Abstract
Interleukin (IL)-25, a member of the IL-17 family of cytokines, is expressed in the brains of normal mice. However, the cellular source of IL-25 and its function in the brain remain to be elucidated. Here, we show that IL-25 plays an important role in preventing infiltration of the inflammatory cells into the central nervous system. Brain capillary endothelial cells (BCECs) express IL-25. However, it is down-regulated by inflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-17, interferon-γ, IL-1β, and IL-6 in vitro, and is also reduced in active multiple sclerosis (MS) lesions and in the inflamed spinal cord of experimental autoimmune encephalomyelitis, an animal model of MS. Furthermore, IL-25 restores the reduced expression of tight junction proteins, occludin, junction adhesion molecule, and claudin-5, induced by TNF-α in BCECs and consequently repairs TNF-α-induced blood-brain barrier (BBB) permeability. IL-25 induces protein kinase Cϵ (PKCϵ) phosphorylation, and up-regulation of claudin-5 is suppressed by PKCϵ inhibitor peptide in the IL-25-stimulated BCECs. These results suggest that IL-25 is produced by BCECs and protects against inflammatory cytokine-induced excessive BBB collapse through a PKCϵ-dependent pathway. These novel functions of IL-25 in maintaining BBB integrity may help us understand the pathophysiology of inflammatory brain diseases such as MS.
Introduction
Interleukin (IL)2-25, originally named IL-17E, is a member of the IL-17 family of cytokines. IL-25 is produced by cells that are associated with allergic immune responses, including activated T helper type 2 (Th2) cells, mast cells, alveolar macrophages, eosinophils, basophils, and lung epithelial cells (1–5). Unlike other IL-17 family cytokines, including IL-17A and IL-17F, IL-25 induces Th2 immune responses such as IL-4, IL-5, and IL-13 production, blood eosinophilia, and IgE production (2). IL-25-deficient mice are more susceptible to parasitic helminthes such as Trichuris muris and Nippostrongylus brasiliensis due to reduced Th2 cytokine production (6, 7). The enforced expression of IL-25 in the lung enhances antigen-induced Th2 cytokine production and airway inflammation (8). Furthermore, transgenic overexpression of IL-25 leads to mucus production and airway infiltration of macrophages and eosinophils (1). Collectively, because IL-25 enhances allergic inflammation by inducing Th2-type immune responses, previous studies have focused on its biological role as a Th2 immune mediator.
Blood-brain barrier (BBB) disintegrity is commonly associated with synaptic and neuronal dysfunction in various disorders, such as multiple sclerosis (MS), Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis (9, 10). The role of BBB disruption has been investigated in these neuronal disorders, especially in MS and its animal model, experimental autoimmune encephalomyelitis (EAE), in which BBB disruption is associated with an increased infiltration of autoreactive inflammatory immune cells, resulting in tissue destruction and neurological impairment (11). Disruption of tight junction (TJ) molecules such as occludin, junctional adhesion molecule (JAM), and claudin-5 by inflammatory cytokines, including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1β, and IL-17, is thought to contribute to the transmigration of inflammatory lymphocytes (12–17). For example, Fabry et al. (13) showed that TNF-α, IFN-γ, and IL-1β significantly up-regulated leukocyte migration through the BBB by reducing occludin. Afonso et al. (12) indicated that TNF-α and IL-1β decreased occludin expression and increased endothelial cell permeability and transcellular migration of T lymphocytes. In addition, Förster et al. (14) demonstrated that TNF-α had an inhibitory effect on occludin and claudin-5 expression in human brain capillary endothelial cells (BCECs). Consistent with these findings, TNF-deficient mice have delayed EAE onset compared with wild-type mice, suggesting that TNF-α plays an important role in inflammatory T cell infiltration into the central nervous system (18). Recently, Th17 cytokines, IL-17 and IL-22, were reported to down-regulate zonula occludens-1 and occludin expression in human BCECs and to promote inflammatory T cell infiltration (15). Taken together, these results suggest that inflammatory cytokines play a key role in enhancing BBB permeability in MS and EAE.
Interestingly, IL-25 mRNA is also detected in the whole brain tissue of normal mice (19), despite the fact that the brain is a fundamentally immunoprivileged site and is not correlated with allergic inflammation. Moreover, it has been reported that IL-25-deficient mice are highly susceptible to EAE because of reduced IL-13 production (20). These findings suggest that IL-25 plays an important role in the maintenance of the central nervous system, especially in the development of MS or EAE. Although IL-25-deficient mice with EAE die (20), those without immunization survive (21) even though they have an increased number of Th17 in periphery. Thus, there may be some suppressive mechanisms that require IL-25 to induce inflammation in the central nervous system.
Here, we show that IL-25 controls BBB disruption induced by TNF-α in the BCEC line, MBEC4, and primary BCECs. A permeability assay and transmigration assay confirm that IL-25 suppresses the TNF-α-induced increase in permeability and T cell migration across the BBB. IL-25 increases TJ proteins, occludin, JAM, and claudin-5, and restores the reduced expression of them, induced by TNF-α through a protein kinase Cϵ (PKCϵ)-mediated pathway. Additionally, RT-PCR and immunohistochemistry indicate that the IL-25-expressing cells are endothelial cells in the brain. Collectively, these results suggest that IL-25 produced by BCECs protects from TNF-α-induced excessive collapse of the BBB and inflammatory CD4+ T cell infiltration across the BBB in MS or EAE.
EXPERIMENTAL PROCEDURES
Reagents
Bisindolylmaleimide I, Gö6976, and PKCϵ inhibitor peptide were all from Merck. The following antibodies were used: anti-mouse von Willebrand factor (vWF) (Millipore); anti-mouse IL-17E and p-PKCϵ (Santa Cruz Biotechnology); anti-mouse PKCϵ and phycoerythrin-conjugated anti-mouse CD31 (BD Biosciences); anti-mouse occludin and claudin-5 (Invitrogen); anti-mouse JAM (R & D Systems); and anti-mouse α-tubulin (Sigma). Recombinant mouse IL-17E, TNF-α, IL-17, IFN-γ, IL-1β, IL-6, transforming growth factor (TGF)-β, IL-4, IL-10, and IFN-β were purchased from R & D Systems. Lipopolysaccharide (LPS) and endothelial cell growth supplement were from Sigma.
Cell Cultures
All animal experiment protocols were approved by the Animal Experiment Committee of Nagoya University. All primary cell cultures were prepared from C57BL/6J mice (Japan SLC).
Primary microglia were isolated from mixed glial cell cultures prepared from newborn mice on day 14 using the “shaking off” method as described previously (22, 23). The purity of the cultures was almost 100%, as determined by immunostaining with anti-CD11b antibodies.
Astrocytes were prepared as described previously (24). Briefly, mixed glial cell cultures were trypsinized after the microglia were collected and replated in Petri dishes. Cultures that had undergone two passages were used as astrocytes. The purity of the cultures was greater than 95% as determined by immunostaining with anti-glial fibrillary acidic protein antibodies.
Primary neurons were prepared from the neocortices of embryonic day 17 embryos as described previously (25, 26). The purity of the cultures was more than 95% as determined by NeuN-specific immunostaining. Primary meningeal fibroblasts were prepared by mincing meninges of newborn mice after removing the blood vessels and then cultured for 10 days before each assay.
Primary BCECs were purified from adult mice as reported by Ge et al. (27) and Ohtsuki et al. (28). In brief, the cerebrums were homogenized and separated by a 16% dextran solution. This homogenate was centrifuged, and the resulting pellet was filtered through a 40-μm cell strainer. The microvessels retained in the cell strainer were then digested in PBS containing 0.5 mg/ml Liberase Blendzyme Type I (Roche Applied Science) and 120 units/ml DNase (Sigma) at 37 °C for 30 min. CD31-positive cells were then sorted using the magnetic-activated cell sorting (MACS) system. The purified cells were washed and resuspended in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 10% horse serum, 50 μg/ml heparin, and endothelial cell growth supplement (Sigma) and then plated onto tissue culture dishes coated with collagen (Roche Applied Science). The purity was >98%, as determined by DiI-labeled acetylated low density lipoprotein uptake.
The BCEC line, MBEC4 (a kind gift from Dr. T. Tsuruo) (29), was maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and used as an established BBB model.
RNA Isolation and Semi-quantitative RT-PCR
Total RNA was extracted from all primary cells and SV40-transduced human BCEC lines (established by T. Kanda, Yamaguchi University, Japan) using the RNeasy mini kit (Qiagen). After 0.2 μg of total RNA was denatured for 5 min at 65 °C, the RT reaction was performed as described previously (22).
Semi-quantitative PCR was performed using AmpliTaq® DNA polymerase (Applied Biosystems) as reported previously (24). Primer sets specific for mouse IL-25, mouse GAPDH, human IL-25, and human GAPDH were as follows: mouse IL-25 forward, 5′-ATGTACCAGGCTGTTGCATTCTTG, and mouse IL-25 reverse, 5′-CTAAGCCATGACCCGGGGCCGCACACACAC; mouse GAPDH forward, 5′-ACTCACGGCAAATTCAACG, and mouse GAPDH reverse, 5′-CCCTGTTTGCTGTAGCCGTA; human IL-25 forward, 5′-CGACCCAGATTAGGTGAGGA, and human IL-25 reverse, 5′-TCCATCTTCACTGGCCCTAC; and human GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT, and Human GAPDH reverse, 5′-TTGATTTTGGAGGGATCTCG.
Real time RT-PCR was performed as described previously (22). Primer sets specific for mouse IL-25 and mouse GAPDH were as follows: mouse IL-25 forward, 5′-GGATGGCCCCCTCAA-CA, and mouse IL-25 reverse, 5′-CGATTCAAGTCCCGTCCAACT; mouse GAPDH forward, 5′-TGTGTCCGTCGTGGATCTGA, and mouse GAPDH reverse, 5′-CCTGCTTCACCACCTTCTTGA.
EAE
Myelin oligodendrocyte glycoprotein (MOG)-induced EAE was generated as described previously (30, 31). In brief, mice were injected subcutaneously with 0.2 ml of emulsion containing 200 μg of MOG-(35–55) in PBS combined with an equal volume of complete Freund's adjuvant (CFA) containing 300 μg of heat-killed Mycobacterium tuberculosis H37Ra. Mice were injected with pertussis toxin intraperitoneally on the day of immunization and 2 days after immunization (200 ng/mouse). EAE mice showed disease onset 14 days after immunization, which peaked 18 days post-immunization as shown in supplemental Fig. 1.
For passive transfer, splenocytes were harvested from C57BL/6 mice 12 days after immunization with MOG and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine, and sodium pyruvate in the presence of MOG-(35–55) for 4 days. Then CD4+ T cells were sorted using CD4+ microbeads (Miltenyi Biotec) with >95%. Dead cells were removed by Ficoll (GE Healthcare). Cells were then washed and injected intravenously into naive C57BL/6 mice (5 × 106 cells/mouse). Animals received 200 ng/mouse pertussis toxin on day 0 and day 2 after transfer. IL-25 was administered at 500 ng/mouse/day from day −2 to day 14 every other day.
BBB Permeability and CD4+ T Cell Transmigration Assay
The permeability of MBEC4 and primary BCEC monolayers was evaluated using the permeability marker, Evan's blue albumin (EBA), as described previously (32) and by measuring transendothelial electrical resistance (TER). Confluent monolayers of MBEC4 cells on the Transwell inserts (3-μm pore size) were incubated with or without 50 ng/ml TNF-α and 50 ng/ml IL-25 for 24 h. The monolayers were then washed with assay buffer (118 mm NaCl, 4.7 mm KCl, 1.3 mm CaCl2, 1.2 mm MgCl2, 1.0 mm NaH2PO4, 25 mm NaHCO3, and 11 mm d-glucose (pH 7.4)). This buffer (1 ml) was added to the outside of the insert (the abluminal side). Assay buffer containing 4% bovine serum albumin (Sigma) mixed with 0.67 mg/ml Evan's blue dye (Sigma) was loaded on the luminal side of the insert for 1 h. The concentration of EBA in the abluminal chamber was determined by measuring the absorbance of aliquots at 630 nm with a microplate reader. Clearance was calculated as follows: volume (μl) = VA × [C]A/[C]L, where VA is the volume of the abluminal side, [C]A is the concentration of EBA on the abluminal side, and [C]L is the concentration of EBA on the luminal side.
TER was measured using a Millicell®-ERS (Millipore). Resistances of blank filters were subtracted from those of filters with cells before final resistances (Ω/cm2) were calculated.
To assess CD4+ T cell transmigration, we sorted CD4+ T cells from the spleens of EAE mice using the MACS system. After 500 μl of RPMI 1640 medium containing 10% fetal bovine serum, 2 mm l-glutamine, and 1 mm sodium pyruvate was added to the abluminal side, sorted CD4+ T cells were then loaded onto the luminal side and incubated for 24 h at 37 °C, 5% CO2. Following migration, 50 μl of 0.5 m EDTA was added to the abluminal side, and the plates were placed on a shaker for 15 min to mobilize cells. Cells in the abluminal side were then harvested and counted by flow cytometry (Cytomics FC500, Beckman Coulter).
Western Blotting
Cells were lysed in TNES buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, and 0.1% SDS) with a protease inhibitor mixture (Roche Applied Science) and a phosphatase inhibitor mixture (Sigma). Thirty μg of protein from the total lysate was examined by Western blotting as described previously (31).
Immunocytochemistry
Primary BCECs were plated onto collagen-coated Aclar® fluoroplastic coverslips (Honeywell International). After fixing with 2% paraformaldehyde for 10 min, cells were incubated with rabbit anti-mouse vWF and then visualized by Alexa 488-conjugated anti-rabbit IgG (Invitrogen) as described previously (22). Cells were examined with a deconvolution fluorescence microscope system (BZ-8000, Keyence).
Immunohistochemistry
Frozen brain and spinal cord sections from normal mice were fixed with 4% paraformaldehyde and stained for IL-17E and vWF followed by Alexa 488-conjugated anti-goat IgG and Alexa 568-conjugated anti-rabbit IgG as described previously (25, 26). The stained sections were examined with a deconvolution fluorescence microscope system (BZ-8000, Keyence) and analyzed by Dynamic Cell Count image analysis program (Keyence).
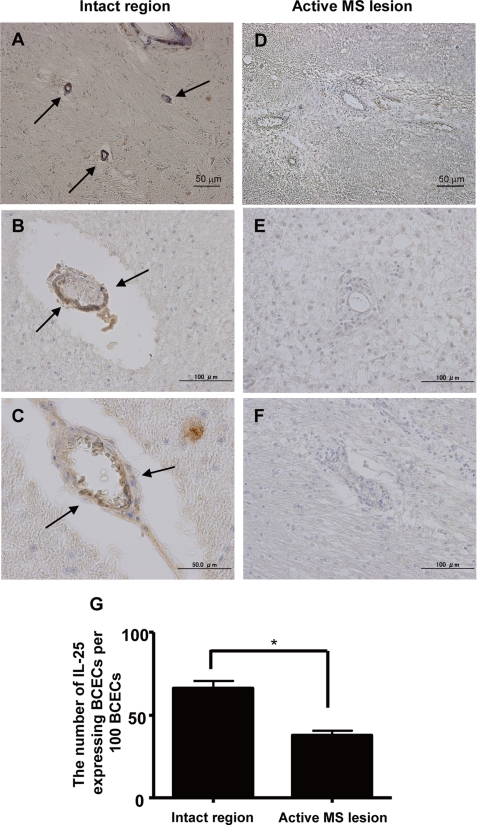
For neuropathologic examination of MS patients, sections from paraffin-embedded blocks of the cerebrum were stained with hematoxylin and eosin and subjected to IL-25 immunostaining with the labeled StreptAvidin-Biotin method. In brief, sections from paraffin-embedded blocks of the cerebrum from three MS patients (46 years old, female with a 3-year history; 54 years old, female with a 24-year history; and 71 years old, female with a 1-month history) were stained with hematoxylin and eosin and subjected to IL-25 immunostaining. Eight-micrometer-thick sections were placed on silanized slides (Dako, Glostrup, Denmark). Immunohistochemistry for IL-25 was performed with monoclonal antibody against mouse IL-17E (dilution, 1:100; Santa Cruz Biotechnology) and the labeled StreptAvidin-Biotin method. After deparaffinization, nonspecific endogenous peroxidase activity was blocked by pretreatment with 3% H2O2 for 5 min. For antigen retrieval, the sections were treated at 100 °C for 30 min in beakers filled with 10 mm citrate buffer. After the temperature had decreased to 60 °C slowly over 1 h, the sections were removed. After a wash in PBS, Fc receptors were blocked with 10% normal nonimmune goat serum (Dako) for 30 min at room temperature prior to incubation with IL-25 antibody. Sections were treated with anti-IL-25 monoclonal antibody for 2 h at room temperature and washed in PBS. Sections were then incubated at room temperature with biotin-conjugated anti-goat IgG for 20 min and washed in PBS, followed by incubation at room temperature with streptavidin-horseradish peroxidase for 20 min. Immunoreactivity was visualized with 3,3′-diaminobenzidine/peroxidase (Wako). IL-25-positive endothelial cells were enumerated in both active MS lesions identified as demyelinated area with cellular infiltration and without these findings (intact area) in hematoxylin and eosin.
Statistical Analysis
Differences between the means of experimental groups were analyzed using the two-tailed Student's t test with Welch's correction.
RESULTS
IL-25 Is Expressed in BCECs
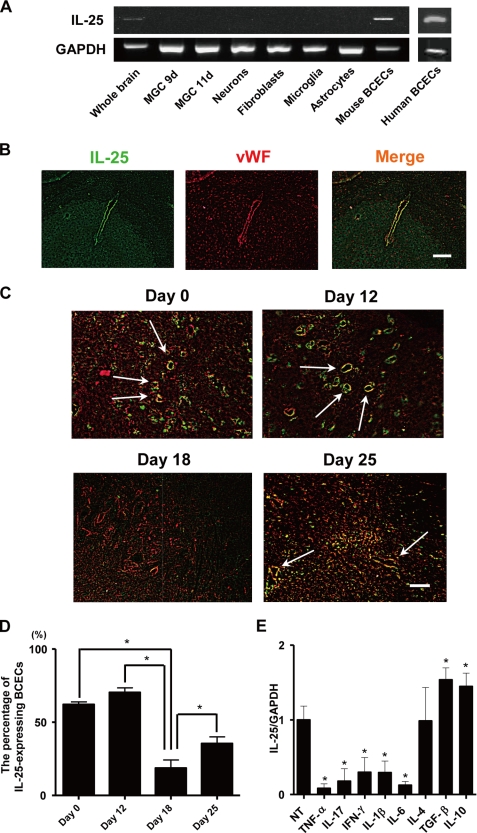
We confirmed that IL-25 mRNA was expressed in whole brain tissue (Fig. 1A). We then identified which cells express IL-25 mRNA using mouse primary cells derived from the brain. Mixed glial cell cultures that had been cultured for 9 days (including oligodendrocyte precursors) and 11 days (including oligodendrocytes), neurons, fibroblasts, astrocytes, and microglia, did not express IL-25 mRNA (Fig. 1A). Although lung macrophages reportedly express IL-25, microglia did not express IL-25 mRNA even after stimulation with LPS, IFN-γ, TNF-α, IL-6, TGF-β, IL-4, or IFN-β (supplemental Fig. 2). However, mouse BCECs expressed IL-25 mRNA. Human BCECs also expressed IL-25 mRNA (Fig. 1A). Immunohistochemistry revealed that IL-25-positive cells in the brain were also vWF-positive, indicating that the IL-25-expressing cells were BCECs (Fig. 1B). IL-25-expressing endothelial cells were clearly observed in the lumbar spinal cord of normal mice and mice before the onset of EAE (12 days after immunization with MOG/CFA) (Fig. 1, C and D). These cells were markedly reduced after the development of EAE (18 days after immunization), but IL-25 expression significantly increased in the EAE recovery phase (25 days after immunization) (Fig. 1, C and D). IL-25 administration ameliorated EAE induced by passive transfer of MOG-reactive CD4+ T cells (supplemental Fig. 3).
FIGURE 1.
IL-25 is expressed in BCECs. A, total RNA was extracted from the whole brain, primary cells, including mixed glial cells cultured for 9 and 11 days (MGC 9d and MGC 11d), neurons, astrocytes, microglia, mouse BCECs, and human BCEC lines. IL-25 and GAPDH mRNA expression was analyzed by RT-PCR. B, frozen brain sections were prepared from normal mice. After fixing with 4% paraformaldehyde, sections were stained against IL-25 (green) and vWF (red). Scale bar, 100 μm. C, frozen sections of the lumbar spinal cord were prepared from mice on the day of MOG/CFA immunization, 12 days (before the onset of EAE), 18 days (after the development of EAE), and 25 days (recovery phase of EAE) after immunization. Arrows show IL-25-expressing endothelial cells. Scale bar, 100 μm. D, results of C were quantified and graphed as the percentage of IL-25-expressing endothelial cells of total endothelial cells. E, MBEC4 cells were treated with or without TNF-α (50 ng/ml), IL-17 (50 ng/ml), IFN-γ (5 ng/ml), IL-1β (20 ng/ml), IL-6 (30 ng/ml), IL-4 (20 ng/ml), TGF-β (5 ng/ml), and IL-10 (20 ng/ml) for 24 h. Following RNA extraction, IL-25 mRNA expression was assessed by real time RT-PCR. The expression levels in untreated cells were set to 1. Data are represented as the means ± S.E. *, p < 0.05; n = 3. NT, not treated.
To further explore whether inflammatory cytokines down-regulated IL-25 expression in BCECs, we assessed IL-25 mRNA expression by real time RT-PCR in the BCEC line, MBEC4. Although TNF-α, IL-17, IFN-γ, IL-1β, and IL-6 reduced IL-25 mRNA expression in MBEC4 cells, TGF-β and IL-10 increased it (Fig. 1E). In addition, a decrease of IL-25 expression in BCECs was also observed in the active MS lesion (Fig. 2, A–F). The number of IL-25-expressing endothelial cells in the severe MS lesion was significantly less than in the normal appearing white matter (Fig. 2G). Thus, these results suggest that IL-25 is expressed by BCECs and may play a novel role in maintaining BBB function to block inflammatory cell infiltration into the central nervous system.
FIGURE 2.
IL-25 expression is also down-regulated in active MS lesion. Human IL-25 expression was examined in the brain sections of active MS with marked inflammation and chronic MS with hypocellular fibrotic lesion and/or well demarcated edge (arrow, brown). We enumerated IL-25-expressing BCECs in normal appearing white matter without accompanying cell infiltration (A–C) and in active MS lesions (D–F). G, results are expressed graphically as the number of IL-25-expressing endothelial cells per 100 endothelial cells. Data are represented as the means ± S.E. *, p < 0.05.
IL-25 Prevents TNF-α-induced Permeability of the BBB
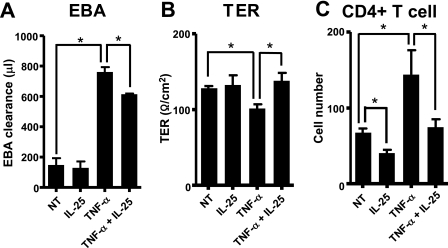
To confirm whether IL-25 rescues TNF-α-disrupted BBB functions, we performed a permeability assay and CD4+ T cell transmigration assay by measuring the levels of EBA, TER, and the number of transmigrated CD4+ T cells through confluent layers of MBEC4 cells. TNF-α increased the permeability of EBA and the transmigration of CD4+ T cells and decreased TER. Adding IL-25 to TNF-α-stimulated BCECs significantly reduced EBA levels on the abluminal side of the well (the outside of the insert) and up-regulated the electrical resistance of BCECs compared with cells treated with TNF-α alone (Fig. 3, A and B). Moreover, the addition of IL-25 significantly decreased TNF-α-induced CD4+ T cell transmigration across the BBB (Fig. 3C). Although IL-25 alone did not significantly change the levels of EBA and TER (Fig. 3, A and B), it significantly suppressed the number of transmigrated CD4+ T cells on the abluminal side compared with the unstimulated control (Fig. 3C). In addition, BCECs expressed the IL-25 receptor, and TNF-α did not change the expression levels (supplemental Fig. 4). These results further support the notion that IL-25 prevents the disturbance of BBB permeability.
FIGURE 3.
IL-25 suppresses TNF-α-induced permeability in MBEC4 cells. MBEC4 cells were treated with TNF-α alone (50 ng/ml), IL-25 alone (50 ng/ml), or both TNF-α and IL-25 for 24 h. A, EBA was loaded onto the luminal side of the insert for 1 h, and then the EBA levels on the abluminal side were analyzed. B, TER was measured using an electrical resistance system. C, CD4+ T cells were loaded onto the luminal side for 24 h. CD4+ T cell numbers on the abluminal side were then analyzed by flow cytometry. Data are represented as the means ± S.E. *, p < 0.05; n = 4. NT, not treated.
IL-25 Blocks the Impaired Expression of TJ Proteins Caused by TNF-α in BCECs
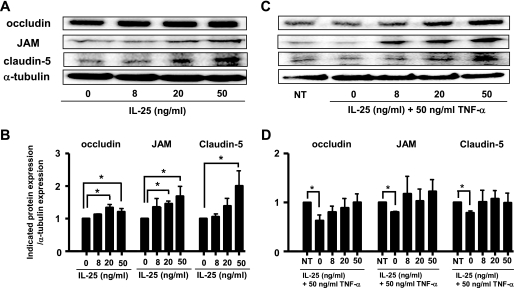
Many reports indicate that TNF-α down-regulates TJ protein expression in human and mouse BCECs (12–14). To elucidate whether IL-25 affects this reduction in TJ protein expression by TNF-α, we assessed the effect of IL-25 on the expression of occludin, JAM, and claudin-5. IL-25 significantly increased their expression levels in a dose-dependent manner (Fig. 4, A and B). Among these proteins, claudin-5 expression was most strongly enhanced by IL-25. Furthermore, IL-25 dose-dependently reversed the TNF-α-induced down-regulation of occludin, JAM, and claudin-5 protein levels (Fig. 4, C and D). IL-25 restored the TNF-α-induced decrease of occludin expression more effectively than any other molecules that have been reported as stabilizers of BBB such as IFN-β, TGF-β, dexamethasone, angiotensin II, and simvastatin (supplemental Fig. 5). IL-25 more effectively restored the TNF-α-induced reduction of JAM and claudin-5 proteins as the restoration of occludin (data not shown).
FIGURE 4.
IL-25 up-regulates the expression of TJ proteins, occludin, JAM, and claudin-5. MBEC4 cells were incubated with or without graded doses of IL-25 alone (A) or with or without graded doses of IL-25 plus TNF-α (C) for 24 h. The expression of occludin, JAM, and claudin-5 in whole cell lysates was analyzed by Western blotting. The results of A and C are expressed graphically in B and D, respectively. The expression levels in untreated cells were set to 1. Data are represented as the means ± S.E. *, p < 0.05; n = 4. NT, not treated.
To further clarify whether IL-25 has the same effect on primary BCECs, these cells were purified using MACS system. The final purity of the primary BCECs was approximately >90%, as determined by morphology, vWF staining, and low density lipoprotein uptake (supplemental Fig. 6, A–C). As found in MBEC4 cells, IL-25 increased the expression of occludin, JAM, and claudin-5 in purified primary BCECs and reversed the TNF-α-induced decrease in their expression levels (supplemental Fig. 6, D and E). Collectively, these results suggest that IL-25 blocks TNF-α-induced disruption of BBB function by up-regulating the expression of occludin, JAM, and claudin-5 in MBEC4 cells and primary BCECs.
Induction of Claudin-5 by IL-25 Occurs through a PKCϵ-dependent Pathway
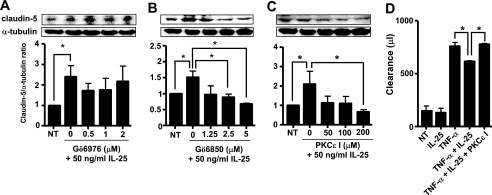
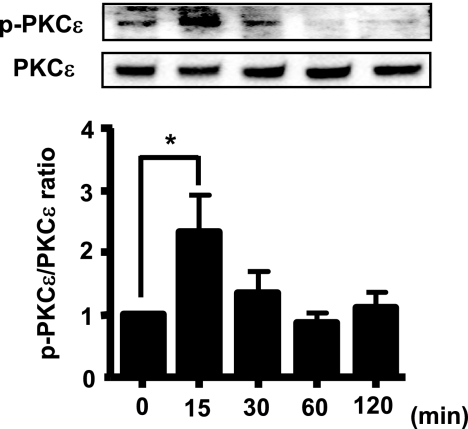
PKC isozymes are reported to be involved in TJ assembly (33). Moreover, BCECs specifically express claudin-5 in large amounts, suggesting that claudin-5 may be associated with BBB formation (34). Thus, to elucidate the IL-25 signaling pathways that augment TJ protein expression, we performed the inhibitory assay using bisindolylmaleimide I (Gö6850) (an inhibitor of PKCα, -βI, -δ, and -ϵ) and Gö6976 (an inhibitor of PKCα, -βI, and -μ). Bisindolylmaleimide I inhibited IL-25-induced claudin-5 expression in a dose-dependent manner, although Gö6976 had no effect, suggesting that IL-25-induced claudin-5 expression was mediated through PKCδ or -ϵ (Fig. 5, A and B, and summarized in supplemental Table 1). PKCϵ inhibitor peptide also dose-dependently suppressed IL-25-induced claudin-5 expression (Fig. 5C). In addition, PKCϵ inhibitor peptide significantly reduced the ability of IL-25 to reverse TNF-α-up-regulated permeability of EBA (Fig. 5D). In fact, stimulation with IL-25 strongly induced PKCϵ phosphorylation (p-PKCϵ) at 15 min (Fig. 6). Collectively, these results suggest that IL-25 rescues TNF-α-induced disruption of the BBB through a PKCϵ-dependent pathway.
FIGURE 5.
Claudin-5 induction by IL-25 is mediated by PKCϵ. A–C, MBEC4 cells were treated with IL-25 (50 ng/ml) for 24 h following pretreatment with graded doses of Gö6976 (A), bisindolylmaleimide I (Gö6850) (B), and PKCϵ inhibitor peptide (C). Claudin-5 expression in whole cell lysates was analyzed by Western blotting. D, MBEC4 cells were pretreated with PKCϵ inhibitor peptide (200 μm) and then stimulated with TNF-α (50 ng/ml) and IL-25 (50 ng/ml) or IL-25 alone for 24 h. EBA was then loaded onto the luminal side of the insert for 1 h, and the levels of EBA on the abluminal side were analyzed. Data are represented as the means ± S.E. *, p < 0.05; n = 4.
FIGURE 6.
IL-25 induces PKCϵ phosphorylation. MBEC4 cells were incubated with IL-25 (50 ng/ml) for 0–120 min. p-PKCϵ and PKCϵ expression levels were analyzed by Western blotting. Data are represented as the means ± S.E. *, p < 0.05; n = 4.
DISCUSSION
IL-25 is known to induce Th2 responses and to be produced by cells related to allergic responses such as Th2 cells, mast cells, and lung macrophages stimulated with anti-CD3/CD28, IgE, and allergens (1, 3, 4). These findings suggest that IL-25 is an inflammatory mediator during Th2-mediated inflammation. On the other hand, a previous study indicated that IL-25 mRNA was expressed in the whole brain tissue of normal mice (19), indicating that IL-25 also plays a distinct role in the central nervous system. Consistent with this hypothesis, in this study we revealed that IL-25 protected excessive disruption of the BBB, resulting in decreased inflammatory T cell infiltration. Kleinschek et al. (20) showed that IL-25-deficient mice immunized with MOG peptide/CFA had increased inflammatory T cell infiltration and a high mortality rate. These findings support our results that IL-25 reverses excessive BBB disruption and inflammatory T cell infiltration. Although Kleinschek et al. (20) also showed that IL-25 was produced by microglia, we found that unstimulated microglia did not express IL-25 mRNA. Additionally, we found that microglia did not express IL-25 mRNA even when stimulated with LPS, IFN-γ, IL-17, TNF-α, IL-6, IL-4, or IFN-β in vitro (supplemental Fig. 2). We also previously reported that microglia did not produce the Th2 cytokine, IL-4 (35). Microglia produced IL-5 at low levels, just above the detection limit (= 5 pg/ml) (36). Thus, microglia probably do not favor Th2 cytokine production, especially under normal conditions. Our results do not exclude the possibility that other factors induce IL-25 in microglia. However, at least in normal mouse brain, IL-25 mRNA is expressed in BCECs. Accordingly, BCECs are able to regulate their own permeability via producing IL-25. Additionally, Kleinschek et al. (20) showed that IL-25 suppressed EAE through inducing production of IL-13 but not IL-4 (20). However, they also showed that IL-13-deficient mice immunized with MOG peptide/CFA survived, although all IL-25-deficient mice immunized died. This implies that IL-25 suppresses EAE not only through an IL-13-mediated pathway but also via another mechanism. It is possible that IL-25 suppresses EAE by ameliorating the disruption of BBB permeability.
Although in this study we showed that IL-25 augments BBB properties, other molecules have also been reported to increase TJ protein expression. TGF-β1 enhances BBB functions in MBEC4 cells (37). Human brain microvascular endothelial cells pretreated with IFN-β, an approved drug for the treatment of MS, decrease Th1 cell migration and suppress increased paracellular permeability of small molecules such as [3H]inulin and [14C]sucrose (38). Angiotensin II, which controls cerebral blood flow, memory retention, and neuronal regeneration, restricts the passage of molecular tracers across the BBB and is required for BBB maintenance (39). Glucocorticoid up-regulates occludin and claudin-5 expression and increases barrier properties (40). Statins, simvastatin and lovastatin, which are 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors and have an anti-inflammatory effect, reduce human BBB permeability (41). All of these molecules were reported previously to have a suppressive effect against MS or EAE or were established as effective drugs for treating these diseases. As IL-25 reportedly has a suppressive effect on EAE (20), and BBB disruption is critical in the development of the disease, these also support our findings that IL-25 restores disrupted BBB permeability. Moreover, IL-25 blocks the TNF-α-induced permeability more effectively than any other molecules by restoring the TNF-α-induced decrease of TJ protein expression (supplemental Fig. 5).
PKC signaling has been shown to affect endothelial barriers. PKC includes 12 isozymes that are classified into three subtypes by differences in their mechanisms of action: conventional (α, βI, βII, and γ), novel (δ, ϵ, θ, η, and μ), and atypical (λ, τ, and ζ). Rat primary BCECs reportedly express PKCα, -β, -δ, -ϵ, and -η isozymes (42). Some of these PKC isozymes appear to be associated with TJ assembly or openings in the BBB (33). Hypoxia/aglycemia-mediated permeability augmentation was inhibited by a conventional PKC inhibitor, Gö6976, suggesting that PKCα or -β signaling may enhance TJ opening (43). Alterations in permeability induced by chemokine monocyte chemoattractant protein-1 (MCP-1)/CCL2 were also mediated by PKCα (44). Furthermore, inhibiting PKCδ protects against hypertensive encephalopathy by preventing a breakdown of the BBB in rats (45), suggesting that PKCδ signaling may lead to BBB disruption. Moreover, it is reported that PKCη is up-regulated following hypoxia, which increases BBB paracellular permeability, suggesting that PKCη may also be involved in BBB disruption (46). Collectively, many of the PKC isozymes are reported to be associated with increased BBB permeability. However, to our knowledge, the role of PKCϵ in BBB maintenance has not been previously reported. In this study, we demonstrated for the first time that the activation of PKCϵ up-regulated claudin-5 and protected the BBB from TNF-α-induced disruption. In Caco-2 cells, a human colorectal carcinoma, epidermal growth factor-mediated activation of PKCϵ reportedly protects TJs from acetaldehyde (47), which support our findings in this study.
The content of the TJ proteins in the tissue or isolated BBB endothelial cells reportedly correlates well with barrier properties (48–51). The high TJ protein levels may account for the maintenance and protection of the BBB integrity. In this study, we showed that IL-25 increased the expression levels of occludin, JAM, and claudin-5 through PKCϵ-dependent signaling. Several pathways are reported as downstream of PKCϵ-dependent signaling. For example, Weiler et al. (52) demonstrated that PKC activation leads to the increased expression of occludin through MEK1/MEK2 signaling. On the other hand, phosphorylation and redistribution of tight junction proteins are reportedly required for the barrier function. Suzuki et al. (47) showed that PKCϵ enhanced epidermal growth factor-mediated redistribution and induction of occludin in Caco-2 cells. In addition, Yoo et al. (53) revealed that PKCϵ-dependent signaling pathway led to phosphorylation of occludin in T84 epithelia. These suggest that IL-25 protects BBB function via up-regulation and probably direct phosphorylation of tight junction proteins through PKCϵ-mediated pathway. In conclusion, our results suggest that IL-25 produced by BCECs protects from TNF-α-induced excessive collapse of the BBB and inflammatory T cell infiltration across the BBB through a PKCϵ-dependent pathway in MS or EAE.
Supplementary Material
Acknowledgment
MBEC4 cells were provided by T. Tsuruo (Japanese Foundation for Cancer Research).
This work was supported in part by Grant-in-aid for Scientific Research Grant C, by the 21st Century COE Program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by Health and Labour Science research grants for research on intractable diseases from the Ministry of Health, Labour, and Welfare of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–6.
- IL
- interleukin
- BBB
- blood-brain barrier
- BCEC
- brain capillary endothelial cell
- CFA
- complete Freund's adjuvant
- EAE
- experimental autoimmune encephalomyelitis
- EBA
- Evan's blue albumin
- JAM
- junctional adhesion molecule
- LPS
- lipopolysaccharide
- MACS
- magnetic-activated cell sorting
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- PKC
- protein kinase C
- TER
- transendothelial electrical resistance
- Th
- helper T cell
- TGF
- transforming growth factor
- TJ
- tight junction
- TNF
- tumor necrosis factor
- vWF
- von Willebrand factor
- IFN
- interferon
- RT
- reverse transcription
- PBS
- phosphate-buffered saline
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
REFERENCES
- 1.Angkasekwinai P., Park H., Wang Y. H., Wang Y. H., Chang S. H., Corry D. B., Liu Y. J., Zhu Z., Dong C. (2007) J. Exp. Med. 204, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fort M. M., Cheung J., Yen D., Li J., Zurawski S. M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., Muchamuel T., Hurst S. D., Zurawski G., Leach M. W., Gorman D. M., Rennick D. M. (2001) Immunity 15, 985–995 [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K., Nakajima H., Suzuki K., Kagami S., Hirose K., Suto A., Saito Y., Iwamoto I. (2003) Blood 101, 3594–3596 [DOI] [PubMed] [Google Scholar]
- 4.Kang C. M., Jang A. S., Ahn M. H., Shin J. A., Kim J. H., Choi Y. S., Rhim T. Y., Park C. S. (2005) Am. J. Respir. Cell Mol. Biol. 33, 290–296 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y. H., Angkasekwinai P., Lu N., Voo K. S., Arima K., Hanabuchi S., Hippe A., Corrigan C. J., Dong C., Homey B., Yao Z., Ying S., Huston D. P., Liu Y. J. (2007) J. Exp. Med. 204, 1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallon P. G., Ballantyne S. J., Mangan N. E., Barlow J. L., Dasvarma A., Hewett D. R., McIlgorm A., Jolin H. E., McKenzie A. N. (2006) J. Exp. Med. 203, 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owyang A. M., Zaph C., Wilson E. H., Guild K. J., McClanahan T., Miller H. R., Cua D. J., Goldschmidt M., Hunter C. A., Kastelein R. A., Artis D. (2006) J. Exp. Med. 203, 843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamachi T., Maezawa Y., Ikeda K., Kagami S., Hatano M., Seto Y., Suto A., Suzuki K., Watanabe N., Saito Y., Tokuhisa T., Iwamoto I., Nakajima H. (2006) J. Allergy Clin. Immunol. 118, 606–614 [DOI] [PubMed] [Google Scholar]
- 9.Zhong Z., Deane R., Ali Z., Parisi M., Shapovalov Y., O'Banion M. K., Stojanovic K., Sagare A., Boillee S., Cleveland D. W., Zlokovic B. V. (2008) Nat. Neurosci. 11, 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlokovic B. V. (2008) Neuron 57, 178–201 [DOI] [PubMed] [Google Scholar]
- 11.Fabis M. J., Scott G. S., Kean R. B., Koprowski H., Hooper D. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5656–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afonso P. V., Ozden S., Prevost M. C., Schmitt C., Seilhean D., Weksler B., Couraud P. O., Gessain A., Romero I. A., Ceccaldi P. E. (2007) J. Immunol. 179, 2576–2583 [DOI] [PubMed] [Google Scholar]
- 13.Fabry Z., Topham D. J., Fee D., Herlein J., Carlino J. A., Hart M. N., Sriram S. (1995) J. Immunol. 155, 325–332 [PubMed] [Google Scholar]
- 14.Förster C., Burek M., Romero I. A., Weksler B., Couraud P. O., Drenckhahn D. (2008) J. Physiol. 586, 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. (2007) Nat. Med. 13, 1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minagar A., Alexander J. S. (2003) Mult. Scler. 9, 540–549 [DOI] [PubMed] [Google Scholar]
- 17.Minagar A., Long A., Ma T., Jackson T. H., Kelley R. E., Ostanin D. V., Sasaki M., Warren A. C., Jawahar A., Cappell B., Alexander J. S. (2003) Endothelium 10, 299–307 [DOI] [PubMed] [Google Scholar]
- 18.Kassiotis G., Pasparakis M., Kollias G., Probert L. (1999) Eur. J. Immunol. 29, 774–780 [DOI] [PubMed] [Google Scholar]
- 19.Pan G., French D., Mao W., Maruoka M., Risser P., Lee J., Foster J., Aggarwal S., Nicholes K., Guillet S., Schow P., Gurney A. L. (2001) J. Immunol. 167, 6559–6567 [DOI] [PubMed] [Google Scholar]
- 20.Kleinschek M. A., Owyang A. M., Joyce-Shaikh B., Langrish C. L., Chen Y., Gorman D. M., Blumenschein W. M., McClanahan T., Brombacher F., Hurst S. D., Kastelein R. A., Cua D. J. (2007) J. Exp. Med. 204, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaph C., Du Y., Saenz S. A., Nair M. G., Perrigoue J. G., Taylor B. C., Troy A. E., Kobuley D. E., Kastelein R. A., Cua D. J., Yu Y., Artis D. (2008) J. Exp. Med. 205, 2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonobe Y., Liang J., Jin S., Zhang G., Takeuchi H., Mizuno T., Suzumura A. (2008) Biochem. Biophys. Res. Commun. 370, 129–133 [DOI] [PubMed] [Google Scholar]
- 23.Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. (1987) J. Neuroimmunol. 15, 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang J., Takeuchi H., Doi Y., Kawanokuchi J., Sonobe Y., Jin S., Yawata I., Li H., Yasuoka S., Mizuno T., Suzumura A. (2008) Brain Res. 1210, 11–19 [DOI] [PubMed] [Google Scholar]
- 25.Mizuno T., Zhang G., Takeuchi H., Kawanokuchi J., Wang J., Sonobe Y., Jin S., Takada N., Komatsu Y., Suzumura A. (2008) FASEB J. 22, 1797–1806 [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi H., Mizuno T., Zhang G., Wang J., Kawanokuchi J., Kuno R., Suzumura A. (2005) J. Biol. Chem. 280, 10444–10454 [DOI] [PubMed] [Google Scholar]
- 27.Ge S., Song L., Serwanski D. R., Kuziel W. A., Pachter J. S. (2008) J. Neurochem. 104, 1219–1232 [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuki S., Yamaguchi H., Katsukura Y., Asashima T., Terasaki T. (2008) J. Neurochem. 104, 147–154 [DOI] [PubMed] [Google Scholar]
- 29.Tatsuta T., Naito M., Oh-hara T., Sugawara I., Tsuruo T. (1992) J. Biol. Chem. 267, 20383–20391 [PubMed] [Google Scholar]
- 30.Sonobe Y., Jin S., Wang J., Kawanokuchi J., Takeuchi H., Mizuno T., Suzumura A. (2007) Tohoku J. Exp. Med. 213, 329–339 [DOI] [PubMed] [Google Scholar]
- 31.Wang J., Takeuchi H., Sonobe Y., Jin S., Mizuno T., Miyakawa S., Fujiwara M., Nakamura Y., Kato T., Muramatsu H., Muramatsu T., Suzumura A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 3915–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohgu S., Nishioku T., Sumi N., Takata F., Nakagawa S., Naito M., Tsuruo T., Yamauchi A., Shuto H., Kataoka Y. (2007) Cell. Mol. Neurobiol. 27, 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Mariscal L., Tapia R., Chamorro D. (2008) Biochim. Biophys. Acta 1778, 729–756 [DOI] [PubMed] [Google Scholar]
- 34.Morita K., Sasaki H., Furuse M., Tsukita S. (1999) J. Cell Biol. 147, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzumura A., Sawada M., Itoh Y., Marunouchi T. (1994) J. Neuroimmunol. 53, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawada M., Suzumura A., Itoh Y., Marunouchi T. (1993) Neurosci. Lett. 155, 175–178 [DOI] [PubMed] [Google Scholar]
- 37.Dohgu S., Yamauchi A., Takata F., Naito M., Tsuruo T., Higuchi S., Sawada Y., Kataoka Y. (2004) Cell. Mol. Neurobiol. 24, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus J., Ling A. K., Hamm S., Voigt K., Oschmann P., Engelhardt B. (2004) Ann. Neurol. 56, 192–205 [DOI] [PubMed] [Google Scholar]
- 39.Wosik K., Cayrol R., Dodelet-Devillers A., Berthelet F., Bernard M., Moumdjian R., Bouthillier A., Reudelhuber T. L., Prat A. (2007) J. Neurosci. 27, 9032–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Förster C., Kahles T., Kietz S., Drenckhahn D. (2007) J. Physiol. 580, 937–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ifergan I., Wosik K., Cayrol R., Kébir H., Auger C., Bernard M., Bouthillier A., Moumdjian R., Duquette P., Prat A. (2006) Ann. Neurol. 60, 45–55 [DOI] [PubMed] [Google Scholar]
- 42.Krizbai I., Szabó G., Deli M., Maderspach K., Lehel C., Oláh Z., Wolff J. R., Joó F. (1995) J. Neurochem. 65, 459–462 [DOI] [PubMed] [Google Scholar]
- 43.Park J. H., Okayama N., Gute D., Krsmanovic A., Battarbee H., Alexander J. S. (1999) Am. J. Physiol. 277, C1066–C1074 [DOI] [PubMed] [Google Scholar]
- 44.Stamatovic S. M., Dimitrijevic O. B., Keep R. F., Andjelkovic A. V. (2006) J. Biol. Chem. 281, 8379–8388 [DOI] [PubMed] [Google Scholar]
- 45.Qi X., Inagaki K., Sobel R. A., Mochly-Rosen D. (2008) J. Clin. Invest. 118, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleegal M. A., Hom S., Borg L. K., Davis T. P. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H2012–H2019 [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T., Seth A., Rao R. (2008) J. Biol. Chem. 283, 3574–3583 [DOI] [PubMed] [Google Scholar]
- 48.Hirase T., Staddon J. M., Saitou M., Ando-Akatsuka Y., Itoh M., Furuse M., Fujimoto K., Tsukita S., Rubin L. L. (1997) J. Cell Sci. 110, 1603–1613 [DOI] [PubMed] [Google Scholar]
- 49.Lu T. S., Avraham H. K., Seng S., Tachado S. D., Koziel H., Makriyannis A., Avraham S. (2008) J. Immunol. 181, 6406–6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronaldson P. T., Demarco K. M., Sanchez-Covarrubias L., Solinsky C. M., Davis T. P. (2009) J. Cereb. Blood Flow Metab. 29, 1084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taddei A., Giampietro C., Conti A., Orsenigo F., Breviario F., Pirazzoli V., Potente M., Daly C., Dimmeler S., Dejana E. (2008) Nat. Cell Biol. 10, 923–934 [DOI] [PubMed] [Google Scholar]
- 52.Weiler F., Marbe T., Scheppach W., Schauber J. (2005) J. Cell. Physiol. 204, 83–86 [DOI] [PubMed] [Google Scholar]
- 53.Yoo J., Nichols A., Mammen J., Calvo I., Song J. C., Worrell R. T., Matlin K., Matthews J. B. (2003) Am. J. Physiol. Cell Physiol. 285, C300–C309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.