Abstract
The paralogous endoribonucleases, RNase E and RNase G, play major roles in intracellular RNA metabolism in Escherichia coli and related organisms. To assay the relative importance of the principal RNA binding sites identified by crystallographic analysis, we introduced mutations into the 5′-sensor, the S1 domain, and the Mg+2/Mn+2 binding sites. The effect of such mutations has been measured by assays of activity on several substrates as well as by an assay of RNA binding. RNase E R169Q and the equivalent mutation in RNase G (R171Q) exhibit the strongest reductions in both activity (the kcat decrease ∼40- to 100-fold) and RNA binding consistent with a key role for the 5′-sensor. Our analysis also supports a model in which the binding of substrate results in an increase in catalytic efficiency. Although the phosphate sensor plays a key role in vitro, it is unexpectedly dispensable in vivo. A strain expressing only RNase E R169Q as the sole source of RNase E activity is viable, exhibits a modest reduction in doubling time and colony size, and accumulates immature 5 S rRNA. Our results point to the importance of alternative RNA binding sites in RNase E and to alternative pathways of RNA recognition.
Introduction
The RNase E/G family of bacterial endoribonucleases is widely distributed among bacteria (1). Both RNase E and RNase G are expressed in Escherichia coli. RNase E was first characterized as an essential processing enzyme required for the maturation of 5 S rRNA2 (2, 3). It is now known also to be involved in processing the 5′-spacer region of 16 S rRNA (4), most tRNA precursors (5, 6), transfer messenger RNA (7), and in the metabolism of many small regulatory RNAs (8, 9). It is also responsible for catalyzing the initial cleavage in the degradation of most mRNAs (10, 11). Furthermore, RNase E is part of a larger complex, the RNA degradosome (12–14). In contrast, RNase G appears to play a more limited role in RNA metabolism. It is responsible for the formation of the mature 5′ terminus of 16 S rRNA (4, 15) and participates in the degradation of a limited set of mRNAs (16, 17). It is not essential, however. Although both enzymes prefer single-stranded substrates, neither displays stringent sequence specificity (18–20). However, both enzymes are 5′-end-dependent; i.e. their activity is stimulated, both in vivo and in vitro by a 5′-monophosphorylated terminus on their substrates (21–26). To explain this observation, it was postulated that a 5′-phosphate binding pocket exists on the surface of these enzymes (24). This idea has been substantially verified by the crystal structure of the catalytic domain of RNase E in complex with a substrate analog (27). These authors showed that RNase E contains a 5′-sensor domain that can interact specifically with a 5′-monophosphorylated substrate via contacts with Gly-124, Val-128, Arg-169, and Thr-170 (27).
Several investigations have identified potential RNA binding surfaces on RNase E in addition to the 5′-sensor, including an arginine-rich region (28–30) and the S1 domain (31, 32). In addition, the active (catalytic) site itself must contribute to substrate binding. The arginine-rich region, however, lies outside the minimal N-terminal domain of RNase E that is sufficient for enzymatic activity (28–30). Several residues in the S1 domain could contribute to RNA binding, but only three, Phe-57, Phe-67, and Lys-112 provide obvious contacts to the substrate (27). Thus, it is not clear to what extent the 5′-sensor contributes to substrate binding. Indeed, it has been suggested that interaction of RNase E or G with a 5′-monophosphorylated substrate increases these enzymes' Vmax, effectively providing activation of these enzymes (25). Because a crystal structure was not available at the time this work was initiated, we examined instead the role of two types of conserved amino acid residue lying between the S1 domain and residue 400 in RNases E and G. In view of the known requirement of RNase E for transition metal ions (3), we tested whether any of five conserved acidic residues that might chelate Mg+2 or Mn+2 would be required for activity or RNA binding. Likewise, we mutated several conserved arginine residues that might be expected to interact with the phosphate backbone of a substrate. We found that the impact of such mutations was generally more severe in RNase G than in RNase E. Quantitative analysis of our data in light of the crystal structure of the N-terminal catalytic domain of RNase E (27) shows that the 5′-phosphate sensor plays a dominant role in substrate binding in vitro yet is not essential for survival in vivo.
EXPERIMENTAL PROCEDURES
Strains
E. coli K12 strain SK9714 (thyA715, λ-, rph-1, srlD300::Tn10, recA56, rne)1018::bla/pSBK1 [CmR, rne+]) and plasmid pQLK26 (KmR, rne+) (33) were obtained from Dr. Sidney Kushner, University of Georgia. The host strain for mutagenesis was E. coli DH5″ (obtained from Invitrogen). The host for enzyme overexpression was E. coli BL21(DE3) originally obtained from Novagen.
The plasmid pQLK26 was modified by replacing its ∼6-kbp insert containing rne with a shortened 3881-bp fragment amplified from pQLK26 to form pSG-Rne (see supplemental Fig. S1, step 1, and supplemental Table 1). The replacement insert contains 600 nucleotides upstream of the rne start codon, the entire coding sequence, and 101 nucleotides downstream from the rne stop codon. All three rne promoters and its transcriptional terminator are included within the 3.8-kbp insert. The empty vector lacks this insert and only contains the backbone from pQLK26 with a single SalI site (this is equivalent to pWSK129 in Ref. 33). To facilitate the construction of mutations in pSG-Rne, the 3.8-kbp insert was further subcloned into pUC19 (see supplemental Fig. S1, step 2). Appropriate fragments containing a mutation of interest were reassembled in pWSK129 to generate a full-length, mutant rne construct (see supplemental Fig. S1, step 4).
Plasmid displacement from strain SK9714 was measured as follows. Approximately 109 competent SK9714 in 0.06 ml were transformed with derivatives of pSG-Rne, allowed to recover in 1.0 ml of LB medium, then diluted 200-fold in LB containing thymidine, kanamycin, tetracycline, and carbenicillin (20 mg/liter each) and allowed to grow to saturation. The saturated culture was diluted 1000-fold into fresh medium also containing selective antibiotics and grown to saturation at 37 °C. Portions of this culture were spread on LB agar plates containing thymidine, kanamycin, tetracycline, and carbenicillin (20 mg/liter each). Colonies were picked onto fresh plates containing thymidine, tetracycline, carbenicillin, and either kanamycin or chloramphenicol. Plasmid displacement was measured as the fraction of kanamycin-resistant colonies that had lost resistance to 20 mg/ml chloramphenicol.
Construction of Rne or Rng Mutants and Purification of Mutant Enzymes
Plasmid pSG-Rne1–529 was constructed in the pET24b (Novagen) backbone between the NdeI and XhoI sites using appropriate PCR primers (see supplemental materials) to amplify DNA corresponding to amino acid residues 1–529 from E. coli DNA. The resultant construct will encode a C-terminal hexahistidine tag. Additional mutants were made as described below with the primers listed in supplementary materials.
Plasmid pDB1, based on pET24b, encodes RNase G with six additional N-terminal histidine residues (34) and was the basis for all further constructions involving RNase G. Mutagenesis was performed using the Stratagene QuikChange® kit. Oligonucleotides used for mutagenesis are listed in supplemental Table 3. Putative mutant plasmids were checked for the desired change by DNA sequence analysis.
His6-RNase E (1–529), His6-RNase G, and derived mutants were purified as described (34). In brief, cell pellets from 1 liter of induced cultures grown to saturation at 20 °C were harvested and lysed in a French pressure cell (Amino). A cleared lysate was passed through a column packed with Talon resin (Clontech). Fractions eluted with >50 mm imidazole were pooled, supplemented with 6 mm 2-mercaptoethanol, and subjected to further chromatography on a column of Source Q (Amersham Biosciences). Fractions containing the desired RNase were pooled, made 1 mm in dithiothreitol, and quantified prior to further characterization.
RNase Assays
Three RNA substrates were used in assays of activity. The first was Ribo-8 (5′-ACAGUAUUUdG), a derivative of BR10 (20) containing a 3′-deoxy-G residue. The other substrates were BR14-FD (25; see Fig. 2A below) and BR13-3′-fluorescein (26). Assays using Ribo-8 contained 50 nm 5′-32P-Ribo-8 and RNase G or its mutants in 25 mm Tris-HCl, pH 7.8, 5 mm MgCl2, 30 mm KCl, 1 mm dithiothreitol, and 5% glycerol. Incubations were initiated by addition of enzyme (8 nm for wild type; see “Effects of Mutation on Enzyme Activity” under “Results”). Samples were removed at timed intervals, denatured in buffered 90% formamide, and boiled for 45 s. Products were separated by electrophoresis on a 15% polyacrylamide gel (29:1 acrylamide:bis acrylamide) containing 8 m urea and detected by phosphorimaging.
FIGURE 2.
Fluorescence assay of RNase G. A, shows a schematic of RNA 1, a 108-nucleotide regulatory RNA that is a model substrate for RNase E. The major cleavage site is denoted by the vertical arrow. BR14FD, a synthetic fluorescent substrate for RNase E or G is based on the 5′-end of RNA 1 (25). B, time course showing the increase in fluorescence as BR14FD is cleaved under conditions described under “Experimental Procedures.” C, Eadie-Hofstee plot of initial rates as a function of concentration of BR14FD. The data for RNase G R171Q are compressed in this graph. D, expansion of the data in C to show the Eadie-Hofstee plot for RNase G R171Q.
The fluorescent substrates, 5′-P-BR14-FD (25) and 5′-P-BR13F (26), were synthesized by the University Core DNA Services at the University of Calgary. Assays were performed at 30 °C in a buffer containing 25 mm Tris-HCl, pH 7.6, 10 mm MgCl2, 30 mm NaCl, 1 mm dithiothreitol, and 5% (v/v) glycerol. For BR14FD, fluorescence at 517 nm was monitored on a Cary Eclipse fluorescence spectrophotometer using excitation at 495 nm. Initial rates were determined by monitoring the increase of fluorescence over the first 5 min following addition of enzyme (i.e. in the linear range). Measurements of initial rates for a given substrate concentration were repeated at least three times, and the average was determined. For assays with BR13F, portions of the assay were withdrawn, quenched in 3 volumes of buffered 90% formamide, and treated as for BR13 above, except that wet gels were scanned for fluorescence using a Typhoon phosphorimaging device. Kinetic constants were extracted from the data using an Eadie-Hofstee plot.
RNA Binding Assays
We adapted the double filter assay of Wong and Lohman (35) to measure the binding of 5′-32P-Ribo-8 to RNase G. Briefly, incubations containing 20 nm RNA oligonucleotide and from 100 nm to 4 mm RNase G were assembled on ice in FAB buffer (20 mm PIPES, 50 mm NaCl, 0.5 mm EDTA, 0.5 mm dithiothreitol, 20 μg/ml acetylated bovine serum albumin (New England Biolabs), pH 6.5). Samples were applied to individual wells in a Bio-Rad 96 well dot-blot apparatus containing a Bio-Rad nitrocellulose membrane over an Amersham Biosciences Hybond-NX nylon membrane, both previously washed with 1× FAB at room temperature. The samples were incubated for 5 min at ambient temperature before being drawn through the membranes with a gentle vacuum and washed with 100 ml of 1× FAB. Membranes were allowed to dry under suction for 5 min and were then exposed to a phosphor storage screen.
Northern Blotting
Total RNA was extracted from exponential cultures grown in LB medium supplemented with 50 μg/ml thymidine and appropriate antibiotics. Samples (3 μg) were dissolved in 8 μl of buffered 80% formamide, separated in a 5% polyacrylamide gel containing 8 m urea in Tris borate-EDTA buffer, and electrophoretically transferred to Hybond NX. Hybridization was performed at 35.5 °C as described before (42) using a 5′-32P-labeled oligonucleotide complementary to 5 S RNA (5′-CGTTTCACTTCTGAGTTCGGC).
RESULTS
Identification and Mutation of Conserved Residues in RNase G
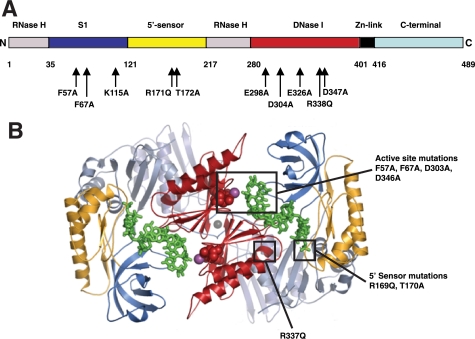
Our goal was the clarification of the role of different potential RNA binding domains in RNase E on this enzyme's activity. However, to avoid some of the complexities of RNase E, including its association with other proteins (12–14) and membranes (36) as well as potential RNA binding sites outside the catalytic domain (28, 29), we initially used RNase G as a surrogate. This enzyme displays 5′-end dependence (22–23), like RNase E (21), and can be assayed against the same substrates (25). We postulated that residues playing important functions in catalysis and substrate binding would be highly conserved in both RNase E and RNase G. To this end, we constructed an alignment of the members of the RNase E and RNase G family similar to published alignments (1, 27). In particular, we identified Glu-298, Asp-304, Glu-326, Asp-347, and Asp-350 in RNase G (Glu-297, Asp-303, Glu-325, Asp-346, and Asp-349, respectively, in RNase E) as conserved acidic residues that could play a role in metal ion chelation. Likewise, Arg-171 and Arg-338 (Arg-169 and Arg-337, respectively, in RNase E) are highly conserved basic residues that could interact with backbone phosphates. Each of these residues was mutated independently to Ala or Gln, and the resultant mutant proteins were expressed, purified, and characterized. Fig. 1A summarizes the mutations used in this study and indicates their location in the presumed domain structure of RNase G. A typical purification of a mutant enzyme, RNase G R171Q, is shown in the supplemental material. The enzyme was substantially pure after immobilized metal ion chromatography and was essentially homogeneous after ion-exchange chromatography (see supplemental Fig. S2B). In several cases, mutant proteins were further characterized by mass spectrometry. Interestingly, for RNase G D347A and D350A, the observed masses of 56,420 and 56,432, respectively, correspond to the expected loss of mass associated with a change from Asp to Ala, but with the retention of the N-formyl group at the N terminus (expected mass, 56,433).
FIGURE 1.
A, schematic map of RNase G. This linear map of RNase G shows the domains inferred from alignment against RNase E. The boundaries are given by the residue numbers below the linear map and correspond to those in RNase E (27). The positions of mutations in RNase G created in this work are noted below the map. B, structure of the N terminus of RNase E. The ribbon diagram was provided by Dr. Ben Luisi and shows the N-terminal region of two monomers of RNase E (27). The positions of substrates (in green) prior to cleavage and the positions of some of the mutations created in this study, are indicated using space-filling atoms. Domains are similarly colored in both A and B.
We also examined several physical properties of some of the mutant enzymes. The mid-point unfolding temperature (Tm) was determined for several mutants using CD (34). Generally, the mutants tested displayed a Tm equal to that of His6-RNase G (50.3 °C (34)). RNase G D350A, however, displayed a somewhat lower Tm (approx 47 °C). Nonetheless, this mutant enzyme cosedimented with His6-RNase G implying that it was still capable of forming dimers (data not shown).
Effects of Mutation on Enzyme Activity
Many of the mutant enzymes were initially assayed on a 519-nucleotide substrate, which mimics the RNase III-processed pre-16 S rRNA (34). Although this assay is rather insensitive, several mutants, including RNase G R171Q, D304A, and D347A exhibited detectable losses in activity (>50%; data not shown). More informative data were obtained by assaying each of the mutant enzymes against 5′-32P-Ribo-8 (Table 1). Although wild-type RNase G could be assayed at limiting enzyme concentrations, most of the mutants had to be assayed in enzyme excess. Nonetheless, RNase G R171Q (5′-sensor domain) and D304A (active site) were virtually inactive. Modest levels of activity could be observed with RNase G F67A and K115A (both in the S1 domain), and R171H and R338Q, especially at high enzyme concentrations. The remaining mutants, including RNase G V131I, E298A, E326A, D347A, and D350A, retained significant levels of activity against this substrate (Table 1). Although Glu-298 and Glu-326 are conserved, they appear not to be required for activity under the conditions tested and were not characterized further. As a control for the validity of the assay, in particular for its sensitivity to the status of the substrate's 5′-end, we repeated several assays with 5′-hydroxyl, 3′-32pCp-BR10 as a substrate to determine the fraction of activity that was 5′-end-independent. His6-RNase G displayed only ≤6% as much activity on this 5′-OH substrate as on 5′-32P-Ribo-8 (data not shown). Moreover, the mutants tested, including R171Q and R338Q, were virtually inactive against this 5′-OH substrate (data not shown).
TABLE 1.
Relative activities and affinities of RNase G point mutants
| Enzyme | Ribo-8a | Kinetic constantsb |
Binding, Kdappc | |
|---|---|---|---|---|
| Km | kcat | |||
| % activity | nm | min−1 | nm | |
| WT (His6) | 100 | 70 | 1.4 | 190 |
| F57A | NDd | 210 | 0.01 | 1200 |
| F67A | ≤5 | 460 | 0.044 | 900 |
| K115A | ≤5 | 110 | 0.046 | 1000 |
| V131I | 50 | ND | ND | ND |
| R171Q | ≤1 | ≥1000e | 0.015e | >5000 |
| R171H | ≤10 | ND | ND | ND |
| E298A | 100 | ND | ND | ND |
| D304A | ≤2 | 330 | 0.014 | 840 |
| E326A | 100 | ND | ND | ND |
| R338Q | 15 | 210 | 0.011 | >1000 |
| D347A | 40 | 300 | 0.046 | 670 |
| D350A | 8 | 230 | 0.80 | 540 |
a Activities were measured with 5′-32P-Ribo-8 and are reported as apparent initial rates of formation of 5-nucleotide product expressed as a percentage of the rate measured for His6-RNase G. Most of the mutants were assayed in enzyme excess to obtain measurable rates while wild type was assayed in substrate excess. Values are the average of duplicates.
b Except as noted in footnote e below, kinetic constants were estimated from Eadie-Hofstee plots of data generated from assays using 5′-P-BR14FD as substrate (“Experimental Procedures”). Each enzyme was assayed in triplicate, and initial rates for each concentration of substrate were averaged. The averaged values were then used to obtain slopes and intercepts.
c Binding data, usually in triplicate, using 32P-Ribo-8 as ligand were obtained from the double filter method (“Experimental Procedures”) and are reported as apparent Kd values.
d ND, not determined.
e Because BR14FD could not be used at concentrations > 800 nm, we employed an in-gel assay with 5′-P-BR13F containing a 3′-fluorescein modification to obtain kinetic parameters for R171Q ((26); see “Experimental Procedures”).
To obtain kinetic parameters, we assayed many of the mutant enzymes against BR14FD, a fluorescent oligoribonucleotide substrate developed by Jiang and Belasco (25). Representative time courses and Eadie-Hofstee plots are shown in Fig. 2, whereas the kinetic data are summarized in Table 1. In general, the results obtained with this assay are qualitatively similar to the data obtained with Ribo-8. The fluorescence assay is, however, far more sensitive and can be conducted at limiting enzyme concentrations. WT (His6) RNase G exhibited a Km of 70 nm and a kcat of 1.4 min−1 (Fig. 2, C and D, and Table 1). These values are similar to those reported by Jiang and Belasco: 230 nm and 2.1 min−1 (25). They also compare favorably to 120 nm and 3.0 min−1, respectively, reported by Jourdan and McDowall (26) using a somewhat different fluorescence assay. To verify the 5′-end dependence of RNase G under these conditions (22, 23, 25), wild-type RNase G was assayed against 5′-OH-BR14. With this substrate, the Km increased to 570 nm, and the kcat fell to 0.03 min−1. This result differs from that reported by Jiang and Belasco (25) who found that the state of phosphorylation of BR14 did not affect Km but only kcat. In this regard, our results more closely agree with those of Jourdan and McDowall (26).
The use of fluorescent substrates permitted an objective comparison of the mutants. The most striking of these is RNase G R171Q for which the kcat drops by almost 100-fold while the Km increases ∼14-fold (Fig. 2D and Table 1). This finding points to the key role in the recognition of some substrates that is played by the phosphate binding pocket in RNase G (see “Discussion”). Interestingly, mutations in three residues in the S1 domain, which line the RNA binding channel, Phe-57, Phe-67, and Lys-115 (27), also affect both kinetic parameters, but not as severely. The Km increases 3- to7-fold while the catalytic efficiency, kcat, falls 30- to 140-fold. Among the putative metal ion chelating residues, mutations in either Asp-304 or Asp-347 affect both Km and kcat, with D304A being the more severe mutation. In contrast, D350A exerts only a modest effect on Km and little effect on kcat (see Table 1). The strong effects of mutations on Asp-304 and Asp-347 agree with the findings of Callaghan et al. (27) for the N-terminal domain of RNase E (see below).
RNA Binding by Mutant RNase G Enzymes
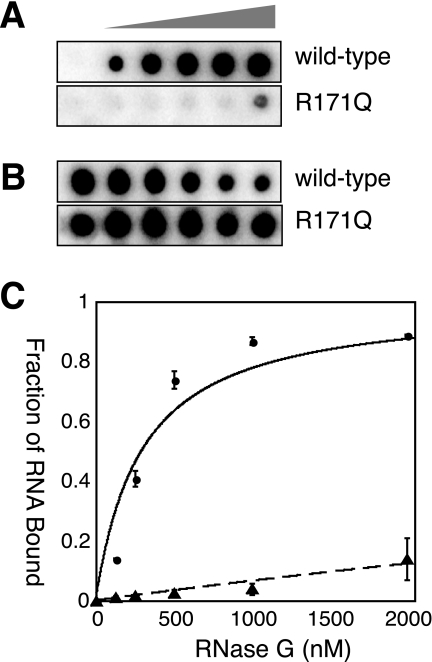
We employed a double filter assay (35) to determine the relative affinities of His6-RNase G and its derivatives for 5′-32P-Ribo-8 (see “Experimental Procedures”). The main challenge was avoidance of contamination by Mg+2 ions and the resultant destruction of the ligand by cleavage. Typical binding isotherms are shown in Fig. 3, and the data are summarized in Table 1. Wild-type RNase G displayed an apparent Kd of 190 nm. This value is close to the Kd of 162 nm reported by Jourdan and McDowall (26) who used fluorescence anisotropy to assess RNA binding to a 2′-O-methyl substrate. In contrast, R171Q exhibited very little binding to this RNA ligand at the protein concentrations tested (Fig. 3C and Table 1). The F57A, F67A, and K115A mutations in the S1 domain also resulted in lowered RNA binding with apparent Kd values of 900–1200 nm, respectively, or 5-fold more than wild type. Interestingly, mutations in the metal binding site, including D304A and D347A, resulted in partial loss of binding to Ribo-8 and elevated Kd values. Nonetheless, the data show that the most severe effect on RNA binding, a >25-fold increase in Kd, is exerted by R171Q. In support of this finding, we tested the binding of 3′-fluorescein-labeled BR13, with or without a 5′-monophosphate, to wild-type RNase G. Binding of 5′-OH-BR13F was much weaker than that of BR13-5′-monophosphate and did not reach 50% at the highest protein concentration tested.
FIGURE 3.
Double filter assay of RNA binding by RNase G. The assay of Wong and Lohman (35) was adapted for RNase G as described under “Experimental Procedures.” A typical set of data is shown. The left hand column in each panel contained no added protein. Protein concentrations ranged from 125 to 2000 nm from left to right. A, nitrocellulose filter (retaining RNA-protein complexes); B, nylon filter with free RNA. The RNase G preparations assayed for binding are listed in the right margin. C, fraction of RNA bound as a function of protein concentration for WT RNase G (●) and RNase G R171Q (▴).
Effect of Selected Mutations on RNase E
To test the implied assumption that the effect of mutations in RNase G could be extrapolated to RNase E, we reconstructed some of the most severe alleles of RNase G in a derivative of RNase E spanning residues 1–529 (i.e. the catalytic domain used for crystallographic analysis (27)). Mutant proteins were expressed and purified as described for RNase G (see “Experimental Procedures”), and then assayed on BR14FD. These data are summarized in Table 2. Several salient observations emerge by comparison to the data in Table 1. First, RNase E is more active than RNase G (i.e. lower Km and higher kcat). This differs from the findings of Jiang and Belasco (25) and may reflect the fact the RNase E construct used by these authors was shorter than the one employed here. Second, mutations in the S1 domain of RNase E (F57A and F67A) exert modest increases in Km (4- to 10-fold) and decreases in kcat (6- to 12-fold). The corresponding mutations in RNase G, however, resulted in 30- to 100-fold reductions in kcat (Table 1). Third, the R169Q mutation in RNase E (5′-sensor domain) increases the Km 10-fold and decreases the kcat almost 40-fold. The change in kcat, in particular, is less severe than observed in the corresponding mutation in RNase G (i.e. R171Q). As a control, we tested the 5′-end dependence of RNase E 1–529. Dephosphorylating the substrate led to a 36-fold increase in Km and a 32-fold decrease in kcat consistent with the effects of the R169Q mutation (see Table 2). Finally, the mutation D303A reduced kcat by 1000-fold, essentially eliminating activity as did D304A in RNase G (Table 1). We were unable to measure the affinity of RNase E 1–529 or any of its derived mutants for Ribo-8 by the double filter method, because binding was too weak and did not reach saturation.
TABLE 2.
Relative activities of RNase E point mutants
| Enzymea | Kinetic constantsb |
|
|---|---|---|
| Km | kcat | |
| nm | min−1 | |
| WT | 33 | 11 |
| WTc | 5100 | 0.95 |
| F57A | 120 | 1.9 |
| F67A | 320 | 1.1 |
| R169Q | 350 | 0.28 |
| D303A | 38 | 0.008 |
| D346A | 77 | 0.007 |
a In all cases, the enzyme includes residues 1–529 of RNase E.
b Except where noted, kinetic constants were estimated from Eadie-Hofstee plots of data generated with 5′-P-BR14FD as substrate (“Experimental Procedures”). Rates for each substrate concentration were determined at least in triplicate, then averaged prior to plotting.
c Data on this line were obtained with 5′-OH- BR14FD as substrate (“Experimental Procedures”).
Effect of Mutations in RNA Binding Residues of RNase E in Vivo
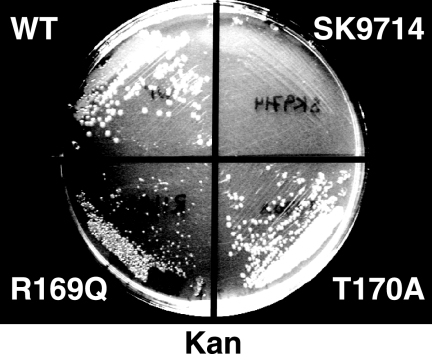
Because RNase G is not essential in E. coli under normal laboratory conditions we instead determined the phenotype of mutations in the corresponding residues in RNase E, because the latter enzyme is essential for viability (10, 33). Mutations were constructed in the rne gene carried on pSG-Rne, a derivative of pQLK26 (KmR), a low copy plasmid capable of complementing rneΔ1018::bla, an otherwise lethal deletion of the chromosomal rne gene (33). Each such construct was tested for its ability to displace a wild-type plasmid, pSBK1 (rne+, CmR), from strain SK9714, which requires a functional rne gene supplied in trans for survival. Data for such experiments are shown in Table 3. The mutants tested fall into two clear classes. The first fails to displace pSBK1 and includes F67A, D303A, D346A, and R337E. Phe-67 interacts with the RNA substrate in the S1 domain close to the catalytic site, while Asp-303 and Asp-346 are believed to chelate an essential Mg+2 ion and participate in catalysis (27). Our data support this view and demonstrate that these residues are essential for RNase E. The deleterious effect of R337E is most likely due to the charge reversal caused by this substitution because R337Q displays almost no phenotype (Table 3). The second class of mutants was able to displace pSBK1 at significant frequencies (Table 3). These mutants, including F57A, R169Q, and T170A, affect residues involved in contacting RNA in the S1 domain (Phe-57) or phosphate binding pocket (27). Although R169Q was able to displace pSBK1 at a significant frequency, the resultant colonies were noticeably smaller (Fig. 4). Transformants of SK9714 containing pSG-Rne-R169Q grew with a doubling time of 32.3 min in rich medium. In contrast, SK9714 containing pSG-Rne (WT) grew significantly more quickly under the same conditions with a doubling time of 24.5 min. Strains transformed with pSG-Rne T170A, which showed no phenotype, also grew well, with a doubling time of 27 min.
TABLE 3.
Complementation of a lethal rne deletion by rne mutants
| Mutation | Plasmida | Percent displacedb |
|---|---|---|
| % | ||
| WT | pSG-Rne | 76 ± 4.1 |
| F57A | pSG-Rne F57A | 93 ± 1.0 |
| F67A | pSG-Rne F67A | 0 |
| R169Q | pSG-Rne R169Q | 47 ± 5.0c |
| T170A | pSG-Rne T170A | 89 ± 2.3 |
| D303A | pSG-Rne D303A | 0 |
| R337E | pSG-Rne R337E | 0 |
| R337Q | pSG-Rne R337Q | 92 ± 1.2 |
| D346A | pSG-Rne D346A | 0 |
a All complementing plasmids were constructed in the backbone from pWSK129 (see “Experimental Procedures” and supplemental Fig. S1).
b The fraction displaced is recorded as the fraction of colonies that have acquired resistance to kanamycin via the mutant plasmid with concomitant loss of resistance to chloramphenicol conferred by pSBK1 after >20 doublings (“Experimental Procedures”).
c Colonies that were resistant to kanamycin but sensitive to chloramphenicol were noticeably smaller than those that retained pSBK1 (also Fig. 4).
FIGURE 4.
Colony Size of SK9714 transformed with different plasmids. Samples of cultures of SK9714 (33) transformed with plasmids of interest (see Table 2) were streaked on plates containing LB, thymidine, carbenicillin, and kanamycin.
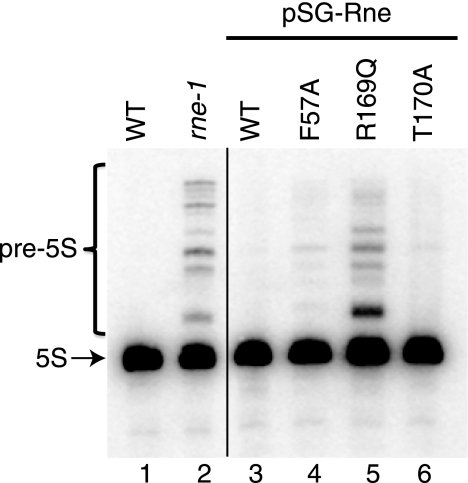
We further characterized the three mutants able to displace WT pSG-Rne by examining the accumulation of 5 S RNA. For comparison, we also included the well known temperature-sensitive rne-1 allele. Fig. 5 shows that precursors to 5 S RNA accumulate at non-permissive temperature in the rne-1 strain, SK5665 (compare lane 2 to lane 1), as expected. A strain containing pSG-Rne R169Q as its only source of RNase E exhibits a pattern of precursor accumulation that is qualitatively identical to that of the rne-1 allele (compare lane 5 to lane 2). In contrast, a strain carrying the T170A allele contains almost no precursors at this exposure (compare lane 6 to lanes 2 and 5), although they are detectable with longer exposures. Interestingly, the F57A allele (lane 4) also accumulates some precursors but to a lesser extent than either rne-1 or R169Q. Processing of the hisR transcript (tRNAHis) from the same strains was also tested. Unlike the case above, it was normal in R169Q and T170A, but defective in rne-1 as expected (6) (data not shown).
FIGURE 5.
Effect of RNase E mutations on stable RNA processing. Total RNA from the following strains was subjected to Northern blotting as described under “Experimental Procedures” using a probe complementary to 5 S rRNA and its precursors: lane 1, MG1693 (WT); lane 2, SK5665 (rne-1); lane 3, SK9714/pSG-Rne [WT]); lane 4, SK9714/pSG-Rne F57A; lane 5, SK9714/pSG-Rne R169Q; and lane 6, SK9714/pSG-Rne T170A. The position of mature 5 S RNA is shown by the arrow in the left margin.
DISCUSSION
This investigation was initiated with the goal of using conserved residues identified by bioinformatic means or subsequently, the crystal structure of RNase E (27), to clarify the contribution of potential RNA binding surfaces to the activity of RNases E and G. A major focus was the interaction with the 5′-phosphate of substrates and its mechanism of stimulation of the activity of RNase E and G. The 5′-end dependence of RNase E (21) is a signature property of RNase E and RNase G (22, 23). Interestingly, the unrelated RNase J1 of Bacillus subtilis also exhibits a preference of 5′-phosphates (37). At least two mechanisms have been invoked to explain why 5′-monophosphorylated RNAs are preferred by RNases E and G. Jiang and Belasco (25) have presented data that suggest that binding of a monophosphorylated oligonucleotide to RNase E or RNase G stimulates Vmax up to 30-fold with little effect on Km. These authors did not measure RNA binding independently of catalysis. In contrast, Jourdan and McDowall (26) reported that the stimulatory effect of a 5′-monophosphate on RNase G is exerted primarily through enhanced substrate binding resulting in a 100-fold improvement in Km. Our data, using the same substrate as Jiang and Belasco, support the view that the primary effect of a 5′-monophosphorylated substrate is enhanced binding. Neutralization of the positive charge on a key residue in the phosphate binding pocket, Arg-171, resulted in a ∼14-fold drop in Km and a 100-fold decrease in kcat by RNase G. Furthermore, measurement of substrate binding in the absence of catalysis showed that the same mutation caused a 25-fold or greater loss in affinity for substrate. Quantitatively, the loss of affinity for RNA by R171Q is greater than by F57A, F67A, or K115A, residues in the S1 domain that line the RNA binding channel. The corresponding residues are known to affect the activity of RNase E as assessed by loss of feedback regulation (31). Nonetheless, our data show that binding of a monophosphorylated substrate to RNase E or RNase G also activates the enzyme as proposed (27, 38). Koslover et al. have shown that binding of a substrate via its 5-monophosphate residue triggers a significant conformational change in RNase E involving both the 5′-sensor and S1 domains (38). In this regard, interactions between substrate and enzyme remote from the catalytic site are known to contribute to the efficiency of catalysis (39). The large decreases in kcat exhibited by RNase E R169Q and by G R171Q are, in this view, obvious consequences of weak substrate binding.
Based on previous data (31), we anticipated that RNase E F57A might be unable to displace pSBK1 from strain SK9714. Nonetheless, displacement was very efficient. Apparently the reduced activity of RNase E F57A is still sufficient to support growth and results in only modest impairment of 5 S rRNA processing. We presume that requirements for autoregulation (31) differ subtly from those for viability.
The physiological effects of mutations in residues in the phosphate binding pocket of RNase E have not previously been reported. To our surprise, both R169Q and T170A are viable, although the former leads to a slow growth phenotype, small colonies, and significant reduction in the rate of 5 S RNA processing. Conversely, mutations D303A and D346A are inviable, consistent with their proposed role in coordinating a hydrated Mg+2 ion that serves as the source of a base in phosphodiester bond cleavage (26, 27). The modest effect of R169Q on RNase E in vivo stands in stark contrast to its effects on both RNase E and RNase G in vitro (26). This dichotomy suggests that the role of the phosphate sensor is more subtle than initially envisaged. We believe that at least two factors should be considered in explaining this outcome. First it is noteworthy that the most pronounced effects of either monophosphorylation or mutations in the phosphate sensor are detected with oligonucleotides such as BR10 and its derivatives or relatively short substrates (e.g. the 9 S RNA precursor to 5 S RNA (246 residues)) (21, 22, 23, 26). Such short substrates may make relatively few contacts with RNase E and thus rely on the phosphate sensor and the S1 domain for binding. Second, at least one additional RNA binding surface is known in full-length RNase E, notably the “arginine-rich region” between residues 610 and 632 (28–30). A comparable region is not found in RNase G. The arginine-rich region may be able to compensate, in part, for deficiencies in 5′-end recognition in R169Q. In this regard, a strain carrying ΔrppH and thus unable to remove the terminal pyrophosphate from RNAs is viable, although the half-lives of many mRNAs are elevated in this strain (40). We infer, therefore, that internal entry (41, 42) may play a greater role in the action of RNase E, particularly on longer RNAs, than hitherto appreciated. The availability of the viable R169Q mutation will greatly facilitate elucidating the physiological role of the 5′-sensor and investigating possible alternative pathways of substrate recognition.
Supplementary Material
Acknowledgments
We thank Dr. Sidney Kushner for his gift of strains, Dr. Ken McDowall for sharing data in advance of publication and for providing comments on the ms, and Dr. Ben Luisi for providing a high resolution image of the catalytic domain of RNase E. The fluorometer was provided through a grant from the Canada Foundation for Innovation and the Michael Smith Foundation to the University of British Columbia Laboratory of Molecular Biophysics.
This work was supported by Grant MOP-5396 from the Canadian Institutes of Health Research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2 and Tables 1–3.
- rRNA
- ribosomal RNA
- WT
- wild type.
REFERENCES
- 1.Condon C., Putzer H. (2002) Nucleic Acids Res. 30, 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghora B. K., Apirion D. (1978) Cell 15, 1055–1066 [DOI] [PubMed] [Google Scholar]
- 3.Misra T. K., Apirion D. (1979) J. Biol. Chem. 254, 11154–11159 [PubMed] [Google Scholar]
- 4.Li Z., Pandit S., Deutscher M. P. (1999) EMBO J. 18, 2878–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Deutscher M. P. (2002) RNA 8, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ow M. C., Kushner S. R. (2002) Genes Dev. 16, 1102–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin-Chao S., Wei C. L., Lin Y. T. (1999) Proc. Nat. Acad. Sci. U.S.A. 96, 12406–12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carpousis A. J. (2003) Genes Dev. 17, 2351–2355 [DOI] [PubMed] [Google Scholar]
- 9.Massé E., Escoria F. E., Gottesman S. (2003) Genes Dev. 17, 2374–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coburn G. A., Mackie G. A. (1999) Prog. Nucleic Acids Res. Mol. Biol. 62, 55–108 [DOI] [PubMed] [Google Scholar]
- 11.Carpousis A. J. (2007) Annu Rev. Microbiol. 61, 71–87 [DOI] [PubMed] [Google Scholar]
- 12.Carpousis A. J., Van Houwe G., Ehretsmann C., Krisch H. M. (1994) Cell 76, 889–900 [DOI] [PubMed] [Google Scholar]
- 13.Miczak A., Kaberdin V. R., Jakobsen J. S., Lin-Chao S., McDowall K. J., von, Gabain A. (1998) Proc. Natl. Acad. Sci U.S.A. 95, 11637–11642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Py B., Higgins C. F., Krisch H. M., Carpousis A. J. (1996) Nature 381, 169–172 [DOI] [PubMed] [Google Scholar]
- 15.Wachi M., Umitsuki G., Shimizu M., Takada A., Nagai K. (1999) Biochem. Biophys. Res. Commun. 259, 483–488 [DOI] [PubMed] [Google Scholar]
- 16.Umitsuki G., Wachi M., Takada A., Hikichi T., Nagai K. (2001) Genes Cells 6, 403–410 [DOI] [PubMed] [Google Scholar]
- 17.Wachi M., Kaga N., Umitsuki G., Clark D. P., Nagai K. (2001) Biochem. Biophys. Res. Commun. 289, 1301–1306 [DOI] [PubMed] [Google Scholar]
- 18.Mackie G. A. (1992) J. Biol. Chem. 267, 1054–1061 [PubMed] [Google Scholar]
- 19.McDowall K. J., Lin-Chao S., Cohen S. N. (1994) J. Biol. Chem. 269, 10790–10796 [PubMed] [Google Scholar]
- 20.McDowall K. J., Kaberdin V. R., Wu S. W., Cohen S. N., Lin-Chao S. (1995) Nature 374, 287–290 [DOI] [PubMed] [Google Scholar]
- 21.Mackie G. A. (1998) Nature 395, 720–723 [DOI] [PubMed] [Google Scholar]
- 22.Jiang X., Diwa A., Belasco J. G. (2000) J. Bacteriol. 182, 2468–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tock M. R., Walsh A. P., Carroll G., McDowall K. J. (2000) J. Biol. Chem. 275, 8726–8732 [DOI] [PubMed] [Google Scholar]
- 24.Spickler C., Stronge V., Mackie G. A. (2001) J. Bacteriol. 183, 1106–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X., Belasco J. G. (2004) Proc. Nat. Acad. Sci. U.S.A. 101, 9211–9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jourdan S. S., McDowall K. J. (2008) Mol. Microbiol. 67, 102–115 [DOI] [PubMed] [Google Scholar]
- 27.Callaghan A. J., Marcaida M. J., Stead J. A., McDowall K. J., Scott W. G., Luisi B. F. (2005) Nature 437, 1187–1191 [DOI] [PubMed] [Google Scholar]
- 28.Cormack R. S., Genereaux J. L., Mackie G. A. (1993) Proc. Nat. Acad. Sci. U.S.A. 90, 9006–9010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaberdin V. R., Walsh A. P., Jakobsen T., McDowall K. J., von Gabain A. (2000) J. Mol. Biol. 301, 257–264 [DOI] [PubMed] [Google Scholar]
- 30.McDowall K. J., Cohen S. N. (1996) J. Mol. Biol. 255, 349–355 [DOI] [PubMed] [Google Scholar]
- 31.Diwa A. A., Jiang X., Schapira M., Belasco J. G. (2002) Mol. Microbiol. 46, 959–969; erratum (2003) 47, 1183 [DOI] [PubMed] [Google Scholar]
- 32.Schubert M., Edge R. E., Lario P., Cook M. A., Strynadka N. C., Mackie G. A., McIntosh L. P. (2004) J. Mol. Biol. 341, 37–54 [DOI] [PubMed] [Google Scholar]
- 33.Ow M. C., Liu Q., Kushner S. R. (2000) Mol. Microbiol. 38, 854–866 [DOI] [PubMed] [Google Scholar]
- 34.Briant D. J., Hankins J. S., Cook M. A., Mackie G. A. (2003) Mol. Microbiol. 50, 1381–1390 [DOI] [PubMed] [Google Scholar]
- 35.Wong I., Lohman T. M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 5428–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khemici V., Poljak L., Luisi B. F., Carpousis A. J. (2008) Mol. Microbiol. 70, 799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de al Sierra Gallay I. L., Zig L., Jamalli A., Putzer H. (2008) Nat. Struct. Mol. Biol. 15, 206–212 [DOI] [PubMed] [Google Scholar]
- 38.Koslover D. J., Callaghan A. J., Marcaida M. J., Garman E. F., Martick M., Scott W. G., Luisi B. F. (2008) Structure 16, 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wicki J., Schloegl J., Tarling C. A., Withers S. G. (2007) Biochemistry 46, 6996–7005 [DOI] [PubMed] [Google Scholar]
- 40.Deana A., Celesnik H., Belasco J. G. (2008) Nature 451, 355–358 [DOI] [PubMed] [Google Scholar]
- 41.Joyce S. A., Dreyfus M. (1998) J. Mol. Biol. 282, 241–254 [DOI] [PubMed] [Google Scholar]
- 42.Baker K. E., Mackie G. A. (2003) Mol. Microbiol. 47, 75–88 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.