Abstract
This study aimed at identifying transcriptional changes associated to neuronal differentiation induced by six distinct stimuli using whole-genome microarray hybridization analysis. Bioinformatics analyses revealed the clustering of these six stimuli into two categories, suggesting separate gene/pathway dependence. Treatment with specific inhibitors demonstrated the requirement of both Janus kinase and microtubule-associated protein kinase activation to trigger differentiation with nerve growth factor (NGF) and dibutyryl cAMP. Conversely, activation of protein kinase A, phosphatidylinositol-3-kinase α, and mammalian target of rapamycin, although required for dibutyryl cAMP-induced differentiation, exerted a negative feedback on NGF-induced differentiation. We identified Polo-like kinase 2 (Plk2) and poliovirus receptor (PVR) as indispensable for NGF-driven neuronal differentiation and αB-crystallin (Cryab) as an inhibitor of this process. Silencing of Plk2 or PVR blocked NGF-triggered differentiation and Cryab down-regulation, while silencing of Cryab enhanced NGF-induced differentiation. Our results position both Plk2 and PVR upstream of the negative regulator Cryab in the pathway(s) leading to neuronal differentiation triggered by NGF.
Introduction
Multiple sclerosis (MS)3 is a chronic inflammatory, neurodegenerative, demyelinating disease of the central nervous system, triggered by the activation of autoreactive T cells, reacting to self-antigens such as the myelin components myelin basic protein or myelin oligodendrocyte glycoprotein (1). MS was originally considered only as an inflammatory disease. However, as a consequence of increasing evidence on the contribution of neurodegenerative processes to the pathology of MS, axonal loss is nowadays considered as an early and persistent event mainly responsible for the neurological deficits (2). Despite this, current therapies for MS consist only of anti-inflammatory and immunomodulatory drugs, which show minimal or no clinical benefit in preventing the progression of neurological disability that characterizes this disease (2). Consequently, elucidation of the molecular mechanisms leading to axonal degeneration as well as of those promoting axonal survival, regeneration, and remyelination is essential for the development of novel therapies based on neuroprotection and/or neuronal regeneration. Moreover, this new class of therapeutics would constitute a breakthrough also for the treatment of other neurodegenerative diseases such as Alzheimer disease, Parkinson disease, Creutzfeldt-Jakob disease, or amyotrophic lateral sclerosis among others. Complex cellular programs, such as those leading to neuronal differentiation require global gene expression changes. The pheochromocytoma PC12 cell line provides a representative model system for studying gene expression and signaling pathways mediating neuronal differentiation (3–6). PC12 cells, originated from a rat adrenochromaffin tumor, can differentiate into sympathetic neuronal-like cells upon treatment with NGF in a process involving growth arrest, neurite extension, and acquisition of electrical excitability (7, 8). Although NGF-driven differentiation is the most studied process, PC12 cells can develop a neuronal phenotype also in response to a variety of other extracellular stimuli such as basic fibroblast growth factor (bFGF), interleukin 6 (IL-6), dbcAMP, pituitary adenylate cyclase-activating polypeptide 38 (PACAP38) or forskolin among others (4, 9–15). Although signal transduction pathways leading to neuronal differentiation have been extensively studied, especially for NGF, the induced gene expression changes are far from complete. Several genome-wide scale approaches have been used to study changes in gene expression required for neuronal differentiation in PC12 cells. Although some studies analyzed the expression profile upon PACAP38 treatment (16–18), the vast majority restricted the investigation to NGF-induced differentiation (3, 19, 20). Only one study extended the analysis to the differential gene expression triggered by PACAP38, dbcAMP, and forskolin as compared with NGF (14). Furthermore, most of the studies have been limited to a single time point despite increasing evidence suggesting a time dependence of regulation of gene expression throughout the differentiation process (3, 19, 21). Mechanisms leading to neuronal differentiation have been proven difficult to sort out by studying differentiation induced with a single stimulus and/or after a unique length of time. Although several lists of genes associated to the acquisition of a neuronal phenotype in PC12 cells have been published, elucidation of a common minimal pattern of global gene changes required for differentiation remains incomplete.
In the present study we sought to investigate this complex process by a more comprehensive systems biology approach. On one side, we analyzed the global transcriptional changes involved in neuronal differentiation using whole-genome microarray hybridization upon differentiation of Neuroscreen-1 (NS1) PC12 cells (3) with NGF, IL-6, bFGF, PACAP38, dbcAMP, and forskolin at 3, 24, and 72 h after each treatment. On the other side, we investigated how the common transcriptional machinery identified was affected by inhibition or enhancement of neuronal differentiation using several small molecule, target-specific inhibitors.
We identified 31 genes whose expression was regulated in common by all stimuli during differentiation. Among them, Plk2 and PVR, two genes up-regulated by all the stimuli leading to neuronal differentiation, were proven to be essential for neurite outgrowth. Silencing of Plk2 and PVR with specific small-interfering RNAs (siRNAs) inhibited this process, deregulated the expression of markers for differentiation, and more interestingly, greatly influenced the overall gene expression machinery identified in the present whole-genome array hybridization study. Moreover, inhibition of neuronal differentiation upon treatments with MEK1/2- or JAK1/2/3-specific inhibitors significantly decreased the up-regulation of Plk2 and PVR observed during differentiation.
Several studies suggest that Cryab plays a role in the pathogenesis of MS. However, whether the specific role of Cryab is beneficial or, rather, detrimental to this pathology is still a matter of debate (22–26). Furthermore, a potential role of crystallins during axonal regeneration has also been recently reported (27, 28). In the present study we uncovered a novel function for Cryab as an inhibitor of neuronal differentiation. Indeed, Cryab was found to be down-regulated by all the stimuli inducing differentiation in NS1 cells as well as by NGF in superior cervical ganglia (SCG) neurons. In addition, transfection of Cryab siRNA resulted in significant enhancement of NGF-induced neurite outgrowth, and inhibition of neurite extension upon silencing of Plk2 or PVR resulted in the up-regulation of Cryab. Taken together, these results identify PVR and Plk2 as two genes essential for the neuronal differentiation process, working upstream of the negative regulator Cryab and regulated in response to MEK and JAK activation, two pathways required for NGF-induced differentiation.
EXPERIMENTAL PROCEDURES
Materials
Flasks and plates were collagen type-I coated (BD Biosciences). RPMI 1640 and Dulbecco's modified Eagle's culture media, l-glutamine, penicillin, streptomycin, and dispase were from GIBCO. Horse serum and fetal calf serum were from Hyclone (Sigma Aldrich). dbcAMP, forskolin, staurosporine. and cytosine β-d-arabinofuranoside hydrochloride were from Sigma. 2.5 S mouse NGF was from Promega AG (Duebendorf, CH), PACAP38 was from Bachem AG (Bubendorf, CH), and rat IL-6 and human bFGF were from Peprotech (London, UK). JAK inhibitor I (2-(1,1-dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one P6), protein kinase A-specific inhibitor H-89, and mTOR inhibitor rapamycin were from Calbiochem. PI3Kα (AS-702630) (52)- and MEK1/2 (AS-702789)-specific inhibitors were from Merck Serono A.S. HitKitTM, and the automated imaging instrumentation and the NeuroscreenTM-1 cells were from Cellomics (Pittsburgh, PA). OFA rats were from Charles River Laboratories (Lyon, FR). siRNAs and primers were from Qiagen AG (Basel, CH). The Amaxa nucleofectorTM II system and cell line nucleofector kit V were from Amaxa (Köln, Germany). RNeasy mini and micro kits, DNase I and quantitative PCR (qPCR) kits (Quantitect and QuantiFast SYBR green), and the reverse transcription-PCR advantage kit was from Clontech (Takara Bio/Clontech, Saint Germain en Laye, FR). qPCRs were performed using the 7900HT Fast real-time PCR system (Applied Biosystems, Rotkreuz, CH). Affymetrix (Bucks, UK) GeneChip Technology used rat Genome 230 2.0 Array, Test3 Array, Hybridization Oven 640, Fluidics Station 450, One-Cycle Target Labeling and Control Reagents, Scanner 3000 7G System, and Operating Software (GCOS) Version 1.1.1. RNA and cRNA quantification was performed in Nanodrop ND-1000 spectrophotometer (Witec AG, Luttau-Luzern, CH). RNA and cRNA quality was checked by RNA6000 Nano Reagents kit and Bioanalyzer 2100 (Agilent Technologies, Basel, CH). Streptavidin phycoerythrin conjugated for the fluorescent labeling were from Invitrogen. Prism 3.0 was from GraphPad (San Diego, CA).
Cell Culture
NS1 cells were chosen because they display several advantages over PC12 cells, including 50–80% faster growth, no tendency toward cell aggregation, and accelerated responsiveness to NGF. NS1 cells were maintained in T75 flasks in RPMI 1640 supplemented with 10% horse serum and 5% fetal calf serum, 2 mm l-glutamine, 25 units/ml penicillin, and 25 μg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were induced to differentiate by the addition of NGF (50 ng/ml and 100 ng/ml for siRNA transfection experiments) for 3 days unless differently specified. To study the concentration-response effects on NOG of extracellular stimuli and inhibitors, cells were plated in 96-well plates (1500 cells/well) 24 h before treatment. For RNA extraction for qPCR analysis, cells were plated in 6-well plates (200,000 cells/well) 24 h before treatment with 10 μm AS-702789, JAK inhibitor I, H-89, AS-702630, or rapamycin or with 10 nm concentrations of non-selective serine/threonine inhibitor staurosporine for 20 min followed by treatment with either NGF or dbcAMP for 3, 24, or 72 h. For the microarray experiments cells were plated in 10-cm dishes at a concentration of 600,000 cells/dish 24 h before treatment with NGF, IL-6, or bFGF or at a concentration of 1,000,000 cells/dish 24 h before treatment with dbcAMP, forskolin, or PACAP38. SCG cells were dissected from postnatal day 1 rat pups and dissociated in 2% dispase for 30 min at 37 °C. Neurons were then mechanically dissociated and plated in 12-well plates and cultured in Dulbecco's modified Eagle's medium containing 15% fetal calf serum, 2 mm l-glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin, 0.1 mm cytosine β-d-arabinofuranoside hydrochloride, and 50 ng/ml NGF for 3 days in a humidified atmosphere containing 5% CO2 at 37 °C.
siRNA Transfection and Quantification of Neurite Length
106 cells were electroporated using Amaxa nucleofectorTM II system (program A-033 and nucleofector kit V) and plated in a 6-well plate. 3 days after electroporation cells were detached, and 106 cells were re-transfected with the same siRNA using the same protocol. Double-transfected cells were plated at a concentration of 250,000 per well and allowed to attach before treatment with 100 ng/ml NGF for 3 additional days. To quantify neurite outgrowth (NOG), phase contrast photographs (10× objective) were taken using a Nikon digital camera CoolPix 4500. For each set of electroporated cells, neurites from a minimum of 100 cells for each of 3 randomly chosen optical fields were measured using the image processing and analysis software ImageJ from JAVA (rsb.info.nih.gov). Neurite length (μm) was analyzed for statistical significance using Prism 3.0. For quantification of NOG by immunocytochemistry, NS1 cells plated in 96-well plates were treated with either increasing concentrations of the different stimuli, as indicated, for 3 days or with increasing concentrations of inhibitors for 20 min followed by a 3-day treatment with a single concentration of NGF or dbcAMP. After fixing the cells with 3.7% formaldehyde and staining with HitKitTM according to the manufacturer's instructions, NOG was visualized by the Cellomics Array Scan HCS system using a 5× objective. Images acquired were analyzed using the NOG BioApplication algorithm and quantified with the automated imaging instrumentation from Cellomics. NOG index represents the percentage of neurons for which the summed length of all neurites is higher than 20 μm.
Quantitative Reverse-transcriptase Polymerase Chain Reactions
Total RNA extraction and DNase digestion were performed according to manufacturer's instructions. Nucleic acid concentrations of the samples were determined using a NanoDrop spectrophotometer. Equal amounts of total RNA were used for cDNA synthesis, and qPCR reactions were performed in triplicate using 2.5 ng of the total cDNA. Values obtained were normalized against those for actin performed in parallel in the same samples.
GeneChip Hybridization
NS1 cells were treated with NGF (50 ng/ml), bFGF (10 μg/ml), IL-6 (5 μg/ml), dbcAMP (500 μm), forskolin (25 μm), or PACAP38 (5 μg/ml) for 3, 24, or 72 h. Total RNA was extracted as described above, and contaminating DNA was removed according to the manufacturer's instructions. Sample concentration and quality were confirmed by Bioanalyzer 2100 (Agilent Technologies) before hybridization. 5 μg of total RNA was used for target synthesis according to the one-cycle target labeling protocol. cRNAs were quantified and qualitatively checked, as described above, before, and after fragmentation. 15 μg of fragmented cRNA was used for GeneChip hybridization (16 h, 45 °C). Chips were washed and labeled in the fluidics station using the protocol EuKGE-WS2v_450. After scanning, CEL files were analyzed using GCOS software to check poly(A) and hybridization controls.
Microarray Analysis
Analysis of microarray data were carried out using R/Bioconductor. Quality control was performed using the Simpleaffy package and principal component analysis (PCA). One outlier was identified and excluded from the rest of the analysis. Normalization was carried out with the gcrma algorithm. Differentially expressed genes for each treatment and time point versus the respective time-matched untreated samples were detected using a modified t test (eBayes method of the Bioconductor Limma package) followed by Benjamini-Hochberg false discovery rate (FDR) correction. For each time point and treatment, gene selection was performed using a FDR cut-off of 0.05 and absolute -fold change larger than 2, except for bFGF, for which a lower -fold change threshold (1.7) was chosen. The final list was obtained by selecting the genes that changed applying the above-mentioned criteria for at least one time point for each extracellular stimulus. Hierarchical cluster analyses were carried out with the SpotFire software using average linkage (UPGMA) with correlation distance.
RESULTS
Systems Biology Approach to Identify Genes and Pathways Essential to Neuronal Differentiation
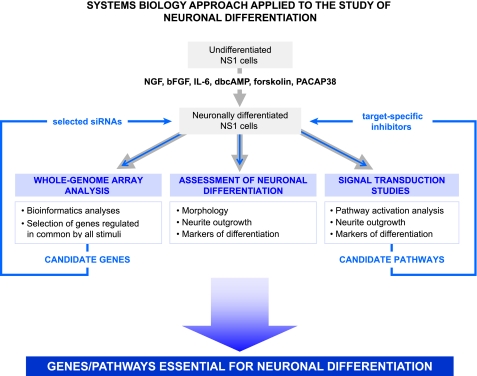
Based on published works on PC12 cells, we selected NGF (3, 4, 6), bFGF (11, 29, 30), IL-6 (9, 10), dbcAMP (12, 31), forskolin (14, 32), and PACAP38 (13, 15, 18, 33) as different extracellular stimuli to induce NS1 cell differentiation. Thereafter, we pursued a comprehensive systems biology approach by which we analyzed the process of neuronal differentiation in NS1 cells from several different aspects to shed light on this complex process. The systems biology approach included, first, a characterization of the neuritogenic capacities of six different stimuli, second, a genome-wide microarray study aimed at the identification of sets of genes relevant to neuronal differentiation, third, the pharmacological treatment with several inhibitors of known targets to dissect the signaling pathways involved in the process, and finally, gene silencing studies to validate some of the genes identified by the microarray analysis. The scheme of this systems biology multiparty approach is shown in Fig. 1.
FIGURE 1.
Scheme of the systems biology approach used for the identification of genes and pathways essential to neuronal differentiation. Undifferentiated NS1 cells were induced to differentiate by treatment with several neuritogenic stimuli as indicated for 3, 24, and 72 h. Whole-genome array hybridizations were performed after extraction of total RNA from differentiated cells. Neuronal differentiation and involvement of several signal transduction pathways were assessed as described under “Experimental Procedures” by quantifying neurite outgrowth and expression of markers for differentiation and by Western blot with specific phospho-antibodies during neuronal differentiation with the stimuli shown in the figure in the absence and presence of specific small molecule inhibitors of known targets. To validate the role of candidate genes identified in the process, specific siRNAs were transfected into NS1 cells, which were then induced to differentiate. The integration of the results obtained from the different approaches described allowed the identification of Plk2 and PVR as essential players for NGF-induced differentiation and regulation of the negative player Cryab and in addition allowed to demonstrate the requirement for JAK kinase activation in addition to the MAPK activation.
We experimentally determined EC50 concentrations for each stimulus to induce differentiation of NS1 cells by performing concentration curves for each of them. We assessed differentiation by morphological evaluation under light microscopy and quantification of NOG as described under “Experimental Procedures.” All the stimuli selected were capable of inducing neuronal differentiation although to a different extent, with NGF and dbcAMP the most potent inducers. Except for bFGF, all the stimuli induced NS1 cell neurite extension in a concentration-dependent manner, as shown in supplemental Fig. 1a. bFGF, although clearly inducing NS1 cells to extend neurites (supplemental Fig. 1a, right panel, d′), did not show concentration dependence when NOG was quantified (data not shown). Remarkably, the shape of the cell bodies and neurites was clearly different in cells treated with the different stimuli. Indeed, differentiation mediated by dbcAMP, forskolin, and PACAP38 was characterized by round-shaped larger cell bodies and shorter and thinner neurites (supplemental Fig. 1a, right panel, e′-g′) when compared with the morphology acquired upon differentiation with NGF, bFGF and IL-6 (supplemental Fig. 1a, right panel, b′–d′). Indeed, NGF-, bFGF-, and IL-6-treated cells showed angular-shaped cell bodies and longer and thicker neurites characteristic of NGF-driven differentiation (3, 4, 6, 11, 29, 30). These observations suggested that pathways and/or genes used by NGF, bFGF, and IL-6 to induce neuronal differentiation may diverge from those used by dbcAMP, forskolin, and PACAP38. To confirm that the morphological changes observed were indeed associated to neuronal differentiation, we performed qPCR analysis to assess the regulation of known markers of NGF-driven differentiation (34–39). Although the vast majority of markers investigated showed up- or down-regulation induced by NGF, as expected, eight among them were regulated also upon treatment with each of the other stimuli (supplemental Fig. 1b). These results further confirmed that the phenotypic changes induced by the treatments under study were effectively associated to neuronal differentiation of NS1 cells. Moreover, our results suggested that markers such as microtubule-associated protein 1b (34, 40), ribosomal protein L19 (35), cyclin D1 (41), acetylcholine receptor 2 (42, 43), clusterin (36, 44), sodium channel type II subunit (37), urokinase plasminogen-activating receptor (38, 45), and growth-associated protein 43 (39, 46) are truly markers for differentiation rather than NGF-associated markers. In addition these markers were regulated by NGF also in SCG primary neurons (data not shown). Interestingly, the degree of gene expression regulation of these differentiation markers correlated well with the degree of NOG induction, as NGF and dbcAMP were also the strongest inducers of marker gene expression (data not shown). Markers such as acetylcholine receptor 2, cyclin D1, microtubule-associated protein 1b, and ribosomal protein L19 showed higher up-regulation upon dbcAMP, forskolin, and PACAP38 treatment as compared with NGF, bFGF, and IL-6 treatments (data not shown). These results further reinforce our hypothesis of these stimuli belonging to two separate categories.
Microarray Gene Expression Study; Experimental Design and Bioinformatics Analyses
After characterization of the differentiating stimuli in NS1 cells and to facilitate the discovery of unique genetic markers that accompany neuronal differentiation, we performed a comprehensive and systematic whole-genome microarray experiment. Total RNA samples were collected upon treatments with NGF, bFGF, IL-6, dbcAMP, forskolin, and PACAP38 for different time lengths. To obtain statistical significance, three biological replicates were prepared for each treatment and time point.
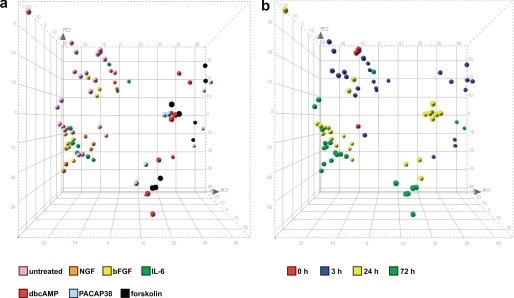
PCA confirmed the coherence of the overall microarray experiment, allowing the identification of one outlier, which was excluded from subsequent analyses. PCA revealed the presence of two principal clusters of treatments. On one side, dbcAMP, forskolin, and PAPAP38 treatments clustered together and clearly separated from untreated samples. On the other side, NGF, bFGF, and IL-6 treatments clustered together and separated from untreated controls and from the first cluster (Fig. 2a). In addition and despite the presence of some outliers, each group of stimuli clustered by time point rather than by treatment when PCA was evaluated with this parameter (Fig. 2 b). These results further confirmed the initial suggestion of two major categories of stimuli based on morphological observations, with NGF, IL-6, and bFGF constituting one category and dbcAMP, forskolin, and PACAP38 composing the other. Hierarchical cluster analysis, which allows the clustering of stimuli based on their similarities, clearly confirmed these results (supplemental Fig. 2).
FIGURE 2.
Microarray experiment; principal component analysis. PCA confirmed the overall coherence of the microarray experiment. PCA showed the expected clustering of untreated control samples versus samples of cells treated with the different stimuli (see “Results”). The figure shows PCA three-dimensional representation where colors represent either different treatments (a) or different time points (b).
Identification of Genes Regulated by All the Stimuli Leading to Neuronal Differentiation
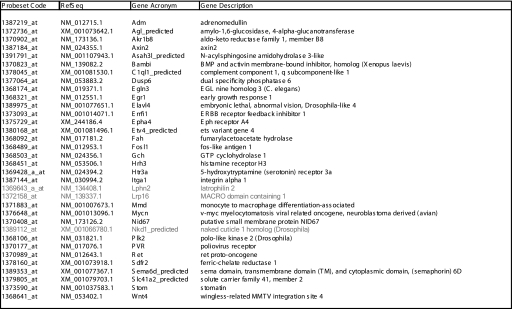
With the aim of investigating transcriptional changes relevant to neuronal differentiation rather than associated to a particular stimulus and/or corresponding signaling pathway, we searched for genes regulated in common by all the stimuli. We selected genes up- or down-regulated by each stimulus at least at one time point with an absolute -fold change greater than 2 and FDR lower than 0.05. Because bFGF displayed weaker NOG and gene regulation for this treatment we considered a -fold change of 1.7 as the threshold. Using these criteria, we identified 34 genes whose expression was regulated upon each of the treatments examined (Table 1). qPCR analysis, performed with specific primers in the same samples, confirmed the differential expression of 31 of these genes (in bold in Table 1). -Fold change and FDR values for this group of 34 genes as well as for all the additional genes regulated by all members of each of the two categories of neuritogenic stimuli are shown in supplemental Table 1.
TABLE 1.
Genes differentially regulated by each extracellular stimulus
The table lists the genes identified by microarray analysis to be up- or down-regulated by all stimuli in at least one time point with an absolute -fold change >2 and an FDR value <0.05. Those genes whose stimulus-induced regulation was not confirmed by qPCR are in gray. For genes in which regulation was observed with two or more probesets, only one probeset is listed. BMP, bone morphogenic protein; EGL, egg-laying protein; ErbB, erythroblastic leukemia viral oncogene homolog; and Eph, ephrin.
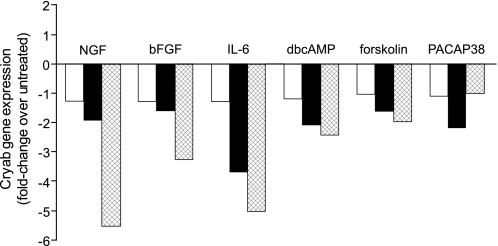
Given the controversial role of the small heat shock protein Cryab in the pathology of MS (22–26) and despite the fact that its corresponding gene was not revealed in the present microarray study, we performed qPCR analysis using specific primers for Cryab in the same samples used for the microarray study. Interestingly, Cryab was down-regulated by all the stimuli investigated (Fig. 3). We, therefore, added this gene to the list of the 31 selected genes and decided to investigate its potential role in the process.
FIGURE 3.
Regulation of Cryab gene expression during differentiation. NS1 cells were left untreated or treated with NGF (50 ng/ml), bFGF (10 μg/ml), IL-6 (5 μg/ml), dbcAMP (500 μm), forskolin (25 μm), or PACAP38 (5 μg/ml) for 3 h (empty bars), 24 h (black bars), or 72 h (crossed bars) as indicated. Cryab gene expression is shown as -fold change over untreated samples.
Effect of Several Target-specific Inhibitors on NGF- and dbcAMP-induced Neuronal Differentiation
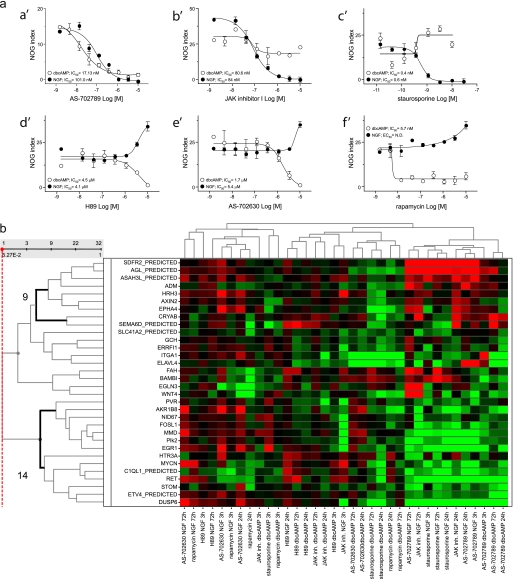
The analyses of the microarray study together with the morphological and biochemical observations suggested a clustering of the neuritogenic stimuli into two categories, most likely because of the usage of different pathways and/or set of genes to induce differentiation. To shed light on the potential signaling pathways involved, we investigated the effect of several small molecule, target-specific inhibitors on neurite outgrowth induced by NGF and dbcAMP as representative of the two groups of stimuli. Among more than 20 small molecule inhibitors tested, only six affected NGF- or dbcAMP-induced neurite outgrowth in a concentration-dependent manner and accordingly to their potency for their respective targets. As expected, inhibition of the MAPK kinase MEK1/2 by the specific inhibitor AS-702789, recently developed by Merck Serono (supplemental Fig. 3), resulted in complete inhibition of both NGF-and dbcAMP-triggered neurite outgrowth, showing IC50 values of 17.13 and 101 nm for dbcAMP- and NGF-induced differentiation, respectively (Fig. 4a, a′). These values are in perfect correlation with the in vitro potency of this inhibitor against MEK1/2 and to its potency in MEK-dependent cellular readouts (supplemental Fig. 3, a and b–d). These results clearly confirmed that, as in PC12 cells (47, 48), also in NS1 cells, both NGF- and dbcAMP-activated pathways converge at least at the level of MAPK activation to induce neuronal differentiation. In agreement with these results, AS-702789 also inhibited both NGF- and dbcAMP-induced ERK phosphorylation, as assessed by Western blot (data not shown).
FIGURE 4.
Effect of distinct target-specific, small molecule inhibitors on neuronal differentiation. a, effect on neurite outgrowth induced by NGF or dbcAMP. NS1 cells were pretreated for 20 min with DMSO or specific inhibitors of known targets as indicated. After pretreatment with inhibitors, 50 ng/ml NGF (full symbols) or 500 μm dbcAMP (empty symbols) were added for 72 h. After immunofluorescence staining, NOG index was quantified using Cellomics software, as described under “Experimental Procedures.” AS-702789, MEK1/2-specific inhibitor; JAK inhibitor I, pan-JAK1/2/3-specific inhibitor; H89, protein kinase A-specific inhibitor; AS-702630, PI3Kα-specific inhibitor. b, effect on differential gene expression induced by NGF and dbcAMP. NS1 cells were pretreated with DMSO or AS-702789 (10 μm), JAK inhibitor I (10 μm), H89 (10 μm), AS-702630 (10 μm), rapamycin (1 μm), or staurosporine (10 nm) before the treatment with NGF or dbcAMP as described in a. Total RNA was extracted after 3, 24, and 72 h, and qPCR analysis of the 31 selected genes as well as for Cryab, was performed using specific primers. Heatmap shows hierarchical cluster analysis of -fold changes in expression (log2 scale). Red, green, and black represent up-, down-, and no-regulation, respectively, in the presence of NGF or dbcAMP and inhibitor compared with NGF or dbcAMP in the absence of inhibitor. Bold lines on the left indicate the two clusters of genes down- or up-regulated by treatments inhibiting differentiation. ND, not determined.
Inhibition of JAK kinases with the specific, non-isoform-selective, JAK kinase inhibitor, JAK inhibitor I, completely blocked NGF-induced differentiation and slightly hampered dbcAMP-induced differentiation (Fig. 4a, b′). This indicates that NGF and, to a lesser extend, dbcAMP use JAK activation to achieve neuronal differentiation. Once again, these results correlate with a clear inhibition of NGF-induced compared with the lack of effect on dbcAMP-induced ERK phosphorylation exerted by this inhibitor (data not shown). It has to be noted that similar to NGF, complete inhibition of IL-6-induced NOG was observed upon both MEK and JAK inhibition under the same conditions, showing IC50 values of 6.6 and 47 nm, respectively (data not shown).
Staurosporine, a non-selective serine/threonine pan protein kinase inhibitor, completely abrogated NGF-driven differentiation, in agreement with previously published data (49, 50). Surprisingly, however, this inhibitor clearly enhanced dbcAMP-induced neurite outgrowth (Fig. 4a, c′). This is in good correlation with an increase in dbcAMP-induced ERK phosphorylation exerted by staurosporine, in contrast to its inhibition of NGF-induced ERK phosphorylation (data not shown). Nevertheless, given the non-selective nature of staurosporine, it is difficult to conclude on the mechanism(s) potentially involved in this activation.
Blockade of protein kinase A with the specific inhibitor H89 inhibited dbcAMP-induced differentiation as predicted (47, 51) but clearly and concentration dependently enhanced NGF-induced differentiation (Fig. 4a, d′). These results point to a negative feedback exerted by dbcAMP-activated signaling pathways on the NGF-activated pathways leading to neuronal differentiation. Moreover, inhibition of PI3Kα and one of its downstream effectors, mTOR, by AS-702630 (52) and rapamycin, respectively, had the same consequences as protein kinase A inhibition, as they blocked dbcAMP-induced differentiation while enhancing NGF-induced differentiation in the same concentration-dependent manner (Fig. 4a, e′ and f′). This effect was specific to the PI3Kα isoform, as inhibitors specific against PI3Kβ, PI3Kγ, or PI3Kδ did not affect either NGF- nor dbcAMP-induced neuronal differentiation (data not shown). Moreover, inhibition of the dbcAMP-induced neuronal differentiation with H89, AS-702630, and rapamycin correlated well with their inhibition of dbcAMP-induced ERK1/2 phosphorylation, whereas, in contrast, they did not affect ERK phosphorylation induced by NGF (data not shown). In the same experiments, AS-702630 showed inhibition of both NGF- and dbcAMP-induced Akt phosphorylation, confirming its expected on-target effect, namely inhibition of PI3K.
These results suggest the existence of a negative feedback exerted on NGF-induced pathways by a cAMP-activated pathway(s) which might involve at least protein kinase A, PI3Kα, and its downstream effector mTOR. Given the fact that protein kinase A, PI3Kα, and mTOR inhibition did not affect NGF-induced ERK phosphorylation, we propose that their negative feedback on NGF-activated pathways leading to differentiation might take place downstream of ERK.
Effect of Several Target-specific Inhibitors on NGF- and dbcAMP-induced Differential Gene Expression
We next investigated the effect of the small molecule, target-specific inhibitors found to affect neuronal differentiation on the regulation of NGF- and dbcAMP-regulated expression of the genes shown in Table 1 and of Cryab. We performed qPCR analysis on total RNA extracted from cells treated with NGF or dbcAMP for 3, 24, and 72 h in the absence or in the presence of inhibitors and analyzed changes in gene expression induced by these inhibitors during NGF or dbcAMP stimulation. Treatment of cells with these inhibitors affected gene expression regulation induced by NGF and dbcAMP for all the genes shown in Table 1, including Cryab. Moreover, hierarchical cluster analysis demonstrated the clustering of treatments such as the blockade of NGF-induced differentiation with staurosporine, MEK, and JAK inhibition and blockade of dbcAMP-induced differentiation by MEK inhibition. Furthermore, clustering of these treatments allowed us to identify two main groups of genes, one composed of 14 genes up-regulated upon inhibitory treatment and a second composed of 9 genes showing down-regulation upon inhibitory treatment (Fig. 4b). In addition, it is important to note that NGF- or dcbAMP-induced gene regulation of most of the genes within these two groups was reversed in the presence of the inhibitors mentioned. These findings are in good correlation to our initial hypothesis sustaining that genes regulated in common by all stimuli are likely to be fundamentally relevant to the process of neuronal differentiation rather than specifically associated to NGF- or dbcAMP-activated pathways. Indeed, when the process is blocked, the expression of these genes is reversed regardless the presence of the stimuli triggering the differentiation. Association of these two sets of genes as a whole with additional treatments that enhanced NGF- or dbcAMP-induced differentiation was less evident (Fig. 4b).
Role of Plk2, PVR, and Cryab on NGF-induced Neuronal Differentiation
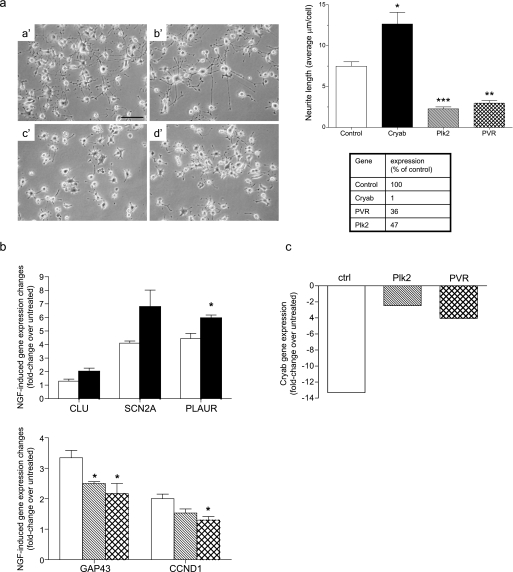
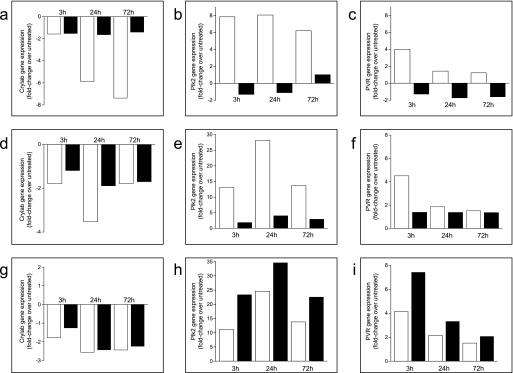
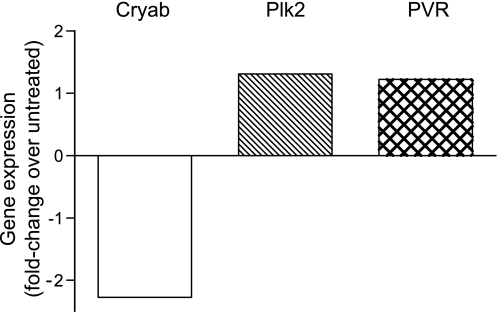
We next investigated the role on neuronal differentiation played by Plk2 and PVR, two genes found up-regulated by each differentiating stimulus, and by Cryab, which was found instead down-regulated by all of them. NS1 cells transfected with Plk2-, PVR-, or Cryab-specific siRNAs were induced to differentiate in the presence of NGF, as described under “Experimental Procedures.” Silencing of Cryab clearly enhanced NGF-induced neuronal differentiation, as shown in Fig. 5a. In contrast, silencing of either Plk2 or PVR strongly inhibited neurite outgrowth induced by NGF (Fig. 5a). The effects on neurite outgrowth were mainly observed in the length rather than in the number of neurites per cell or in the degree of neurite ramification. Interestingly, silencing of Cryab enhanced NGF-induced up-regulation of the differentiation markers clusterin, sodium channel type II subunit, and urokinase plasminogen-activating receptor, whose expression was not affected by silencing of Plk2 and PVR. In contrast, silencing of the latter two genes decreased the NGF-induced down-regulation of a different set of differentiation markers, namely, cyclin D1 and growth-associated protein 43 (Fig. 5b). These results further confirmed the opposite and crucial roles on neuronal differentiation played by Plk2 and PVR on one side and by Cryab on the other. Given that the expression of Plk2 and PVR is regulated during differentiation in an opposite way than that of Cryab and that silencing their respective expressions clearly affected this process in opposite ways, we subsequently investigated whether silencing of Plk2 and/or PVR affected the expression of Cryab or vice versa in the attempt to confirm or rule out their involvement in a unique pathway leading to neuronal differentiation. Interestingly, whereas silencing of Cryab did not significantly affect the expression of either Plk2 or PVR but affected the regulated expression of most of the other genes shown in Table 1 (data not shown), silencing of both Plk2 and PVR significantly decreased NGF-induced Cryab down-regulation (Fig. 5c). In contrast, silencing of Plk2 or PVR did not significantly affect their respective NGF-regulated expressions (data not shown). These results suggest that Cryab is regulated by and, thus, located downstream of Plk2 and PVR in a single pathway or in converging pathways mediating NGF-induced differentiation. Moreover, inhibition of NGF-induced differentiation by inhibiting MEK and JAK kinases resulted in a dramatic decrease or even reversion of the up-regulation of both Plk2 and PVR triggered during differentiation (Fig. 6, b, c, e, and f). Conversely, enhanced NGF-driven differentiation upon inhibition of PI3Kα resulted in the increase of NGF-induced up-regulation of these two genes (Fig. 6, h and i). In contrast, NGF-induced down-regulation of Cryab was significantly decreased upon JAK and MEK inhibition (Fig. 6, a and d). These results are in agreement with a role of Cryab as a negative regulator of neuronal differentiation whose expression is modulated by Plk2 and PVR, which are indeed required for this process and are regulated downstream of MEK and JAK in response to NGF. The relevance of these novel findings is reinforced by the fact that NGF-induced up-regulation of Plk2 and PVR as well as the NGF-induced down-regulation of Cryab were also confirmed in primary SCG neurons isolated from rat newborns (Fig. 7).
FIGURE 5.
Cryab, Plk2, and PVR are critical for neuronal differentiation and NGF-induced gene regulation. NS1 cells were transfected with either a control siRNA targeting an mRNA not present in rat cells, or with siRNAs-specific against Cryab, Plk2, or PVR. 72 h after transfection, cells were re-transfected with the same siRNAs for a second time and induced to differentiate with 100 ng/ml NGF for an additional 72 h. a, effect of silencing Cryab, Plk2, and PVR on neurite outgrowth. Left, phase-contrast photomicrographs from representative cultures of NGF-treated cells transfected with control (a′), Cryab (b′), Plk2 (c′), or PVR (d′) siRNAs. The graph shows the quantification of the effect of siRNA on neurite outgrowth, expressed as the average of neurite length (μm) per cell and quantified as described under “Experimental Procedures” for the experiment exemplified in the photomicrographs shown on the left. This experiment is representative of at least three other experiments with identical results. Per each condition, neurites were measured from ≥100 cells spread in to 3 different optic fields chosen randomly, as described under “Experimental Procedures” (p < 0.05, as calculated by unpaired t test). The scale bar represents 100 μm. The table shows the percentage of residual mRNA expression for each gene after its silencing with the specific siRNA, as quantified by qPCR in the experiment shown in the graph. b, effect of Cryab, Plk2, and PVR silencing on NGF-induced regulation of the expression of differentiation markers. Expression of eight differentiation markers (supplemental Fig. 1b) was determined by qPCR with specific primers on total RNA samples from cells treated with siRNA and induced to differentiate. The upper panel shows the effect of Cryab silencing on the NGF-induced regulation of the expression of clusterin (CLU), sodium channel type II subunit (Scn2a), and urokinase plasminogen-activating receptor (PLAUR) (full bars) as compared with transfection with a control siRNA (empty bars) (p < 0.05 as calculated by unpaired t test). The lower panel shows the effects of Plk2 (hashed bars) or PVR (crossed bars) silencing on NGF-induced regulation of growth-associated protein 43 (GAP43) and cyclin D1 as compared with control siRNA (empty bars) (p < 0.05 as calculated by unpaired t test). The experiment shown is representative of at least three experiments with identical results. c, effect of Plk2 and PVR silencing on the regulation of Cryab induced by NGF. Silencing of both Plk2 (hashed bar) and PVR (crossed bar) significantly decreased the down-regulation of Cryab gene expression exerted by NGF as compared with control cells transfected with a control siRNA (empty bar) and induced to differentiate with NGF as described for a and b and determined by qPCR. ctrl, control.
FIGURE 6.
Effect of the inhibition of JAK, MEK, and PI3Kα on Cryab, Plk2, and PVR gene expression. NS1 cells were pretreated with 10 μm concentrations of either AS-702789, JAK inhibitor I, AS-702630, or DMSO for 20 min before the addition of NGF (50 ng/ml) for 3, 24, and 72 h. Expression levels of Cryab, Plk2, and PVR were quantified by qPCR and are expressed as the -fold change relative to untreated cells. Empty and full bars represent expression levels in the absence and presence of inhibitors, respectively. a, b, and c, treatment with JAK inhibitor I; d, e, and f, treatment with AS-702789; g, h, and i, treatments with AS-702630. Cryab gene expression (a, d, and g), Plk2 gene expression (b, e, and h), and PVR gene expression (c, f, and i) is shown.
FIGURE 7.
Regulation of the expression of Cryab, Plk2, and PVR by NGF in rat superior cervical ganglia primary neurons. After dissection, SCG neurons were left untreated or treated with 50 ng/ml NGF for 72 h. NGF-regulated expression of Plk2, PVR, and Cryab is shown as -fold change over untreated samples, as indicated.
DISCUSSION
In the present study we have applied a systems biology approach by combining biochemical, whole-genome array, bioinformatics, and gene silencing as well as pharmacological studies to provide compelling evidence of the requirement of several signaling pathways leading to the global gene regulation associated to neuronal differentiation. In addition to the recognized MEK activation, our results suggest the involvement of other transduction pathways such as JAK, PI3Kα, mTOR, and protein kinase A. Analysis of gene expression changes during differentiation of NS1 cells with NGF, bFGF, IL-6, dbcAMP, forskolin, and PACAP38 has led to the identification of 31 genes showing regulated expression by each of the stimuli. The presence of genes such as Egr1, Fosl1, and Dusp6, found in several other studies during NGF-induced neuronal differentiation (3, 53, 54), further confirmed the good quality of the experimental design and of the overall whole-genome array study.
Hierarchical cluster and principal component analyses revealed the existence of two main categories of stimuli comprising NGF, bFGF, and IL-6 on one hand and dbcAMP, forskolin, and PACAP38 on the other. Indeed, gene expression changes induced by stimuli belonging to one or the other categories clustered together and separated from each other and from untreated samples. Thus, suggesting that each category of stimuli uses divergent sets of genes and/or pathways to induce neuronal differentiation. This is further supported by our findings showing both dissimilar differentiation-associated cell morphologies and regulation of differentiation markers, induced by these two categories of stimuli. Using selective inhibitors during differentiation of NS1 cells with NGF or dbcAMP as representative of the two categories of stimuli, we demonstrated that the expression of the 31 genes identified as regulated by all stimuli and of Cryab is consistently deregulated upon inhibition or enhancement of neuronal differentiation, hence further validating the critical involvement of these genes in the process of neuronal differentiation.
Among these genes, we identified Plk2 and PVR as strongly up-regulated by each stimulus. These two genes have been found also in recent microarray studies performed on NGF-treated PC12 cells (3, 55). Plk2 is a member of the highly conserved Polo-like kinase family (56), playing a role on cell cycle progression (57). However, Plk2 is not expressed in proliferating tissues such as thymus, liver, or intestine but is expressed in brain, although its function is not fully understood (58). PVR/CD155 is an adhesion molecule acting as a virus receptor for the central nervous system. Although PVR has been suggested to play a role during cellular migration (59, 60), its cellular functions have not been clearly established.
On the other hand, the small heat shock protein Cryab, although not present in the microarray results, was down-regulated during differentiation by each of the stimuli investigated, when the samples were analyzed by qPCR. This down-regulation has never been reported before despite the increasing attention recently paid to this protein because of its controversial contribution to the pathogenesis of MS. Indeed, Cryab, which is a component of brain myelin, was found as the most abundant unique transcript in MS plaques. Furthermore, it has been shown that peripheral blood mononuclear cells from MS patients contain large amounts of Cryab specific auto-reactive T cells (22–25). Recently, however, a protective role of Cryab during experimental autoimmune encephalomyelitis, the animal model mirroring MS, has been published (26). In addition, β- and γ-crystallin family members, have been suggested to play a role during axonal regeneration (27, 28).
Using specific siRNAs to silence Plk2, PVR, and Cryab, we prove their critical and opposing roles on neuronal differentiation. On one hand we show that Plk2 and PVR are essential for NGF-induced differentiation, which does not take place in the absence of their expression. On the other hand, we demonstrate that Cryab plays a negative role in this process, as its silencing enhances NGF-induced differentiation. In addition, we show that inhibition of MEK and JAK kinases not only blocks NGF- and dbcAMP-induced differentiation but also results in the down-regulation of Plk2 and PVR and in the concomitant up-regulation of Cryab. Moreover, silencing of Plk2 and PVR inhibits neuronal differentiation while inducing the up-regulation of Cryab.
Furthermore, we demonstrate for the first time the negative role exerted by PI3Kα on NGF-induced differentiation, showing that its inhibition clearly enhances NGF-induced neurite outgrowth. Our results also suggest that, in order for differentiation to take place, activation of MEK upon treatment with NGF leads to partial inhibition of PI3Kα activity. Indeed, blockade of NGF-induced differentiation by inhibition of MEK resulted in enhanced Akt phosphorylation, and inhibition of PI3Kα led to enhanced NGF-induced differentiation. Although the prevailing view considers ERK and PI3K as involved in two distinct pathways, their cross-talk has already been proposed previously (61, 62).
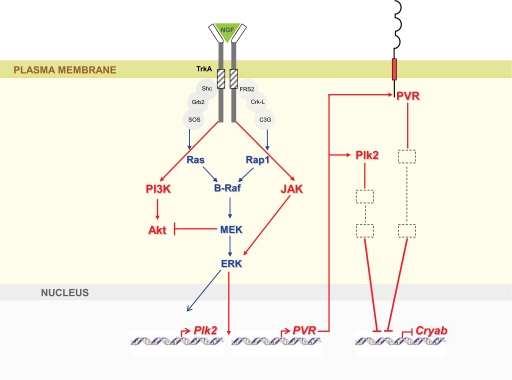
Our results, schematized in Fig. 8, provide evidence that NGF-induced neuronal differentiation requires, in addition to ERK, activation of one or more isoforms of JAK kinase and a parallel inhibition of the negative player PI3Kα. Finally, NGF stimulation results in the up-regulation of Plk2 and PVR, two genes identified in the present study as essentially required for neuronal differentiation, and in the down-regulation of the downstream negative effector of differentiation, Cryab (Fig. 8).
FIGURE 8.
Scheme of a model outlining the proposed relationship between the newly revealed genes Plk2, PVR, and Cryab during the NGF differentiation program. Blue arrows indicate activation of known NGF-driven pathways. Red arrows represent the genes/pathways discovered in the present study. NGF leads to the activation of the Ras-Braf-MAPK and Rap1-Braf-MAPK pathways, which result in ERK1/2 activation and gene transcription. In addition, stimulation of NS1 cells by NGF triggers the activation of PI3Kα and its consequent Akt phosphorylation. Akt phosphorylation is in turn negatively regulated by MEK in a NGF-induced negative feedback loop. By an as yet unknown mechanism, NGF-driven ERK activation results in the expression of Plk2 and PVR. Plk2 (cytosolic) and PVR (transmembrane) proteins lead to the down-regulation of Cryab, which functions as a downstream suppressor of NGF-induced neurite outgrowth.
Supplementary Material
Acknowledgments
We are grateful to Rob Hooft for critical reading of the manuscript and to Isabelle Martinou for technical support.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
- MS
- multiple sclerosis
- bFGF
- basic FGF
- Cryab
- αB-crystallin
- dbcAMP
- dibutyryl cAMP
- FDR
- false discovery rate
- IL-6
- interleukin-6
- NOG
- neurite outgrowth
- NS1
- Neuroscreen-1 PC12 cell line
- PACAP38
- pituitary adenylate cyclase-activating polypeptide 38
- PC12
- pheochromocytoma cell line
- PCA
- principal component analysis
- Plk2
- Polo-like kinase 2
- PVR
- poliovirus receptor
- qPCR
- quantitative PCR
- SCG
- superior cervical ganglion
- NGF
- nerve growth factor
- siRNA
- small interfering RNA
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- JAK
- Janus kinase
- MAPK
- microtubule-associated protein kinase
- PI3K
- phosphatidylinositol 3-kinase
- Cryab
- αB-crystallin
- ERK
- extracellular signal-regulated kinase
- mTOR
- mammalian target of rapamycin.
REFERENCES
- 1.Zamvil S. S., Steinman L. (2003) Neuron 38, 685–688 [DOI] [PubMed] [Google Scholar]
- 2.Dutta R., Trapp B. D. (2007) Neurology 68, Suppl. 3, 22–31 and 43–54 [DOI] [PubMed] [Google Scholar]
- 3.Dijkmans T. F., van Hooijdonk L. W., Schouten T. G., Kamphorst J. T., Vellinga A. C., Meerman J. H., Fitzsimons C. P., de Kloet E. R., Vreugdenhil E. (2008) J. Neurochem. 105, 2388–2403 [DOI] [PubMed] [Google Scholar]
- 4.Marampon F., Casimiro M. C., Fu M., Powell M. J., Popov V. M., Lindsay J., Zani B. M., Ciccarelli C., Watanabe G., Lee R. J., Pestell R. G. (2008) Mol. Biol. Cell 19, 2566–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obermeier A., Bradshaw R. A., Seedorf K., Choidas A., Schlessinger J., Ullrich A. (1994) EMBO J. 13, 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willard M. D., Willard F. S., Li X., Cappell S. D., Snider W. D., Siderovski D. P. (2007) EMBO J. 26, 2029–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene L. A., Tischler A. S. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peunova N., Enikolopov G. (1995) Nature 375, 68–73 [DOI] [PubMed] [Google Scholar]
- 9.Ihara S., Iwamatsu A., Fujiyoshi T., Komi A., Yamori T., Fukui Y. (1996) J. Biochem. 120, 865–868 [DOI] [PubMed] [Google Scholar]
- 10.Wu Y. Y., Bradshaw R. A. (2000) J. Biol. Chem. 275, 2147–2156 [DOI] [PubMed] [Google Scholar]
- 11.Shin E. Y., Shin K. S., Lee C. S., Woo K. N., Quan S. H., Soung N. K., Kim Y. G., Cha C. I., Kim S. R., Park D., Bokoch G. M., Kim E. G. (2002) J. Biol. Chem. 277, 44417–44430 [DOI] [PubMed] [Google Scholar]
- 12.Kiermayer S., Biondi R. M., Imig J., Plotz G., Haupenthal J., Zeuzem S., Piiper A. (2005) Mol. Biol. Cell 16, 5639–5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravni A., Bourgault S., Lebon A., Chan P., Galas L., Fournier A., Vaudry H., Gonzalez B., Eiden L. E., Vaudry D. (2006) J. Neurochem. 98, 321–329 [DOI] [PubMed] [Google Scholar]
- 14.Ravni A., Vaudry D., Gerdin M. J., Eiden M. V., Falluel-Morel A., Gonzalez B. J., Vaudry H., Eiden L. E. (2008) Mol. Pharmacol. 73, 1688–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Duan W., Cheung N. S., Huang Z., Shao K., Li Q. T. (2007) J. Neurochem. 103, 1157–1167 [DOI] [PubMed] [Google Scholar]
- 16.Vaudry D., Chen Y., Ravni A., Hamelink C., Elkahloun A. G., Eiden L. E. (2002) J. Neurochem. 83, 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grumolato L., Elkahloun A. G., Ghzili H., Alexandre D., Coulouarn C., Yon L., Salier J. P., Eiden L. E., Fournier A., Vaudry H., Anouar Y. (2003) Endocrinology 144, 2368–2379 [DOI] [PubMed] [Google Scholar]
- 18.Ravni A., Eiden L. E., Vaudry H., Gonzalez B. J., Vaudry D. (2006) J. Neurochem. 98, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Töröcsik B., Angelastro J. M., Greene L. A. (2002) J. Neurosci. 22, 8971–8980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uittenbogaard M., Chiaramello A. (2004) J. Neurochem. 91, 1332–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard D. G., Ziff E. B., Greene L. A. (1987) Mol. Cell. Biol. 7, 3156–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Noort J. M., van Sechel A. C., Bajramovic J. J., el Ouagmiri M., Polman C. H., Lassmann H., Ravid R. (1995) Nature 375, 798–801 [DOI] [PubMed] [Google Scholar]
- 23.Bajramović J. J., Plomp A. C., Goes A., Koevoets C., Newcombe J., Cuzner M. L., van Noort J. M. (2000) J. Immunol. 164, 4359–4366 [DOI] [PubMed] [Google Scholar]
- 24.Stoevring B., Vang O., Christiansen M. (2005) Clin. Chim. Acta 356, 95–101 [DOI] [PubMed] [Google Scholar]
- 25.van Noort J. M., Verbeek R., Meilof J. F., Polman C. H., Amor S. (2006) Mult. Scler. 12, 287–293 [DOI] [PubMed] [Google Scholar]
- 26.Ousman S. S., Tomooka B. H., van Noort J. M., Wawrousek E. F., O'Connor K. C., Hafler D. A., Sobel R. A., Robinson W. H., Steinman L. (2007) Nature 448, 474–479 [DOI] [PubMed] [Google Scholar]
- 27.Fischer D., Hauk T. G., Müller A., Thanos S. (2008) Mol. Cell Neurosci. 37, 471–479 [DOI] [PubMed] [Google Scholar]
- 28.Liedtke T., Schwamborn J. C., Schröer U., Thanos S. (2007) Mol. Cell. Proteomics 6, 895–907 [DOI] [PubMed] [Google Scholar]
- 29.Shin K. S., Shin E. Y., Lee C. S., Quan S. H., Woo K. N., Soung N. K., Kwak S. J., Kim S. R., Kim E. G. (2002) Exp. Mol. Med. 34, 172–176 [DOI] [PubMed] [Google Scholar]
- 30.Hadari Y. R., Kouhara H., Lax I., Schlessinger J. (1998) Mol. Cell. Biol. 18, 3966–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunning P. W., Landreth G. E., Bothwell M. A., Shooter E. M. (1981) J. Cell Biol. 89, 240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen T. O., Rehfeld J. F., Nielsen F. C. (2003) Neurosci. Lett. 347, 57–61 [DOI] [PubMed] [Google Scholar]
- 33.Hernandez A., Kimball B., Romanchuk G., Mulholland M. W. (1995) Peptides 16, 927–932 [DOI] [PubMed] [Google Scholar]
- 34.Goold R. G., Gordon-Weeks P. R. (2001) J. Cell Sci. 114, 4273–4284 [DOI] [PubMed] [Google Scholar]
- 35.Angelastro J. M., Töröcsik B., Greene L. A. (2002) BMC Neurosci. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S. W., Shin Y. J., Shim Y. J., Jeong S. Y., Park I. S., Min B. H. (2005) Exp. Cell Res. 309, 305–315 [DOI] [PubMed] [Google Scholar]
- 37.Fanger G. R., Erhardt P., Cooper G. M., Maue R. A. (1993) J. Neurochem. 61, 1977–1980 [DOI] [PubMed] [Google Scholar]
- 38.Farias-Eisner R., Vician L., Silver A., Reddy S., Rabbani S. A., Herschman H. R. (2000) J. Neurosci. 20, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strittmatter S. M., Fankhauser C., Huang P. L., Mashimo H., Fishman M. C. (1995) Cell 80, 445–452 [DOI] [PubMed] [Google Scholar]
- 40.Goold R. G., Gordon-Weeks P. R. (2005) Mol. Cell Neurosci. 28, 524–534 [DOI] [PubMed] [Google Scholar]
- 41.Yan G. Z., Ziff E. B. (1997) J. Neurosci. 17, 6122–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson L. P., Gdovin M. J., Liu C., Gardner P. D., Maue R. A. (1994) J. Neurosci. 14, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ifune C. K., Steinbach J. H. (1990) Brain Res. 506, 243–248 [DOI] [PubMed] [Google Scholar]
- 44.Gutacker C., Klock G., Diel P., Koch-Brandt C. (1999) Biochem. J. 339, 759–766 [PMC free article] [PubMed] [Google Scholar]
- 45.Farias-Eisner R., Vician L., Reddy S., Basconcillo R., Rabbani S. A., Wu Y. Y., Bradshaw R. A., Herschman H. R. (2001) J. Neurosci. Res. 63, 341–346 [DOI] [PubMed] [Google Scholar]
- 46.Aigner L., Arber S., Kapfhammer J. P., Laux T., Schneider C., Botteri F., Brenner H. R., Caroni P. (1995) Cell 83, 269–278 [DOI] [PubMed] [Google Scholar]
- 47.Vossler M. R., Yao H., York R. D., Pan M. G., Rim C. S., Stork P. J. (1997) Cell 89, 73–82 [DOI] [PubMed] [Google Scholar]
- 48.York R. D., Yao H., Dillon T., Ellig C. L., Eckert S. P., McCleskey E. W., Stork P. J. (1998) Nature 392, 622–626 [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto S., Hagino A. (1989) J. Neurochem. 53, 1675–1685 [DOI] [PubMed] [Google Scholar]
- 50.Nye S. H., Squinto S. P., Glass D. J., Stitt T. N., Hantzopoulos P., Macchi M. J., Lindsay N. S., Ip N. Y., Yancopoulos G. D. (1992) Mol. Biol. Cell 3, 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. (1990) J. Biol. Chem. 265, 5267–5272 [PubMed] [Google Scholar]
- 52.Rueckle T., Jiang X., Gaillard P., Church D., Vallotton T. (January20, 2004) U. S. Patent 2,0040,07491 [Google Scholar]
- 53.Camps M., Chabert C., Muda M., Boschert U., Gillieron C., Arkinstall S. (1998) FEBS Lett. 425, 271–276 [DOI] [PubMed] [Google Scholar]
- 54.Levkovitz Y., Baraban J. M. (2002) J. Neurosci. 22, 3845–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen T. O., Borup R., Marstrand T., Rehfeld J. F., Nielsen F. C. (2008) J. Neurochem. 104, 1450–1465 [DOI] [PubMed] [Google Scholar]
- 56.Llamazares S., Moreira A., Tavares A., Girdham C., Spruce B. A., Gonzalez C., Karess R. E., Glover D. M., Sunkel C. E. (1991) Genes Dev. 5, 2153–2165 [DOI] [PubMed] [Google Scholar]
- 57.Lee K. H., Ryu C. J., Hong H. J., Kim J., Lee E. H. (2005) Neurochem. Res. 30, 533–540 [DOI] [PubMed] [Google Scholar]
- 58.Seeburg D. P., Pak D., Sheng M. (2005) Oncogene 24, 292–298 [DOI] [PubMed] [Google Scholar]
- 59.Sloan K. E., Eustace B. K., Stewart J. K., Zehetmeier C., Torella C., Simeone M., Roy J. E., Unger C., Louis D. N., Ilag L. L., Jay D. G. (2004) BMC Cancer 4, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagamatsu Y., Rikitake Y., Takahashi M., Deki Y., Ikeda W., Hirata K., Takai Y. (2008) J. Biol. Chem. 283, 14532–14541 [DOI] [PubMed] [Google Scholar]
- 61.York R. D., Molliver D. C., Grewal S. S., Stenberg P. E., McCleskey E. W., Stork P. J. (2000) Mol. Cell. Biol. 20, 8069–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson T. R., Blader I. J., Hammonds-Odie L. P., Burga C. R., Cooke F., Hawkins P. T., Wolf A. G., Heldman K. A., Theibert A. B. (1996) J. Cell Sci. 109, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.