Abstract
Objective
To use a biomechanical model to explore how impairment of the pubovisceral portion of the levator ani muscle and/or the apical vaginal suspension might interact to affect anterior vaginal wall prolapse severity.
Method
A biomechanical model of the anterior vaginal wall and its support system was developed and implemented. The anterior vaginal wall and main muscular and connective tissue support elements, namely the levator plate, pubovisceral muscle, cardinal and uterosacral ligaments, were included and their geometry based on mid-sagittal plane magnetic resonance scans. Material properties were based on published data. The change in the sagittal profile of the anterior vaginal wall during a maximum Valsalva was then simulated when different combinations of muscle and connective tissue impairment were present.
Results
Under raised intra-abdominal pressure, the magnitude of anterior vaginal wall prolapse was shown to be a combined function of both pubovisceral muscle and uterosacral and/or cardinal ligament (“apical supports”) impairment. Once a certain degree of pubovisceral impairment was reached, the genital hiatus opened and a prolapse developed. The larger the pubovisceral impairment, the larger the anterior wall prolapse became. A 90% impairment of apical support led to an increase in anterior wall prolapse from 0.3 cm to 1.9 cm (a 530% increase) at 60% pubovisceral muscle impairment, and from 0.7 cm to 2.4 cm (a 240% increase) at 80% pubovisceral muscle impairment.
Conclusions
These results suggest that a prolapse can develop as a result of impairment of the muscular and apical supports of the anterior vaginal wall.
Introduction
Pelvic organ prolapse is a common disease that prevents women from enjoying a full and active life. In the US, approximately 200,000 women (1) have surgery to repair prolapse annually with a cost to society that exceeds 1 billion dollars per year (2). Anterior vaginal wall prolapse, clinically known as cystocele, is the most common form of pelvic organ prolapse. (3)
At present, our understanding of anterior vaginal wall support mechanisms is focused primarily on paravaginal attachments. (4,5) Recently two additional observations have been made. First, levator ani muscle damage is seen in women with prolapse (6,7,8,9). Second, anterior compartment prolapse is highly correlated (R = 0.73) with loss of apical support (10). How these observations help explain anterior compartment failure has been unclear and is a goal of the present paper.
A common approach to analyzing how a complex biologic system works is to represent the behavior of interest with a mathematic model. Such models can yield useful mechanistic insights and even predict system responses with reasonable accuracy. (11) One class of such models is the “lumped parameter” model in which the relevant features of the biologic system are divided into relatively few subunits. Through simplifying assumptions, the overall properties of each subunit can be approximated by an equation. Hence, the relationship between the tensile force and length of the pubovisceral muscle can most simply be represented by a lumped parameter representing the development of active contractile force and another representing the passive resistance of the muscle to stretch. Overall system behavior is then predicted by assembling and solving the system of equations that represent the various subunits and how they physically interact with one another. The aim of this study, therefore, was to develop and test a first-generation model to investigate how pelvic floor muscle and apical connective tissue interact to affect anterior vaginal wall support under conditions of raised intra-abdominal pressure.
Methods
A sagittally-symmetric, lumped parameter, biomechanical model of the anterior vaginal wall and its support system was developed based on our previous anatomical work (5,12,13,14). As a first step, the dimensions and orientation of the anterior vaginal wall and its support system were measured from mid-sagittal plane magnetic resonance scans of 10 healthy volunteers. This sample size was judged sufficient, based on the authors’ previous experience, to yield representative geometry. These women were recruited from 2001 to 2003 as controls in an IRB-approved case-control study comparing findings in women with normal support to women with pelvic organ prolapse. They denied having incontinence or prolapse symptoms and had demonstrated normal support on POP-Q examination (15) (no vaginal wall point lower than one centimeter above the hymen). These women had a mean age of 58.8 years (± standard deviation of 12.2 years) and parity of 2.45 (± 1.03). Axial, sagittal and coronal magnetic resonance images of the pelvic floor region were taken at 5 mm intervals as previously described (16). The dimensions and orientation of the anterior vaginal wall and levator plate were measured in the mid-sagittal plane at rest. The mean length of the vaginal wall was 7.0 (± 1.0) cm, with a mean vaginal inclination angle of 62.4 °(± 7.8). The mean length of the levator plate was 10.4 (± 0.9) cm with a mean levator inclination angle of 47.9 ° (± 4.8).
A 3-D model of the levator ani muscles, vagina, rectum, cardinal and uterosacral ligaments (“apical supports”), pubic bone and sacrum was created from the most representative subject’s MR images using 3-D-Slicer version 2.1b1, as previously described (17,18). The attachments of the levator ani muscle to the pubic bone and the lines-of-action of the apical supports were located based on 3-dimensional reconstructions. A two-dimensional (2-D), sagittally-symmetric, lumped parameter, biomechanical model was then created by projecting the 3-D geometry of anterior vagina wall and its support system onto the mid-sagittal plane (Figure 1). The anterior vaginal wall was modeled as a deformable and stretchable membrane lying on the rectum, which was supported by the levator plate. The anterior vaginal wall was fixed at the perineal membrane which was assumed to be stationary during Valsava. For simplicity, the levator plate was modeled as a rigid “trap door” hinged on the sacrum (SAC). To isolate the anterior vaginal wall effects, no relative motion was assumed to occur between the rectum and the levator plate. The pubovisceral portion of the levator ani muscle (PVM) was considered to control levator plate inclination with respect to the pelvis and was modeled using both an active contractile element and a passive elastic spring element (Fpvm =Factive + Fpassive)
Figure 1.
Model Development. A: mid-sagittal MR image. B: modeled element traced or projected on mid-sagittal MR image. C: lumped parameter biomechanical model. Pubovisceral muscle (PVM) is modeled as a spring in parallel with an active force generator. PS: pubic symphysis; SAC: sacrum; PM: perineal membrane; LA plate: levator plate; R: rectum; V: vagina; CL: cardinal ligament spring; US: uterosacral ligament spring; B: bladder; UT: uterus. Copyright Biomechanics Research Lab, University of Michigan, 2006
Apical support, provided to the vagina in Level I by the cardinal ligament and the uterosacral ligament, was represented by two passive elastic springs (Figure 1) attached to the top of the vaginal wall. An exponential relationship between force and elongation for anterior vaginal wall membrane and all passive springs (including passive material property of pubovisceral muscle and the apical supports) was assumed to present the nonlinear material property of biological tissue, that is
where C1 and C2 are the material property parameters, l is the current length and l0 is the initial length. The lower end of the vagina was considered fixed at its anterior attachment to the perineal membrane. The material property parameters of the vaginal wall were held constant through these simulations to focus on the interaction of apical supports and levator activity.
The vaginal wall, pubovisceral muscle and apical supports material properties used were based on values from existing literature (1920). Table 1 shows the dimensions and material properties of each model element.
Table 1.
Dimension and material property of each element of the 2-D biomechanical model
| Original length (cm) | Original inclination (relative to horizontal in degrees) | Material property (F in Newton and l in cm) | ||
|---|---|---|---|---|
| Vaginal wall | 7 | 62 |
|
|
| LA plate (R*) | 10 | 48 | Rigid plate | |
| PVM | 9.4 | 152 |
|
|
| Cardinal | 7.6 | 81 |
|
|
| Uterosacral | 4.8 | 25 |
|
R is modeled as rigid spacer allowing no movement relative to the model levator ani plate. LA: levator ani; PVM: pubovisceral muscle
The model was implemented in Matlap (The Math-Works Inc., Natick, MA). To model a Valsalva maneuver, the model abdomen was pressurized with 70 cmH2O of intra-abdominal pressure in order to represent a maximum Valsalva maneuver (21). In a woman with normal support, clinical observations show that a maximum Valsalva maneuver causes little displacement of the pelvic floor, if the pelvic floor muscles are actively contracted so as to equilibrate the downward intra-abdominal pressure acting on the levator plate (22). Therefore, in our model we assumed that the intact pubovisceral muscle can increase its contractile force sufficiently above the baseline resting tone so as to maintain hiatus closure under increased intra-abdominal pressure, as observed in women with normal support. Therefore, the model anterior vaginal wall was considered to be completely supported by the rectum and levator plate, without tension on the cardinal and uterosacral ligaments. The mean maximum vaginal closure force was assumed to be 2.8 Newtons (N) which is the average value reported in the literature (23, 24). (Figure 2a) Since the force exerted by the pubovisceral muscles holds the trap door or urogenital hiatus closed, intra-abdominal pressure cannot induce inferior-superior tension in the anterior vaginal wall because there is no pressure differential being generated.
Figure 2.
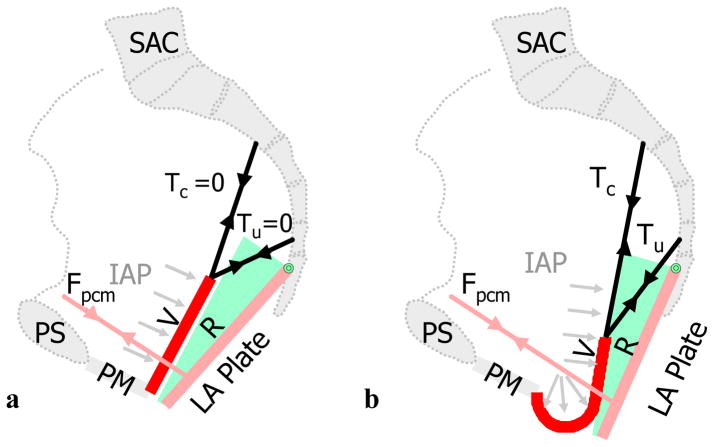
Force diagram showing the loading of the anterior vaginal wall and its support system. A: loading anterior vaginal wall with normal muscular support; B: loaded pelvic floor with defective muscular support and part of vaginal wall exposed to intra-abdominal pressure (light grey arrows). IAP denotes intra-abdominal pressure; Fpvm: tensile force generated by pubovisceral muscle between the projection of its origin on the pelvic side wall and insertion on the levator plate; Tc and Tu: tensile forces generated by the cardinal and uterosacral ligaments; D, the decent of the most dependent point of vaginal wall from end of perineal membrane (PM) which is used as the measurement of prolapse size in the simulation. Note descent of the vaginal apex as well as vaginal wall protrusion. Copyright Biomechanics Research Lab, University of Michigan, 2006
To use the model to simulate the two mechanisms underlying prolapse, we modeled the pubovisceral impairment (indicated by Defect%) by simulating loss of both the pubovisceral muscle’s active contractile force (Factive) and also its passive spring constant (derived its passive resistance to stretch in the uncontracted state), that is
Impairment of the connective tissue apical support (indicated by Defect%) was modeled as a decrease in their elastic properties, that is
Model simulations were run with impairments of 0%, 20%, 40%, 60%, 80% decrements in the maximal force that the pubovisceral muscle could generate and 0%, 50%, 90% increases in the elasticity of the apical support. Since we focused on the interaction between pubovisceral muscle and apical supports, the material property of the vaginal wall was held constant in all simulations. The primary outcome measure was the change in anterior vaginal wall deformation, and secondary outcome measures were the downward angular displacement of the levator plate and the downward translation of the top of the anterior vaginal wall under the action of 70 cm H2O intra-abdominal pressure. We used the descent of most dependent point of the vaginal wall as a measure of prolapse size, as shown in Figure 2b.
Results
General model behavior under load is shown in Figure 2b. When the system with impaired muscle and connective tissue was loaded with maximum intra-abdominal pressure (Valsalva), downward rotation of the levator plate occurred when the defective pubovisceral muscle could no longer generate sufficient force to counterbalance the abdominal pressure increase. In this situation, the model trap-door begins to ‘open’ such that the lower part of the anterior vaginal wall is no longer supported by the levator ani plate. The unsupported distal region of vaginal wall becomes the structure that lies between a higher intra-abdominal pressure zone and a lower atmospheric pressure zone. This pressure differential causes the downward ‘bulging’ (deformation) of the distal anterior vaginal wall in the areas not supported by the levator ani muscle. This, in turn, induces a superior-inferior tension force in the upper anterior vaginal wall. The apical supports are now placed under tension as they resist the downward movement of the upper vaginal wall until a new mechanical equilibrium is achieved in the system.
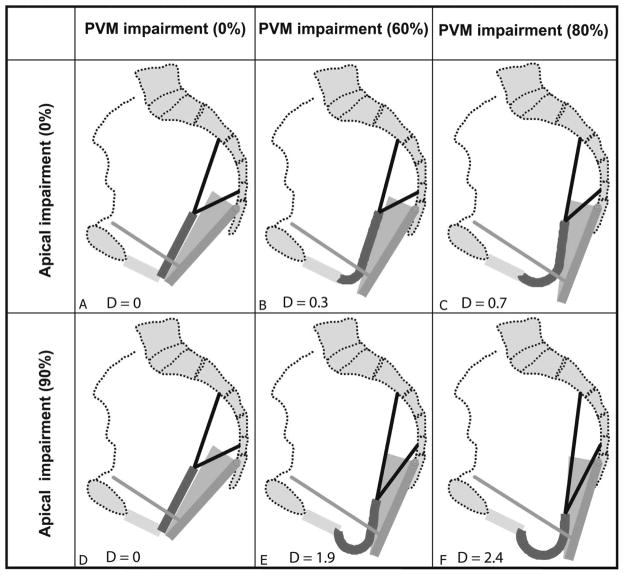
The specific simulation results with different combinations of pubovisceral and apical support impairment are shown in Figure 3. Here, the degree of anterior vaginal wall descent is shown as the distance below the perineal membrane, which lies at the level of the hymenal ring. For the results in the top row (Figure 3a, 3b, and 3c), the apical supports had the normal material parameter constants. The bottom row shows the results with 90% apical support impairment. In the first column (Figure 3a and 3d), the pubovisceral muscle has normal active contractile function and normal passive material properties, while the second and third columns show the effects of increasing, 60% and 90%, pubovisceral muscle impairment, respectively.
Figure 3.
Simulated deformation of the model anterior vaginal wall, and its support system, under maximal Valsalva with various degrees of pubovisceral muscle (PVM) and cardinal and uterosacral ligament impairment (indicated in percent). D presents the size of prolapse measured as the decent of the most dependent point of vaginal wall from end of perineal membrane. Copyright Biomechanics Research Lab, University of Michigan, 2006
When the pubovisceral muscle has normal properties (Figure 3a), no deformation is seen. With pubovisceral impairment (Figure 3b & 3c), some degree of anterior vaginal prolapse occurs. With normal muscles, but impaired apical support (Figure 3d), no displacement occurs because the vaginal wall is not subjected to a pressure differential.
The interaction between pubovisceral muscle defect and apical support impairment is shown in the bottom row (Figure 3e and 3f). When the apical support impairment was superimposed on the pubovisceral muscle impairment there was a loss of resistance to the descent of apical anterior vaginal wall, resulting in a much larger prolapse. The prolapse size, D, defined as the largest descent of the most independent point, is also shown in Figure 3. A 90% impairment of apical support led to an increase in anterior wall prolapse from 0.3 cm to 1.9 cm (a 530% increase) at 60% pubovisceral muscle impairment (Figure 3c compared with 3f), and from 0.7 cm to 2.4 cm (a 240% increase) at 80% pubovisceral muscle impairment (Figure 3b compared with 3e).
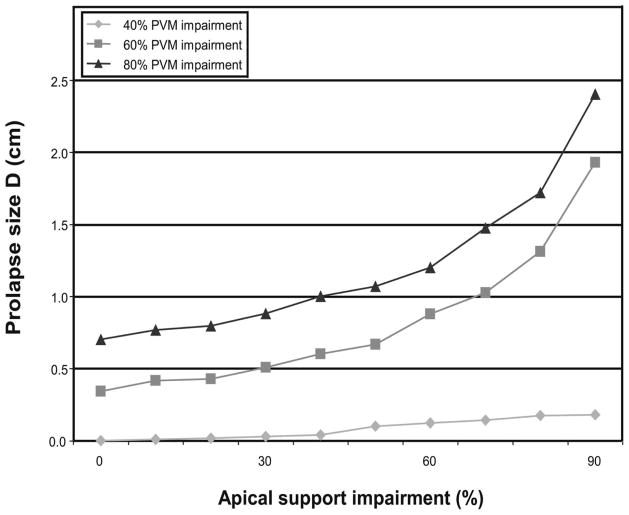
The prolapse size is shown as a function of apical support impairment for different degree of pubovisceral impairment in Figure 4. The prolapse size increases exponentially with increasing apical support impairment.
Figure 4.
Prolapse size measured by the most dependent point on the vaginal wall as a function of apical support impairment for different degrees of pubovisceral muscle (PVM) impairment. Copyright Biomechanics Research Lab, University of Michigan, 2006
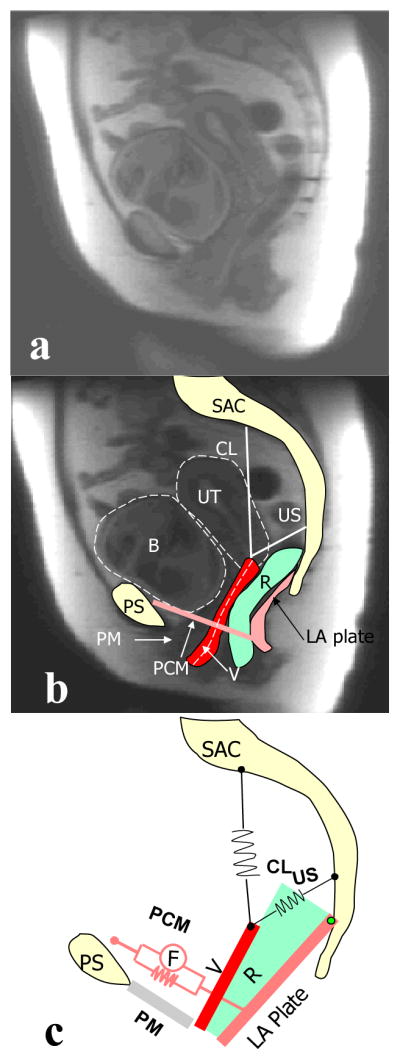
To verify that simulation results captured interactions between connective tissue and muscle support of vaginal wall, these findings were compared to a typical magnetic resonance image of a patient with cystocele in both resting state as well as under a maximum Valsalva (Figure 5). In the resting state, the patient has a relatively normal configuration. But with increasing intra-abdominal pressure associated with a Valsalva maneuver, the levator plate is pushed posteriorly and downwards to a more vertical orientation, the anterior vaginal wall is pushed inferiorly via the hiatus, while the uterus is pulled down by anterior vaginal wall. This scenario matches well with our model simulation thereby helping to validate the behavior of our model.
Figure 5.
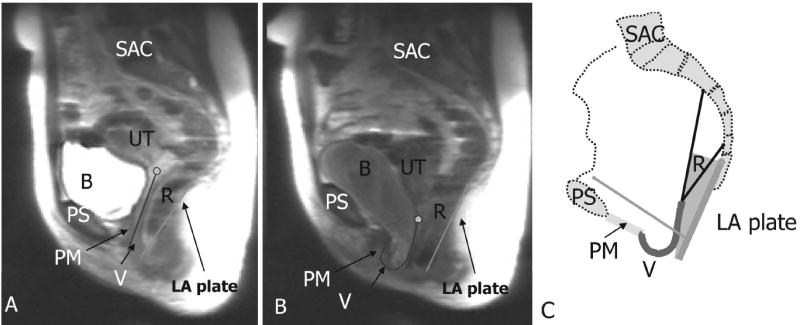
Validation of the model. a: mid-sagittal MR image of resting status; b: mid-sagittal MR image at maximum Valsava; c: sample model simulation result having a similar configuration as in (b). In this figure PS denotes pubic symphysis; SAC: sacrum; PM: perineal membrane; PVM: pubovisceral muscle; LA plate: levator plate; R: rectum; V: vagina; CL: cardinal ligament; US: uterosacral ligament; B: bladder; and UT: uterus Copyright Biomechanics Research Lab, University of Michigan, 2006
Discussion
This paper presents the development of a simple 2-D model that was used to investigate how anterior vaginal wall muscular and connective tissue supports affect the size of anterior vaginal wall prolapse. The model is suitable for examining first-order effects of the interaction between these supportive elements in the presence of impairments in the pubovisceral muscle and apical support tissues. The model is focused on anterior vaginal wall prolapse because it is the most common form of pelvic organ prolapse (3) and a form that can be used to initiate meaningful investigation into muscle/connective tissue interaction. We chose to focus on the apical connective tissue supports as a result of recent data (10) showing a strong correlation between apical descent and anterior compartment prolapse, recognizing that further work will be needed to add other elements of the support system. This model shows important qualitative results on how a combination of muscular and connective tissue impairments affects prolapse magnitude. The resulting anterior wall prolapses also mimic the ones seen clinically, thereby providing validity for our model.
The clinical importance of this article lies in its demonstration of the interaction between apical connective tissue supports and the levator ani muscles that result in anterior vaginal wall prolapse. This links research showing that the levator muscles are abnormal in women with pelvic organ prolapse (6,7,8,9) with observations of connective tissue abnormalities in pelvic organ prolapse (4,14,25). Our simulations also suggest that to some degree normal muscle and normal connective tissue function can compensate for injury to the other system. It is when both systems have impairments that the larger anterior vaginal wall prolapse is predicted. This can partly explain why women with levator ani muscle defect are more likely to have prolapse (9), but not all women with levator ani muscle defects have developed anterior vaginal wall prolapse.
When the intact pubovisceral muscle maintains urogenital hiatus closure, there is no tension in the vaginal wall because it is well supported by the levator plate and therefore no pressure differential across it. This assumption is based on clinical observations in nulliparous women that reveal minimal descent of the pelvic floor during Valsalva when the muscles are contracted. When the pubovisceral muscle is defective and its contractile force reduced, and the model predicted that less hiatus closure force is generated, which agrees with clinical measurements showing a 30% reduction in force among women with stress incontinence (24). The muscles are no longer able to maintain hiatal closure, and a pressure differential occurs between the high abdominal pressure zone and the lower atmospheric pressure. This pressure differential deforms the vaginal wall, driving it inferiorly towards the low pressure region. The behavior of our model extends previous theoretical work of the concept of the “pelvic valve” (26).
It is important to recognize that there are inherent limitations in this type of research. In this first simulation we chose to focus the analysis on the anterior vaginal wall and have made some simplifying assumptions. For example, it was assumed there is no deformation of levator plate or rectal movement relative to the levator plate. Second, this model is focused on the interaction between muscular support and apical suspension on anterior vaginal wall support. In this 2-D model, the paravaginal supports of the anterior vaginal wall that attach laterally to the pelvic sidewall have not specifically been modeled because this requires a more complex, 3-dimensional, approach. From a structural mechanics point of view, paravaginal support adds a reinforcing element to the mid-portion of the vaginal wall which will reduce its descent through the hiatus. In our model, we found that we needed to adjust the vaginal wall material property to ten fold stiffer than published experimental results in order to produce reasonable model results. This need may reflect this lack of paravaginal support. Also, stretch is not necessarily uniform along each element of the model as assumed; it can vary locally along and across the elements, especially if the thickness varies. The effect of nonuniform stretch in these structures requires further investigation, particularly if systematic regional variations in properties are found. Finally, the elements in the model are assumed having hyperelastic material property and no time dependent behavior was represent. Despite these simplifications, we believe this simple model captures the qualitative behavior of the system and yields useful predictions. In the future, distributed parameter models, such as finite element models, may prove to be more accurate and better for the 3-D analyses that are needed. This is a first attempt to use biomechanical modeling to study the mechanics of pelvic organ support. This model offers an opportunity to analyze fundamental concepts related to how injury of levator ani muscle and impairment of connective tissue can lead to anterior vaginal wall prolapse.
Acknowledgments
We gratefully acknowledge support of Public Health Service grant number R01 HD038665 from the Office of Women’s Health and NICHD’ Sex and Gender Factors Affecting Women’s Health SCOR P50 HD 44406
References
- 1.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 2.Subak LL, Waetjen LE, van den Eden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2002;98:646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 3.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002 Jun;186(6):1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 4.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976 Nov 1;126(5):568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 5.DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–1728. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 6.Tunn R, Paris S, Fischer W, Hamm B, Kuchinke J. Static magnetic resonance imaging of the pelvic floor muscle morphology in women with stress urinary incontinence and pelvic prolapse. Neurourol Urodyn. 1998;17(6):579–89. doi: 10.1002/(sici)1520-6777(1998)17:6<579::aid-nau2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Singh K, Jakab M, Reid WM, Berger LA, Hoyte L. Three-dimensional magnetic resonance imaging assessment of levator ani morphologic features in different grades of prolapse. Am J Obstet Gynecol. 2003;188(4):910–5. doi: 10.1067/mob.2003.254. [DOI] [PubMed] [Google Scholar]
- 8.Hoyte L, Schierlitz L, Zou K, Flesh G, Fielding JR. Two- and 3-dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am J Obstet Gynecol. 2001 Jul;185(1):11–9. doi: 10.1067/mob.2001.116365. [DOI] [PubMed] [Google Scholar]
- 9.Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gynecol. 2004 Sep;191(3):856–61. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 10.Summers A, Winkel LA, Hussain H, DeLancey JOL. The Relationship between Anterior and Apical Compartment Support. Am J Obstet Gynecol. 2006 doi: 10.1016/j.ajog.2006.01.057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander RM. Modelling approaches in biomechanics. Phil Trans Roy Soc Lond B Biol Sci. 2003;358(1437):1429–35. doi: 10.1098/rstb.2003.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–1720. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 13.DeLancey JOL. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol. 1999;180:815–23. doi: 10.1016/s0002-9378(99)70652-6. [DOI] [PubMed] [Google Scholar]
- 14.DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93–8. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 15.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 16.Chou Q, DeLancey JO. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J ObstetGynecol. 2001;185(1):44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Hsu Y, Ashton-Miller JA, Delancey JO. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynaecol Obstet. 2006 Jan 24; doi: 10.1016/j.ijgo.2005.12.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu Y, Chen L, Delancey JO, Ashton-Miller JA. Vaginal thickness, cross-sectional area, and perimeter in women with and those without prolapse. Obstet Gynecol. 2005;105:1012–7. doi: 10.1097/01.AOG.0000158127.97690.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada H. Strength of Biological Materials. Waverly Press, INC; Baltimore, Maryland, 21202, USA: 1970. pp. 205–270. [Google Scholar]
- 20.Bartscht KD, Delancey JOL. A technique to study the passive supports of the uterus. Obstet Gynecol. 1988 Dec;72(6):940–3. doi: 10.1097/00006250-198812000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Howard D, Miller JM, DeLancey JOL, Ashton-Miller JA. Differential Effects of Cough, Valsalva, and Continence Status on Vesical Neck Movement. Obstet Gyenecol. 2000;95:535–540. doi: 10.1016/s0029-7844(99)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafik A, Doss S, Asaad S. Etiology of the resting myoelectric activity of the levator ani muscle: physioanatomic study with a new theory. World J Surg. 2003 Mar;27(3):309–14. doi: 10.1007/s00268-002-6584-1. [DOI] [PubMed] [Google Scholar]
- 23.Morgan DM, Kaur G, Hsu Y, Fenner DE, Guire K, Miller J, Ashton-Miller JA, Delancey JO. Does vaginal closure force differ in the supine and standing positions? Am J Obstet Gynecol. 2005 May;192(5):1722–8. doi: 10.1016/j.ajog.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Morin M, Bourbonnais D, Gravel D, Dumoulin C, Lemieux MC. Pelvic floor muscle function in continent and stress urinary incontinent women using dynamometric measurements. Neurourol Urodyn. 2004;23(7):668–74. doi: 10.1002/nau.20069. [DOI] [PubMed] [Google Scholar]
- 25.Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–61. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 26.Porges R, Porges J, Blinick G. Mechanisms of Uterine Support and the Pathogenesis of Uterine Prolapse. Obstetrics and Gynecology. 1960 Jun;15(6):711–726. [PubMed] [Google Scholar]