Abstract
The ability of insulin-like growth factor I (IGF-I) to stimulate cartilage matrix synthesis is reduced in aged and osteoarthritic cartilage. Aging and osteoarthritis are associated with an increase in reactive oxygen species, which we hypothesized would interfere with normal IGF-I signaling. We compared IGF-I signaling in normal and osteoarthritic human articular chondrocytes and investigated the effects of oxidative stress induced by tert-butylhydroperoxide (tBHP). In normal human chondrocytes, IGF-I initiated a strong and sustained phosphorylation of IRS-1 (Tyr-612) and Akt (Ser-473) and transient ERK phosphorylation. In contrast, in osteoarthritic chondrocytes, which possessed elevated basal IRS-1 (Ser-312) and ERK phosphorylation, IGF-I failed to stimulate IRS-1 (Tyr-612) or Akt phosphorylation. In normal human chondrocytes, tBHP triggered strong IRS-1 (Ser-312 and Ser-616) and ERK phosphorylation and inhibited IGF-I-induced IRS-1 (Tyr-612) and Akt phosphorylation. Lentivirus-mediated overexpression of constitutively active (CA) Akt significantly enhanced proteoglycan synthesis, whereas both dominant negative Akt and CA MEK inhibited proteoglycan synthesis. CA Akt also promoted type II collagen and Sox9 expression, whereas tBHP treatment and CA MEK inhibited aggrecan, collagen II, and Sox9 mRNA expression. In osteoarthritic chondrocytes, the antioxidants Mn(III) tetrakis(4-benzoic acid)porphyrin and N-acetylcysteine increased the ratio of Akt to ERK phosphorylation and promoted IGF-I-mediated proteoglycan synthesis. Chemical inhibition of ERK significantly enhanced IGF-I phosphorylation of Akt and alleviated tBHP inhibition of Akt phosphorylation. These results demonstrate opposing roles for phosphatidylinositol 3-kinase-Akt and MEK-ERK in cartilage matrix synthesis and suggest that elevated levels of reactive oxygen species cause chondrocyte IGF-I resistance by altering the balance of Akt to ERK activity.
Introduction
Insulin-like growth factor I (IGF-I)2 plays a critical role in regulating normal growth and tissue formation during both embryonic and postnatal development through its ability to promote mitogenesis, cell migration, survival, and protein synthesis. Aberrant IGF-I expression or signaling has been implicated in various human diseases, including certain growth disorders (1), type II diabetes and cardiovascular disease (2), osteoarthritis (OA) (3), and cancer (4), as well as in cell senescence (5). Importantly, an age-related decline in response to IGF-I stimulation has been noted in musculoskeletal tissues, including bone (6, 7) and cartilage (8–10), but the underlying mechanisms for this decline are not completely understood.
IGF-I functions through binding to its cognate cell membrane receptor, IGF-I receptor, which is activated by autophosphorylation and then recruits and activates several signaling intermediates, including members of the insulin-receptor substrate (IRS) family and Shc. Activation of IRS leads downstream to activation of the PI 3-kinase-Akt pathway that activates mammalian target of rapamycin (mTOR), p70S6K, and 4E-BP-1 (eIF-4E-binding protein), key factors regulating mRNA translation and protein synthesis. Activation of Shc leads to activation of the ERK MAPK pathway through Grb2/SOS, Ras, Raf, and the MAPK/ERK kinase (MEK) (4). MEK1/2 phosphorylates and activates ERK1/2, which, in turn, phosphorylates and activates other kinases and several nuclear factors, such as p90RSK/c-Fos and Elk1. Depending on the cell type and context in which IGF-I stimulation occurs, activation of the PI 3-kinase-Akt pathway most often promotes cell growth, protein synthesis, and/or cell survival, whereas activation of the ERK MAPK pathway promotes cell proliferation and/or gene transcription.
Aging is a major risk factor for the development of osteoarthritis (OA), the most common joint disease that affects about half of the population over the age of 65 years. OA is characterized by the progressive destruction and loss of the articular cartilage matrix due to unbalanced metabolic activities of the chondrocyte. Compared with those from normal young tissue, chondrocytes isolated from older adult or osteoarthritic cartilage have been found to produce much lower levels of collagen and proteoglycan in response to IGF-I (3, 8, 9). Evidence is accumulating to support a pathogenic role of disrupted redox balance in OA onset and progression, although the mechanisms responsible have not been fully defined (11–13). There is evidence for increased oxidative stress with aging in human chondrocytes (13), and chondrocytes from OA tissue have been reported to possess lower levels of anti-oxidant enzymes and increased production of reactive oxygen species (ROS) when compared with normal cartilage (12, 14–17). Nitrotyrosine, a marker for oxidative damage, has been detected in articular cartilage from older adults and in diseased tissue from people with osteoarthritis, and this correlated with a reduced ability of the articular chondrocytes to produce proteoglycans in response to IGF-I stimulation (18).
Because IGF-I utilizes many of the same signaling intermediates as the insulin signaling pathway, shared mechanisms may exist between IGF-I resistance and insulin resistance, the latter of which has been well studied in the context of type II diabetes. An important mechanism contributing to insulin resistance is the inhibition of IRS-1 activation through phosphorylation at several inhibitory serine residues that include Ser-312 and Ser-616 (19, 20). Serine phosphorylation of IRS-1 acts to inhibit insulin-mediated tyrosine phosphorylation, the latter of which is required for the ability of IRS-1 to activate the PI 3-kinase pathway. Several mechanisms exist for the induction of IRS-1 serine phosphorylation resulting in insulin resistance, and many of these have inflammation and oxidative stress as common mediators (21).
To further investigate mechanisms of IGF-I resistance in articular chondrocytes and the potential role of ROS, we compared the activation of key IGF-I signaling intermediates in cells isolated from normal and osteoarthritic cartilage and in normal cells treated with tert-butylhydroperoxide (tBHP). tBHP is a substrate for glutathione peroxidase and, when added to cells, causes oxidative stress by increasing the levels of GSSG at the expense of GSH (22). The signaling intermediates studied include members of the PI 3-kinase/Akt pathway and the ERK MAPK pathway. Our results show that the balance of PI 3-kinase-Akt and MEK-ERK activity regulates chondrocyte matrix synthesis, and this balance is modulated by oxidative stress that results from elevated levels of ROS.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Collagenase-P was purchased from Roche Applied Science. Pronase was from Calbiochem. Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 (1:1), antibiotics, and fetal bovine serum were from Invitrogen. IGF-I was from Austral Biologicals (San Ramon, CA). tBHP and N-acetyl-l-cysteine (NAC) were from Sigma. U0126 was from Cell Signaling Technology (Beverly, MA), Mn(III) tetrakis(4-benzoic acid) porphyrin (MnTBAP) was from Calbiochem, [35S]sulfate was from Amersham Biosciences, and PicoGreen double-stranded DNA assay reagent was from Invitrogen. Antibodies and their sources were as follows: IRS-1 (Ser(P)-312 and total) were from Upstate Biotechnology, Inc. (Lake Placid, NY); IRS-1 (Tyr(P)-612) was from Invitrogen; IRS-1 (Ser(P)-616), Akt (Ser(P)-473, Thr(P)-308, and total), MEK1/2 (Ser(P)-217/Ser(P)-221 and total), ERK1/2 (Thr(P)-202/Tyr(P)-204 and total), p70S6K (Thr(P)-389 and total), and HA tag were from Cell Signaling Technology. Anti-β-actin was from Abcam (Cambridge, MA).
Chondrocyte and Cartilage Explant Preparation and Culture
Normal human ankle cartilage was obtained from tissue donors with no prior history of arthritis through the Rush University Medical Center (Chicago, IL), from the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL), or from the National Disease Research Interchange (Philadelphia, PA). Each cartilage specimen was graded for degenerative status based on the five-point Collins scale, as modified by Muehleman et al. (23); cartilage of grade less than 2 was used as normal samples. The OA cartilage was discarded tissue obtained after knee replacement surgery performed at the Wake Forest University Baptist Medical Center (Winston-Salem, NC). Cartilage was dissected from the joint surfaces, digested with 0.2% Pronase for 1 h and then with 0.025% Collagenase-P (Roche Applied Science) overnight, as described previously (24). High density monolayer cultures were established by plating cells in 6-well plates at 1 × 106 cells/ml (2 ml/well) in DMEM/Ham's F-12 medium supplemented with 10% fetal bovine serum. Plates were maintained for 5–7 days until they reached confluence prior to experimental use. For explant cultures, full-thickness cartilage discs were obtained using a 4-mm biopsy punch and cultured for 3–5 days in serum-free DMEM/Ham's F-12 supplemented with 1% mini-ITS plus ascorbate (5 nm insulin, 2 μg/ml transferrin, 2 ng/ml selenous acid, 25 μg/ml l-ascorbic acid phosphate magnesium salt n-hydrate (Wako, Richmond, VA), 420 μg/ml bovine serum albumin, and 2.1 μg/ml linoleic acid) for recovery before experimental treatments.
Chondrocyte Signaling Studies
Chondrocytes were cultured in serum-free DMEM/Ham's F-12 medium overnight before being stimulated. Unless otherwise noted, the following standard concentrations of reagents were used: 250 μm tBHP, 250 μm MnTBAP, and 50 ng/ml IGF-I. The effects of these reagents on chondrocyte survival were monitored using the LIVE/DEAD® cell assay (Molecular Probes) as described previously (25). Immunoblotting was performed as described previously with slight modification (26). In brief, total cellular protein was prepared using cell lysis buffer (Cell Signaling Technology) supplemented with Phosphatase Inhibitor Mixture 2 (Sigma) and 1 mm phenylmethylsulfonyl fluoride. Proteins were separated by SDS acrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Bio-Rad) and probed by antibodies as indicated. The ECL detection kit (Amersham Biosciences) was used for blot visualization. Band densitometry analysis was performed using Eastman Kodak Co. 1D 3.6 image analysis software.
Lentivirus Generation and Chondrocyte Infection
The lentiviral constructs expressing constitutively activated (CA) and dominant negative (DN) Akt were kindly provided by Michael B. Robinson (Children's Hospital of Philadelphia, Pennsylvania). CA Akt has the N-terminal (amino acids 4–129) truncated and replaced by a Src myristoylation signal. DN Akt has K179A, T308A, and S473A mutations. The construct of CA MEK1 was generated by subcloning MEK1-R4F (ΔN3/S218E/S222D) (27) into the PmeI site of the pHAGE vector. All constructs contained an HA tag for identification. Lentivirus was generated by transfecting 293FT cells using these constructs together with ViraPower Packaging Mix (Invitrogen) according to the manufacturer's instructions. Concentration of the lentivirus was achieved by centrifugation of the lentiviral supernatant at 15,000 × g at 4 °C for 2 h; the lentivirus pellet was resuspended in medium of one-tenth volume of the supernatant, aliquoted, and kept at −80 °C. Infection of chondrocytes was performed by culturing chondrocytes in the concentrated lentivirus solution supplemented with 6 μg/ml Polybrene for 2 days. The infection was confirmed by immunocytochemistry using antibody against the HA tag expressed by the constructs (data not shown) and by immunoblotting for HA (Fig. 3A).
FIGURE 3.
MEK-ERK and Akt differentially regulate IRS-1-Akt signaling and proteoglycan production. Normal human chondrocytes were incubated for 2 days with lentivirus expressing CA MEK, CA Akt, and DN Akt or a control vector. A, the infected cells were serum-starved overnight. IGF-I at 50 ng/ml IGF-I was added for the last 30 min of culture where indicated. The expression of the HA tag and the activation of IRS-1, Akt, p70S6K, and ERK1/2 (T/Y, Thr-202/Tyr-204) were determined by immunoblotting. β-Actin was used as an additional protein loading control. B, the infected cells were cultured overnight with or without 100 ng/ml IGF-I before being incubated for 4 h with [35S]sulfate to measure proteoglycan (PG) synthesis using the sulfate incorporation assay corrected for cell numbers by DNA measurement. The data are presented as -fold change relative to the cells infected with the control vector. Normal human chondrocytes were treated with 25 μm tBHP for 4 h or vehicle for the same time period as control (Ctrl) (C) or infected for 2 days with lentivirus expressing CA MEK, CA Akt, or control vector (D). Aggrecan mRNA expression was determined by quantitative real-time PCR using expression of the TATA box-binding protein as a control. The data are the mean ± S.D. of three independent experiments. *, p < 0.05; ***, p < 0.005.
Proteoglycan Synthesis Assay
The [35S]sulfate incorporation assay was performed to measure proteoglycan synthesis. Depending on the experimental condition, chondrocytes were cultured in serum-free medium in monolayer or explants with or without overnight stimulation using 100 ng/ml IGF-I. The next day the medium was replaced with fresh serum-free medium 1 h prior to incubation with [35S]sulfate for another 4 h. [35S]sulfate incorporation was measured using the Alcian blue precipitation method (26) and normalized to DNA content. DNA was quantitated by PicoGreen double-stranded DNA assay according to the manufacturer's protocol.
Enzyme-linked Immunosorbent Assay for Collagen II
Normal human chondrocytes were infected with lentivirus expressing control vector, CA MEK, or CA Akt and then cultured in serum-free DMEM/Ham's F-12 supplemented with 1% mini-ITS plus ascorbate for 2 days. Uninfected cells were treated with 25 μm tBHP for 2 days. The cell layers were collected and analyzed for collagen II levels using an enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (MD Biosciences Inc., St. Paul, MN).
Quantitative Real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's protocol. RNA concentrations were determined by a DU 730 Life Science UV-visible spectrophotometer (Beckman). 2 μg of total RNA was used to synthesize cDNA using oligo(dT)15 as reverse primer. Equivalent amounts of cDNA were used for real-time PCR in a 25-μl reaction mixture with 12.5 μl of 2× SYBR Green PCR Mastermix and 1 μl of specific primer pair. Reactions were run in triplicate with 40 cycles of amplification on an ABI Prism 7000 real-time PCR machine (Applied Biosystems, Foster City, CA). The sequences of primers used were as follows: aggrecan, sense (5′-AGAATCCACCACCACCAG-3′) and antisense (5′-ATGCTGGTGCTGATGACA-3′); TATA box-binding protein, sense (5′-TGCACAGGAGCCAAGAGTGAA-3′) and antisense (5′-CACATCACAGCTCCCCACCA-3′) (28); collagen II, sense (5′-TGCTGCCCAGATGGCTGGAGGA-3′) and antisense (5′-TGCCTTGAAATCCTTGAGGCCC-3′) (29); Sox9, sense (5′-CACACTACAGCCCCTCCTAC-3′) and antisense (5′-CCTCCTCAAGGTCGAGTGAG-3′) (30). The expression levels of aggrecan, collagen II, and Sox9 mRNA were normalized relative to the expression of TATA box-binding protein measured in parallel samples.
Statistical Analysis
Data were expressed as mean ± S.D. and subjected to analysis of variance by using StatView 5.0 software (SAS Institute, Cary, NC). A level of p < 0.05 was considered to be significant.
RESULTS
OA Chondrocytes Possess a High Basal Level of IRS-1 Serine and ERK Phosphorylation and Reduced IGF-I Activation of Akt Phosphorylation
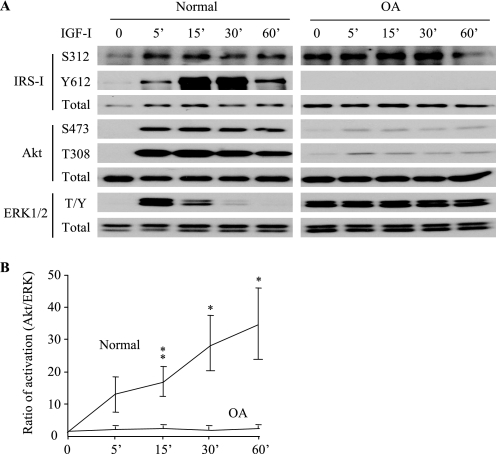
A time course study was performed to compare IGF-I signal transduction in chondrocytes isolated from normal human articular and osteoarthritic cartilage. In normal chondrocytes, 50 ng/ml IGF-I induced rapid (<5 min) and strong activation of IRS-1, which peaked at 30 min, as evidenced by the phosphorylation of Tyr-612 (Fig. 1A), a site essential for IRS-1 activation and the generation of a docking site for the downstream PI 3-kinase (19, 20, 31). IGF-I also induced phosphorylation of IRS-1 at Ser-312, which serves as a negative feedback regulator by inhibiting Tyr-612 phosphorylation (19, 20). Similar to previous results (26), IGF-I stimulated Akt phosphorylation at Ser-473 and Thr-308. Notably, the IGF-I-induced Akt phosphorylation was very strong and sustained at high levels during the whole test period (60 min). Besides stimulating the PI 3-kinase pathway, IGF-I also triggered the ERK MAPK signaling pathway in normal chondrocytes, but the induced ERK1/2 Thr202/Tyr204 phosphorylation was transient and decreased to the basal level within 30–60 min (Fig. 1A). In contrast to chondrocytes isolated from normal cartilage, OA chondrocytes exhibited a relatively high basal level of IRS-1 Ser-312 and ERK1/2 phosphorylation (Fig. 1A). In these cells, IGF-I failed to trigger detectable IRS-1 Tyr-612 phosphorylation, and the induced Akt phosphorylation was very weak. The high basal ERK1/2 phosphorylation in OA cells did not increase further after the addition of IGF-I. Comparison of the ratio of IGF-I-induced Akt to ERK phosphorylation revealed significantly greater Akt relative to ERK phosphorylation in normal compared with OA chondrocytes (Fig. 1B).
FIGURE 1.
The balance of IGF-I activation of Akt and ERK1/2 is altered in osteoarthritic chondrocytes. Chondrocytes from normal and OA human cartilage were serum-starved overnight and treated with 50 ng/ml IGF-I for the indicated times. A, the activation of IRS-1, Akt and ERK1/2 (T/Y, Thr-202/Tyr-204) were determined by immunoblot analysis using phosphospecific antibodies. Note that any two sections in one row were from the same blot with identical length of exposure. The results are representative of four normal tissue donors and three OA patients. B, ratios of the IGF-I-induced phosphorylation of Akt to ERK1/2 (normalized to their total proteins) were determined by densitometric analysis of the bands on the immunoblots. Results represent the mean ± S.D. of four normal donors (ages 74, 79, 66, and 82 years) and three OA patients (ages 65, 70, and 66 years). *, p < 0.05; **, p < 0.01.
Oxidative Stress Inhibits IGF-I-mediated IRS-1-Akt Signaling but Activates MEK-ERK
We hypothesized that increased levels of endogenous ROS due to oxidative stress, previously shown to be present in OA cells (11, 14, 17, 18), might act to inhibit IGF-I signaling. To test this, we induced oxidative stress in normal human chondrocytes by incubating cultures for 30 min with 250 μm tBHP. A cell survival assay did not detect any cytotoxic effect at this dose and time point, whereas a glutathione assay showed a significant increase in oxidized glutathione as evidence of oxidative stress (data not shown). The addition of tBHP induced IRS-1 phosphorylation at Ser-312 and Ser-616 (Fig. 2A). In addition, tBHP also significantly activated the MEK-ERK pathway, as indicated by the phosphorylation of MEK1/2 at Ser-217/221 and ERK1/2 at Thr-202/Tyr-204 (Fig. 2, A and C). Pretreatment with tBHP significantly inhibited the IGF-I-induced phosphorylation of IRS-1 Tyr-612 and Akt Ser-473 by 71 and 54%, respectively (Fig. 2, A and B), indicating an inhibitory effect of oxidative stress on IGF-I signaling. In contrast, treatment with tBHP followed by IGF-I did not notably alter the activation of ERK1/2 (Fig. 2, A and C). Thus, similar to what was noted in OA chondrocytes, oxidative stress in normal chondrocytes resulted in increased MAPK activation and resistance to IGF-I stimulation of the IRS-1-Akt pathway.
FIGURE 2.
Oxidative stress activates ERK MAPK signaling but inhibits IGF-I activation of Akt in normal human chondrocytes. Chondrocytes cultured in monolayer were treated with 250 μm tBHP for 30 min, followed by a 30-min stimulation with 50 ng/ml IGF-I. A, immunoblotting was performed to determine the phosphorylation of IRS-1, Akt, p70S6K, MEK1/2 (S/S, Ser-217/Ser-221), and ERK1/2 (T/Y, Thr-202/Tyr-204) using antibodies specific to their phosphorylated forms. B and C, the relative phosphorylation levels (normalized to their total proteins) of IRS-1, Akt, and ERK1/2 were determined by densitometric analysis of the bands and were expressed as a percentage of the IGF-I-stimulated sample. The results shown represent the means ± S.D. of three normal donors. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Akt Promotes, whereas MEK-ERK Inhibits, Basal and IGF-I-induced Proteoglycan Synthesis
Using chemical inhibitors, we have previously shown that PI 3-kinase activity is required for IGF-I induction of proteoglycan synthesis by human chondrocytes, whereas inhibition of MEK-ERK increased proteoglycan synthesis, suggesting that the ERK pathway might function as a negative regulator (26). To further determine the roles of PI 3-kinase-Akt and MEK-ERK pathways in basal and IGF-I-induced proteoglycan synthesis, we infected normal human chondrocytes with lentivirus expressing CA MEK, CA Akt, DN Akt, or the empty control virus. Immunoblotting with antibody specific for the HA tag in the lentivirus constructs confirmed the successful infection (Fig. 3A). Immunocytochemistry with anti-HA antibody revealed >90% infection efficiency (data not shown). Overexpression of CA MEK, as expected, led to strong and persistent phosphorylation of the downstream ERK1/2 without stimulating phosphorylation of Akt or p70S6K (Fig. 3A). Interestingly, CA MEK expression also led to a notable increase of the phosphorylation of two IRS-1 serine residues (Ser-312 and Ser-616), which are known to negatively regulate IRS-1 activation (32). In cells expressing CA Akt (a truncated form, seen as a band running lower than the native Akt), Akt Ser-473 phosphorylation was increased. DN Akt (with three point mutations but the same size as native Akt) was expressed at a low level (note weaker HA immunostaining) presumably due to the essential role of Akt for cell functioning and survival, so that cells expressing very high levels of DN Akt may not have survived. Expression of DN Akt weakly inhibited the IGF-I-induced phosphorylation of the downstream p70S6K. Similar to that seen in normal chondrocytes in Fig. 1, IGF-I triggered strong Akt activation in control cells but weak ERK activation compared with that triggered by the CA MEK expression.
To determine the role of MEK-ERK and Akt activity in the regulation of chondrocyte proteoglycan synthesis, we cultured the lentivirus-infected cells in the absence or presence of 100 ng/ml IGF-I overnight, followed by incubation with 35S-labeled sulfate to measure proteoglycan production. Compared with vector control, the basal level of proteoglycan synthesis was inhibited by the expression of CA MEK and DN Akt by 53 and 51%, respectively, whereas the expression of CA Akt significantly enhanced proteoglycan synthesis by over 2-fold (Fig. 3B). IGF-I-induced proteoglycan synthesis was completely inhibited by the expression of CA MEK or DN Akt, indicating a negative regulation by MEK-ERK activation and a requirement for Akt activation in both basal and IGF-I-induced proteoglycan production.
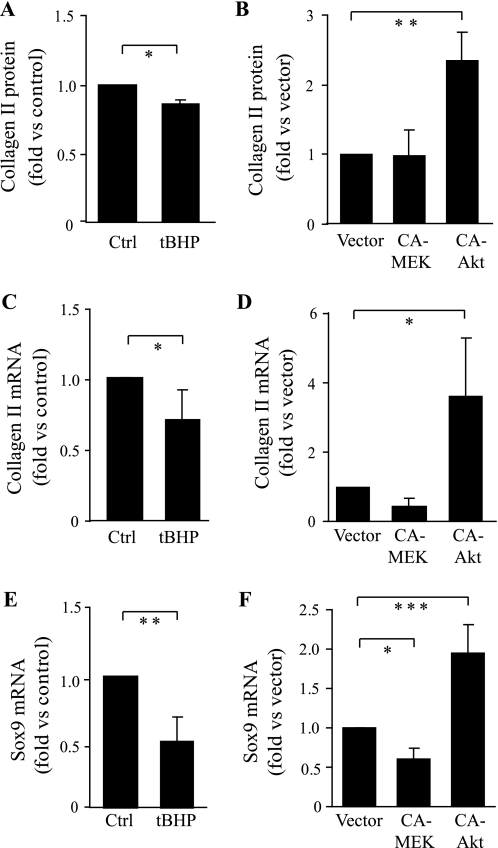
Regulation of Aggrecan, Collagen II, and Sox9 Expression by Oxidative Stress, MEK-ERK, and Akt
MEK/ERK signaling is mainly known to regulate gene transcription, whereas the effect of oxidative stress on proteoglycan production could be either at the level of protein synthesis or mRNA expression. Quantitative real-time PCR revealed that tBHP treatment or CA MEK expression in normal human chondrocytes significantly inhibited aggrecan mRNA expression by 50 and 68%, respectively (Fig. 3, C and D). tBHP also inhibited collagen type II mRNA expression, collagen type II protein production, and expression of Sox-9 (Fig. 4, A, C, and E), the latter being a master transcriptional regulator of the differentiated chondrocyte phenotype, including expression of type II collagen and aggrecan (33–35). CA MEK did not decrease type II collagen protein levels but did reduce type II collagen and Sox9 expression, with only the latter reaching statistical significance (Fig. 4, B, D, and F).
FIGURE 4.
Effects of oxidative stress, Akt, and MEK-ERK on collagen II and Sox9 expression. Normal human chondrocytes were treated with 25 μm tBHP (added twice a day) for 2 days in DMEM/Ham's F-12 supplemented with 1% mini-ITS plus ascorbate (A, C, and E) or infected for 2 days with lentivirus expressing CA MEK or CA Akt or control vector (B, D, and F). A and B, cell lysates were collected, and the collagen II protein levels (normalized to DNA) were analyzed by enzyme-linked immunosorbent assay and presented as -fold change relative to the untreated control (Ctrl) (A) or vector (B); the data are the mean ± S.D. of three independent experiments. C–F, total RNA was isolated, and quantitative real-time PCR was performed to determine mRNA expression of collagen II (C and D) and Sox9 (E and F) using the TATA box-binding protein as a control. The mRNA expression is presented as -fold change relative to the untreated (control) or vector; the data are the mean ± S.D. of four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
Depending on the cell type and protein of interest, Akt has been found to regulate both mRNA transcription and protein translation. In primary human chondrocytes where CA Akt stimulated proteoglycan synthesis, it did not change the levels of aggrecan mRNA (Fig. 3D). However, CA Akt did stimulate type II collagen protein production as well as increased levels of mRNA for type II collagen and Sox-9 (Fig. 4).
Antioxidants Promote IGF-I-IRS-1-Akt Signaling and Proteoglycan Synthesis
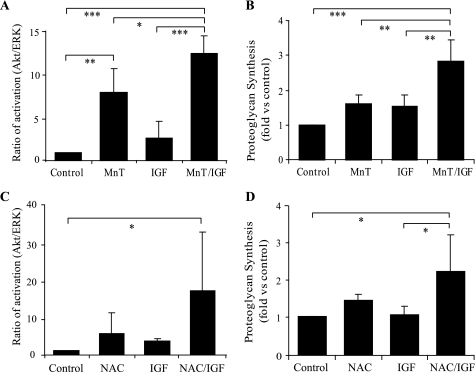
In order to further determine the role of increased ROS due to oxidative stress in the inhibition of IGF-I signaling and proteoglycan synthesis, we tested the effects of an antioxidant, MnTBAP, which can scavenge superoxide radicals and peroxides (36, 37) as well as peroxynitrite (38). We also tested NAC, which is a general thiol anti-oxidant. For signaling experiments, osteoarthritic chondrocytes were incubated with 250 μm MnTBAP or 10 mm NAC for 30 min, followed by IGF-I treatment (50 ng/ml) for 30 min. Because our results suggested that the ratio of Akt to ERK phosphorylation was altered in OA cells and the relative activation of these two pathways regulates proteoglycan synthesis, immunoblotting was performed to determine the phosphorylation status of Akt and ERK1/2 expressed as a ratio as shown in Fig. 1. MnTBAP treatment alone significantly induced Akt phosphorylation at Ser-473 with no change in ERK1/2 phosphorylation, resulting in a significant increase in the ratio of Akt to ERK phosphorylation (Fig. 5A). This finding suggests that the existence of endogenous ROS production in OA cells is sufficient to inhibit autocrine IRS-1 and Akt activation. There was a further increase in the Akt to ERK phosphorylation ratio when IGF-I was added to cells treated with MnTBAP. NAC alone caused a modest but not significant increase in the Akt to ERK phosphorylation ratio and significantly improved the response to IGF-I (Fig. 5C).
FIGURE 5.
Antioxidants increase the ratio of Akt to ERK phosphorylation and promote IGF-I-stimulated proteoglycan synthesis in osteoarthritic chondrocytes. A and C, OA chondrocytes in serum-free medium were pretreated for 30 min with 250 μm MnTBAP or 10 mm NAC prior to incubation with 50 ng/ml IGF-I for 30 min. Cell lysates were prepared and used for immunoblotting with antibodies to phosphorylated Akt (Ser-473) and ERK and for total Akt and ERK. The ratios of Akt to ERK1/2 phosphorylation normalized to the total Akt and ERK protein bands were determined by densitometric analysis, as shown in Fig. 1. B and D, cartilage explants (B) and monolayer cultures (D) from OA tissue were incubated overnight with 100 ng/ml IGF-I in the presence of 250 μm MnTBAP (B) or 10 mm NAC (D). Proteoglycan synthesis was measured using the sulfate incorporation. The relative proteoglycan synthesis (corrected by the DNA amount) is presented as -fold change relative to the untreated (control) sample. The data are the mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.005.
To determine whether the antioxidant treatment could lead to a change in proteoglycan production, we pretreated OA cartilage explants with either MnTBAP or NAC for 30 min before overnight incubation with IGF-I. Proteoglycan synthesis was modestly but not significantly increased by either MnTBAP or NAC alone. However, when IGF-I was added to cultures treated with either anti-oxidant, the response to IGF-I was significantly better than to IGF-I or anti-oxidant alone, demonstrating that anti-oxidant inhibition of ROS can promote proteoglycan production and improve the chondrocyte response to IGF-I in OA chondrocytes (Fig. 5, B and D). Because quantitative real-time PCR did not reveal any effect of MnTBAP on aggrecan mRNA expression (data not shown), it is likely that MnTBAP increased proteoglycan synthesis via promoting mRNA translation as expected with activation of the PI 3-kinase-Akt pathway. These findings are consistent with a hypothesis that the balance of Akt to ERK1/2 activity is important in the regulation of proteoglycan production, that anti-oxidants improve the balance favoring Akt phosphorylation, and that this in turn is associated with increased proteoglycan synthesis.
Inhibition of MEK-ERK Promotes IGF-I Activation of IRS-I-Akt Signaling
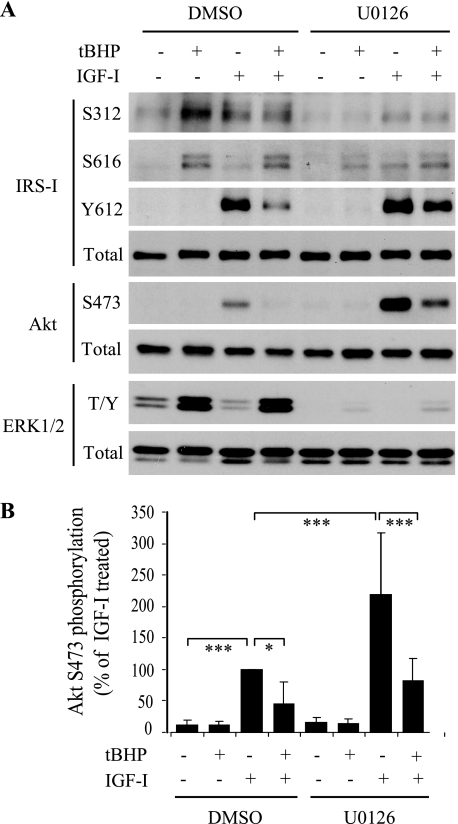
Activation of ERK1/2 in response to oxidative stress could inhibit IGF-I stimulation of IRS-1-Akt signaling because this MAPK can phosphorylate IRS-1 on inhibitory serine residues. In chondrocytes infected with lentivirus expressing CA MEK, we had noted increased phosphorylation at both Ser-312 and Ser-616 (Fig. 3). In order to test if these sites were phosphorylated in response to MAPK activation by increased levels of ROS, we preincubated normal chondrocytes with the MEK1/2-specific inhibitor U0126 (10 μm) for 30 min prior to induction of oxidative stress with tBHP. Similar to Fig. 2A, tBHP treatment induced IRS-1 phosphorylation at both Ser-312 and Ser-616, increased ERK phosphorylation, and inhibited IGF-I activation of IRS-1 Tyr-612 and Akt Ser-473 (Fig. 6). Inhibition of MEK activation of ERK with U0126 prevented tBHP-induced IRS-1 serine phosphorylation at either site and significantly enhanced IGF-I-induced IRS-1 Tyr-612 phosphorylation and Akt Ser-473 phosphorylation (Fig. 6, A and B).
FIGURE 6.
MEK-ERK inhibition blocks oxidative stress-induced inhibition of IGF-I stimulation of IRS-I and Akt signaling. Normal human chondrocytes were serum-starved overnight and pretreated for 30 min with the MEK1/2 inhibitor U0126 (10 μm) or control vehicle (0.1% DMSO) followed by 250 μm tBHP for 30 min and then 50 ng/ml IGF-I for 30 min as indicated. A, immunoblotting was performed to determine the activation of IRS-1, Akt, and ERK1/2 (T/Y, Thr-202/Tyr-204) using antibodies specific to their phosphorylated forms. B, the relative Akt (Ser-473) phosphorylation level (normalized to the total Akt protein) in the above treated samples was determined by densitometric analysis and expressed as a percentage of the DMSO control IGF-I-stimulated sample. The results represent the means ± S.D. of three independent experiments. *, p < 0.05; ***, p < 0.005.
DISCUSSION
This study delineated the roles of two major IGF-I-stimulated signaling pathways, IRS-1-PI 3-kinase-Akt and ERK MAPK, in the regulation of proteoglycan and type II collagen production by human articular chondrocytes and provided evidence that oxidative stress and increased levels of ROS can alter the balance in the activity of these pathways, leading to IGF-I resistance (Fig. 7). Using lentiviral constructs to express CA Akt and CA MEK, we found that Akt activation was sufficient to mediate an increase in proteoglycan synthesis, expression of type II collagen, and type II collagen protein production, whereas activated MEK-ERK was inhibitory for aggrecan expression and proteoglycan synthesis. Although in normal chondrocytes, IGF-I stimulated activation of both the IRS-1-PI 3-kinase-Akt pathway and the MEK-ERK pathway, the phosphorylation of ERK1/2 was more transient relative to Akt. Importantly, the balance in the level of activity of these two pathways was disrupted in chondrocytes isolated from osteoarthritic cartilage such that excessive basal activation of MEK-ERK and an inability of IGF-I to stimulate Akt phosphorylation resulted in an inability of IGF-I to stimulate proteoglycan production. The hypothesis that this imbalance in signaling may result from the excessive levels of ROS that have been observed in OA cartilage (14, 17, 18) was supported by the findings that induction of oxidative stress in normal chondrocytes with tBHP reproduced the changes seen in OA cells, whereas treatment of OA cells with anti-oxidants improved the balance of Akt to ERK phosphorylation and improved the anabolic response of the cells to IGF-I.
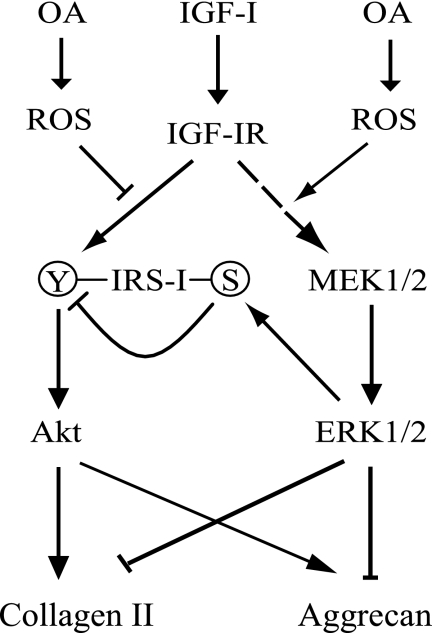
FIGURE 7.
Hypothetical model for the effect of oxidative stress on IGF-I regulation of chondrocyte proteoglycan (aggrecan) and collagen II production. In this model, the balance of Akt and ERK1/2 activation determines the final effect of IGF-I on proteoglycan production and collagen expression. In normal chondrocytes, IGF-I activates the IGF-I receptor (IGF-IR), which induces IRS-1 activation through tyrosine phosphorylation and subsequently activates the PI 3-kinase-Akt pathway, which leads downstream to increased aggrecan synthesis and collagen II expression. Increased activation of MEK-ERK signaling, stimulated by oxidative stress and increased ROS levels present in OA cartilage, inhibits aggrecan and collagen gene transcription. Oxidative stress also activates MEK-ERK signaling, which, through inducing IRS-1 serine phosphorylation, negatively regulates IGF-I activation of PI 3-kinase-Akt signaling and proteoglycan synthesis.
The mechanism for IGF-I resistance in OA cells as well as in normal cells with tBHP induced oxidative stress appeared to be through an inhibition of IRS-1 activation. Studies in chondrocytes from transgenic mice with an IRS-1 deletion have demonstrated that IRS-1 activity is required for IGF-I stimulation of Akt but not ERK1/2 (39). We noted an increase in phosphorylation of IRS-1 at Ser-312 and Ser-616 in OA cells and in normal cells treated with tBHP. This was associated with a decrease in phosphorylation of IRS-1 at Tyr-612 in response to IGF-I. Activation of IRS-1 requires tyrosine phosphorylation (in particular Tyr-612) (31), whereas serine phosphorylation at a variety of sites has been shown to be inhibitory (19, 20). Depending on the specific serine residue, phosphorylation can be mediated through a variety of kinases, including protein kinase C (40, 41), IκB kinase (42), mTOR (43), MEK-ERK (44, 45), and JNK1/2 (19, 46).
Increased activity of MAPKs, including ERK1/2 and JNK1/2, has been reported in OA chondrocytes (47–49). We focused the current study on the role of ERK. Increased basal ERK phosphorylation was noted in OA chondrocytes when compared with chondrocytes isolated from normal tissue, and the addition of tBHP to normal cells resulted in ERK phosphorylation. Importantly, a role for ERK in the inhibition of IRS-I-PI 3-kinase-Akt signaling was demonstrated using the MEK1/2 inhibitor U0126 as well as CA MEK expression. MEK1/2 inhibition reduced the level of IRS-I Ser phosphorylation induced by tBHP and increased IGF-I-stimulated IRS-I Tyr and Akt phosphorylation, whereas CA MEK had the opposite effect.
Our results suggest that increased activity of the ERK MAPK pathway may not only inhibit activation of Akt by IGF-I and thereby inhibit IGF-I-mediated proteoglycan synthesis, but also prolonged ERK activity can inhibit proteoglycan production through down-regulation of aggrecan core protein expression. Aggrecan is the most abundant proteoglycan in articular cartilage and is produced by the addition of glycosaminoglycan chains to the aggrecan core protein. Infection of chondrocytes with lentivirus expressing CA MEK reduced both aggrecan mRNA expression and proteoglycan protein synthesis, as did activation of MEK-ERK using tBHP. Negative regulation of proteoglycan production by activated MEK-ERK has been previously observed during chondrogenesis in embryonic chick limb bud cultures (50), and inhibition of MEK-ERK has been found to promote chondrocytic differentiation of ATDC5 cells (51) and aggrecan expression in immortalized rat chondrocytes (52). These findings are in contrast to a study of mechanically stimulated bovine calf chondrocytes, where inhibition of MEK-ERK either reduced aggrecan expression or had no effect, depending on the stimulation conditions (53). Thus, the role of MEK-ERK as a positive or negative regulator of chondrocyte aggrecan expression may depend on the stimulus and the contribution of additional signaling pathways that would be stimulus-specific.
CA MEK also reduced expression of Sox-9, which was accompanied by a modest reduction in type II collagen expression but not collagen protein levels. It should be noted that we measured the total amount of type II collagen protein present in the cell layer rather than new collagen synthesis, so this measurement would include any type II collagen that had already accumulated in the cell layer during the 5 days of culture prior to infection with lentivirus expressing CA MEK.
Our findings indicate that the relative balance of Akt to ERK activation is important in the ability of IGF-I to stimulate proteoglycan synthesis by adult human articular chondrocytes. The activation of Akt increased proteoglycan synthesis without an increase in aggrecan mRNA levels, suggesting that Akt regulation of proteoglycan synthesis occurs at either the level of protein translation or through regulation of the expression of one or more of the enzymes required for the addition of glycosaminoglycans to the aggrecan core protein or sulfation of the glycosaminoglycan chains. IGF-I activation of the PI 3-kinase-Akt pathway is known to result in an activation of mTOR, a master regulator of mRNA translation and protein synthesis (54), and inhibition of mTOR with rapamycin was previously found to inhibit IGF-I-stimulated proteoglycan synthesis (26).
We did observe a significant increase in collagen II and Sox9 mRNA levels in chondrocytes expressing CA Akt, and this was accompanied by an increase in the amount of type II collagen deposited in the matrix. This finding is consistent with the well documented function of Sox-9 as a key transcriptional regulator of the type II procollagen gene (34, 55). Because proteoglycan synthesis and collagen II expression are characteristic of the differentiated chondrocyte phenotype, it is possible that Akt promotes and helps in maintaining a differentiated chondrocyte phenotype. This is consistent with a recent study that found that Akt positively regulates chondrocyte maturation and cartilage matrix production during skeletal development (56).
This study for the first time demonstrated that, in adult human primary chondrocytes, oxidative stress and ERK activation inhibit aggrecan mRNA expression. These findings are consistent with previous studies that demonstrated inhibition of aggrecan expression by H2O2 in juvenile bovine chondrocytes (57) and negative regulation of aggrecan expression by ERK in embryonic chick limb mesenchyme (50). The ERK inhibition of aggrecan is probably through the activation of its downstream target, c-Fos, which has been found to directly reduce aggrecan expression in the chondrosarcoma cell line HCS 2/8 cells (58). The down-regulation of Sox-9 by CA MEK suggests another possible mechanism because Sox-9, along with l-Sox-5 and Sox-6, has recently been shown to regulate aggrecan expression (35). Further studies will be needed to determine more precisely how ERK negatively regulates aggrecan transcription in primary human chondrocytes.
Another open question is how oxidative stress activates the MEK-ERK signaling pathway in chondrocytes. ROS have been found to regulate cell signaling at multiple steps in MAPK pathways through activation of a host of kinases as well as through inhibition of various phosphatases (59). In rat vascular smooth muscle cells, EGFR transactivation has been shown to be necessary for the ROS activation of ERK1/2 (60). In our study, pretreatment with an EGFR inhibitor, AG1478, failed to block tBHP activation of ERK1/2 in human chondrocytes.3 Further studies are needed to define the mechanism for ROS activation of ERK1/2 in chondrocytes because inhibition of this pathway would be expected to improve the ability to produce proteoglycans in response to IGF-I.
In summary, we provide evidence that in osteoarthritic chondrocytes and in normal chondrocytes with oxidative stress induced by tBHP, there is an imbalance in IGF-I signaling, resulting in reduced activation of the IRS-1-PI 3-kinase-Akt pathway relative to activation of the MEK-ERK MAPK pathway. Because activation of Akt results in increased expression of Sox9 and type II collagen and increased synthesis of proteoglycans, whereas activation of ERK is inhibitory, this signaling imbalance can have profound effects on chondrocyte matrix production. Additional studies are indicated to determine the mechanism for ROS activation of the MEK-ERK pathway in chondrocytes and how elevated MEK-ERK activity inhibits aggrecan and Sox9 expression. Inhibition of MEK-ERK was found to reduce the loss of cartilage matrix in an animal model of osteoarthritis (61), suggesting that these findings have in vivo relevance. Another strategy for stimulation of matrix synthesis in OA may be to promote the activity of Akt in chondrocytes and improve the balance of Akt to ERK signaling.
Acknowledgments
We thank Yiwen Zhao for technical assistance, Michael Robinson for Akt lentiviral constructs, Natalie Ahn for CA MEK1, and Richard Mulligan for the pHAGE vector. We thank the National Disease Research Interchange (Philadelphia, PA) and the Gift of Hope Organ and Tissue Donor Network (Elmhurst, IL) and Drs. Arkady Margolis and Marcello Del Carlo for providing normal donor tissue and Dr. David Martin (Department of Orthopedic Surgery, Wake Forest University School of Medicine) for assistance in obtaining osteoarthritic tissue.
This work was supported, in whole or in part, by National Institutes of Health Grant AG-16697. This work was also supported by an Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Fellowship.
W. Yin and R. F. Loeser, unpublished results.
- IGF-I
- insulin-like growth factor-I
- ROS
- reactive oxygen species
- OA
- osteoarthritis
- IRS
- insulin receptor substrate
- MAPK
- mitogen-activated protein kinase
- PI 3-kinase
- phosphatidylinositol 3-kinase
- ERK
- extracellular signal-regulated kinase
- MEK
- MAPK/ERK kinase
- mTOR
- mammalian target of rapamycin
- p70S6K
- p70 S6 kinase
- MnTBAP
- Mn(III) tetrakis(4-benzoic acid) porphyrin
- NAC
- N-acetyl-l-cysteine
- tBHP
- tert-butylhydroperoxide
- DMEM
- Dulbecco's modified Eagle's medium
- HA
- hemagglutinin
- CA
- constitutively active
- DN
- dominant negative.
REFERENCES
- 1.Baker J., Liu J. P., Robertson E. J., Efstratiadis A. (1993) Cell 75, 73–82 [PubMed] [Google Scholar]
- 2.Ezzat V. A., Duncan E. R., Wheatcroft S. B., Kearney M. T. (2008) Diabetes Obes. Metab. 10, 198–211 [DOI] [PubMed] [Google Scholar]
- 3.Martel-Pelletier J., Di Battista J. A., Lajeunesse D., Pelletier J. P. (1998) Inflamm. Res. 47, 90–100 [DOI] [PubMed] [Google Scholar]
- 4.Samani A. A., Yakar S., LeRoith D., Brodt P. (2007) Endocr. Rev. 28, 20–47 [DOI] [PubMed] [Google Scholar]
- 5.Cristofalo V. J., Phillips P. D., Sorger T., Gerhard G. (1989) J. Gerontol. 44, 55–62 [DOI] [PubMed] [Google Scholar]
- 6.D'avis P. Y., Frazier C. R., Shapiro J. R., Fedarko N. S. (1997) Biochem. J. 324, 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J. J., Kurimoto P., Boudignon B., Rosen C., Lima F., Halloran B. P. (2007) J. Bone Miner. Res. 22, 1271–1279 [DOI] [PubMed] [Google Scholar]
- 8.Martin J. A., Ellerbroek S. M., Buckwalter J. A. (1997) J. Orthop. Res. 15, 491–498 [DOI] [PubMed] [Google Scholar]
- 9.Loeser R. F., Shanker G., Carlson C. S., Gardin J. F., Shelton B. J., Sonntag W. E. (2000) Arthritis Rheum. 43, 2110–2120 [DOI] [PubMed] [Google Scholar]
- 10.Messai H., Duchossoy Y., Khatib A. M., Panasyuk A., Mitrovic D. R. (2000) Mech. Ageing Dev. 115, 21–37 [DOI] [PubMed] [Google Scholar]
- 11.Henrotin Y. E., Bruckner P., Pujol J. P. (2003) Osteoarthritis Cartilage 11, 747–755 [DOI] [PubMed] [Google Scholar]
- 12.Yudoh K., Nguyen T., Nakamura H., Hongo-Masuko K., Kato T., Nishioka K. (2005) Arthritis Res. Ther. 7, R380–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlo M. D., Jr., Loeser R. F. (2003) Arthritis Rheum. 48, 3419–3430 [DOI] [PubMed] [Google Scholar]
- 14.Shah R., Raska K., Jr., Tiku M. L. (2005) Arthritis Rheum. 52, 2799–2807 [DOI] [PubMed] [Google Scholar]
- 15.Regan E., Flannelly J., Bowler R., Tran K., Nicks M., Carbone B. D., Glueck D., Heijnen H., Mason R., Crapo J. (2005) Arthritis Rheum. 52, 3479–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aigner T., Fundel K., Saas J., Gebhard P. M., Haag J., Weiss T., Zien A., Obermayr F., Zimmer R., Bartnik E. (2006) Arthritis Rheum. 54, 3533–3544 [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Romero C., Calamia V., Mateos J., Carreira V., Martinez-Gomariz M., Fernandez M., Blanco F. J. (2009) Mol. Cell Proteomics 8, 172–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeser R. F., Carlson C. S., Del Carlo M., Cole A. (2002) Arthritis Rheum. 46, 2349–2357 [DOI] [PubMed] [Google Scholar]
- 19.Aguirre V., Werner E. D., Giraud J., Lee Y. H., Shoelson S. E., White M. F. (2002) J. Biol. Chem. 277, 1531–1537 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. F., Herschkovitz A., Boura-Halfon S., Ronen D., Paz K., Leroith D., Zick Y. (2004) Mol. Cell. Biol. 24, 9668–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellen K. E., Hotamisligil G. S. (2005) J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurz D. J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J. D. (2004) J. Cell Sci. 117, 2417–2426 [DOI] [PubMed] [Google Scholar]
- 23.Muehleman C., Bareither D., Huch K., Cole A. A., Kuettner K. E. (1997) Osteoarthritis Cartilage 5, 23–37 [DOI] [PubMed] [Google Scholar]
- 24.Loeser R. F., Todd M. D., Seely B. L. (2003) J. Rheumatol. 30, 1565–1570 [PubMed] [Google Scholar]
- 25.Del Carlo M., Jr., Loeser R. F. (2002) Arthritis Rheum. 46, 394–403 [DOI] [PubMed] [Google Scholar]
- 26.Starkman B. G., Cravero J. D., Delcarlo M., Loeser R. F. (2005) Biochem. J. 389, 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis T. S., Hunt J. B., Aveline L. D., Jonscher K. R., Louie D. F., Yeh J. M., Nahreini T. S., Resing K. A., Ahn N. G. (2000) Mol. Cell 6, 1343–1354 [DOI] [PubMed] [Google Scholar]
- 28.Pombo-Suarez M., Calaza M., Gomez-Reino J. J., Gonzalez A. (2008) BMC Mol. Biol. 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henrotin Y. E., Deberg M. A., Crielaard J. M., Piccardi N., Msika P., Sanchez C. (2006) J. Rheumatol. 33, 1668–1678 [PubMed] [Google Scholar]
- 30.Sen M., Cheng Y. H., Goldring M. B., Lotz M. K., Carson D. A. (2004) Arthritis Rheum. 50, 488–497 [DOI] [PubMed] [Google Scholar]
- 31.Esposito D. L., Li Y., Cama A., Quon M. J. (2001) Endocrinology 142, 2833–2840 [DOI] [PubMed] [Google Scholar]
- 32.Gual P., Le Marchand-Brustel Y., Tanti J. F. (2005) Biochimie 87, 99–109 [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre V., de Crombrugghe B. (1998) Matrix Biol. 16, 529–540 [DOI] [PubMed] [Google Scholar]
- 34.Bi W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (1999) Nat. Genet. 22, 85–89 [DOI] [PubMed] [Google Scholar]
- 35.Han Y., Lefebvre V. (2008) Mol. Cell. Biol. 28, 4999–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasternack R. F., Banth A., Pasternack J. M., Johnson C. S. (1981) J. Inorg. Biochem. 15, 261–267 [DOI] [PubMed] [Google Scholar]
- 37.Day B. J., Fridovich I., Crapo J. D. (1997) Arch. Biochem. Biophys. 347, 256–262 [DOI] [PubMed] [Google Scholar]
- 38.Szabó C., Day B. J., Salzman A. L. (1996) FEBS Lett. 381, 82–86 [DOI] [PubMed] [Google Scholar]
- 39.Shimoaka T., Kamekura S., Chikuda H., Hoshi K., Chung U. I., Akune T., Maruyama Z., Komori T., Matsumoto M., Ogawa W., Terauchi Y., Kadowaki T., Nakamura K., Kawaguchi H. (2004) J. Biol. Chem. 279, 15314–15322 [DOI] [PubMed] [Google Scholar]
- 40.Liu Y. F., Paz K., Herschkovitz A., Alt A., Tennenbaum T., Sampson S. R., Ohba M., Kuroki T., LeRoith D., Zick Y. (2001) J. Biol. Chem. 276, 14459–14465 [DOI] [PubMed] [Google Scholar]
- 41.Moeschel K., Beck A., Weigert C., Lammers R., Kalbacher H., Voelter W., Schleicher E. D., Häring H. U., Lehmann R. (2004) J. Biol. Chem. 279, 25157–25163 [DOI] [PubMed] [Google Scholar]
- 42.Gao Z., Hwang D., Bataille F., Lefevre M., York D., Quon M. J., Ye J. (2002) J. Biol. Chem. 277, 48115–48121 [DOI] [PubMed] [Google Scholar]
- 43.Gual P., Grémeaux T., Gonzalez T., Le Marchand-Brustel Y., Tanti J. F. (2003) Diabetologia 46, 1532–1542 [DOI] [PubMed] [Google Scholar]
- 44.Rui L., Aguirre V., Kim J. K., Shulman G. I., Lee A., Corbould A., Dunaif A., White M. F. (2001) J. Clin. Invest. 107, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman J. A., Berg A. H., Lewis R. Y., Lisanti M. P., Scherer P. E. (2000) Mol. Endocrinol. 14, 1557–1569 [DOI] [PubMed] [Google Scholar]
- 46.Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000) J. Biol. Chem. 275, 9047–9054 [DOI] [PubMed] [Google Scholar]
- 47.Clancy R., Rediske J., Koehne C., Stoyanovsky D., Amin A., Attur M., Iyama K., Abramson S. B. (2001) Osteoarthritis Cartilage 9, 294–299 [DOI] [PubMed] [Google Scholar]
- 48.Fan Z., Söder S., Oehler S., Fundel K., Aigner T. (2007) Am. J. Pathol. 171, 938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boileau C., Martel-Pelletier J., Brunet J., Schrier D., Flory C., Boily M., Pelletier J. P. (2006) Ann. Rheum. Dis. 65, 573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bobick B. E., Kulyk W. M. (2004) J. Biol. Chem. 279, 4588–4595 [DOI] [PubMed] [Google Scholar]
- 51.Phornphutkul C., Wu K. Y., Yang X., Chen Q., Gruppuso P. A. (2004) J. Endocrinol. 183, 477–486 [DOI] [PubMed] [Google Scholar]
- 52.Yagi R., McBurney D., Horton W. E., Jr. (2005) J. Biol. Chem. 280, 30517–30525 [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald J. B., Jin M., Chai D. H., Siparsky P., Fanning P., Grodzinsky A. J. (2008) J. Biol. Chem. 283, 6735–6743 [DOI] [PubMed] [Google Scholar]
- 54.Hay N., Sonenberg N. (2004) Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 55.Lefebvre V., Huang W., Harley V. R., Goodfellow P. N., de Crombrugghe B. (1997) Mol. Cell Biol. 17, 2336–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rokutanda S., Fujita T., Kanatani N., Yoshida C. A., Komori H., Liu W., Mizuno A., Komori T. (2009) Dev. Biol. 328, 78–93 [DOI] [PubMed] [Google Scholar]
- 57.Martin G., Andriamanalijaona R., Mathy-Hartert M., Henrotin Y., Pujol J. P. (2005) Osteoarthritis Cartilage 13, 915–924 [DOI] [PubMed] [Google Scholar]
- 58.Tsuji M., Funahashi S., Takigawa M., Seiki M., Fujii K., Yoshida T. (1996) FEBS Lett. 381, 222–226 [DOI] [PubMed] [Google Scholar]
- 59.Monteiro H. P., Arai R. J., Travassos L. R. (2008) Antioxid. Redox Signal. 10, 843–889 [DOI] [PubMed] [Google Scholar]
- 60.Meng D., Shi X., Jiang B. H., Fang J. (2007) Free Radic. Biol. Med. 42, 1651–1660 [DOI] [PubMed] [Google Scholar]
- 61.Pelletier J. P., Fernandes J. C., Brunet J., Moldovan F., Schrier D., Flory C., Martel-Pelletier J. (2003) Arthritis Rheum. 48, 1582–1593 [DOI] [PubMed] [Google Scholar]