch S., Poniachick J., Avendaño M., Csendes A., Burdiles P., Smok G., Diaz J. C., de la Maza M. P. (2005) Nutrition 21, 137–141 [DOI] [PubMed] [Google Scholar]
- 13.Zhang K., Kaufman R. J. (2004) J. Biol. Chem. 279, 25935–25938 [DOI] [PubMed] [Google Scholar]
- 14.Zhang K., Kaufman R. J. (2006) Neurology 66, S102–109 [DOI] [PubMed] [Google Scholar]
- 15.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marciniak S. J., Ron D. (2006) Physiol. Rev. 86, 1133–1149 [DOI] [PubMed] [Google Scholar]
- 17.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 18.Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 19.Perlmutter D. H. (2002) J. Clin. Invest. 110, 1579–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tardif K. D., Mori K., Siddiqui A. (2002) J. Virol. 76, 7453–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Jhaveri R., Huang J., Qi Y., Diehl A. M. (2007) Lab. Invest. 87, 927–937 [DOI] [PubMed] [Google Scholar]
- 22.Kim D. S., Jeong S. K., Kim H. R., Kim D. S., Chae S. W., Chae H. J. (2007) Biochem. Biophys. Res. Commun. 363, 140–145 [DOI] [PubMed] [Google Scholar]
- 23.Wang D., Wei Y., Pagliassotti M. J. (2006) Endocrinology 147, 943–951 [DOI] [PubMed] [Google Scholar]
- 24.Outinen P. A., Sood S. K., Liaw P. C., Sarge K. D., Maeda N., Hirsh J., Ribau J., Podor T. J., Weitz J. I., Austin R. C. (1998) Biochem. J. 332, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mato J. M., Lu S. C. (2007) Hepatology 45, 1306–1312 [DOI] [PubMed] [Google Scholar]
- 26.Henning S. M., McKee R. W., Swendseid M. E. (1989) J. Nutr. 119, 1478–1482 [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Kramer P. M., Yang S., Pereira M. A., Tao L. (2001) J. Chromatogr. B Biomed. Sci. Appl. 762, 59–65 [DOI] [PubMed] [Google Scholar]
- 28.Liu R., Pan X., Whitington P. F. (2009) Liver Int. 29, 337–343 [DOI] [PubMed] [Google Scholar]
- 29.Rizki G., Arnaboldi L., Gabrielli B., Yan J., Lee G. S., Ng R. K., Turner S. M., Badger T. M., Pitas R. E., Maher J. J. (2006) J. Lipid Res. 47, 2280–2290 [DOI] [PubMed] [Google Scholar]
- 30.Yang H., Sadda M. R., Li M., Zeng Y., Chen L., Bae W., Ou X., Runnegar M. T., Mato J. M., Lu S. C. (2004) Hepatology 40, 221–231 [DOI] [PubMed] [Google Scholar]
- 31.Rahman S. M., Schroeder-Gloeckler J. M., Janssen R. C., Jiang H., Qadri I., Maclean K. N., Friedman J. E. (2007) Hepatology 45, 1108–1117 [DOI] [PubMed] [Google Scholar]
- 32.Ji C., Shinohara M., Kuhlenkamp J., Chan C., Kaplowitz N. (2007) Hepatology 46, 1586–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O K., Lynn E. G., Chung Y. H., Siow Y. L., Man R. Y., Choy P. C. (1998) Biochim. Biophys. Acta 1393, 317–324 [DOI] [PubMed] [Google Scholar]
- 34.Shivapurkar N., Wilson M. J., Hoover K. L., Mikol Y. B., Creasia D., Poirier L. A. (1986) J. Natl. Cancer Inst. 77, 213–217 [PubMed] [Google Scholar]
- 35.Igolnikov A. C., Green R. M. (2006) J. Hepatol. 44, 586–592 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.