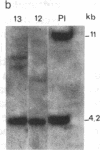
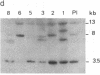
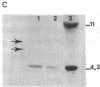
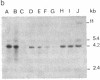
Abstract
Synovial membranes in patients with rheumatoid arthritis as well as other types of chronic destructive inflammatory arthritis contain infiltrates of activated T lymphocytes that probably contribute to the pathogenesis of the disease. In an effort to elucidate the nature of these infiltrates, interleukin 2 (IL-2)-responsive T lymphocytes were grown out of synovial fragments from 14 patients undergoing surgery for advanced destructive inflammatory joint disease. Eleven of the samples examined were from patients with classical rheumatoid arthritis, while three others were obtained from individuals with clinical osteoarthritis. Southern blot analysis of T-cell receptor (TCR) beta-chain genes in 13 of 14 cultures showed distinct rearrangements, indicating that each culture was characterized by the predominance of a limited number of clones. T-cell populations from peripheral blood stimulated with a variety of activators and expanded with IL-2 did not demonstrate evidence of similar clonality in long-term culture. These results suggest that a limited number of activated T-cell clones predominate at the site of tissue injury in rheumatoid synovial membranes as well as in other types of destructive inflammatory joint disease. Further characterization of these T-cell clones may aid our understanding of the pathogenesis of these rheumatic disorders.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Froland S. S., Natvig J. B., Pahle J. Elution and characterization of lymphocytes from rheumatoid inflammatory tissue. Scand J Immunol. 1975;4(8):823–830. doi: 10.1111/j.1365-3083.1975.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Decker J. L., Malone D. G., Haraoui B., Wahl S. M., Schrieber L., Klippel J. H., Steinberg A. D., Wilder R. L. NIH conference. Rheumatoid arthritis: evolving concepts of pathogenesis and treatment. Ann Intern Med. 1984 Dec;101(6):810–824. doi: 10.7326/0003-4819-101-6-810. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy R. J., Marrari M., Hackbarth S., Zeevi A. Serological and cellular definition of a new HLA-DR associated determinant, MC1, and its association with rheumatoid arthritis. Hum Immunol. 1984 Jul;10(3):165–176. doi: 10.1016/0198-8859(84)90037-5. [DOI] [PubMed] [Google Scholar]
- Fink P. J., Matis L. A., McElligott D. L., Bookman M., Hedrick S. M. Correlations between T-cell specificity and the structure of the antigen receptor. Nature. 1986 May 15;321(6067):219–226. doi: 10.1038/321219a0. [DOI] [PubMed] [Google Scholar]
- Flug F., Pelicci P. G., Bonetti F., Knowles D. M., 2nd, Dalla-Favera R. T-cell receptor gene rearrangements as markers of lineage and clonality in T-cell neoplasms. Proc Natl Acad Sci U S A. 1985 May;82(10):3460–3464. doi: 10.1073/pnas.82.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Førre O., Thoen J., Lea T., Dobloug J. H., Mellbye O. J., Natvig J. B., Pahle J., Solheim B. G. In situ characterization of mononuclear cells in rheumatoid tissues, using monoclonal antibodies. No reduction of T8-positive cells or augmentation in T4-positive cells. Scand J Immunol. 1982 Oct;16(4):315–319. doi: 10.1111/j.1365-3083.1982.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Goverman J., Minard K., Shastri N., Hunkapiller T., Hansburg D., Sercarz E., Hood L. Rearranged beta T cell receptor genes in a helper T cell clone specific for lysozyme: no correlation between V beta and MHC restriction. Cell. 1985 Apr;40(4):859–867. doi: 10.1016/0092-8674(85)90345-9. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hochgeschwender U., Simon H. G., Weltzien H. U., Bartels F., Becker A., Epplen J. T. Dominance of one T-cell receptor in the H-2Kb/TNP response. Nature. 1987 Mar 19;326(6110):307–309. doi: 10.1038/326307a0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Sorge J., Fong S., Heitzmann J. G., Curd J. G., Chen P. P., Goldfien R., Carson D. A. Cloning and sequence determination of a human rheumatoid factor light-chain gene. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2195–2199. doi: 10.1073/pnas.83.7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Simon L. S. Rheumatoid arthritis: clinical features and pathogenetic mechanisms. Med Clin North Am. 1986 Mar;70(2):263–284. doi: 10.1016/s0025-7125(16)30953-1. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Kurnick J. T., Kradin R. L., Blumberg R., Schneeberger E. E., Boyle L. A. Functional characterization of T lymphocytes propagated from human lung carcinomas. Clin Immunol Immunopathol. 1986 Mar;38(3):367–380. doi: 10.1016/0090-1229(86)90247-3. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Levinson A., Silver D., Seed B. Minimal size plasmids containing an M13 origin for production of single-strand transducing particles. J Mol Appl Genet. 1984;2(6):507–517. [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Mayer T. G., Fuller A. A., Fuller T. C., Lazarovits A. I., Boyle L. A., Kurnick J. T. Characterization of in vivo-activated allospecific T lymphocytes propagated from human renal allograft biopsies undergoing rejection. J Immunol. 1985 Jan;134(1):258–264. [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Minden M. D., Toyonaga B., Ha K., Yanagi Y., Chin B., Gelfand E., Mak T. Somatic rearrangement of T-cell antigen receptor gene in human T-cell malignancies. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1224–1227. doi: 10.1073/pnas.82.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Panayi G. S., Hobbs S., Raftery M. J., Janossy G. Activated T lymphocytes of the synovial membrane in rheumatoid arthritis and other arthropathies. Scand J Immunol. 1985 Dec;22(6):683–690. doi: 10.1111/j.1365-3083.1985.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm N. W., Carbone A., Kushnir E., Taylor B. A., Riblet R. J., Marrack P., Kappler J. W. The major histocompatibility complex-restricted antigen receptor on T cells: the genetics of expression of an allotype. J Immunol. 1985 Sep;135(3):2176–2182. [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Toyonaga B., Yanagi Y., Suciu-Foca N., Minden M., Mak T. W. Rearrangements of T-cell receptor gene YT35 in human DNA from thymic leukaemia T-cell lines and functional T-cell clones. 1984 Sep 27-Oct 3Nature. 311(5984):385–387. doi: 10.1038/311385a0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]