Abstract
To initiate and sustain an infection in mammals, bacterial pathogens must acquire host iron. However, the host's compartmentalization of large amounts of iron in heme, which is bound primarily by hemoglobin in red blood cells, acts as a barrier to bacterial iron assimilation. Bacillus anthracis, the causative agent of the disease anthrax, secretes two NEAT (near iron transporter) proteins, IsdX1 and IsdX2, which scavenge heme from host hemoglobin and promote growth under low iron conditions. The mechanism of heme transfer from these hemophores to the bacterial cell is not known. We present evidence that the heme-bound form of IsdX1 rapidly and directionally transfers heme to IsdC, a NEAT protein covalently attached to the cell wall, as well as to IsdX2. In both cases, the transfer of heme is mediated by a physical association between the donor and recipient. Unlike Staphylococcus aureus, whose NEAT proteins acquire heme from hemoglobin directly at the bacterial surface, B. anthracis secretes IsdX1 to capture heme in the extracellular milieu and relies on NEAT-NEAT interactions to deliver the bound heme to the envelope via IsdC. Understanding the mechanism of NEAT-mediated iron transport into pathogenic Gram-positive bacteria may provide an avenue for the development of therapeutics to combat infection.
Introduction
The acquisition of iron by pathogenic bacteria is necessary for their survival in mammalian hosts (1). The host, however, sequesters most iron in the porphyrin heme, which in turn is bound to proteins like hemoglobin, myoglobin, and hemopexin (2–4). Circulating hemoglobin is an iron reservoir that is exploited by rapidly growing bacteria in infected hosts, but, after hemolysis, the precise mechanisms of heme extraction, transport, and assimilation remain less clear (5–8). In Gram-negative bacteria, outer membrane, periplasmic, and inner membrane proteins most likely form a coordinated network to transfer heme into the cell (9, 10). Gram-positive bacteria, however, lack the outer membrane and periplasm but instead contain a thick cell wall (11, 12). Thus, the movement of heme into Gram-positive bacteria is distinct from their Gram-negative counterparts (6, 13) and likely involves heme transfer from globins to proteins attached to the cell wall (8, 14–16). A mechanistic understanding of how Gram-positive pathogens import heme will lead to the development of new anti-infectives to combat infections caused by these bacteria (17, 18).
Bacillus anthracis is a spore-forming, Gram-positive bacterium that causes anthrax disease and is an agent of bioterrorism (19, 20). The current model of anthrax pathogenesis suggests infectious spores enter a host via inhalation, ingestion, or a wound (21, 22). Following entry, spores are phagocytosed by macrophages and transported to regional lymph nodes, where germination of the spores leads to the vegetative form of B. anthracis (23, 24). Vegetative cells elucidate several virulence determinants whose collective action leads to bacterial growth, dissemination to major organ systems, and eventually host death (24–26). In the intervening weeks as the host decays, vegetative cells undergo sporulation, and the new spores are dispersed by environmental forces (27). Although the natural incidence of human infection is a few thousand people worldwide per year, the intentional release of B. anthracis spores into heavily populated areas is a serious public health concern (19).
Recent studies indicate heme acquisition in several pathogenic Gram-positive bacteria proceeds via a specialized iron-uptake module termed the near iron transporter (NEAT)3 domain (28–38). In Staphylococcus aureus, proteins harboring a NEAT domain partake in heme acquisition while being covalently attached to the cell wall (34, 39). B. anthracis secretes two of its NEAT proteins, IsdX1 and IsdX2, into the extracellular milieu (38). IsdX1 and IsdX2 appear to extract heme directly from hemoglobin and they contribute to the growth of B. anthracis on hemoglobin as the sole source of iron (38, 40). We hypothesized that these NEAT-containing hemophores likely pass heme to a recipient localized on the bacterial envelope, an event that would be expected to initiate heme import into B. anthracis. In this report, we provide evidence that the cell wall-anchored protein IsdC serves as the heme recipient for these NEAT hemophores and that heme transfer proceeds through a contact-dependent mechanism.
EXPERIMENTAL PROCEDURES
Protein Purification
IsdX1, IsdC, and IsdX2 were purified as previously described (36, 38). Briefly, Escherichia coli XL-1 Blue harboring expression plasmids pgst-isdX1, pgst-isdC, and pgst-isdX2 were propagated in 50 ml of LB with ampicillin (0.1 mg/ml) overnight at 37 °C. Bacteria were next inoculated into 1 liter of fresh LB plus ampicillin and rotated at 250 rpm at 37 °C. After 2 h of incubation, 1.5 mm isopropyl-β-d-thiogalactopyranoside was added, and cultures were incubated for an additional 2 h. Bacteria were sedimented by centrifugation at 6,000 × g for 8 min, suspended in phosphate-buffered saline (PBS: 137 mm NaCl, 2.7 mm KCl, 10 mm sodium phosphate dibasic, 2 mm potassium phosphate monobasic, pH 7.4), and cells were broken with two passes through a French press at 14,000 p.s.i. Bacterial lysates were centrifuged at 30,000 × g for 20 min, and soluble protein in supernatants was passed through a 0.45-μm pore-size cellulose filter. Filtrates were subjected to affinity chromatography on 2-ml glutathione-Sepharose (Amersham Biosciences) and the column pre-equilibrated with 20 ml of PBS. Columns were washed with 40 ml of PBS, and target proteins were eluted with 2 ml of PBS with 25 mm reduced glutathione. Purified proteins were dialyzed against 4 liters of PBS for 24 h to remove the glutathione. When necessary, the GST tag was removed from IsdX1, IsdC, or IsdX2 by thrombin digestion according to the manufacturer's instructions (Amersham Biosciences). Endogenous bound heme was removed from hemoproteins by briefly lowering the pH to 2.0 and extracting free heme with methyl ethyl ketone as described (41). Protein concentrations were either determined by the bicinchoninic acid method (Pierce) or by SDS-PAGE (42). All protein preparations were stored at −20 °C.
Creation of Holo-proteins
GST-IsdX1 and GST-IsdC were prepared from E. coli as described above. Increasing amounts of hemin, solubilized in 0.1 m NaOH, was incubated with GST-IsdX1 (20 μm) and GST-IsdC (20 μm) until saturation, defined as when the difference between the test (GST-IsdC/IsdX2 + hemin) and control (hemin alone) absorbance (404 nm) plateaued. Approximately 1 ml of the test mixture was next incubated with 500 μl of glutathione-Sepharose for 30 min at 25 °C with gentle shaking. Bound GST-NEAT protein was washed with 40 ml of PBS (pH 7.4) and incubated with 50 units of thrombin in 1 ml for 1 h at 25 °C with gentle shaking. After centrifugation, supernatants harboring GST-free NEAT proteins were added to 500 μl of aminobenzamidine-Sepharose (to remove thrombin) for 30 min at 25 °C with gentle shaking. After centrifugation, supernatants containing holo-NEAT protein were stored at −20 °C. This procedure resulted in a 6- to 8-fold enrichment of heme-bound NEAT protein (as determined by the A404/A280 ratio) when compared with untreated controls.
Heme Transfer Using Affinity Chromatography
Heme recipients (apo forms of GST-IsdC, GST-IsdX2, GST, and GST-IsdX1) were prepared as described above. 150 μl of each recipient (Cf = 10 μm) was incubated with glutathione-Sepharose for 30 min at 25 °C, washed 3× with 1 ml of PBS, and incubated with either 150 μl of holo-IsdX1 or holo-IsdC (Cf = 10 μm) for 1 h at 25 °C. Reactions were centrifuged to generate a sediment (recipient) and supernatant (donor) fractions, the sediment was washed 3× with 1 ml of PBS, and recipients were eluted with 125 μl of 25 mm reduced glutathione in PBS. Both fractions were subjected to absorbance spectroscopy at 404 nm, followed by subtraction of a mock (no donor added) sample equivalent of each recipient: heme content = A404(test) − A404(mock).
Heme Transfer Using Stopped-flow and Conventional Spectrophotometry
Transfer from holo-IsdX1 to apo-IsdC/apo-IsdX2 (200 μl of holo-IsdX1 (4 μm) was mixed with 200 μl of apo-IsdC (20 or 40 μm) or apo-IsdX2 (6.4 μm or 10 μm)) at 25 °C and spectral changes at 404 nm relative to 580 nm were monitored over 100 ms in an RSM-1000 (OLIS, Bolgart, GA) stopped-flow spectrophotometer (1000 spectra per second). The dead time of the instrument was ∼3 ms, and the light path was 20 mm. Transfer from holo-IsdX1 to apo-Mb (200 μl of holo-IsdX1 (5.4 μm) was mixed with apo-Mb (62.8 μm) in PBS) at 25 °C and spectral changes at 390 nm relative to 418 nm were monitored over 48 h in a DU800 spectrophotometer (Beckman-Coulter, London, UK).
Heme Transfer Using Size-exclusion Chromatography
Transfer from holo-IsdX1 to apo-IsdX2 (holo-IsdX1 (17 μm), apo-IsdX2 (64 μm), or holo-IsdX1 (17 μm)/apo-IsdX2 (64 μm) mixtures) was applied to a G-100 Sephadex size-exclusion column at 4 °C, and 1-ml fractions were collected. Heme and protein content was measured by subtracting the absorbance at 404 and 280 nm, respectively, from a reference absorbance at 700 nm.
Association of NEAT Proteins
The interaction of holo-IsdX1 with apo-IsdC or apo-IsdX2 was analyzed using a BIAcore 3000 biosensor (Amersham Biosciences) via surface plasmon resonance (SPR) spectroscopy (43, 44). Apo-IsdC or apo-IsdX2 in HBS (10 mm HEPES, pH 7.4, 0.15 m NaCl, 50 mm EDTA, 0.05% Tween 20) were covalently coupled to a CM5 sensor chip at 25 °C to a density of 2100 (for apo-IsdC) and 6000 (for apo-IsdX2) response units (RUs) using amine chemistry as described before (45, 46). Holo-IsdX1 (0.75–10 μm for apo-IsdC interactions or 2.5–100 nm for apo-IsdX2 interactions) in HBS was injected at 20 μl/min for 300 s, and dissociation followed after HBS injection for another 300 s at 25 °C. Data were prepared by “double referencing,” where parallel injections of analyte and buffer are flowed over a control dextran surface and then immobilized apo-IsdC or apo-IsdX2. In the derived sensogram, association and dissociation were fitted locally and globally using BIAevaluation 4.1 software (Amersham Biosciences). Affinity constants were estimated by fitting the data to a simple 1:1 binding model (47) and analyzed using dR/dt = kaC(Rmax − R) − kdR, where R is the SPR signal (in response units), ka is the association rate constant (in m−1 s−1), kd is the dissociation rate constant (in s−1), C is the concentration of holo-IsdX1 (in molar), Rmax is the maximum holo-IsdX1 binding capacity (in response units), and dR/dt is the rate of change of the SPR signal. The equilibrium binding constants (KD) of the complexes were determined as the ratio of ka/kd.
RESULTS
Generation of Heme-bound IsdX1
The reconstitution of holo (heme-bound) hemoproteins from their apo forms is complicated by heme insolubility, nonspecific binding to proteins, and the difficulty of removing excess heme by dialysis (48). We developed an affinity chromatography-based method to generate holo-IsdX1 from protein expressed in Escherichia coli (Fig. 1A). Briefly, GST-(apo)IsdX1 was titrated with purified hemin (the oxidized form of heme) until saturation (Fig. 1, A (steps 1 and 2) and B). GST-(holo)IsdX1 was bound to glutathione-Sepharose (Glut. Seph.), washed, and incubated with thrombin to release holo-IsdX1 into the supernatant (Fig. 1A, steps 3–7). Approximately 30–40% of GST-free holo-IsdX1 was recovered (Fig. 1C, compare protein levels after steps 3 and 7). To remove thrombin, the sample was incubated with aminobenzamidine-Sepharose (Fig. 1A, step 7). As judged by SDS-PAGE (Fig. 1C, step 8) and the light-brown color imparted by heme in hemoprotein samples (Fig. 1D, step 8), this methodology generates a pure preparation of holo-IsdX1. Holo-IsdX1 was used as a reagent to study heme transfer between NEAT proteins from B. anthracis.
FIGURE 1.
Generation of heme-bound (holo) IsdX1. A, a schematic demonstrating a procedure for the purification of holo-IsdX1. The highlighted numbers refer to key steps in the purification and can be followed in B–D. B, purified GST-IsdX1 was incubated with increasing amounts of hemin and binding measured by quantitating the difference in the absorption maxima (404 nm) between the test (IsdX1 + hemin) and control (hemin) samples. C, 10 μl of sample after each step was analyzed by SDS-PAGE, followed by gel staining with Coomassie Blue. D, the Eppendorf tubes after the indicated steps were photographed to highlight the light-brown color of the samples, suggestive of the presence of heme.
Heme Transfer to Apo-IsdC and Apo-IsdX2
IsdX1 is located in a gene cluster that has similarities to the iron-regulated surface-determinant locus (Isd) found in S. aureus (6). A component of this system is IsdC, a NEAT-domain protein that is covalently tethered to the cell wall in both B. anthracis and S. aureus and may serve as a heme recipient from holo forms of IsdX1 (36). To test this hypothesis, we purified B. anthracis IsdC from E. coli (36), removed endogenous heme by acid treatment/methyl ethyl ketone extraction to generate the apo form (41), and used this reagent in heme transfer studies with holo-IsdX1. Heme transfer between the NEAT proteins was monitored by an affinity chromatography-based method as described previously (38). In this assay, heme recipients are separated from heme donors by capture on glutathione-Sepharose, the sample is centrifuged, and heme content is quantified by measuring the absorbance at ∼404 nm of the sediment (recipient) versus the supernatant (donor) (Fig. 2A).
FIGURE 2.
Heme transfer to apo-IsdC and apo-IsdX2. A, a schematic demonstrating a procedure to monitor heme transfer from holo-IsdX1 to apo proteins. The highlighted numbers refer to the mixture of holo-IsdX1 (10 μm) or a PBS control solution with either apo forms of GST-IsdC (1), GST-IsdX2 (2), GST (3), or GST-IsdX1 (4). B, holo-IsdX1 or PBS were mixed with equimolar amounts of apo proteins 1–4 from A, mixtures were separated by centrifugation, and heme content in the sediment and supernatant fractions was assessed by measuring the absorbance at ∼404 nm, followed by subtraction of the PBS control values. The mean ± S.D. values of three independent experiments are shown. C, 20 μl of each fraction for both the holo-IsdX1- and PBS-treated samples was subjected to SDS-PAGE, and the resultant gel was stained with Coomassie Blue. D, holo-IsdX1 was generated using the methodology described in Fig. 1 but with hemoglobin substituted as the ligand. Holo-IsdX1 (10 μm) was mixed with equimolar amounts of apo-proteins 1–4, and heme transfer was assessed as described in A. The mean ± S.D. values of three independent experiments are shown. E, 10 μl of each fraction for both the holo-IsdX1- and PBS-treated samples was subjected to SDS-PAGE, and the resultant gel was stained with Coomassie Blue.
When holo-IsdX1 was incubated with equimolar amounts of GST-(apo)IsdC (conjugated to glutathione-Sepharose), ∼60% of the heme was transferred to the sediment (Fig. 2B, scenario 1). When holo-IsdX1 was incubated with GST-(apo)IsdX2 (also conjugated), ∼80% of the heme was transferred to the sediment (Fig. 2B, scenario 2). The amounts of recipient and donor proteins were approximately equivalent in this experiment (Fig. 2C). As a control, when holo-IsdX1 was incubated with only GST, >90% of the heme remained in the supernatant (i.e. associated with IsdX1 (Fig. 2B, scenario 3)). Finally, only 30% of the heme was associated with the sediment when holo-IsdX1 was incubated with GST-(apo)IsdX1 (Fig. 2B, scenario 4). This latter control indicates the movement of heme from the supernatant to sediment fractions in scenarios 1 and 2 cannot be explained by simple dissociation of heme from donor proteins followed by the scavenging of free heme by recipients. Taken together, these data suggest that holo-IsdX1 transfers heme to both apo-IsdC and apo-IsdX2.
The above experiments were performed with IsdX1 that had been loaded with purified, commercially available hemin. As a control we examined if the same transfer reactions would occur if IsdX1 had acquired its heme from hemoglobin. Apo-IsdX1 was incubated with holo-hemoglobin to generate holo-IsdX1 as previously described (38). Purified holo-IsdX1 was next incubated individually with apo-IsdC or apo-IsdX2, and the heme transfer assays were performed as described in Fig. 2A. As demonstrated in Fig. 2D, holo-IsdX1 efficiently transferred heme to both GST-(apo)IsdC and GST-(apo)IsdX2 (scenarios 1 and 2, respectively). The levels of donor and recipient were approximately equimolar in these experiments, as demonstrated in Fig. 2E. These data indicate that, after acquiring heme from hemoglobin, IsdX1 can transfer its heme to apo-IsdC and apo-IsdX2.
Considering that most of IsdX2 is found in the secreted fraction (38), the finding that another secreted protein, IsdX1, can transfer heme to IsdX2 was surprising. As a second, independent measure of heme transfer between IsdX1 and IsdX2, we performed size-exclusion chromatography of holo-IsdX1, apo-IsdX2, and holo-IsdX1/apo-IsdX2 mixtures. Both the heme (A402–700 nm) and protein (A280–700 nm) content was assayed for each fraction collected. Chromatography of each individual protein yielded holo-IsdX1 in fraction 15 and apo-IsdX2 in fraction 5 (Fig. 3, A and B). However, upon mixing holo-IsdX1 with apo-IsdX2, there was an 85% reduction in the heme signal associated with IsdX1 (Fig. 3A, fraction 15) and a corresponding 80% increase in the heme signal associated with IsdX2 (Fig. 3A, fraction 5), demonstrating that heme was transferred from IsdX1 to IsdX2. A smaller (∼38%) increase in the protein signal for fraction 5 may indicate a portion of IsdX1 is bound to IsdX2 (Fig. 3B). Taken together, the experiments in Figs. 2 and 3 demonstrate that IsdX1 can deliver heme to both IsdC and IsdX2, indicating these NEAT proteins probably serve as heme recipients on or near the surface of B. anthracis.
FIGURE 3.
Size-exclusion chromatography of holo-IsdX1/apo-IsdX2 mixtures. Holo-IsdX1 (17 μm), apo-IsdX2 (64 μm), or holo-IsdX1 (17 μm)/apo-IsdX2 (64 μm) mixtures were applied to a Sephadex G-100 size-exclusion column, and 1-ml fractions were assayed for both heme (A) or protein (B) content.
Directional Heme Transfer from IsdX1 to IsdC
The transfer of heme from IsdX1 to IsdC appears to be directional. To test this hypothesis, we generated holo-IsdC using the methods described in Fig. 1A. GST-IsdC was saturated with heme (supplemental Fig. S1A), thrombin-cleaved, and holo-IsdC-purified (supplemental Fig. S1, B and C). Using the heme transfer assay presented in Fig. 2, we assessed if holo-IsdC can transfer heme to GST-(apo)IsdX1 (Fig. 4A). When holo-IsdC was incubated with GST-tagged apo-IsdX1, ∼75% of the heme was retained in the supernatant, which was similar to the GST control (Fig. 4B, scenarios 2 and 3). These data indicate holo-IsdC retains its heme when incubated with apo-IsdX1, a finding consistent with net transfer at equilibrium from holo-IsdX1 to apo-IsdC. Thus, the transfer of heme from IsdX1 to IsdC is unidirectional. Surprisingly, when holo-IsdC was incubated with GST-(apo)IsdC, ∼50% of the heme was found in the sediment fraction (Fig. 4B, scenario 1), which suggests IsdC can exchange heme with itself. An equivalent amount of donor and recipient proteins was present in these experiments (Fig. 4C).
FIGURE 4.
Directional heme transfer from holo-IsdX1 to apo-IsdC. A, a schematic demonstrating a procedure to monitor heme transfer from holo-IsdC (10 μm) to apo proteins. The highlighted numbers refer to the mixture of holo-IsdC with either apo forms of GST-IsdC (1), GST (2), or GST-IsdX1 (3). B, holo-IsdC was mixed with equimolar amounts of apo proteins 1–3 and heme transfer assessed as described in Fig. 2. The mean ± S.D. values of three independent experiments are shown. C, 20 μl of each fraction for both the holo-IsdC- and PBS-treated samples was subjected to SDS-PAGE, and the resultant gel was stained with Coomassie Blue.
Kinetics of Heme Transfer from Holo-IsdX1 to Apo-IsdC and Apo- IsdX2
The transfer of heme can occur by spontaneous heme disassociation from the donor and then uptake from solution by the acceptor or via complex formation between the two proteins. To differentiate between these possibilities, the rates of heme transfer from holo-IsdX1 to apo-IsdC and apo-IsdX2 were measured using stopped-flow spectrophotometry and compared with the rate of hemin dissociation from holo-IsdX1 using an apo-myoglobin (apo-Mb)-scavenging reagent (49). This methodology is possible because the Soret (heme signal) absorbance peaks and intensities are slightly different for holo-IsdX1 compared with holo-IsdC and holo-IsdX2. Time courses for heme transfer can be obtained by measuring absorbance changes in the region of the heme donor or acceptor maxima. Mixing of 2 μm holo-IsdX1 with 20 μm apo-IsdC or 5.5 μm apo-IsdX2 produced rapid absorbance increases at 404 nm on a time scale of seconds (Fig. 5, A and B). No changes were observed when holo-IsdX1 was mixed with apo-IsdX1 (not shown). The time courses of heme transfer were biphasic and fit to a two-exponential expression to derive the apparent pseudo first order rate constants for heme transfer from holo-IsdX1 to apo-IsdC (average rates of 13.1 and 2.4 s−1) and apo-IsdX2 (16.7 and 3.6 s−1). For both transfer reactions, when the concentration of heme donor was held constant and the recipient apo-protein concentration was decreased, there was a proportional decrease in the observed rate. Thus, at these low protein concentrations, the rate-limiting step in the transfer reaction involves the formation of bimolecular complexes. Further work will be required to establish a more detailed kinetic mechanism.
FIGURE 5.
Kinetics of heme transfer to apo-IsdC and apo-IsdX2. 2 μm holo-IsdX1 was mixed with 20 μm apo-IsdC (A) or 5.5 μm apo-IsdX2 (B), and spectral changes at 404 nm were monitored using a stopped-flow spectrophotometer. C, 2.7 μm holo-IsdX1 was mixed with 31.4 μm apo-myoglobin (Mb), and spectral changes at 390 nm were measured using a conventional spectrophotometer. One of two independent determinations are shown. The apparent rate constants derived from these experiments are indicated in the text.
In contrast to the rapid transfer of heme from holo-IsdX1 to the acceptor apo-proteins, the spontaneous dissociation of heme from holo-IsdX1, as measured by heme transfer to the heme-scavenging reagent apo-Mb, is extremely slow, taking ∼2 days to complete with a first order rate constant of ≤0.0001 s−1 (Fig. 5C). Thus, the rate of heme transfer from IsdX1 to IsdC and IsdX2 is ∼10,000-fold faster than that expected for a simple hemin-dissociation mechanism. This result implies that movement of heme from IsdX1 to B. anthracis surface proteins is an active process that involves direct protein-protein interactions.
Interaction of Holo-IsdX1 with Apo-IsdC and Apo-IsdX2
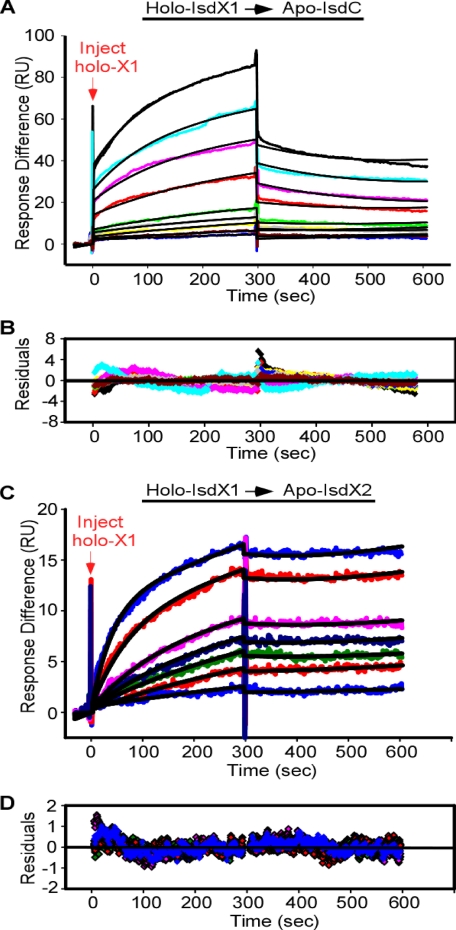
The data presented in Figs. 2–5 indirectly suggest heme transfer is mediated by NEAT protein-protein interactions. To test the hypothesis that holo-IsdX1 can associate with the apo forms of IsdC and IsdX2, we used SPR spectroscopy, which uses light diffraction changes of two potentially interacting molecules to determine the strength and kinetics of their association (43, 44). Heme recipients (apo-IsdC and apo-IsdX2) were covalently coupled to saturation on the surface of a CM5 sensor chip. Next, various amounts of holo-IsdX1 were infused over the surface, and interactions were quantified by measuring the response units with time. Infusion of holo-IsdX1 over 1.5 nmol of apo-IsdC produced a dose-dependent increase in RUs that was complete at ∼300 s (Fig. 6A, inject holo-X1). After the infusion of buffer, the signal decreased and returned to near baseline values over the next 1000 s (not shown). Infusion of holo-IsdX1 over 6 nmol of apo-IsdX2 also produced a dose-dependent increase in RUs but with faster association kinetics (Fig. 6B and Table 1). The estimated Kd values for holo-IsdX1 binding to apo-IsdC and apo-IsdX2 were ∼5 and 0.01 μm, respectively (Table 1). When this experiment was repeated using the apo form of IsdX1 at 100 μm (the highest concentration of holo-IsdX1 tested in these experiments), there was no increase in the RU values, which suggests there was no interaction with either apo-IsdC or apo-X2 (not shown). These results support the hypothesis that heme transfer between holo-IsdX1 and apo-IsdC/apo-IsdX2 is via a direct-contact mechanism that is facilitated by the donor being in the heme-bound state.
FIGURE 6.
The interaction of holo-IsdX1 with apo-IsdC and apo-IsdX2. A, holo-IsdX1 was injected at concentrations ranging from 0.75 to 10 μm (lower to upper curves) at a constant flow rate of 20 μl/min over 2100 RU of immobilized apo-IsdC. The association and dissociation phases were each monitored for 300 s by following the change in response units (RUs) with time. B, residuals from a single-site binding model are shown. C, holo-IsdX1 was injected at concentrations ranging from 2.5 to 100 nm at a constant flow rate of 20 μl/min over 6000 RU of immobilized apo-IsdX2. D, residuals from a single-site binding model are shown. The black curves represent a fit of the data to a single-site binding model, as described under “Experimental Procedures.”
TABLE 1.
Association parameters for the interaction of holo-IsdX1 with apo-IsdC and apo-IsdX2
SPR experiments were performed as described in the legend to Fig. 6. Data were fit to a single-site interaction model using BIAevaluation version 4.1 as described under “Experimental Procedures.” The values represent the mean of nine different holo-IsdX1 concentrations for each interaction with apo-IsdC or apo-IsdX2.
| Holo-IsdX1 → apo-IsdC | Holo-IsdX1 → apo-IsdX2 | |
|---|---|---|
| Ka (m−1s−1) | 722 | 2.04 × 105 |
| kd (s−1) | 3.57 × 10−3 | 2.04 × 10−3 |
| Kd (m) | 4.95 × 10−6 | 1.34 × 10−8 |
DISCUSSION
The development of antibiotic resistance in resident microbiomes, the emergence of new species of pathogenic bacteria, and the use of the most virulent agents for bioterrorism have created the need to identify and validate novel bacterial targets for drug discovery (50, 51). Because the assimilation of iron is necessary for bacterial pathogens to sustain an infection, iron-uptake systems represent promising candidates for anti-infective development. Understanding the molecular mechanism of bacterial iron import provides the necessary conceptual and structural coordinates to design inhibitors that block such systems during infection.
Both Gram-positive and -negative bacteria utilize heme as a source of iron (1). However, compositional and structural differences in their envelopes imply they use distinct mechanisms to transport heme. In Gram-negative bacteria, heme passes through an outer membrane, a periplasm, and inner membrane prior to entry into the cytoplasm (52). Gram-positive bacteria, however, do not have an outer membrane and periplasm and instead must transport heme through a thick cell wall (6). This process is currently thought to consist of three steps: (i) red blood cell hemolysis and diffusion of released hemoglobin to the bacterial envelope, (ii) the extraction of hemin from hemoglobin by cell wall-localized proteins, and (iii) the passage of extracted heme to ABC transporters in the bacterial membrane by cell wall proteins.
In S. aureus, these steps are manifested at the bacterial surface via an eight-gene system termed the iron-regulated surface determinant locus (isdA, -B, -C, -D, -E, -F, -G, and srtB), and an additional gene (isdH/harA) located elsewhere in the genome (34, 53). The first step, extraction of heme from hemoglobin, is most likely performed by IsdH and IsdB, two hemoglobin receptors covalently attached to the cell wall by the enzyme sortase (29, 37, 54, 55). Heme is removed through an undefined mechanism from hemoglobin by IsdH and passed to IsdA, which is also anchored to the cell wall (8, 30, 34, 53, 56–59). A fourth cell wall-anchored protein, IsdC, receives the heme from IsdA and transfers the heme to the membrane protein IsdE (8, 59, 60). IsdE is next believed to transport heme to IsdG, where enzymatic degradation of the hemin yields free iron and biliverdin (61, 62). Alternative routes of heme transit also have been observed; for example, IsdH can pass heme directly to IsdC, which suggests multiple pathways are used in vivo (8, 59). IsdH, -B, -A, and -C share in common one or more versatile yet specialized heme/hemoglobin binding modules termed the NEAT (near iron transporter) domain (28). Thus, in S. aureus, cell wall-attached NEAT proteins bridge the interface between heme sources (e.g. hemoglobin) in the extracellular milieu and the bacterial cell surface.
B. anthracis, however, seems to have evolved a different strategy to attain heme iron, one that may be reflective of fundamental and unappreciated differences in the bacterial envelope as compared with staphylococci and streptococci. Instead of immobilizing NEAT domains on the bacterial surface, B. anthracis secretes two of them (IsdX1 and IsdX2) (38). Following a transient interaction of IsdX1 and IsdX2 with hemoglobin, heme is acquired and presumably imported into B. anthracis (38). To understand how secreted NEAT proteins deliver heme to the bacterial surface of B. anthracis, we investigated the relationship between IsdX1, IsdX2, and the only known cell wall-anchored heme-binding protein in B. anthracis, IsdC (36).
Using affinity chromatography and stopped-flow spectroscopy, we have shown that holo-IsdX1 can transfer heme unidirectionally to apo-IsdC. Thus, unlike S. aureus, it seems likely that heme uptake in B. anthracis is initiated following the engagement of secreted, free hemophores with the bacterial cell wall. Interestingly, holo-IsdX1 can also transfer heme to apo-IsdX2 and with similar kinetics as that observed for holo-IsdX1 transfer to apo-IsdC. The movement of heme from one extracellular secreted protein to another would seem counterproductive. However, the five NEAT domains present in IsdX2 imply a multifunctional, perhaps storage role in heme transport that is not currently appreciated. Indeed, a fraction of IsdX2 is cell-associated and thus may serve a similar role in heme uptake as IsdA in S. aureus (38). Having multiple and distinct routes of heme import may be a mechanism for pathogenic bacteria to control the rate of iron assimilation under varying conditions of heme availability. These hypotheses require testing in future studies.
At least three functionally distinct attributes have been ascribed to NEAT proteins: (i) heme and hemoglobin binding, (ii) heme extraction from hemoglobin, and (iii) NEAT to NEAT transfer of heme. Lei, Stillman, and coworkers have provided strong, but indirect evidence that NEAT proteins from S. aureus and Shp, Shr, and HtsA from S. pyogenes form complexes upon the transfer of heme; however, there is no direct evidence for the equilibrium formation of donor-acceptor complexes (8, 59, 60). The slow rates of dissociation of heme from IsdX1 (hours), combined with the finding that heme transfer from holo-IsdX1 to apo-IsdC and apo-IsdX2 is rapid (a few seconds), suggests that heme transfer in B. anthracis is mediated by an active protein-protein interaction mechanism. The SPR results in Fig. 6 demonstrate that holo-IsdX1 can bind rapidly, transiently, and with high affinity to apo-IsdC and apo-IsdX2, providing the first direct evidence of complex formation between NEAT proteins that are involved in heme transport.
The interaction of a secreted hemophore like IsdX1 with a surface-attached recipient has no precedent in Gram-positive bacteria. However, in Gram-negative bacteria, Serratia marcescens secretes HasA, a hemophore that transfers its heme to an outer membrane protein termed HasR (63–65). This transfer is also mediated by protein-protein interactions and is independent of other cellular factors (66). Thus, for both the HasA and IsdX proteins, it seems all the structural requirements for association and transfer are intrinsic to the donor:recipient pair. Indeed, a recent structure of the HasA-HasR complex suggests association of the two proteins leads to steric clashes between amino acid side chains and heme, which may facilitate transfer by displacing the heme long enough to be captured by the recipient (67). However, unlike the Has system, where the structures of HasA and HasR are quite distinct, the heme transfer pair in B. anthracis (IsdX1 and IsdC) most likely harbor similar structural folds by virtue of both being principally composed of a NEAT domain (37% identity and 56% similarity). Thus, the kinetics of transfer, the available structural data, and the direct interactions described herein suggest heme transfer between NEAT proteins proceeds via a ligand-exchange mechanism following NEAT-NEAT association (8, 29–31, 59, 68).
We now propose a pathway for heme acquisition and transport via secreted hemophores in B. anthracis (Fig. 7). IsdX1, following its secretion into extracellular tissues or blood, extracts heme from free hemoglobin. Holo-IsdX1 interacts with growing bacilli through a NEAT-NEAT physical interaction with apo-IsdC, a process that facilitates the transfer of heme to the bacterial envelope. Holo-IsdC most likely transfers the heme to an IsdE homolog in the membrane, which subsequently imports the heme to the cytosol where it is degraded to release free iron by the monooxygenase IsdG. Alternatively, heme may be passed from IsdX1 to IsdX2; however, whether this occurs at the bacterial envelope or in the extracellular space, and what the downstream recipients of this exchange are, is currently not understood. The collective action and relative contribution of these pathways to heme uptake in the context of an infection is also not known and will require reagents that allow for the in vivo visualization of such events.
FIGURE 7.
A current model for heme acquisition and transport into B. anthracis. First, secreted IsdX1 extracts heme from hemoglobin (Hb). Once IsdX1 has diffused to the bacterial cell, heme is rapidly transferred to cell wall-attached IsdC through a NEAT-NEAT interaction mechanism. Alternatively, IsdX1 may transfer heme to secreted or cell-associated IsdX2; however, the location and downstream heme recipient in this auxiliary pathway are not known. IsdC may next pass heme to an ABC-type transporter with homology to S. aureus IsdE in the membrane (not shown). Finally, IsdG will receive and degrade the heme in the cytosol to liberate the iron. EC, extracellular; CW, cell wall; M, membrane; and C, cytosol.
This study used several methodologies to establish: (i) holo-IsdX1 readily transfers heme to both apo-IsdX2 and apo-IsdC, (ii) the transfer is directional, (iii) the transfer rate is ∼10,000 times greater than the rate at which heme is spontaneously lost from holo-IsdX1, and (iv) transfer is mediated by a protein-protein complex mechanism that is dependent on the donor being heme-bound. An evaluation of the structure, mechanism, and biologic function of heme acquisition in Gram-negative and Gram-positive bacteria will promote studies aimed at validating these systems as targets for therapeutic intervention.
Supplementary Material
Acknowledgments
We thank Olaf Schneewind for reading the manuscript and Yael Tarlovsky and Erin Honsa for suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grants AI069697 (to A. W. M.), GM035649 and HL047020 (to J. S. O.), and GM84348 (to M. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- NEAT
- near iron transporter
- Isd
- iron-regulated surface determinant
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- SPR
- surface plasmon resonance
- RU
- response unit(s)
- Mb
- myoglobin.
REFERENCES
- 1.Crosa J. H., Mey A. R., Payne S. M. (2004) Iron Transport in Bacteria, American Society of Microbiology Press, Washington, DC [Google Scholar]
- 2.Heinemann I. U., Jahn M., Jahn D. (2008) Arch. Biochem. Biophys. 474, 238–251 [DOI] [PubMed] [Google Scholar]
- 3.De Domenico I., McVey Ward D., Kaplan J. (2008) Nat. Rev. Mol. Cell Biol. 9, 72–81 [DOI] [PubMed] [Google Scholar]
- 4.Wittenberg J. B., Wittenberg B. A. (1990) Annu. Rev. Biophys. Biophys. Chem. 19, 217–241 [DOI] [PubMed] [Google Scholar]
- 5.Genco C. A., Dixon D. W. (2001) Mol. Microbiol. 39, 1–11 [DOI] [PubMed] [Google Scholar]
- 6.Maresso A. W., Schneewind O. (2006) Biometals 19, 193–203 [DOI] [PubMed] [Google Scholar]
- 7.Stojiljkovic I., Perkins-Balding D. (2002) DNA Cell Biol. 21, 281–295 [DOI] [PubMed] [Google Scholar]
- 8.Zhu H., Xie G., Liu M., Olson J. S., Fabian M., Dooley D. M., Lei B. (2008) J. Biol. Chem. 283, 18450–18460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandersman C., Stojiljkovic I. (2000) Curr. Opin. Microbiol. 3, 215–220 [DOI] [PubMed] [Google Scholar]
- 10.Wilks A., Burkhard K. A. (2007) Nat. Prod. Rep. 24, 511–522 [DOI] [PubMed] [Google Scholar]
- 11.Navarre W. W., Schneewind O. (1999) Microbiol. Mol. Biol. Rev. 63, 174–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neidhardt F. C., Ingraham J. L., Schaechter M. (1990) Physiology of the Bacterial Cell, Sinauer, Sunderland, MA [Google Scholar]
- 13.Wandersman C., Delepelaire P. (2004) Annu. Rev. Microbiol. 58, 611–647 [DOI] [PubMed] [Google Scholar]
- 14.Liu M., Lei B. (2005) Infect. Immun. 73, 5086–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygaard T. K., Blouin G. C., Liu M., Fukumura M., Olson J. S., Fabian M., Dooley D. M., Lei B. (2006) J. Biol. Chem. 281, 20761–20771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ran Y., Zhu H., Liu M., Fabian M., Olson J. S., Aranda R., 4th, Phillips G. N., Jr., Dooley D. M., Lei B. (2007) J. Biol. Chem. 282, 31380–31388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maresso A. W., Schneewind O. (2008) Pharmacol. Rev. 60, 128–141 [DOI] [PubMed] [Google Scholar]
- 18.Reniere M. L., Torres V. J., Skaar E. P. (2007) Biometals 20, 333–345 [DOI] [PubMed] [Google Scholar]
- 19.Inglesby T. V., O'Toole T., Henderson D. A., Bartlett J. G., Ascher M. S., Eitzen E., Friedlander A. M., Gerberding J., Hauer J., Hughes J., McDade J., Osterholm M. T., Parker G., Perl T. M., Russell P. K., Tonat K. (2002) JAMA 287, 2236–2252 [DOI] [PubMed] [Google Scholar]
- 20.Koch R. (1876) Beitraege zur Biologie der Pflanzen 2, 277–310 [Google Scholar]
- 21.Ross J. M. (1957) J. Path. Bact. 73, 485–494 [Google Scholar]
- 22.Abramova F. A., Grinberg L. M., Yampolskaya O. V., Walker D. H. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2291–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lincoln R. E., Hodges D. R., Klein F., Mahlandt B. G., Jones W. I., Jr., Haines B. W., Rhian M. A., Walker J. S. (1965) J. Infect. Dis. 115, 481–494 [DOI] [PubMed] [Google Scholar]
- 24.Smith H., Keppie J., Stanley J. L. (1954) Lancet 267, 474–476 [DOI] [PubMed] [Google Scholar]
- 25.Smith H., Keppie J., Stanley J. L. (1955) Br. J. Exp. Pathol. 36, 460–472 [PMC free article] [PubMed] [Google Scholar]
- 26.Smith H., Keppie H. S., Stanley J. I. (1953) Br. J. Exp. Pathol. 34, 477–485 [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen G. B., Hansen B. M., Eilenberg J., Mahillon J. (2003) Environ. Microbiol. 5, 631–640 [DOI] [PubMed] [Google Scholar]
- 28.Andrade M. A., Ciccarelli F. D., Perez-Iratxeta C., Bork P. (2002) Genome Biol. 3, research 0047.1–0047.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilpa R. M., Fadeev E. A., Villareal V. A., Wong M. L., Phillips M., Clubb R. T. (2006) J. Mol. Biol. 360, 435–447 [DOI] [PubMed] [Google Scholar]
- 30.Grigg J. C., Vermeiren C. L., Heinrichs D. E., Murphy M. E. (2007) Mol. Microbiol. 63, 139–149 [DOI] [PubMed] [Google Scholar]
- 31.Sharp K. H., Schneider S., Cockayne A., Paoli M. (2007) J. Biol. Chem. 282, 10625–10631 [DOI] [PubMed] [Google Scholar]
- 32.Mack J., Vermeiren C., Heinrichs D. E., Stillman M. J. (2004) Biochem. Biophys. Res. Commun. 320, 781–788 [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M., Tanaka Y., Suenaga A., Kuroda M., Yao M., Watanabe N., Arisaka F., Ohta T., Tanaka I., Tsumoto K. (2008) J. Biol. Chem. 283, 28649–28659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazmanian S. K., Skaar E. P., Gasper A. H., Humayun M., Gornicki P., Jelenska J., Joachmiak A., Missiakas D. M., Schneewind O. (2003) Science 299, 906–909 [DOI] [PubMed] [Google Scholar]
- 35.Skaar E. P., Schneewind O. (2004) Microbes Infect. 6, 390–397 [DOI] [PubMed] [Google Scholar]
- 36.Maresso A. W., Chapa T. J., Schneewind O. (2006) J. Bacteriol. 188, 8145–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dryla A., Hoffmann B., Gelbmann D., Giefing C., Hanner M., Meinke A., Anderson A. S., Koppensteiner W., Konrat R., von Gabain A., Nagy E. (2007) J. Bacteriol. 189, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maresso A. W., Garufi G., Schneewind O. (2008) PLoS. Pathog. 4, e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skaar E. P., Humayun M., Bae T., DeBord K. L., Schneewind O. (2004) Science 305, 1626–1628 [DOI] [PubMed] [Google Scholar]
- 40.Gat O., Zaide G., Inbar I., Grosfeld H., Chitlaru T., Levy H., Shafferman A. (2008) Mol. Microbiol. 70, 983–999 [DOI] [PubMed] [Google Scholar]
- 41.Ascoli F., Fanelli M. R., Antonini E. (1981) Methods Enzymol. 76, 72–87 [DOI] [PubMed] [Google Scholar]
- 42.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 43.Myszka D. G. (2000) Methods Enzymol. 323, 325–340 [DOI] [PubMed] [Google Scholar]
- 44.Morton T. A., Myszka D. G. (1998) Methods Enzymol. 295, 268–294 [DOI] [PubMed] [Google Scholar]
- 45.Murphy M., Jason-Moller L., Bruno J. (2006) Curr. Protoc. Protein Sci., Chapter 19, Unit 19.14 [DOI] [PubMed] [Google Scholar]
- 46.Howell S., Kenmore M., Kirkland M., Badley R. A. (1998) J. Mol. Recognit. 11, 200–203 [DOI] [PubMed] [Google Scholar]
- 47.Dementieva I. S., Tereshko V., McCrossan Z. A., Solomaha E., Araki D., Xu C., Grigorieff N., Goldstein S. A. (2009) J. Mol. Biol. 387, 175–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alison G., Smith M. W. (2002) Heme, Chlorophyll, and Bilins Methods and Protocols, Humana Press, New York, NY [Google Scholar]
- 49.Hargrove M. S., Singleton E. W., Quillin M. L., Ortiz L. A., Phillips G. N., Jr., Olson J. S., Mathews A. J. (1994) J. Biol. Chem. 269, 4207–4214 [DOI] [PubMed] [Google Scholar]
- 50.Bonomo R. A. (2007) Enzyme-mediated Resistance to Antibiotics; Mechanisms, Dissemination and Prospects for Inhibition, The American Society of Microbiology Press, Washington, DC [Google Scholar]
- 51.Grey M. R., Spaeth K. R. (2006) The Bioterrorism Sourcebook, McGraw-Hill Co., Columbus, OH [Google Scholar]
- 52.Braun V. (2001) Int. J. Med. Microbiol. 291, 67–79 [DOI] [PubMed] [Google Scholar]
- 53.Taylor J. M., Heinrichs D. E. (2002) Mol. Microbiol. 43, 1603–1614 [DOI] [PubMed] [Google Scholar]
- 54.Dryla A., Gelbmann D., von Gabain A., Nagy E. (2003) Mol. Microbiol. 49, 37–53 [DOI] [PubMed] [Google Scholar]
- 55.Torres V. J., Pishchany G., Humayun M., Schneewind O., Skaar E. P. (2006) J. Bacteriol. 188, 8421–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vermeiren C. L., Pluym M., Mack J., Heinrichs D. E., Stillman M. J. (2006) Biochemistry 45, 12867–12875 [DOI] [PubMed] [Google Scholar]
- 57.Pluym M., Muryoi N., Heinrichs D. E., Stillman M. J. (2008) J. Inorg. Biochem. 102, 480–488 [DOI] [PubMed] [Google Scholar]
- 58.Pilpa R. M., Robson S. A., Villareal V. A., Wong M. L., Phillips M., Clubb R. T. (2009) J. Biol. Chem. 284, 1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muryoi N., Tiedemann M. T., Pluym M., Cheung J., Heinrichs D. E., Stillman M. J. (2008) J. Biol. Chem. 283, 28125–28136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu M., Tanaka W. N., Zhu H., Xie G., Dooley D. M., Lei B. (2008) J. Biol. Chem. 283, 6668–6676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skaar E. P., Gaspar A. H., Schneewind O. (2004) J. Biol. Chem. 279, 436–443 [DOI] [PubMed] [Google Scholar]
- 62.Skaar E. P., Gaspar A. H., Schneewind O. (2006) J. Bacteriol. 188, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cescau S., Cwerman H., Létoffé S., Delepelaire P., Wandersman C., Biville F. (2007) Biometals 20, 603–613 [DOI] [PubMed] [Google Scholar]
- 64.Létoffé S., Ghigo J. M., Wandersman C. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 9876–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Létoffé S., Nato F., Goldberg M. E., Wandersman C. (1999) Mol. Microbiol. 33, 546–555 [DOI] [PubMed] [Google Scholar]
- 66.Izadi-Pruneyre N., Huché F., Lukat-Rodgers G. S., Lecroisey A., Gilli R., Rodgers K. R., Wandersman C., Delepelaire P. (2006) J. Biol. Chem. 281, 25541–25550 [DOI] [PubMed] [Google Scholar]
- 67.Krieg S., Huché F., Diederichs K., Izadi-Pruneyre N., Lecroisey A., Wandersman C., Delepelaire P., Welte W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aranda R., 4th, Worley C. E., Liu M., Bitto E., Cates M. S., Olson J. S., Lei B., Phillips G. N., Jr. (2007) J. Mol. Biol. 374, 374–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.