Abstract
ASIC3 is an acid-sensing ion channel expressed in sensory neurons, where it participates in acidic and inflammatory pain. In addition to the “classical” transient current, ASIC3 generates a sustained current essential for pain perception. Using chimeras between the ASIC3 and ASIC1a channels we show that the first transmembrane domain (TM1), combined with the N-terminal domain, is the key structural element generating the low pH (<6.5)-evoked sustained current. The TM1 domain also modulates the pH-dependent activation of the fast transient current thus contributing to a constitutive window current, another type of sustained current present near physiological pH. The C-terminal and the TM2 domains negatively regulate both types of sustained current, and the extracellular loop affects its kinetics. These data provide new information to aid understanding the mechanisms of the multifaceted pH gating of ASIC3. Together with the peak current, both components of the sustained current (window and sustained at pH <6.5) allow ASIC3 to adapt its behavior to a wide range of extracellular pH variations by generating transient and/or sustained responses that contribute to nociceptor excitability.
Introduction
Ischemia, inflammation, tumors, or tissue damages are associated with a decrease in extracellular pH (1). Activation of nociceptive sensory nerve endings by tissue acidosis correlates with pain sensations in human and rodents (1–4). The acid-sensing ion channel 3 (ASIC3)2 is predominantly expressed in sensory neurons and has been recently shown to be a sensor of acid-mediated and primary inflammatory pain in rats (4). ASIC3 belongs to the acid-sensing ion channel family, which comprises at least seven isoforms encoded by four different genes (5–7). These isoforms, which comprise cytosolic N and C termini, two transmembrane helices, and a disulfide-rich, multidomain extracellular region, can associate into homo- or heterotrimers (8, 9). ASICs are amiloride-sensitive voltage-independent cationic channels that are activated by a decrease in extracellular pH. Protons trigger a transient inward current that desensitizes rapidly in all of the ASICs except ASIC3, which carries in addition to the transient current a sustained current that does not fully inactivate while the pH remains acidic (10–12). For moderate extracellular acidification (between pH 7.3 and 6.7 for the rat ASIC3 channel), the sustained current results from the window of overlap between inactivation and activation of the fast transient current (4, 13), whereas the sustained current that is activated by lower pH seems to involve a different, uncharacterized mechanism. Despite the important role proposed for the ASIC3-sustained current in pain perception (4, 13, 14), very little is known about the molecular mechanisms underlying this current. This paper identifies the key structural elements involved in the generation and in the modulation of the window current (Iwindow) observed at modest acidifications near the physiological pH and of the low pH (<6.5)-evoked sustained current (Isustained-pH5.0). Both types of current are important for producing a persistent ASIC3-induced depolarization proposed to be essential for pain perception. We also propose an integrated model for the gating of ASIC3.
EXPERIMENTAL PROCEDURES
Xenopus Oocyte Preparation, DNA Injection, and Electrophysiological Measurements
Animal handling and experiments fully conformed to French regulations and were approved by local governmental veterinary services (authorization B 06-152-5 delivered by the Ministère de l'Agriculture, Direction des services vétérinaires). Xenopus laevis oocyte preparation was done as described previously (15). Briefly, animals were anesthetized by exposure for 20 min to a 0.2% solution of 3-aminobenzoic acid ethyl ester (MS-222). Oocytes were surgically removed and dissociated with collagenase type IA (Sigma). pCI-ratASIC1a (200 pg) and the derived chimeras, or pCI-ratASIC3 (500 pg) and the derived chimeras were injected into Xenopus oocyte nuclei. Oocytes were kept at 19 °C in ND96 solution containing 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 2 mm MgCl2 and 5 mm HEPES (pH 7.4 with NaOH); ND96 solution was supplemented with penicillin (6 μg·ml−1) and streptomycin (5 μg·ml−1). Currents were recorded 3 days after DNA injection under voltage clamp (Dagan TEV 200 amplifier; Dagan Corporation, Minneapolis, MN) using two standard glass microelectrodes (1–2.5 MΩ) filled with a 3 mm KCl solution. Stimulation, data acquisition, and analysis were performed using pCLAMP 9.2 software (Axon Instruments, Union City, CA). All experiments were performed at 19–21 °C in ND96 solution. Changes in extracellular pH were induced by a microperfusion system that allowed local and rapid changes of solutions. HEPES was replaced by MES to buffer solutions with a pH between 6.4 and 5.0.
Plasmid Constructions and Mutagenesis
The coding sequences of rat ASIC1a, rat ASIC3 (GenBank accession numbers U94403 and AF013598, respectively), and the related chimeras were subcloned into the NheI/NotI restriction sites of the pCI vector (Promega). Chimeras were obtained by recombinant PCR strategies as described previously (15).
Statistical Analysis
Data are represented as means ± S.E., and the statistical significance of differences between sets of data was estimated using the unpaired or paired Student's t test or the one-way analysis of variance followed by a Tukey's post hoc test, when appropriate (*, p < 0.05; **, p < 0.01; GraphPad Prism 4.03 software).
RESULTS
pH 5.0 Activation of ASIC3 Evokes a Slowly Inactivating Current That Is Supported by a Long-lived Inactivated State
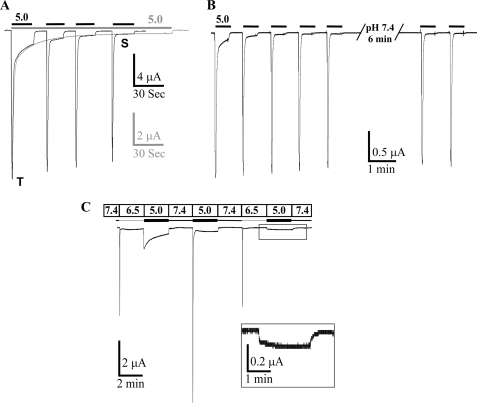
ASIC3 generates a biphasic current with a fast transient component and a second phase that corresponds to a very slow inactivation of the channel (Fig. 1A). This slow inactivation process is triggered by the first pulse at pH 5.0 and is not dependent on any subsequent pH 5.0 pulse, the plateau phase of ASIC3 decreasing slowly and continuously regardless of the number of pulses (Fig. 1A). The channel is not able to recover from this slow inactivation process even after a long exposure to pH 7.4 (Fig. 1B).
FIGURE 1.
Activation of the pH 5.0-evoked ASIC3 sustained current. A, typical traces of the ASIC3 currents recorded at −50 mV from Xenopus oocytes injected with ASIC3 after continuous (gray bar above the current traces) or repetitive (black bars above the current traces) application of a pH 5.0 solution from a holding pH of 7.4. Both traces have been superimposed to compare the time course of the effect in each condition. The sustained current is associated with a very slow inactivating process, which is not dependent on the nature of the stimulation. T and S, transient and sustained components, respectively. B, typical ASIC3 current trace induced by repetitive pulse at pH 5.0 solution, illustrating the absence of recovery of the sustained current after up to 6 min at pH 7.4. C, differential activation of the transient and the sustained currents. The first pH drop from 7.4 to 6.5 specifically activates the transient current, and the following drop to pH 5.0 specifically activates the sustained current. Maximal activation of the sustained current is only seen after the first pulse at pH 5.0, subsequent pulses at pH 5.0 are only associated with a small plateau current (inset).
We have taken advantage of the very different pH sensitivity of the transient and pH 5.0-activated plateau current of ASIC3 (12) to analyze this phenomenon in more detail. Fig. 1C shows that the first pulse at pH 6.5 only activates the transient current, which then fully inactivates. Then, a subsequent pulse at pH 5.0 only activates the plateau phase current with slow inactivating kinetics. After a recovery period at pH 7.4, a new pulse at pH 5.0 gives again a biphasic ASIC3 current corresponding to the sum of the fast transient and the slowly inactivating plateau currents. If the same sequence of pH pulses (7.4/6.5/5.0/7.4) is applied again, the pH 5.0 pulse triggers only a very small plateau current, indicating as in Fig. 1B the lack of recovery of the sustained current after the first activation by pH 5.0. The slow sustained current is thus characterized by a long lived inactivated state triggered by the initial acidification at pH 5.0, and the pattern observed when the pH is changed for the first time from 7.4 to 5.0 (Fig. 1A) results from the combination of this current with the fast transient current.
Extracellular Loop of ASIC3 Contributes to Influence Kinetics but Has No Role in Generation of Sustained Currents
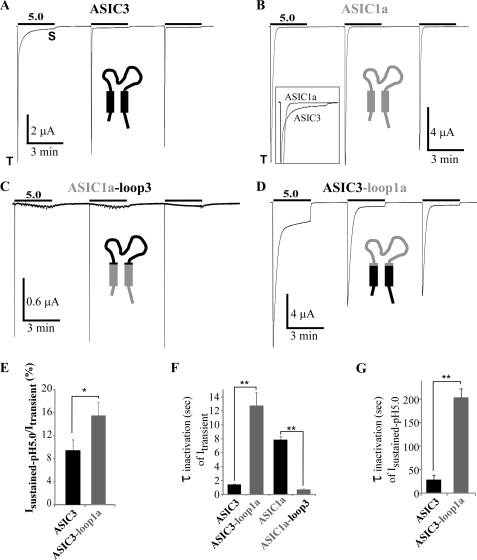
To identify the structural elements involved in the ASIC3 sustained current generation, we have constructed chimeras between ASIC3 and ASIC1a, another ASIC subunit that is known to generate a transient current with slower kinetics compared with ASIC3 and no sustained current (5) (Fig. 2B). The cytosolic, N- and C-terminal, the first and the second transmembrane domains (TM1 and TM2), and the extracellular loop have been swapped (supplemental Fig. 1).
FIGURE 2.
Analysis of the role of the extracellular loop in generating sustained current in ASIC1a/3 and ASIC3/1a chimeras. A and B, representative current traces of pH 5. 0-activated currents recorded at −50 mV from Xenopus oocytes injected with ASIC3 and ASIC1a. Three pH pulses of 3 min each are made at 3-min intervals from a holding pH of 7.4. T and S, transient and sustained components, respectively. Inset in B, traces corresponding to the first pH pulse shown in A and B have been superimposed to illustrate the differences in the current kinetics between ASIC3 and ASIC1a. C and D, representative current traces of pH 5.0-activated currents recorded at −50 mV after swapping the extracellular loops of ASIC1a and ASIC3 (ASIC1a-loop3 and ASIC3-loop1a, respectively). Note the “oscillatory” current associated with the ASIC1a-loop3 chimera after activation of the peak current, probably related to a significant Ca2+ permeability of this ASIC1a-based chimera and the activation of endogenous calcium-activated channels. E, average relative pH 5.0-evoked sustained current measured 45 s after the peak activated by the first pH pulse as shown in A and D, for ASIC3 and ASIC3-loop1a (n = 28 and 8, respectively). F, kinetics of inactivation (τinact) of the transient current generated by ASIC3, ASIC3-loop1a, ASIC1a, and ASIC1a-loop3 (n = 18, 8, 12, and 10, respectively). G, kinetics of inactivation (τinact) of the pH 5.0-activated sustained current generated by ASIC3 and ASIC3-loop1a, (n = 4 and 5, respectively). Kinetics of the sustained current have been measured after activation of the current from pH 6.5 to 5.0 as shown on Fig. 1C, to exclude any influence of the transient current.
The properties of the transient and sustained currents were assessed by three successive pulses to pH 5.0. The first pulse was used to measure reference amplitude of the transient and the sustained currents. Under the experimental conditions used, the rundown of ASIC1a is absent or very slow (Fig. 2B). Transferring the extracellular loop of ASIC3 to ASIC1a (ASIC1a-loop3 chimera) does not confer to ASIC1a any significant pH 5.0-evoked sustained current (Fig. 2C) nor pH 7.0-evoked window current (not shown). On the other hand, replacing the transmembrane and cytosolic domains of ASIC1a with those of ASIC3 (ASIC3-loop1a chimera) induces a biphasic current with a plateau phase, which is even larger in amplitude than the one of ASIC3 itself (Isustained-pH5.0/Itransient = 9.4 ± 1.8% and 15.5 ± 2.3% for the ASIC3 and ASIC3-loop1a, respectively; Fig. 2, A, D, and E). The kinetics of the transient current in these chimeras are directly related to the nature of the extracellular loop (i.e. fast for ASIC3 and slower for ASIC1a; see Fig. 2A and B), in good agreement with previous data (16). When the loop of ASIC3 is transferred into ASIC1a, it induces a 9.9-fold faster inactivation (τinact = 0.8 ± 0.1 s and 7.9 ± 0.5 s for ASIC1a-loop3 and ASIC1a, respectively; Fig. 2, C and F), and when the ASIC1a loop is transferred into ASIC3, it leads to a 8.6-fold slower inactivation (τinact = 12.9 ± 1.9 s and 1.5 ± 0.1 s for ASIC3-loop1a and ASIC3, respectively; Fig. 2, D and F). The extracellular loop also influences the kinetics of the pH 5.0-activated sustained current (τinact = 29 ± 9 s and 204 ± 18 s for ASIC3 and ASIC3-loop1a, respectively; Fig. 2, D and G). Altogether, these data indicate that the extracellular loop of ASIC3 does not significantly contribute to the generation of the sustained current but is central for determining the inactivation kinetics of the transient and sustained currents.
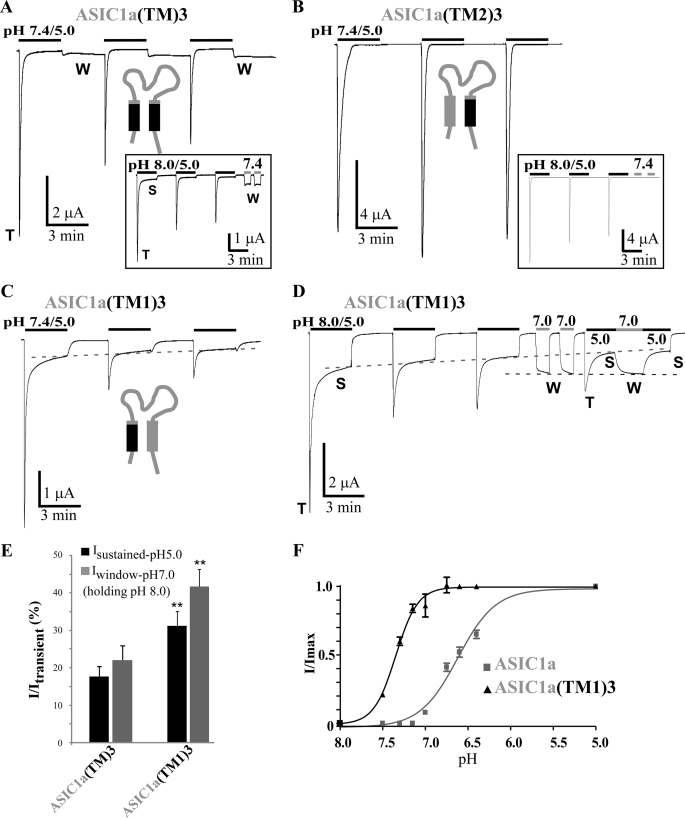
First Transmembrane Domain of ASIC3 Is Important for Generation of Sustained Current at pH 5.0 and Window Current at pH 7.0
We have tested the participation in the sustained current of the transmembrane domains of ASIC3 by first transferring both the TM1and TM2 domains of ASIC3 into ASIC1a (ASIC1a(TM)3 chimera). This chimera displays a significant sustained current (Isustained-pH5.0/Itransient = 17.7 ± 2.7%) measured 45 s after the transient current with a pH pulse from 8.0 to 5.0 (Fig. 3, A and E). A strong steady-state inward current, which is time-independent and clearly distinct from the pH 5.0-activated sustained current, is evoked when the pH is shifted back from 5 to 7.4 after the usual pH pulse from 7.4 to 5.0 (Fig. 3A). This persistent current is attributable to a window current, which is suppressed when the holding pH was at pH 8.0 instead of pH 7.4 to maintain the channel in its closed state (Fig. 3A, inset). In these conditions, which allow a clear discrimination between Isustained-pH5.0 and Iwindow, the ASIC1a(TM)3 chimera generates a pH 5.0-activated transient current with slow kinetics followed by a plateau similar to the one observed in ASIC3, and a persistent window current can be activated at pH 7.4 (Fig. 3A, inset). We have measured this window current at pH 7.4 and at pH 7.0 (pulse from pH 8.0) (Iwindow-pH7.0/Itransient = 22.1 ± 3.8% and Iwindow-pH7.4/Itransient = 17.8 ± 4.2%; Fig. 3, A and E).
FIGURE 3.
Analysis of the role of the transmembrane domains in generation of the sustained current in ASIC1a/3 chimeras. A, B, and C, representative current traces of pH 5. 0-activated currents recorded at −50 mV from Xenopus oocytes injected with ASIC1a(TM)3 (A), ASIC1a(TM2)3 (B), and ASIC1a(TM1)3 (C). Three pH pulses of 3 min each are made at 3-min intervals from a holding pH of 7.4. Inset in A and B, Similar recordings made from a holding pH of 8.0 instead of 7.4. Two successive pH pulses from 8.0 to 7.4 have been used at the end of the recordings to check for the presence of a window current. T, S, and W, transient, sustained, and window components, respectively. D, typical current trace of the ASIC1a(TM1)3 chimera recorded as in C but from a holding pH of 8.0 instead of 7.4, followed by the activation of the window current at pH 7.0 and of the pH 5.0-evoked sustained current. The dashed lines illustrate the slow decrease of the pH 5.0-evoked sustained current and the steady-state of the pH 7.0-activated window current. E, average relative pH 5.0-evoked sustained current and pH 7.0-evoked window currents (holding pH of 8.0). pH 5.0-evoked sustained current were measured 45 s after the peak activated by the first pH pulse as shown in A–C, for ASIC1a(TM)3 and ASIC1a(TM1)3 (n = 30 and 35, respectively). Average relative pH 7.0-evoked window currents for ASIC1a(TM)3 and ASIC1a(TM1)3 (n = 22 and 35, respectively) are shown. The ASIC1a(TM2)3 chimera, which is lacking both pH 5.0-activated and pH 7.0- and pH 7.4-evoked window currents, is not included in this graph. **, p < 0.01, significantly different from ASIC1a(TM)3. F, pH-dependent activation curves of the transient current associated with ASIC1a and the ASIC1a(TM1)3 chimera. pH has been decreased from pH 8.0 to the indicated pH values (7.5/7.3/7.15/7.0/6.75/6.6/6.4/5.0) and normalized to pH 5.0. The pH0.5 values for activation of ASIC1a and ASIC1a(TM1)3 are 6.62 ± 0.02 and 7.34 ± 0.02, n = 8 and 7, respectively.
The TM1 and TM2 domains have then been individually transferred from ASIC3 to ASIC1a to build the ASIC1a(TM1)3 and ASIC1a(TM2)3 chimeras. The ASIC1a(TM2)3 chimera has almost no pH 5.0-evoked sustained current (Isustained-pH5.0/Itransient = 1.2 ± 0.4%) and no window current, as shown by raising the holding pH from 7.4 to 8.0 to allow the necessary discrimination between Isustained-pH5.0 and Iwindow (Fig. 3B, inset). The ASIC1a(TM1)3 chimera generates both an Isustained-pH5.0 current (Isustained-pH5.0/Itransient = 31.2 ± 3.8%; 45 s after the peak) and an Iwindow current (Iwindow-pH7.0/Itransient = 41.7 ± 4.5%; Fig. 3, C–E), indicating an important role for the TM1 domain in the generation of the ASIC3-sustained currents. Some of the chimeras like ASIC1a(TM)3 and ASIC1a(TM1)3 have sustained currents significantly larger than ASIC3, which could be explained by the presence of the extracellular domain of ASIC1a. This is suggested by the much larger pH 5.0-activated sustained current of the ASIC1a(TM)3 chimera (Fig. 3, A and E; extracellular domain of ASIC1a) compared with the ASIC3(NC)1a chimera (see Fig. 5, D and E; extracellular domain of ASIC3), the only difference between the two chimeras being their extracellular loops. This is also suggested by the properties of the ASIC3-loop1a chimera (extracellular domain of ASIC1a), which has a larger pH 5.0-activated sustained current compared with ASIC3 (Fig. 2, A, D, and E).
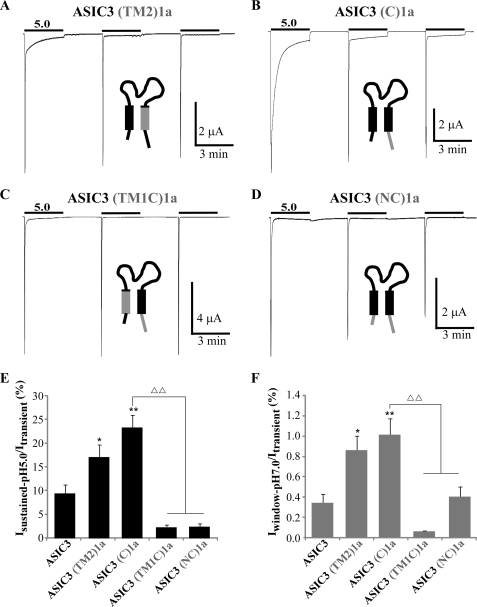
FIGURE 5.
Analysis of the role of the transmembrane and intracellular domains in generation and modulation of the sustained current in ASIC3/1a chimeras. A–D, representative current traces of pH 5.0-activated currents recorded at −50 mV from Xenopus oocytes injected with ASIC3(TM2)1a (A), ASIC3(C)1a (B), ASIC3(TM1C)1a (C), and ASIC3(NC)1a (D). Three pH pulses of 3 min each are made at 3-min intervals from a holding pH of 7.4. E and F, average relative pH 5.0-evoked sustained current (E) and pH 7.0-evoked window current (F) recorded for ASIC3, ASIC3(TM2)1a, ASIC3(C)1a, ASIC3(TM1C)1a, and ASIC3(NC)1a (n = 28, 16, 15, 15 and 15 for Isustained-pH5.0, and 10, 16, 15, 15, and 15 for Iwindow-pH7.0, respectively). The pH 5.0-evoked sustained currents have been measured 45 s after the peak activated by the first pH pulse as shown in A–D. *, p < 0.05; **, p < 0.01, significantly different from ASIC3; ΔΔ, p < 0.01, significantly different from ASIC3(C)1a.
A specific protocol was used to discriminate clearly between Isustained-pH5.0 and Iwindow in the ASIC1a(TM1)3 chimera (Fig. 3D). A pH shift from 8.0 to 7.0 following the first three successive pulses to pH 5.0 activates a relatively large window current, which does not display a substantial decrease over time (Fig. 3D, dotted line). Shifting again the pH to 5.0 activates a small peak current because of the strong rundown, as well as a sustained current with a decreased amplitude (Fig. 3D, dotted line) as expected. Moving back the pH from 5.0 to 7.0 activates the window current again and confirms a lack of significant decay in current amplitude. Another pulse from pH 7.0 to 5.0 shifts the current from the window to the sustained type. It is thus possible to activate the persistent pH 7.0-evoked window current and the slowly inactivating pH 5.0-evoked sustained current independently and differentially.
Isustained-pH5.0 and Iwindow are both larger in the ASIC1a(TM1)3 chimera compared with the ASIC1a(TM)3 chimera (Fig. 3E), suggesting a negative role for the TM2 domain of ASIC3. The pH-dependent activation of the ASIC1a(TM1)3 chimera (Fig. 3F) shows a large shift toward more alkaline pH compared with ASIC1a (pH0.5 = 6.62 ± 0.02 and 7.34 ± 0.02 for ASIC1a and ASIC1a(TM1)3, respectively). This large shift in the pH dependence of activation explains the substantial window current associated with this chimera. This result is consistent with the data obtained with the “reverse” chimera ASIC3(TM1C)1a (supplemental Fig. 2, C and D) showing a shift of the pH-dependent activation curve in the opposite way, i.e. toward more acidic pH, although with a less dramatic effect. Surprisingly, the ASIC1a(TM1)3 chimera appears to be more pH-sensitive than either ASIC1a or ASIC3 parent channels, which could be attributable to the different molecular context surrounding the ASIC3 TM1 domain in this ASIC1-based chimera. It was not possible to estimate the pH-dependent inactivation of this chimera because of both the strong rundown of the peak current and the important window current generated at the different conditioning pH values. The amplitude of the peak current cannot be determined when it becomes smaller than the amplitude of the window current.
The first transmembrane domain of ASIC3 is thus a major structural element for the generation of the sustained current at pH 5.0. The same domain is also important to produce the window current at pH 7.0.
Intracellular N- and C-terminal Domains of ASIC3 Also Contribute to Generation and Modulation of Sustained Currents
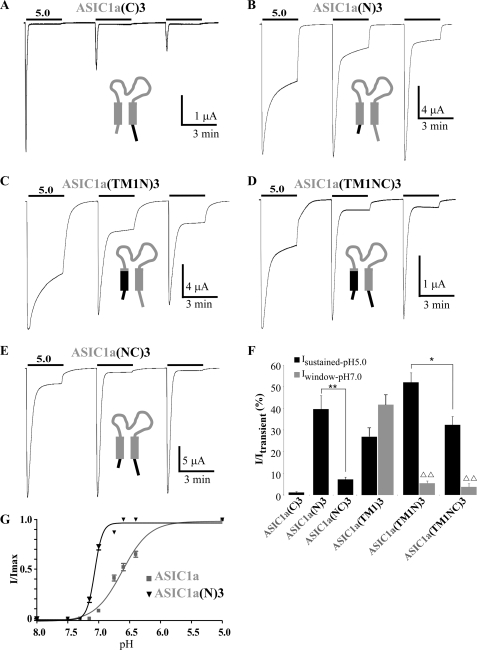
Changing the C-terminal domain of ASIC1a by the C-terminal domain of ASIC3 (ASIC1a(C)3 chimera) was associated with a strong rundown of the channel activity, but it did not induce a pH 5.0-evoked sustained current (Fig. 4A). No window current has been recorded at pH 7.0 for this chimera either (data not shown). The intracellular C-terminal domain of ASIC3 does not contribute to the sustained current nor to the window current. Transferring the N-terminal domain of ASIC3 into ASIC1a (ASIC1a(N)3 chimera) generates a large pH 5.0-evoked sustained current (Isustained-pH5.0/Itransient = 39.6 ± 6.0%; Fig. 4, B and F), but no window current at pH 7.0 (data not shown). The intracellular N-terminal domain of ASIC3 is thus also important for the pH 5.0-evoked sustained current. The N-terminal domain chimera clearly indicates that the pH 5.0-evoked sustained current and the pH 7.0-evoked window current are generated by different mechanisms, as already suggested by data shown in Fig. 3D. The threshold for pH activation of the ASIC1a(N)3 chimera is not significantly shifted compared with ASIC1a (Fig. 4G), which is consistent with the absence of window current in this chimera.
FIGURE 4.
Analysis of the role of the intracellular N- and C-terminal domains in generation and modulation of the sustained current in ASIC1a/3 chimeras. A–E, representative current traces of pH 5. 0-activated currents recorded at −50 mV from Xenopus oocytes injected with ASIC1a(C)3 (A), ASIC1a(N)3 (B), ASIC1a(TM1N)3 (C), ASIC1a(TM1NC)3 (D), or ASIC1a(NC)3 (E). Three pH pulses of 3 min each are made at 3-min intervals from a holding pH of 7.4. F, average relative pH 5.0-evoked sustained current and pH 7.0-evoked window currents recorded for ASIC1a(C)3, ASIC1a(N)3, ASIC1a(NC)3, ASIC1a(TM1)3, ASIC1a(TM1N)3, and ASIC1a(TM1NC)3 (n = 11, 15, 8, 22, 23, and 14, respectively). The pH 5.0-evoked sustained currents have been measured 2 min after the peak activated by the first pH pulse as shown in A–D. ΔΔ, p < 0.01, significantly different from ASIC1a(TM1)3. G, pH-dependent activation curves of the transient current associated with ASIC1a and the ASIC1a(N)3 chimera. pH has been decreased from pH 8.0 to the indicated values (7.5/7.3/7.15/7.0/6.75/6.6/6.4/5.0) and normalized to pH 5.0. The pH0.5 values for activation of ASIC1a and ASIC1a(N)3 are 6.62 ± 0.02 and 7.06 ± 0.01, n = 8 and 7, respectively. Hill coefficients (n) calculated for ASIC1a and ASIC1a(N)3 are 1.96 and 6.96, respectively.
Replacing together the cytosolic N-terminal domain and the TM1 domain of ASIC1a with those of ASIC3 (ASIC1a(TM1N)3 chimera) leads to a biphasic current with a pH 5.0-evoked sustained component that is not significantly different (p = 0.08) from the current generated by the ASIC1a(N)3 chimera (Isustained-pH5.0/Itransient = 52.2 ± 4.2% and 39.6 ± 6.0%, respectively; Fig. 4, B, C, and F). This new chimera compared with the ASIC1a(TM1)3 chimera displays a significantly decreased window current, with no current generated at pH 7.4 and a smaller current generated at pH 7.0 (Iwindow-pH7.0/Itransient = 5.5 ± 1.1% versus 41.7 ± 4.4% for ASIC1a(TM1N)3 and ASIC1a(TM1)3, respectively; Fig. 4F), and a significantly increased pH 5.0-evoked sustained current (Isustained-pH5.0/Itransient = 52.2 ± 4.2% and 26.9 ± 4.1%, for the ASIC1a(TM1N)3 and ASIC1a(TM1)3 chimeras, respectively; p < 0.01; Fig. 4F). However, the absence of an additive or synergistic effect between the TM1 and the N-terminal domains of ASIC3 suggests a common mechanism for the pH 5.0-evoked sustained current in both cases.
To test the effect of the C-terminal domain of ASIC3 on the sustained current generated by the N-terminal and the TM1 domains, we have transferred this domain in the ASIC1a(TM1N)3 chimera (ASIC1a(TM1NC)3 chimera). The C-terminal domain of ASIC3 decreases the pH 5.0-evoked sustained current by 1.6-fold compared with the ASIC1a(TM1N)3 chimera (Fig. 4, C, D, and F). It also drastically decreases (5.5-fold) the pH 5.0-evoked sustained current associated with the ASIC1a(N)3 chimera (ASIC1a(NC)3 chimera; Fig. 4, B, E, and F). These data suggest that the C-terminal domain of ASIC3, although not contributing by itself to the generation of the pH 5.0-evoked sustained current, acts as a negative modulator of this current.
N-terminal Domain and TM1 Domain Are Both Necessary to Generate ASIC3-sustained Currents
To confirm the role in ASIC3 of the domains identified through their transfer in ASIC1a, we have replaced the corresponding ASIC3 domains by their ASIC1a counterparts. When the TM2 or the C-terminal domains of ASIC3 were exchanged by corresponding domains of ASIC1a (ASIC3(TM2)1a and ASIC3(C)1a chimeras; Fig. 5, A and B), a significant increase of both the sustained current at pH 5.0 and of the window current at pH 7.0 was observed (Isustained-pH5.0/ Itransient = 9.4 ± 1.8%, 17.2 ± 2.5% and 23.4 ± 2.6% and Iwindow-pH7.0/Itransient = 0.34 ± 0.09%, 0.86 ± 0.14 and 1.01 ± 0.16%, for ASIC3, ASIC3(TM2)1a, and ASIC3(C)1a, respectively; Fig. 5, E and F). This confirms that these domains in ASIC3 probably have an inhibitory effect on sustained currents.
Replacement of the TM1 or the N-terminal domain of ASIC3 by the corresponding domains of ASIC1a to construct the ASIC3(TM1)1a or the ASIC3(N)1a chimeras led to nonfunctional channels. The TM1 domain of ASIC3 has then been replaced by the TM1 domain of ASIC1a in the ASIC3(C)1a chimera to produce the ASIC3(TM1C)1a chimera. The pH 5.0- and pH 7.0-evoked sustained currents are both largely decreased in this chimera (Isustained-pH5.0/Itransient = 23.4 ± 2.6% and 2.4 ± 0.4%, and Iwindow-pH7.0/Itransient = 1.01 ± 0.16% and 0.06 ± 0.01%, for ASIC3(C)1a and ASIC3(TM1C)1a, respectively; Fig. 5, C, E, and F), confirming the importance of the TM1 domain to generate both the sustained current at pH 5.0 and the window current at pH 7.0. Similarly, replacing the N-terminal domain in the ASIC3(C)1a chimera by the N-terminal domain of ASIC1a (ASIC3(NC)1a chimera) suppresses the pH 5.0-evoked sustained current (Isustained-pH5.0/Itransient = 23.4 ± 2.6% and 2.5 ± 0.6% for ASIC3(C)1a and ASIC3(NC)1a, respectively; Fig. 5, D and E), showing that the TM1 domain and the N-terminal domain are necessary together for the generation of the pH 5.0-evoked sustained current in ASIC3.
These data taken together confirm the participation of the N-terminal and the TM1 domains in the generation of ASIC3 pH 5.0-evoked sustained current and the central role of the TM1 domain in generation of the window current. They also confirm the inhibitory role of the TM2 and the C-terminal domains on these currents.
DISCUSSION
Our data clearly underline the very remarkable capacity of ASIC3 to adopt its response to a wide range of extracellular pH variations. ASIC3 is capable of adapting three different modes of functioning depending on the pH conditions. In the first mode, rapid pH variations of the extracellular medium generate a fast transient current, which by itself is large enough to depolarize the membrane and trigger action potentials (14). In the second mode, sustained and modest variations of the extracellular pH, which all remain near the physiological pH, generate a window current, which produces a persistent depolarization that appears to be important for pain perception in conditions related to inflammation (4). In the third mode, more severe sustained acidifications like those described in a number of pathological situations, such as hematomas and bone cancer (17), activate a plateau current through a different mechanism, also producing a persistent depolarization. ASIC3 thus emerges as a very sensitive pH sensor that is able to respond to extracellular acidosis ranging from modest variations near the physiological pH to severe decrease in pH and generate transient and sustained currents that can affect neuronal excitability by triggering action potential generation and most probably by controlling both the slope and the number of these action potentials. In nociceptors, this property is directly relevant to the role proposed for ASIC3 in detection of noxious acidosis associated with inflammation, ischemia, tumors, or tissue damages (4, 18, 19).
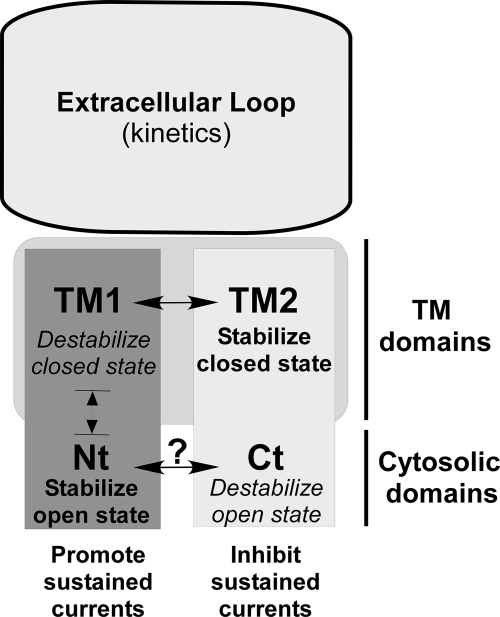
We propose a model that summarizes our data illustrating the influence of the TM and cytosolic domains on the gating of ASIC3. The extracellular domain of ASIC channels has been described as a “clenched hand” comprising a disulfide bond-rich “thumb” domain contacting the TM domains, a “finger” and a “knuckle” domain located above a large seven-strand sheet “palm” domain that directly connect to TM1 and TM2, as well as to the thumb domain (8, 9). The extracellular loop influences both the kinetics and the pH dependence of the transient current (15, 16, 20) as well as the kinetics of the sustained current as shown in this paper. The loop binds protons to both the acidic pocket, a region enriched in acidic residues (8), and to the β-sheet region localized in the palm domain (8, 20–22). The respective roles of each of these two H+-binding domains are not yet completely clear. Upon acidification, resulting conformational changes in the extracellular loop are transmitted to the transmembrane domains (23, 24), leading to the rapid opening and closing of the channel and to the generation of the transient current. Higher proton concentrations (<pH 6.5) also induce conformational changes, generating a very slowly inactivating current, which is associated with a long-lived inactivated state of the channel. It is not clear whether intracellular protonation of the channel could contribute to this effect, as suggested for the rundown of ASIC1a (25). The TM1 domain is important for this low pH effect in association with the N-terminal domain. Our model (Fig. 6) proposes that the TM1 and the N-terminal regions contribute to the sustained current and also probably to the transient current by promoting the open state in two different ways. On the one hand, we suggest that the TM1 domain tends to destabilize the closed state, possibly by acting on the TM2 domain (both domains are in close proximity in the tridimensional structure of ASIC1 (8, 9)). This interpretation stems from our data showing (i) a significant modification of the pH threshold when the TM1 domain of ASIC3 is introduced into ASIC1a (ASIC1a(TM1)3 chimera; Fig. 3F), consistent with a destabilization of the closed state, but (ii) no modification of the slope and shape of the activation curve once the channel is open, consistent with an absence of effect on the open state. In good agreement with this model, the activation curve of the transient current is also shifted toward more acidic pH when the TM1 domain of ASIC3 is replaced by the TM1 domain of ASIC1a in the ASIC3(C)1a chimera, without any effect on the inactivation of the channel (supplemental Fig. 2, C and D). On the other hand, the N-terminal domain of ASIC3 would tend to stabilize the open state of the channel. When this N-terminal domain is introduced in ASIC1a (ASIC1a(N)3 chimera; Fig. 4G), the pH threshold is not modified, consistent with an absence of effect on the closed state, but the slope of the activation curve is significantly increased, as expected if this domain stabilizes the open state of the channel. In the model we propose, the TM2 domain and the C-terminal domain tend to prevent the generation of the sustained current by promoting the closed state through its stabilization by the TM2 domain and the destabilization of the open state by the C-terminal domain (Fig. 6). This view is supported by our present data showing a shift of the inactivation curve of the transient current toward more acidic pH when the C-terminal domain of ASIC3 is replaced by the C-terminal domain of ASIC1a, without any effect on the pH-dependent activation of the channel (supplemental Fig. 2, A and B), consistent with a destabilization of the open state, and by previous observations that mutations at a particular position (the Deg position) located in the TM2 domain of ASIC2 dramatically reduce channel closing (26). This first demonstration of an involvement of the C-terminal domain in gating of ASIC channels is an important observation in relation with the regulation of the ASIC3 channel. We have indeed previously shown that association of the NHERF-1 (Na+/H+ exchanger regulatory factor-1) adaptor protein with the C-terminal domain of ASIC3 produces a strong up-regulation of the sustained component of the ASIC3 current (27). We propose that the C-terminal domain of ASIC3 is normally hampering the generation of sustained currents but that this role can become positive when binding of accessory proteins such as NHERF-1 to the C-terminal domain of ASIC3 relieves the inhibitory effect and favors sustained currents upon acidification.
FIGURE 6.
Model summarizing the influence of the transmembrane and cytosolic domains on ASIC3 gating. The extracellular loop does not significantly contribute to the generation of the sustained current but is central for determining the inactivation kinetics of both the transient and sustained currents. The TM1 and N-terminal (Nt) domains are the key structural elements generating the sustained currents, whereas the C-terminal (Ct) and the TM2 domains act as negative regulators. Our data suggest that TM1 and TM2 participate in the gating of ASIC3 essentially through stabilization (TM2) or destabilization (TM1) of the closed state, whereas the cytosolic domains (Nt and Ct) act via stabilization (Nt) or destabilization (Ct) of the open state. The structure and the organization of the cytosolic domains are not known, and it is not clear whether these domains can interact with each other. They can be affected by post-translational modifications (31, 32), associated proteins (27, 33–35), or variation in the intracellular pH (36), with important consequences on the gating of the channel.
In conclusion, the present work provides new structural information to help in an understanding of the mechanisms of the multifaceted pH gating of ASIC3 (and probably more generally of ASIC channels). This is an important issue considering the role of these channels in pain (4) and in a growing number of pathological conditions (28–30).
Supplementary Material
Acknowledgments
We thank Drs. Anne Baron, Emmanuel Deval, Xavier Gasull, and Jacques Noel for reading the manuscript and valuable comments; Dr. Sylvie Diochot for helpful discussion; V. Friend for excellent technical assistance; and C. Chevance for secretarial assistance.
This work was supported by the CNRS, the Agence Nationale de la Recherche, the Association Française contre les Myopathies, the Association pour la Recherche sur le Cancer, and the Institut UPSA de la Douleur.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- ASIC3
- acid-sensing ion channel 3
- MES
- 2-morpholinoethanesulfonic acid
- TM
- transmembrane.
REFERENCES
- 1.Reeh P. W., Steen K. H. (1996) Prog. Brain Res. 113, 143–151 [DOI] [PubMed] [Google Scholar]
- 2.Steen K. H., Issberner U., Reeh P. W. (1995) Neurosci. Lett. 199, 29–32 [DOI] [PubMed] [Google Scholar]
- 3.Issberner U., Reeh P. W., Steen K. H. (1996) Neurosci. Lett. 208, 191–194 [DOI] [PubMed] [Google Scholar]
- 4.Deval E., Noël J., Lay N., Alloui A., Diochot S., Friend V., Jodar M., Lazdunski M., Lingueglia E. (2008) EMBO J. 27, 3047–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. (1997) Nature 386, 173–177 [DOI] [PubMed] [Google Scholar]
- 6.Lingueglia E. (2007) J. Biol. Chem. 282, 17325–17329 [DOI] [PubMed] [Google Scholar]
- 7.Wemmie J. A., Price M. P., Welsh M. J. (2006) Trends Neurosci. 29, 578–586 [DOI] [PubMed] [Google Scholar]
- 8.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 9.Gonzales E. B., Kawate T., Gouaux E. (2009) Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babinski K., Lê K. T., Séguéla P. (1999) J. Neurochem. 72, 51–57 [DOI] [PubMed] [Google Scholar]
- 11.de Weille J. R., Bassilana F., Lazdunski M., Waldmann R. (1998) FEBS Lett. 433, 257–260 [DOI] [PubMed] [Google Scholar]
- 12.Waldmann R., Bassilana F., de Weille J. R., Champigny G., Heurteaux C., Lazdunski M. (1997) J. Biol. Chem. 272, 20975–20978 [DOI] [PubMed] [Google Scholar]
- 13.Yagi J., Wenk H. N., Naves L. A., McCleskey E. W. (2006) Circ. Res. 99, 501–509 [DOI] [PubMed] [Google Scholar]
- 14.Deval E., Baron A., Lingueglia E., Mazarguil H., Zajac J. M., Lazdunski M. (2003) Neuropharmacology 44, 662–671 [DOI] [PubMed] [Google Scholar]
- 15.Salinas M., Rash L. D., Baron A., Lambeau G., Escoubas P., Lazdunski M. (2006) J. Physiol. 570, 339–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coric T., Zhang P., Todorovic N., Canessa C. M. (2003) J. Biol. Chem. 278, 45240–45247 [DOI] [PubMed] [Google Scholar]
- 17.Mantyh P. W., Clohisy D. R., Koltzenburg M., Hunt S. P. (2002) Nat. Rev. Cancer 2, 201–209 [DOI] [PubMed] [Google Scholar]
- 18.Yen Y. T., Tu P. H., Chen C. J., Lin Y. W., Hsieh S. T., Chen C. C. (2009) Mol. Pain, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland S. P., Benson C. J., Adelman J. P., McCleskey E. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paukert M., Chen X., Polleichtner G., Schindelin H., Gründer S. (2008) J. Biol. Chem. 283, 572–581 [DOI] [PubMed] [Google Scholar]
- 21.Baron A., Schaefer L., Lingueglia E., Champigny G., Lazdunski M. (2001) J. Biol. Chem. 276, 35361–35367 [DOI] [PubMed] [Google Scholar]
- 22.Smith E. S., Zhang X., Cadiou H., McNaughton P. A. (2007) Neurosci. Lett. 426, 12–17 [DOI] [PubMed] [Google Scholar]
- 23.Li T., Yang Y., Canessa C. M. (2009) J. Biol. Chem. 284, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwood T. W., Askwith C. C. (2008) J. Biol. Chem. 283, 1818–1830 [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Gründer S. (2007) J. Physiol. 579, 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champigny G., Voilley N., Waldmann R., Lazdunski M. (1998) J. Biol. Chem. 273, 15418–15422 [DOI] [PubMed] [Google Scholar]
- 27.Deval E., Friend V., Thirant C., Salinas M., Jodar M., Lazdunski M., Lingueglia E. (2006) J. Biol. Chem. 281, 1796–1807 [DOI] [PubMed] [Google Scholar]
- 28.Ziemann A. E., Schnizler M. K., Albert G. W., Severson M. A., Howard M. A., 3rd, Welsh M. J., Wemmie J. A. (2008) Nat. Neurosci. 11, 816–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friese M. A., Craner M. J., Etzensperger R., Vergo S., Wemmie J. A., Welsh M. J., Vincent A., Fugger L. (2007) Nat. Med. 13, 1483–1489 [DOI] [PubMed] [Google Scholar]
- 30.Ettaiche M., Deval E., Pagnotta S., Lazdunski M., Lingueglia E. (2009) Invest. Ophthalmol. Vis. Sci. 50, 2417–2426 [DOI] [PubMed] [Google Scholar]
- 31.Bashari E., Qadri Y. J., Zhou Z. H., Kapoor N., Anderson S. J., Meltzer R. H., Fuller C. M., Benos D. J. (2009) Am. J. Physiol. Cell Physiol. 296, C372–C384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deval E., Salinas M., Baron A., Lingueglia E., Lazdunski M. (2004) J. Biol. Chem. 279, 19531–19539 [DOI] [PubMed] [Google Scholar]
- 33.Anzai N., Deval E., Schaefer L., Friend V., Lazdunski M., Lingueglia E. (2002) J. Biol. Chem. 277, 16655–16661 [DOI] [PubMed] [Google Scholar]
- 34.Hruska-Hageman A. M., Benson C. J., Leonard A. S., Price M. P., Welsh M. J. (2004) J. Biol. Chem. 279, 46962–46968 [DOI] [PubMed] [Google Scholar]
- 35.Donier E., Rugiero F., Okuse K., Wood J. N. (2005) J. Biol. Chem. 280, 38666–38672 [DOI] [PubMed] [Google Scholar]
- 36.Wang W. Z., Chu X. P., Li M. H., Seeds J., Simon R. P., Xiong Z. G. (2006) J. Biol. Chem. 281, 29369–29378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.