Abstract
Objective
To test the hypothesis that hyporesponsiveness to ghrelin due to reduced growth hormone (GH) contributes to the aging-related hyperinflammatory state in sepsis.
Summary Background Data
Sepsis and septic shock are a serious problem particularly in the geriatric population. Ghrelin is an endogenous ligand for the GH secretagogue receptor 1a (GHSR1a, i.e., ghrelin receptor). The decline in GH with age is directly associated with many adverse changes that occur with aging. However, the role of GH, ghrelin, and GHSR1a in the age-associated vulnerability to sepsis remains unknown.
Methods
Male Fischer-344 rats (young: 3-months; aged: 24-months) were used. Plasma GH levels, ghrelin receptor expression and neuronal activity in the parasympathostimulatory nuclei of the brain stem in normal young and aged animals were measured. Endotoxemia was induced by intravenous injection of lipopolysaccharide (LPS, 15 mg/kg BW).
Results
While LPS-induced release of proinflammatory cytokines from macrophages isolated from aged rats decreased, LPS injection caused an in vivo hyperinflammatory state. GH levels were lower in aged rats, which was associated with lower expression of GHSR1a in the dorsal vagal complex (DVC) and a decrease in parasympathostimulatory neuronal activity. GHSR1a antagonist elevated LPS-induced cytokine release in young rats. GH increased GHSR-1a expression in the DVC in aged rats. Co-administration of ghrelin and GH, but not ghrelin alone or GH alone, markedly reduced cytokine levels and organ injury after endotoxemia in aged rats, which was associated with significantly elevated parasympathostimulatory neuronal activity.
Conclusions
These findings suggest that the reduced central (brain) responsiveness to ghrelin due to the decreased GH, plays a major role in producing the hyperinflammatory state, resulting in severe organ injuries and high mortality after endotoxemia in aged animals. Ghrelin and GH can be developed as a novel therapy for sepsis in the geriatric population.
Keywords: Aging, sepsis, inflammation, ghrelin, growth hormone
INTRODUCTION
Sepsis, a life-threatening inflammatory disorder representing the immune response to an infection, is a common disorder affecting nearly 750,000 people annually in the United States.1 It is particularly a serious problem in the geriatric population. A recent study has shown that the elderly (> or = 65 years of age) account for 12% of the U.S. population and 65% of sepsis cases, yielding a relative risk of 13.1 compared with younger patients.2 Case-fatality rates increase linearly by age; age is an independent predictor of mortality in an adjusted multivariable regression.2 Although this problem is increasingly recognized, the underlying mechanism(s) responsible for this age-associated vulnerability remain largely unknown.
The vagus nerve is an important link between the autonomic nervous system and immune cells, which has been suggested for more than 70 years.3 The majority of efferent vagus nerve fibers originates from the dorsal motor nucleus (DMN) of the vagus within the dorsal vagal complex (DVC) of the brain stem.4 The DVC consists of the DMN, the nucleus tractus solitarius (NTS), and the area postrema (AP), being parasympathostimulatory. This ‘parasympathetic’ nerve emanates from the cranium and innervates all major organs in a subconscious way. Tracey and associates found that after administration of LPS in the rat, electrical stimulation of the vagus nerve via the activation of nicotinic acetylcholine receptors (α7 receptors), prevented both the release of tumor necrosis factor (TNF) from macrophages and death.5–8
Ghrelin, a novel gastrointestinal hormone, was first identified in 1999 as an endogenous ligand for the growth hormone secretagogue receptor type 1a (GHSR-1a, i.e., ghrelin receptor).9 In addition to its growth hormone-releasing properties10, ghrelin has now been proved to possess other endocrine and non-endocrine activities including anti-inflammation.11–14 Ghrelin can cross the blood-brain barrier.15–17 It has been demonstrated that ghrelin activates the vagus nerve and vagal blockade abolishes ghrelin-induced growth hormone secretion.18 The stimulatory effect of ghrelin on the vagus nerve is mainly mediated via the central nervous system.19–21 This is not surprising, because ghrelin receptors are expressed at a high density in the brain22,23, especially in the DMN and NTS.24,25 Our recent study has clearly demonstrated that ghrelin’s direct effect on inflammatory cytokine release from macrophages is negligible.26 On the other hand, vagotomy prevents the downregulatory effect of ghrelin on inflammatory cytokines in rat models of polymicrobial sepsis and intestinal ischemia-reperfusion.26,27 Although the detailed central and visceral neuronal signaling evoked by systemic ghrelin administration remains obscure, these findings suggest that ghrelin may be an endogenous mediator regulating the immune system through the vagus nerve. In humans, GH levels decline approximately 15% per decade after age 25.28 The decline in GH with age is directly associated with many adverse changes that occur with aging.28,29 However, the role of GH, ghrelin, and GHSR1a in the age-associated vulnerability to sepsis remains unknown. The purpose of the current study was to test the hypothesis that hyporesponsiveness to ghrelin due to reduced GH plays a major role in aging-related hyperinflammatory state in septic shock.
MATERIALS AND METHODS
Experimental animals
Male Fischer-344 rats (young: 3-month-old; aged: 24-month-old) were provided by the National Institute on Aging, and housed in a temperature controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. Prior to the induction of endotoxemia, rats were fasted overnight but allowed water ad libitum. All experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Animal model of endotoxemia
Rats were anesthetized with isoflurane inhalation, the inguinal region was shaved and washed with 10% povidone-iodine and a short subinguinal incision was made. The femoral vein was carefully separated from the artery and cannulated with a catheter (PE-50 tubing). A bolus injection of lipopolysaccharide (LPS, 15 mg/kg BW; E. coli 055:B5 in 200-μ1 normal saline; Sigma, St. Louis, MO) was given through the femoral vein catheter. Blood and tissue samples were collected 4 h after LPS injection.
Determination of plasma ghrelin, GH, TNF-α, and Interleukin-6 (IL-6)
Plasma levels of ghrelin, GH, TNF-α, and IL-6 were quantified using an enzyme-linked immunosorbent assay (ELISA) kit specifically for ghrelin (Linco Research, Inc, St. Charles, MO), rat/mouse GH (Diagnostic Systems Laboratories, Webster, TX), rat TNF-α or IL-6 (BD Biosciences, San Diego, CA). The assay was carried out according to the instructions provided by the manufacturer.
Determination of plasma transaminases, lactate, and bilirubin
Plasma concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate, and bilirubin were determined by using assay kits according to the manufacturer’s instructions (Pointe Scientific, Lincoln Park, MI).
Cell culture
Peritoneal macrophages and Kupffer cells were isolated from normal young (3-month-old) and aged (24-month-old) rats. Briefly, peritoneal macrophages were isolated by peritoneal lavage with Hanks’ balanced salt solution (HBSS) as we described previously.26 Kupffer cells were isolated by collagenase perfusion of the liver, isopycnic sedimentation in a Percoll gradient, and selective adherence.30 The isolated peritoneal macrophages and Kupffer cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum, 10 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin at the concentration of 106 cells/ml and plated at a density of 5×105/well in 24-well cell culture plates. After being washed twice with HBSS, the cells were cultured in culture medium containing various concentrations of LPS (0, 1, 10, and 100 ng/ml) for a period of 24 h. The supernatant levels of cytokines (TNF-α and IL-6) were measured by ELISA as described above.
RNA extraction and determination of GHSR-1a genes
The dorsal vagal complex (DVC) was isolated from the brain stem of aged and young rats. Total RNA was extracted from DVC by Tri-Reagent (Molecular Research Center, Cincinnati, OH). RNA concentration and purity were determined by measuring the absorbance at 260 and 280 nm. Five μg of RNA from each tissue was reverse-transcribed in a 20 μl reaction volume containing 50 mM KCl, 10 mM Tris-HCl, 5 mM MgCl2, 1 mM dNTP, 20 U RNase inhibitor, 2.5 mM oligo d(T)16 primer and 50 U reverse transcriptase. The reverse transcription reaction solution was incubated at 42°C for 1 h, followed by heating at 95°C for 5 min. One μl cDNA was amplified with 0.15 μM each of 3′ and 5′ primers, specific for rat GHSR-1a 31 (AB001982: 5′ GAG ATC GCT CAG ATC AGC CAG TAC 3′, 5′ TAA TCC CCA AAC TGA GGT TCT GC 3′) and rat glyceraldehydes-3-phosphate-dehydrogenase (G3PDH) 32 (M17701: 5′ TGA AGG TCG GTG TCA ACG GAT TTG GC 3′, 5′ CAT GTA GGC CAT GAG GTC CAC CAC 3′) in 25 μl of PCR mixture containing 50 mM KCl, 10 mM Tris-HCl, 2 mM MgCl2, 0.2 mM dNTP and 0.7 U AmpliTaq DNA polymerase. PCR was carried out in a Bio-Rad thermal cycler. Following RT-PCR, 5 μl of the reaction mixture was electrophoresed in 1.2% TBE-agarose gel containing 0.22 μg/ml ethidium bromide. The gel was then developed and band intensities were normalized by G3PDH using the Bio-Rad image system (Hercules, CA).
Western blotting analysis of GHSR-1a
Tissue samples (DVC) were lysed and homogenized in lysis buffer (10 mM TBS, 1 mM EDTA, 1 mM EGTA, 2 mM Na Orthovanadate, 0.2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1% Triton-X 100) for 30 min on ice, and cleared by centrifugation at 14,000 rpm for 15 min at 4 °C. Fifty μg protein were fractionated on 4–12% Bis-Tris gel and transferred to 0.2 μm-nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% milk for 1 h. Blots were incubated with rabbit anti-rat GHSR-1a IgG (1:5,000, Alpha diagnostic International, San Antonio, TX) overnight at 4 °C. The blots were then washed in TBST 5 times for 10 min. Blots were incubated with HRP labeled goat anti-rabbit IgG for 1 h at room temperature, then washed 5 times in TBST for 10 min. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences) was applied according to the manufacturer’s instructions, and the membranes were exposed briefly to X-ray film. The band densities were normalized by β-actin using the Bio-Rad image system.
c-Fos immunohistochemistry
Expression of the immediate-early gene product c-fos has been widely used as a marker of neural activity in the brain.33 To determine whether aging is associated with decreased neuronal activity in the DVC, the rat brain samples were fixed in 10% buffered formalin solution and processed for paraffin sections. Serial sections of the brain were collected. The paraffin sections were dewaxed and rehydrated, followed by microwave antigen retrieval procedure. Slides were soaked in 20% citric acid buffer, pH 6.0 (Vector Labs, Burlingame, CA) and heated in the microwave oven and maintain the temperature at 95 °C for 15 min. The slides were cooled in room temperature for 5 min and then rinsed with Tris buffer saline (TBS, PH 7.5). Endogenous peroxidase was blocked by 2% H2O2 in 60% methanol for 20 min. Normal goat serum (2%) was used to block the nonspecific binding sites. The sections were then incubated in 1:400 rabbit anti-rat ghrelin polyclonal antibodies (Phoenix Pharmaceuticals, Belmont, CA) for 1.5 h at room temperature. After washing with TBS, the sections were reacted in 1:200 biotinylated anti-rabbit IgG (Vector Labs, Burlingame, CA) for 0.5 h. Vectastain ABC reagent and DAB kit (Vector, Burlingame, CA) were used to reveal the immunohistochemical reaction. For the negative control, the primary antibody was substituted by normal rabbit IgG.
Administration of ghrelin receptor antagonist [D-Arg1 D-Phe5 D-Trp7,9 Leu11]-substance P
To define the role of ghrelin receptor downregulation in producing hyperinflammatory responses during the aging process, a specific and potent ghrelin receptor antagonist, [D-Arg1 D-Phe5 D-Trp7,9 Leu11]-substance P (0.4 μmol/kg BW in 1 ml normal saline, Bachem, Torrance, CA)34, was administered to 3-month old Fischer-344 rats over a period of 30 min, starting 1 h before LPS injection (15 m g/kg BW). Plasma levels of TNF-α, IL-6, ALT, AST, and lactate were measured at 4 h after LPS injection as described above.
Administration of ghrelin and GH
A femoral vein was cannulated with a PE-50 tubing under anesthesia (isoflurane inhalation). Rat ghrelin alone (20 nmol/kg BW, Phoenix Pharmaceuticals, Belmont, CA), rat GH alone (25 μg/kg BW, ProSpec-Tany TechnoGene, Israel), ghrelin and GH in combination (20 nmol/kg BW and 25 μg/kg BW, respectively), or vehicle (0.5 ml normal saline) was administered intravenously as a bolus at 30 min prior to LPS injection (15 mg/kg BW) in 24-month-old (aged) rats. Plasma levels of TNF-α, IL-6, ALT, and AST were measured at 4 h after LPS injection as described above.
Statistical analysis
All data are expressed as mean ± standard error (SE) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test for multiple group analysis or Student’s t-test for two group analysis. Differences in values were considered significant if P<0.05.
RESULTS
Aging exacerbates the proinflammatory response and worsens tissue injury in endotoxemia
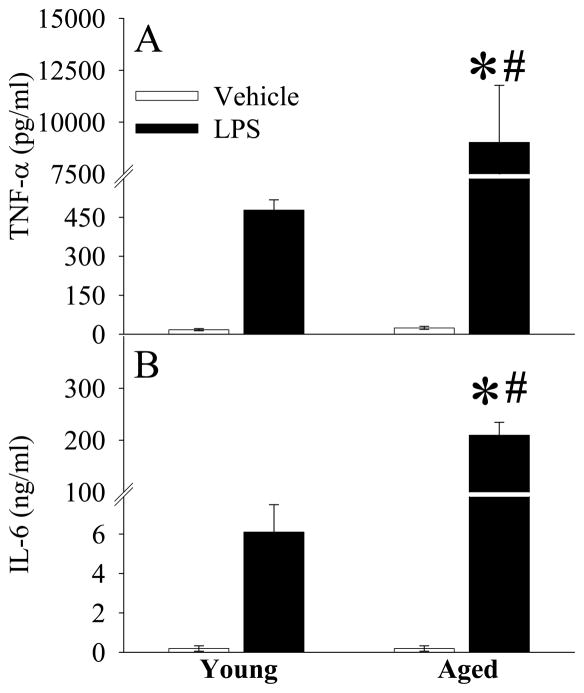
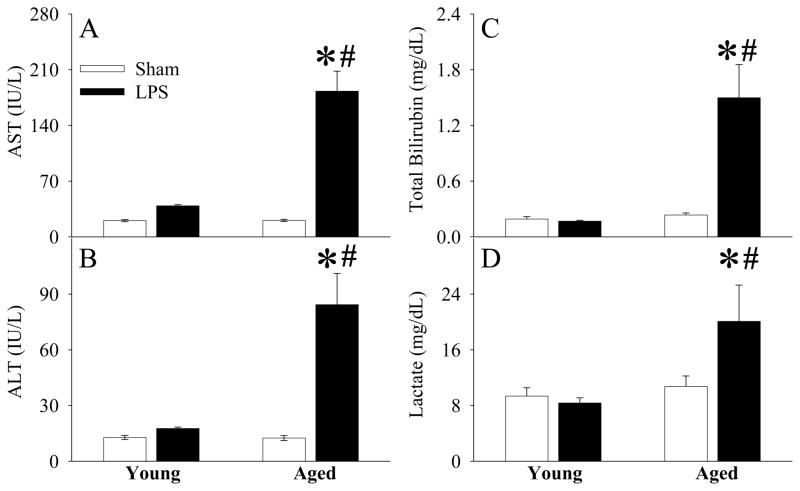
As shown in Figures 1A–B, while plasma levels of TNF-α and IL-6 increased at 4 h after LPS injection in young animals, the elevation of these cytokines was much greater in aged animals (P<0.05). This result indicates that a more severe hyperinflammatory response occurs in aged animals in response to the same dosage of LPS. The aged animals were also associated with severe tissue injury at 4 h after LPS injection, as evidenced by significantly elevated levels of circulating AST (P<0.05, Fig. 2A), ALT (P<0.05, Fig. 2B), total bilirubin (P<0.05, Fig. 2C), and lactate (P<0.05, Fig. 2D).
Figure 1.
Alterations in plasma levels of TNF-α (A) and IL-6 (B) in young (3-month-old) or aged (24-month-old) rats at 4 h after normal saline (Vehicle) or LPS (LPS) injection. Data are presented as means ± SE (n=5–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Vehicle group; #P < 0.05 versus Young LPS group.
Figure 2.
Alterations in plasma levels of AST (A), ALT (B), total bilirubin (C), and lactate (D) in young (3-month-old) or aged (24-month-old) rats at 4 h after normal saline (Vehicle) or LPS (LPS) injection. Data are presented as means ± SE (n=5–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Vehicle group; #P < 0.05 versus Young LPS group.
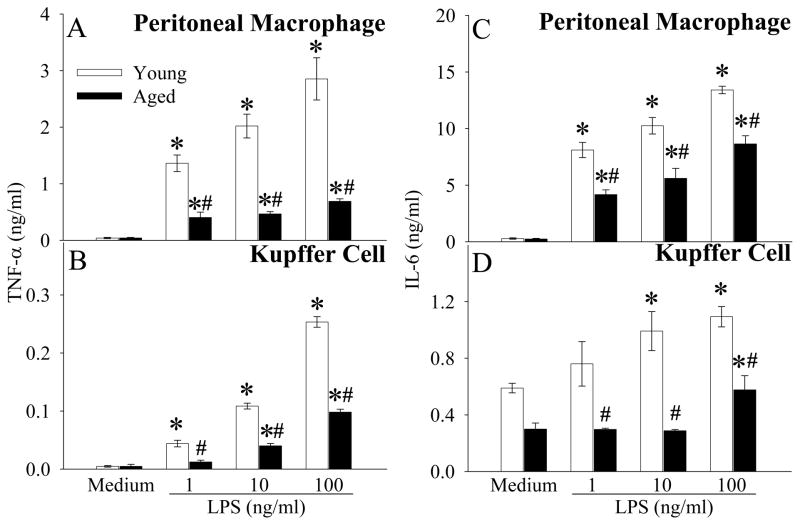
Reduced production of proinflammatory cytokines from isolated macrophages in response to LPS in aged animals
Although proinflammatory cytokines TNF-α and IL-6 increased significantly in vivo after LPS injection in aged vs. young animals, to our surprise, TNF-α production/release was significantly lower in both peritoneal macrophages (Fig. 3A) and Kupffer cells (Fig. 3B) collected from aged rats than those from young rats at 24 h after LPS stimulation in. Similarly, LPS-induced IL-6 release was also significantly lower in peritoneal macrophages and Kupffer cells isolated from aged animals at 24 h after LPS stimulation, as compared to the cells from young animals (Figs. 3C–D). A short incubation time (4 h) also resulted in a similarly lower TNF-α and IL-6 release from both LPS-stimulated peritoneal macrophages and Kupffer cells isolated from aged animals (data not shown).
Figure 3.
Dose-dependent effects of LPS on TNF-α (A, B) or IL-6 (C, D) secretion in peritoneal macrophages or Kupffer cells isolated from young (3-month-old) or aged (24-month-old) rats. Cells were cultured in DMEM containing various concentrations of LPS (0, 1, 10, and 100 ng/ml) for a period of 24 h. The supernatant levels of cytokines (TNF-α and IL-6) were measured by ELISA. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls test: * P<0.05 versus Medium group; # P<0.05 versus corresponding Young group.
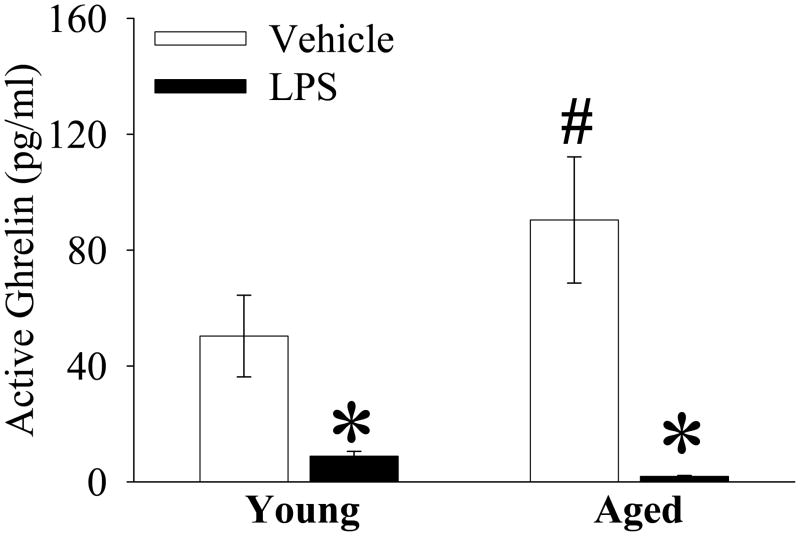
Plasma ghrelin is further reduced in aged animals in endotoxemia
As indicated in Figure 4, basal plasma levels of ghrelin are 79.6% higher in aged as compared to young rats (Sham, P<0.05). At 4 h after LPS injection, plasma ghrelin decreased by only 82.4% in young, but decreased by 97.9% in aged animals (P<0.05, Fig. 4).
Figure 4.
Alterations in plasma levels of active ghrelin in young (3-month-old) or aged (24-month-old) rats at 4 h after normal saline (Vehicle) or LPS (LPS) injection. Data are presented as means ± SE (n=5–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Vehicle group; #P < 0.05 versus Young Vehicle group.
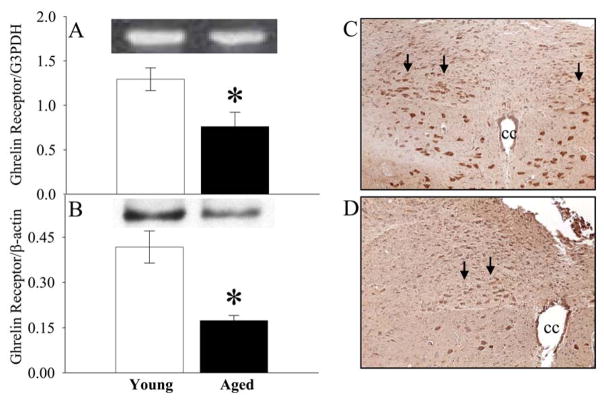
Aging reduces ghrelin receptor expression and neuronal activity in the parasympathostimulatory nuclei of the brain stem in normal animals
To determine whether aging is associated with decreased brain ghrelin receptor expression and neuronal activity, the dorsal vagal complex (DVC) was isolated from the brain stem of aged and young rats. The DVC contains several nuclei involving in parasympathetic activity (i.e., DMN: dorsal motor nucleus of the vagus; NTS: nucleus tractus solitarius; and AP: area postrema), which control the vagus nerve efferent output. As shown in Figures 5A–B, both the gene expression and protein levels of the ghrelin receptor were significantly decreased in the DVC in aged as compared to young rats. Immunohistochemistry showed that c-fos staining in the DMN is decreased in aged rats (Fig. 5D, indicated by arrows) vs. young rats (Fig. 5C). Thus, aging is accompanied by reduction in both brain ghrelin receptor expression and neuronal activity in the parasympathostimulatory nuclei under normal conditions.
Figure 5.
Alterations in gene expression (A) and protein levels (B) of GHSR1a (i.e., ghrelin receptor) in the dorsal vagal complex (DVC) of normal young (3-month-old) or aged (24-month-old) rats. Representative blots are presented. Data are presented as means ± SE (n=5–6) and compared by one-way ANOVA and Student-Newman-Keuls test: * P<0.05 versus Young group. Immunohistochemical stainings of c-fos in the dorsal motor nucleus of the vagus (DMN) in normal young (C) or aged rats (D). cc: central canal. Original magnification, × 200.
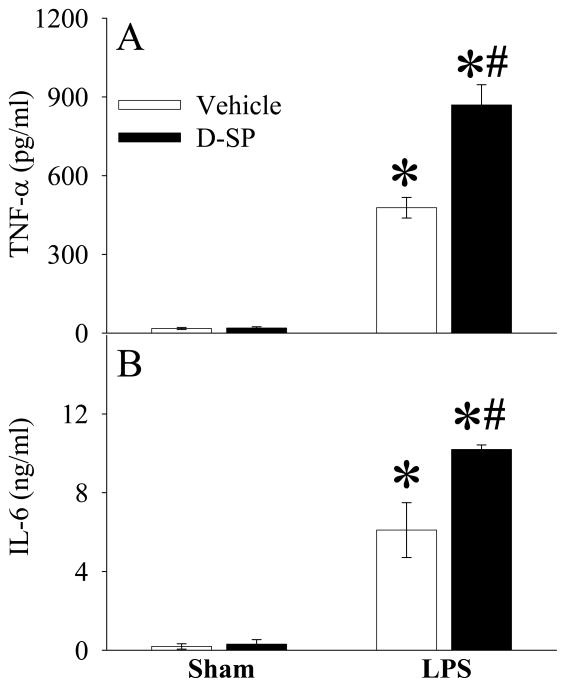
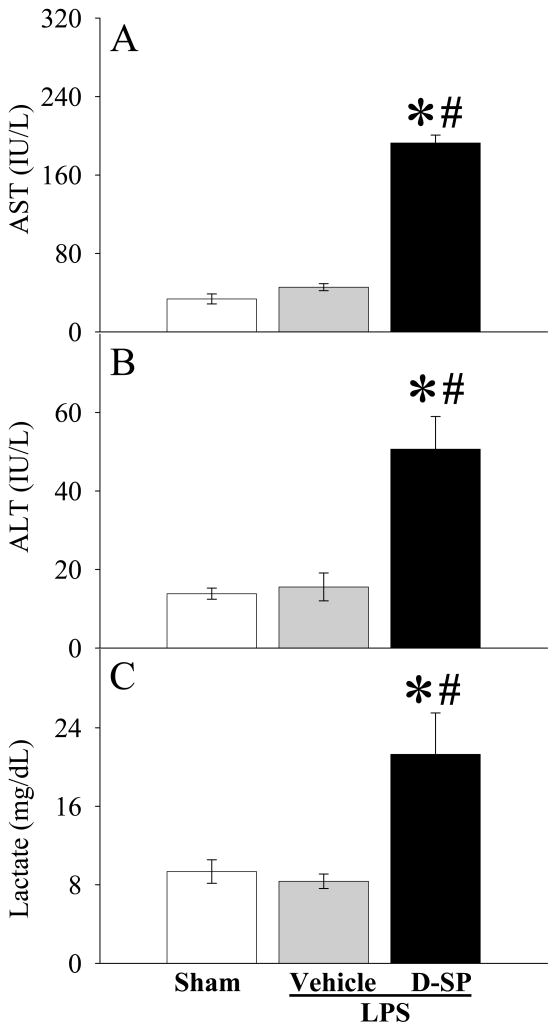
Ghrelin receptor blockade exacerbates inflammatory responses and organ injury in young rats after LPS injection
To further define the role of ghrelin receptor downregulation in producing hyperinflammatory responses during the aging process, a specific ghrelin receptor antagonist, [D-Arg1 D-Phe5 D-Trp7,9 Leu11]-substance P34, was administered to young animals before LPS injection. As shown in Figures 6A–B, ghrelin receptor blockade further upregulated LPS-induced TNF-α and IL-6 production in young animals. Similarly, ghrelin receptor blockade further increased plasma levels of AST (P<0.05, Fig. 7A), ALT (P<0.05, Fig. 7B) and lactate (P<0.05, Fig. 7C) after LPS injection in young animals.
Figure 6.
Alterations in plasma levels of TNF-α (A) and IL-6 (B) in young (3-month-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal saline (Vehicle) or [D-Arg1 D-Phe5 D-Trp7,9 Leu11]-substance P (D-SP). Data are presented as means ± SE (n=3–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus LPS Vehicle group.
Figure 7.
Alterations in plasma levels of AST (A), ALT (B), and Lactate (C) in young (3-month-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal saline (Vehicle) or [D-Arg1 D-Phe5 D-Trp7,9 Leu11]-substance P (D-SP). Data are presented as means ± SE (n=3–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus LPS Vehicle group.
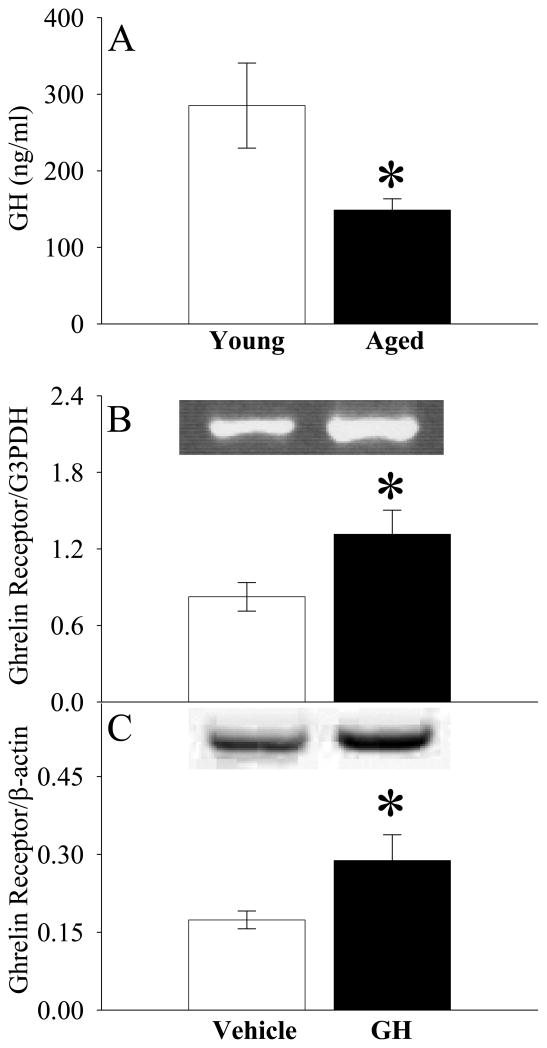
Growth hormone (GH) sensitizes ghrelin’s activity by increasing the central ghrelin receptor expression in aged animals
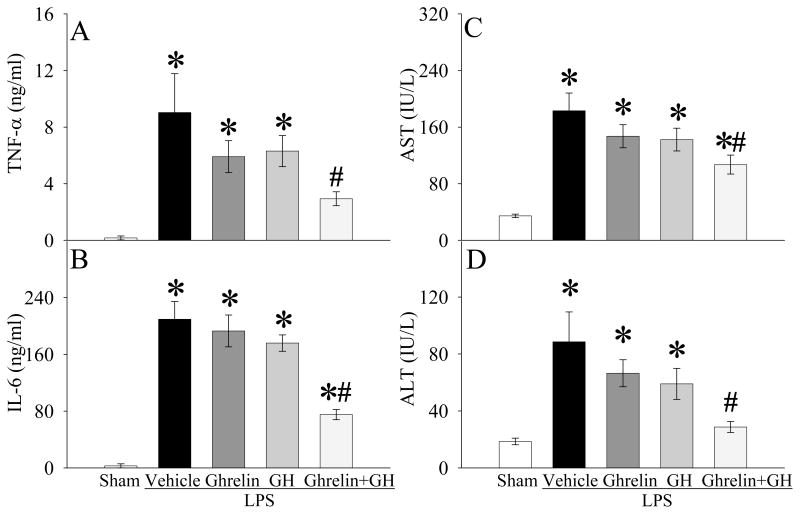
As indicated in Figure 8A, normal plasma GH levels were 48% lower in aged rats than young rats (P<0.05). Interesting, GH administration increased ghrelin receptor gene expression and protein levels in the DVC of aged rats by 59% and 66%, respectively (P<0.05, Figs. 8B–C). To determine whether GH sensitizes ghrelin’s anti-inflammatory effects, we administered GH alone, ghrelin alone, or GH and ghrelin in combination at 30 min prior to LPS injection in aged rats. As shown in Figures 9A–B, neither ghrelin alone nor GH alone significantly decreased plasma levels of TNF-α and IL-6 at 4 h after LPS injection. In contrast, co-administration of ghrelin and GH markedly reduced TNF-α and IL-6 levels (P<0.05, Figs. 9A–B). Similarly, organ injury indicators (i.e., AST and ALT) were attenuated only following co-administration of ghrelin and GH after LPS injection in aged animals (P<0.05, Figs. 9C–D). Regarding the neuronal activity, c-fos expression in the DMN markedly decreased (Fig. 10B, indicated by arrows) as compared to the sham control (Fig. 10A) at 4 h after LPS injection in aged rats. Ghrelin alone increased c-fos expression to a certain degree (Fig. 10C). Similarly, GH also increased c-fos expression in LPS-injected rats (Fig. 10D). Co-administration of GH and ghrelin after LPS injection, however, significantly upregulated DMN’s c-fos expression (Fig. 10E), suggesting a synergistic effect of ghrelin and GH in aged animals.
Figure 8.
Differences in plasma levels of growth hormone (GH) between normal young (3-month-old) and aged (24-month-old) rats (A) and alterations in gene expression (B) and protein levels (C) of GHSR1a (i.e., ghrelin receptor) in the dorsal vagal complex (DVC) of aged (24-month-old) rats treated with normal saline (Vehicle) or growth hormone (GH). Representative blots are presented. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls test: * P<0.05 versus Young group or Vehicle group.
Figure 9.
Alterations in plasma levels of TNF-α (A), IL-6 (B), AST (C), and ALT (D) in aged (24-month-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal saline (Vehicle), ghrelin (Ghrelin), growth hormone (GH), or ghrelin and growth hormone in combination (Ghrelin+GH). Data are presented as means ± SE (n=4–6/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus Sham group; #P < 0.05 versus LPS Vehicle group.
Figure 10.
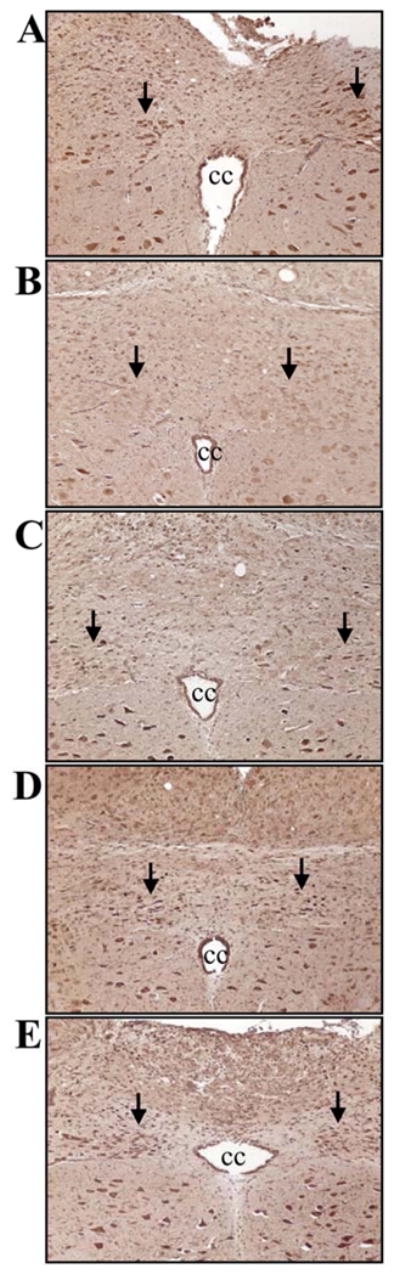
Immunohistochemical stainings of c-fos in the dorsal motor nucleus of the vagus (DMN) in aged (24-month-old) rats at 4 h after normal saline (A) or LPS injection treated with normal saline (B), ghrelin (C), growth hormone (D), or ghrelin and growth hormone in combination (E). cc: central canal. Original magnification, × 200.
DISCUSSION
Sepsis is particularly a serious problem in the geriatric population. The incidence of sepsis increases from <0.05% in 20–29 year-old to 1.0–1.5% in 70–79 year-old and up to 2.6% in >85-year-old persons.1 Nearly 60% of all cases of sepsis occur in patients older than 65 years of age, and 80% of septic deaths occur in this population.1,2,35 The mortality rate also significantly increases with age, from 10–15% in patients 20–29 year-old to 38.4% in those over 85-year-old.1 Although it is assumed that the increased incidence and mortality rate of sepsis in the elderly is the result of the impaired immune response, the effects of aging on the immune system are not well understood. Here, we showed that while LPS-induced release of proinflammatory cytokines is lower in macrophages isolated from aged than young rats, LPS injection causes an in vivo hyperinflammatory state in aged animals, characterized by further increased proinflammatory cytokines, and more severe tissue injury. Those “conflicting” findings reflect the complexity of the aging process and its influence on inflammation and inflammatory mediators. The reduced proinflammatory cytokine release under in vitro conditions might be the result of the low toll like receptor 4 (TLR4) gene expression in macrophages of aged mice, rendering the macrophage less responsive to LPS.36 For the in vivo hyperinflammatory response to LPS in aged animals, it is possible that different cells may be responsible for cytokine production. However, we and others have shown that reduction of the number of macrophages (including Kupffer cells) prior to the insult significantly decreases plasma levels of proinflammatory cytokines.37–40 In this regard, we speculate that the regulation of cytokine production appears to be impaired in aged animals, leading to the unrestricted proinflammatory cytokine production.
Activation of the vagus nerve during systemic stress confers a protective advantage to the host by restraining a potentially adverse peripheral immune response.41 An underlying defect in the activity of the cholinergic anti-inflammatory pathway may trigger an overwhelming and self-destructive immune response to an otherwise innocuous immunological stimulus and thus contribute to disease pathogenesis.41,42 Although it is not currently known whether vagus nerve activity in the heart correlates with the activation of the cholinergic anti-inflammatory pathway, there have been reports of decreased cardiac vagus nerve activity in diseases associated with exaggerated inflammatory responses.41 Clinical observations implicate a significant correlation between decreased instantaneous heart rate variability and increased morbidity and/or mortality in sepsis, rheumatoid arthritis, lupus, sarcoidosis, inflammatory bowel diseases, and trauma.43–46 In the current study, we showed that parasympathostimulatory neuronal activity in the DMN decreased in aged rats, as demonstrated by the reduced c-fos expression. Thus, the impaired cholinergic anti-inflammatory pathway may be responsible for the increased morbidity and mortality in sepsis in elderly patients.
Interestingly, the reduced parasympathostimulatory neuronal activity is associated with the downregulated ghrelin receptor expression in the DVC in aged rats. Administration of a specific ghrelin receptor antagonist further increases proinflammatory cytokines and worsens tissue injury in young rats after LPS injection. Ghrelin is an orexigenic hormone, and can stimulate the vagus nerve through central ghrelin receptors.19–21 Despite some controversial reports47,48, evidence has indicated that under normal conditions, gastric ghrelin gene expression and its levels in the stomach and plasma increase during the aging process.49,50 Moreover, aging is associated with reduced responsiveness to ghrelin stimulation.51 In addition to the downregulated ghrelin receptor expression, we showed that although the basal levels of plasma ghrelin are much higher in aged rats, ghrelin is reduced to a much greater extent after endotoxemia compared to young rats. Thus, reduction of the central ghrelin signals occurs during the aging process, which may in turn impair the cholinergic anti-inflammatory pathway.
Our recent study has indicated that administration of ghrelin in sepsis improves cardiovascular function, attenuates organ injury, and reduces mortality in young animals.14,52 Ghrelin’s beneficial effect in sepsis in young animals is associated with its anti-inflammatory properties requiring the intact vagus nerve.26 The beneficial effect of ghrelin has been confirmed by others in a rat model of endotoxemia.53 As indicated in this study, however, administration of ghrelin failed to protect aged rats with endotoxemia. On the other hand, a low dose of GH upregulates ghrelin receptor and c-fos expression in the DVC in aged animals. Moreover, treatment with ghrelin and GH in combination significantly attenuates proinflammatory cytokines and organ injury after LPS injection in aged animals, suggesting GH sensitizes ghrelin responsiveness under such conditions. The decline in GH with aging is directly associated with many symptoms of aging including wrinkles, grey hair, hair loss, decreased energy, decreased sexual function, loss of muscle mass, increase in body fat, depression, cardiovascular disease, osteoporosis, and overall lower life expectancy.28 To the best of our knowledge, our current study is the first one to link the age-related decline in GH with the increased incidence and mortality rate of sepsis in the elderly.
Human GH is widely used as an anti-aging therapy, although its use for this purpose has not been approved by the U.S. Food and Drug Administration and its distribution as an anti-aging agent is illegal in the United States.54 Randomized, controlled trials evaluating GH therapy in the healthy elderly suggest that it is associated with small changes in body composition and increased rates of adverse events.29,54 Although it cannot be recommended as an anti-aging therapy, our current study suggests that a low dose of GH may be used as a ghrelin sensitizing agent for sepsis treatment in the geriatric population. A recent clinical trail has shown that large doses of GH (i.e., 100 μg/kg BW daily for up to 21 days; maximum dosage at 2,100 μg/kg BW) are not beneficial for critically ill adults.55 Since high levels of GH (>200 fold higher than the normal value) have been shown to decrease ghrelin receptor expression in the pituitary in young rats56, a reduced central responsiveness to ghrelin in those patients may contribute to the failure of the previous clinical trial.55 Moreover, the use of GH alone instead of both GH and ghrelin may also play a role in the failure. The dosage of ghrelin used in this study was based on our previous experience in rat models of sepsis 13 and gut ischemia reperfusion injury.27 The dosage of GH used in this study was based on our preliminary study in which 5 to 10 nM GH significantly increased ghrelin receptor expression in differentiated neuroblastoma SH-SY5Y cells. Most importantly, this dose of GH significantly elevated ghrelin receptor expression in the DVC of aged rats. Nevertheless, a dose response curve of GH and ghrelin will be determined in our future studies.
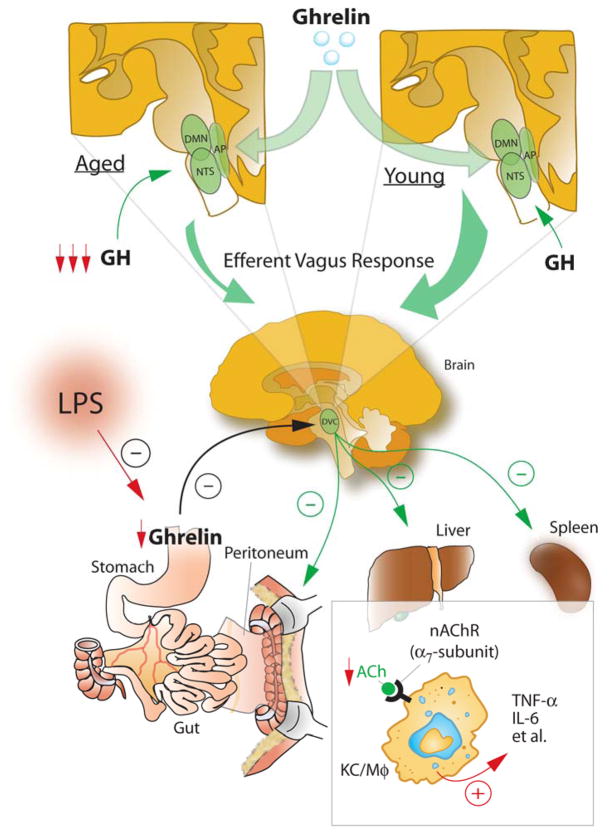
As summarized in Figure 11, we have shown that aging-related hyperinflammation is associated with central hyporesponsiveness to ghrelin, reducing parasympathostimulatory neuronal activity in the DVC of the brain stem. This hyporesponsiveness is caused by ghrelin receptor downregulation. Plasma levels of ghrelin are reduced to a much greater extent in aged rats with endotoxemia. Administration of a specific ghrelin receptor antagonist further increases cytokines and worsens tissue injury in young rats with endotoxemia. Moreover, aged rats are associated with reduced GH release. A low dose of GH upregulates ghrelin receptor expression in the DVC in aged rats. Treatment with ghrelin and GH reduces cytokine release and attenuates tissue injury in aged rats. Please note that the main purpose of this study was to explore the molecular mechanisms of aging-related alterations in systemic inflammation. Endotoxemia is a valuable animal model in elucidating pathophysiologic mechanisms responsible for cell and organ dysfunction observed in sepsis. As such, the rat model of endotoxemia was used in this study. However, the therapeutic potential and the optimal dose of GH and ghrelin in sepsis in aging will be evaluated in more clinically relevant models of sepsis such as cecal ligation and puncture in our future studies. In conclusion, the reduced central (brain) responsiveness to ghrelin due to the decreased GH, plays a major role in producing the hyperinflammatory state, resulting in severe organ injuries and high mortality after endotoxemia in aged animals. Administration of ghrelin and GH in combination may be a novel approach to reduce sepsis-induced lethality in the geriatric population.
Figure 11.
Balanced inflammatory responses are essential elements of a successful host response after injury. However, excessive and sustained inflammatory responses can lead to severe tissue damage. Stimulation of the vagus nerve can rapidly attenuate systemic inflammatory responses through inhibiting the activation of Kupffer cells (KC)/macrophages (Mφ). This physiological mechanism, termed ‘the cholinergic anti-inflammatory pathway’, can reflexively monitor and adjust the inflammatory response to prevent excessive inflammation. The majority of efferent vagus nerve fibers originates from the dorsal motor nucleus (DMN) of the vagus within the dorsal vagal complex (DVC) of the brain stem. The DVC consists of the DMN, the nucleus tractus solitarius (NTS), and the area postrema (AP), being parasympathostimulatory. In aged animals, the reduced responsiveness to ghrelin in the DVC due to decreased GH impairs the efficacy of the cholinergic anti-inflammatory pathway, thereby leading to the hyperinflammatory state and subsequent local and remote organ injuries. (ACh: acetylcholine; nAChR: nicotinic acetylcholine receptor; TNF-α: tumor necrosis factor-α, IL: interleukin)
Acknowledgments
This study was supported by National Institutes of Health grants R01 AG028352 and R01 GM053008 (PW). Dr. Rongqian Wu was supported by a Postdoctoral Fellowship from the American Heart Association (the Heritage Affiliate) #0325802T.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 3.Floto RA, Smith KG. The vagus nerve, macrophages, and nicotine. Lancet. 2003;361:1069–1070. doi: 10.1016/S0140-6736(03)12902-9. [DOI] [PubMed] [Google Scholar]
- 4.Pavlov VA, Wang H, Czura CJ, et al. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 6.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 7.Borovikova LV, Ivanova S, Nardi D, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 10.Arvat E, Di Vito L, Broglio F, et al. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–495. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- 11.Cowley MA, Grove KL. Ghrelin--Satisfying a Hunger for the Mechanism. Endocrinol. 2004;145:2604–2606. doi: 10.1210/en.2004-0346. [DOI] [PubMed] [Google Scholar]
- 12.Wu JT, Kral JG. Ghrelin: integrative neuroendocrine peptide in health and disease. Ann Surg. 2004;239:464–474. doi: 10.1097/01.sla.0000118561.54919.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu R, Zhou M, Das P, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–E1702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 14.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks WA, Tschop M, Robinson SM, et al. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Zhou M, Cui X, et al. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. Int J Mol Med. 2003;12:777–781. [PubMed] [Google Scholar]
- 17.Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 18.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 19.Date Y, Murakami N, Kojima M, et al. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem Biophys Res Commun. 2000;275:477–480. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- 20.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 21.Date Y, Nakazato M, Murakami N, et al. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 22.Shuto Y, Shibasaki T, Wada K, et al. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci. 2001;68:991–996. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 23.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Matsumura K, Fukuhara M, et al. Ghrelin acts at the nucleus of the solitary tract to decrease arterial pressure in rats. Hypertension. 2004;43:977–82. doi: 10.1161/01.HYP.0000122803.91559.55. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Lin TR, Hu Y, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu R, Dong W, Cui X, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Dong W, Ji Y, et al. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE. 2008;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherlock M, Toogood AA. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007;10:189–203. doi: 10.1007/s11102-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 29.Nass R, Park J, Thorner MO. Growth hormone supplementation in the elderly. Endocrinol Metab Clin North Am. 2007;36:233–245. doi: 10.1016/j.ecl.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Zhou M, Wang P. Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells. Regul Pept. 2003;112:19–26. doi: 10.1016/s0167-0115(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya N, Kojima M, Uematsu M, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1483–R1487. doi: 10.1152/ajpregu.2001.280.5.R1483. [DOI] [PubMed] [Google Scholar]
- 32.Tso JY, Sun XH, Kao TH, et al. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- 34.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw M, Rockwell J, Engleman C, et al. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 37.Koo DJ, Chaudry IH, Wang P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J Surg Res. 1999;83:151–157. doi: 10.1006/jsre.1999.5584. [DOI] [PubMed] [Google Scholar]
- 38.Luster MI, Germolec DR, Yoshida T, et al. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480–488. [PubMed] [Google Scholar]
- 39.Motoyama K, Kamei T, Nakafusa Y, et al. Donor treatment with gadolinium chloride improves survival after transplantation of cold-stored livers by reducing Kupffer cell tumor necrosis factor production in rats. Transplant Proc. 1995;27:762–764. [PubMed] [Google Scholar]
- 40.Ikejima K, Enomoto N, Iimuro Y, et al. Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol. 1998;274:G669–G676. doi: 10.1152/ajpgi.1998.274.4.G669. [DOI] [PubMed] [Google Scholar]
- 41.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tracey KJ. Fat meets the cholinergic antiinflammatory pathway. J Exp Med. 2005;202:1017–1021. doi: 10.1084/jem.20051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evrengul H, Dursunoglu D, Cobankara V, et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol Int. 2004;24:198–202. doi: 10.1007/s00296-003-0357-5. [DOI] [PubMed] [Google Scholar]
- 44.Lindgren S, Stewenius J, Sjolund K, et al. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand J Gastroenterol. 1993;28:638–642. doi: 10.3109/00365529309096103. [DOI] [PubMed] [Google Scholar]
- 45.Laversuch CJ, Seo H, Modarres H, et al. Reduction in heart rate variability in patients with systemic lupus erythematosus. J Rheumatol. 1997;24:1540–1544. [PubMed] [Google Scholar]
- 46.Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med. 2007;14:392–397. doi: 10.1197/j.aem.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Rigamonti AE, Pincelli AI, Corra B, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–R5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- 48.Sturm K, MacIntosh CG, Parker BA, et al. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J Clin Endocrinol Metab. 2003;88:3747–3755. doi: 10.1210/jc.2002-021656. [DOI] [PubMed] [Google Scholar]
- 49.Englander EW, Gomez GA, Greeley GH., Jr Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech Ageing Dev. 2004;125:871–875. doi: 10.1016/j.mad.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Qi X, Reed J, Englander EW, et al. Evidence that growth hormone exerts a feedback effect on stomach ghrelin production and secretion. Exp Biol Med (Maywood) 2003;228:1028–1032. doi: 10.1177/153537020322800907. [DOI] [PubMed] [Google Scholar]
- 51.Broglio F, Benso A, Castiglioni C, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537–1542. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 52.Wu R, Dong W, Zhou M, et al. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Chang L, Zhao J, Yang J, et al. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur J Pharmacol. 2003;473:171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 55.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 56.Nass R, Gilrain J, Anderson S, et al. High plasma growth hormone (GH) levels inhibit expression of GH secretagogue receptor messenger ribonucleic acid levels in the rat pituitary. Endocrinol. 2000;141:2084–2089. doi: 10.1210/endo.141.6.7503. [DOI] [PubMed] [Google Scholar]