Abstract
Rheumatoid arthritis (RA) is a chronic debilitating autoimmune disease that results in inflammation and structural destruction of the joints. A hallmark of RA pathogenesis is an imbalance of the osteoblast–osteoclast axis driven by inflammatory processes, resulting in elevated bone resorption by osteoclasts. Current therapies used to treat this disease have focused on inhibition of synovitis, but such treatments do not adequately repair damaged bone. A key pathway of osteoclast formation involves the receptor activator of NF-κB ligand pathway acting on myeloid progenitor cells. The Wnt pathway has been shown to be important for the differentiation of osteoblasts from mesenchymal lineage precursors, and endogenous Wnt inhibitors such as Dickkopf1 and sclerostin might have important roles in osteoclast dysregulation in RA. Inhibition of the receptor activator of NF-κB ligand pathway, or blockade of Dickkopf1 and sclerostin, might serve to restore the osteoblast–osteoclast balance and repair bone erosion in RA joints. Such treatments, in combination with anti-inflammatory therapies, could stabilize and repair damaged joints and have the potential to be valuable additions to the armory of RA treatments.
Background
In addition to inflammation of the synovium, a major clinical manifestation of rheumatoid arthritis (RA) is the progressive destruction of bone and cartilage structures in the joints of patients, leading to radiographically defined features such as bone erosion and joint space narrowing. Typical pathogenic processes in the synovium of patients with RA include a thickened hyperplastic synovial layer, neoangiogenesis, and ongoing migration of macrophages and autoreactive lymphocytes into the joints resulting from the action of chemokines and proinflammatory cytokines 1. These inflammatory processes have been intensively studied, and are known to activate bone and cartilage destruction via the action of tumor necrosis factor (TNF) and receptor activator of NF-κB ligand (RANKL)-mediated signaling, amongst other signaling pathways, on bone resorptive osteoclast cell formation and activation of synovial fibroblasts 2. Therapies used in the clinic for the successful treatment of RA target various aspects of these inflammatory pathways (e.g. corticosteroids, methotrexate, anti-TNF agents, interleukin [IL]-1β and IL-6 pathway blockade, B-cell depletion via targeting of CD20, and blockade of lymphocyte co-stimulation via cytotoxic T lymphocyte antigen 4 1,3,4,5,6,7,8,9). It is also noteworthy that bisphosphonates are also used to target bone destruction in RA resulting from inflammatory processes as well as from commonly used anti-inflammatory treatments, particularly glucocorticoids. These are pyrophosphate analogues that target osteoclasts, and are currently considered the standard of care for reducing bone loss in postmenopausal osteoporosis. Although anecdotal reports of bisphosphonate use in RA patients are widespread, there is a dearth of appropriately designed clinical trials that specifically investigate the effects of bisphosphonates in RA 10,11. Therefore, while most of these therapies have been shown to slow progressive joint damage as determined by X-ray imaging 5,9,12,13, not all patients respond robustly to these therapies with respect to bone erosion. Hence, in addition to targeting synovitis, it is highly desirable to find mechanisms to stop and ultimately reverse bone erosion in RA.
The normal mechanism by which bones are formed and resorbed is mediated via the interaction between two cell lineages, bone-forming osteoblasts and bone-resorbing osteoclasts (Figure 1). Osteoblasts differentiate from the mesenchymal cell lineage under the control of key signals such as parathyroid hormone, the canonical Wnt–β catenin pathway and the bone morphogenetic protein (BMP) pathway 14,15. These signaling pathways produce key bone matrix products that are subsequently mineralized. Osteoclasts differentiate from myeloid lineage precursors under the control of the key pathways involving colony stimulating factor 1 and the RANKL–RANK axis, which act at early and terminal differentiation stages respectively 2,16,17. These cells degrade bone via expression of effector molecules such as cathepsins, matrix metalloproteinases, and local production of hydrogen ions. Thus, these two types of effector cells, derived from independent precursor lineages and with opposing functions, act in concert to maintain normal bone metabolism. Cross regulation of these cell types can occur; for example, the RANKL decoy receptor osteoprotegerin (OPG) is expressed by osteoblasts, induced by Wnt signaling 18, and acts to repress the osteoclast axis by regulating RANK signaling. Much effort has been dedicated to the study of these cell lineages given their important contribution to human diseases such as osteoporosis, osteoarthritis, ankylosing spondylitis and RA 2,19,20.
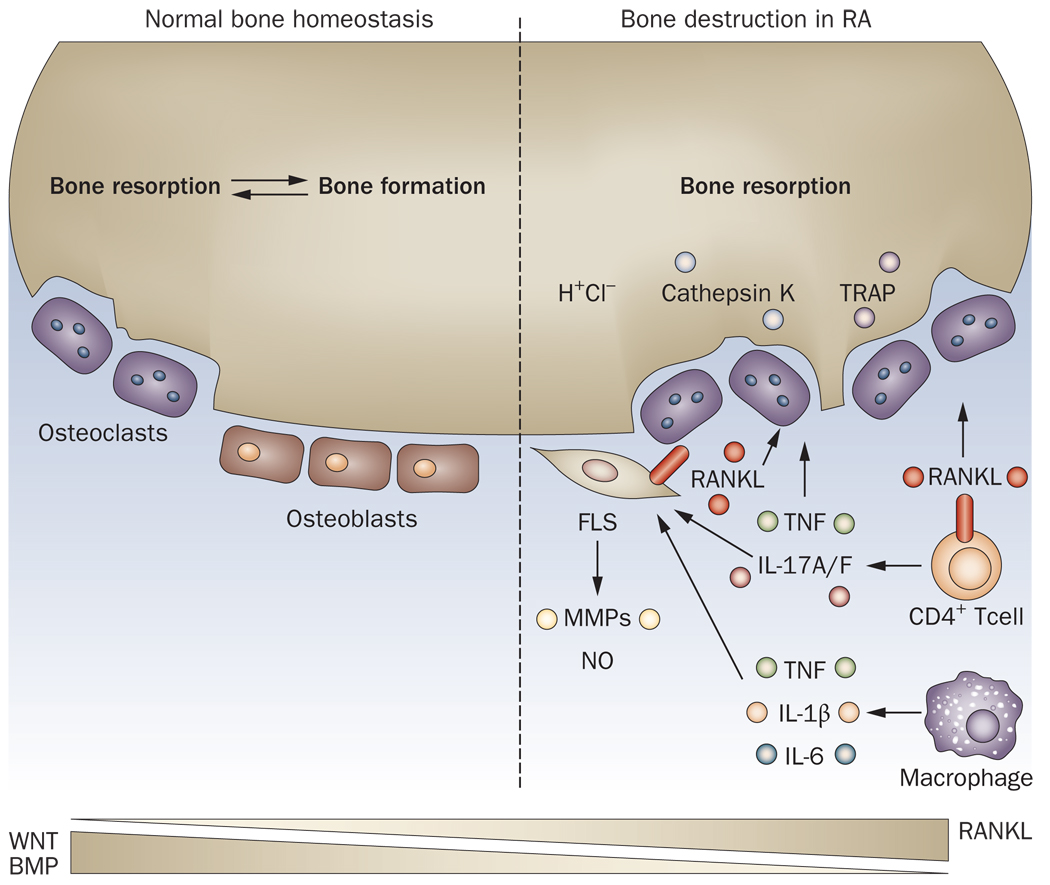
Figure 1. Bone homeostasis in healthy and RA joints.
In normal joints, bone formation and bone resorption are maintained by the balanced function of osteoblasts and osteoclasts. The molecular basis of this homeostasis is controlled in part by the opposing actions of Wnt and BMP pathways on osteoblasts and the RANKL pathway on osteoclasts. Under the inflammatory conditions of RA, activity of infiltrating macrophages and CD4+ T cells results in expression of proinflammatory cytokines such as TNF that drive osteoclast formation via induction of RANKL in the synovium. In addition, RANKL is expressed on synovial fibroblasts and infiltrating T cells. The resulting osteoclasts, and associated local production of H+ ions and cathepsin K, result in increased bone resorption and joint destruction.
BMP, bone morphogenetic protein; FLS, fibroblast-like synoviocyte; IL, interleukin; MMPs, matrix metalloproteinases; NO, nitric oxide; RA, rheumatoid arthritis; RANKL, Receptor activator of NF-κB Ligand; TNF, tumor necrosis factor; TRAP, tartrate-resistant acid phosphastase
In the case of RA, the osteoblast–osteoclast axis is severely disrupted due to ongoing inflammatory processes resulting in enhanced osteoclast function 21,22. Key macrophage-derived and T cell-derived proinflammatory cytokines such as TNF, IL-1β, IL-6 and IL-17 act in a pleiotropic network to induce expression of RANKL by synovial fibroblasts, osteoblasts and bone marrow stromal cells leading to enhanced osteoclast differentiation 23,24,25,26,27. In addition, RANKL is expressed by activated CD4+ T cells infiltrating the synovium, providing other cellular sources of this osteoclast-generating protein under inflammatory conditions 23,28,29. TNF also acts directly on osteoclast formation via signaling through TNFRSF1A (tumor necrosis factor receptor superfamily, member 1A), and IL-1βcan upregulate the expression of RANK on osteoclast progenitors 30,31. Increased signaling through RANK on differentiating osteoclasts results in recruitment of TNF receptor-associated factor 6 and subsequent activation of the transcription factor NFATc1 (nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 1), which is a master regulator of osteoclastogenesis 32,33. This inflammation-driven activation of the RANKL–RANK pathway increases osteoclast levels in the joints and thus drives bone destruction in RA.
Perturbations to the osteoblast axis have also been implicated in RA pathogenesis because of the lack of bone repair activities after formation of erosions in this disease. Indeed, a recent murine arthritis study showed that inflammation within arthritic bone resulted in a decrease in mature osteoblast lineage cells and concomitant bone formation 34. The BMP and canonical Wnt–β catenin pathways have been implicated in osteoblast function and bone formation 35,36,37. BMPs signal through heteromeric BMP type I and type-II serine-threonine kinase receptor complexes, and activate downstream osteoblast transcriptional pathways via phosphorylation of receptor-regulated Smad complexes 15,37. Wnt proteins bind to the membrane receptors low density lipoprotein receptor-related protein (LRP)5 and LRP6 together with the coreceptor frizzled (Figure 2). Formation of this transmembrane complex results in activation of an intracellular signaling pathway that releases β-catenin, via changes in its phosphorylation status, from a destruction complex comprising the proteins axin, glycogen synthase kinase 3 (GSK3) and adenomatosis polyposis coli (APC) via activation of disheveled (DVL). β-catenin accumulates in the cell and translocates to the nucleus, where it induces expression of target genes including alkaline phosphatase and CCN family genes via the β-catenin–T cell factor/lymphoid enhancer binding factor 1 complex and drives differentiation of mesenchymal cells into osteoblasts 18,38,39,40.
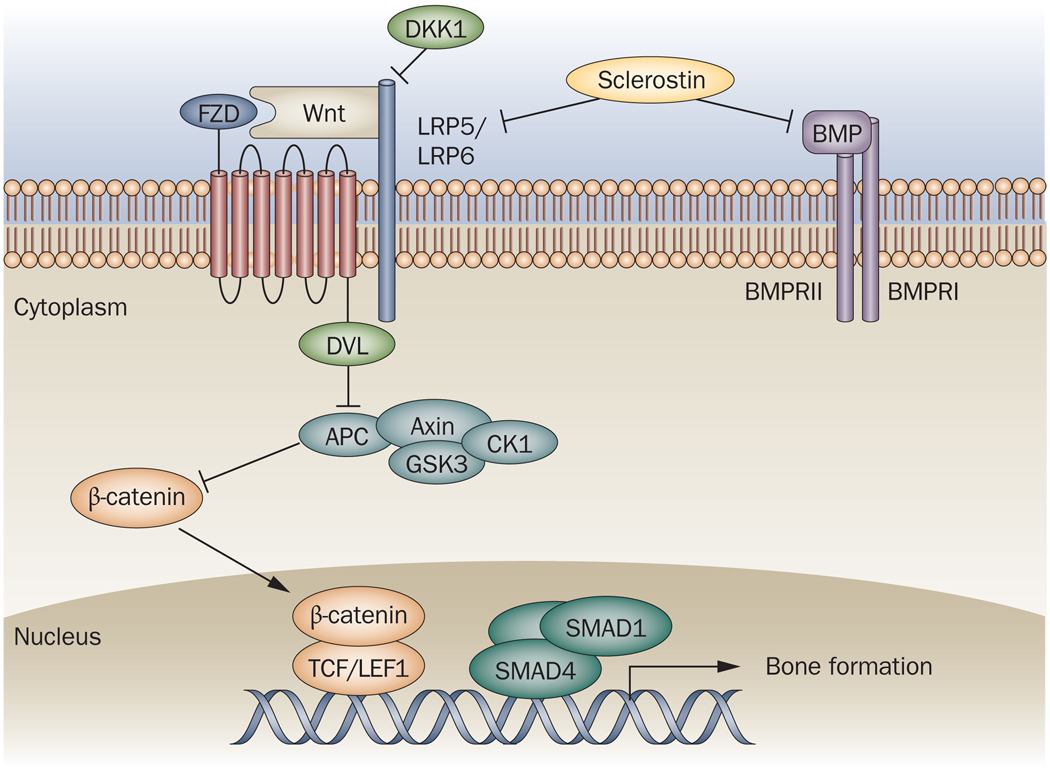
Figure 2. Osteoblast differentiation pathways.
Osteoblast formation from mesenchymal progenitors is controlled by the canonical Wnt–β catenin and the BMP pathways. Engagement of plasma membrane Wnt receptors LRP5 and LRP6 and the coreceptor FZD by Wnt leads to activation of DVL, which blocks a protein complex comprising axin, APC and GSK3. This allows the translocation of β-catenin to the nucleus, where it complexes with TCF/LEF1 and binds DNA. In addition, binding of BMP to its membrane receptor complex activates a signaling cascade involving heteromeric SMAD protein complexes, which translocate to the nucleus and bind DNA. Both of these transcriptional complexes then drive osteoblastogenic gene expression programs. DKK1 and sclerostin are endogenous proteins that inhibit osteoblast differentiation by binding to the LRP receptors and blocking Wnt docking. In addition, sclerostin binds BMPs and so can potentially block this pathway too.
APC, adenomatosis polyposis coli; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; CK1, casein kinase 1; DKK1, Dickkopf1; DVL, human homolog of the Drosophila dishevelled gene; FZD, frizzled; GSK3, glycogen synthase kinase 3; LRP, LDL receptor-related protein; SMAD, TCF/LEF1, T cell factor/lymphoid enhancer binding factor 1.
Importantly, there are several endogenous inhibitors of the canonical Wnt pathway that regulate osteoblast function. Dickkopf1 (DKK1), a member of a family of cysteine-rich proteins, functions as an inhibitor of Wnt signaling via competitive binding to the LRP5/LRP6 receptor complex, and by recruiting kringle containing transmembrane protein 1, a negative coreceptor 41,42. Levels of DKK1 are increased in both experimental arthritis and in human RA synovial tissue and serum, and expression of DKK1 is induced by TNF signaling 43. Another protein of interest, sclerostin, is a secreted glycoprotein of the evolutionary conserved differential screening-selected gene aberrant in neuroblastoma family of proteins containing a cysteine knot motif, and has been implicated as an inhibitor of both the Wnt and BMP pathways driving osteoblast formation 44,45. Unlike DKK1 and other Wnt antagonists, sclerostin is unique in that it is produced by osteocytes and so is an endogenous mediator in the bone formation/destruction axis 46. Sclerostin binds to BMPs as well as to LRP5/LRP6, thus antagonizing BMP and Wnt signaling and hence osteoblast formation 47,48,49. Inherited genetic mutation of the SOST gene (which encodes sclerostin in man) results in sclerosteosis, a progressive bone dysplasia disorder 50.
Given the important roles of the RANKL–RANK pathway in bone resorption, and the Wnt and BMP pathways in bone formation and repair, strategies targeting both RANK signaling as well as targeting antagonists of the Wnt and BMP pathways, such as DKK1 and sclerostin, might provide avenues both to inhibit bone destruction and potentially to repair erosions in RA.
Pre-clinical data
Inhibition of RANK signaling has been shown to be beneficial in several animal models of arthritis. In a TNF-induced model of arthritis, inflammation and bone erosion could be blocked by simultaneous treatment with anti-TNF and the RANKL decoy receptor OPG versus anti-TNF alone 51; OPG treatment alone slowed joint destruction, but did not affect inflammation 52,53. In a rat adjuvant-induced arthritis model, treatment with OPG at time of disease onset resulted in decreased bone erosion, but did not affect inflammatory progression 28. Using the K/BxN mouse model of serum transferred arthritis, RANKL-deficient mice were protected against bone loss but not inflammation 54. Similarly, in a rat adjuvant-induced arthritis model, treatment with OPG resulted in decreased loss of bone mineral density, but did not affect inflammation 55. Blockade of intracellular signaling pathways downstream of RANK also prevented bone loss or destruction in inflammation-induced bone destruction and ovariectomy-induced bone loss mouse models 56. Therefore, inhibition of RANK signaling is beneficial for protection against bone damage in multiple animal arthritis models, but has little benefit in inhibiting the inflammatory axis of the disease.
Blockade of DKK1 using an antibody resulted in a decrease in bone erosion but did not affect inflammatory signs of disease in the TNF-induced mouse arthritis model and the collagen-induced mouse arthritis model 43. Interestingly, blockade of DKK1 also resulted in new bone formation due to formation of osteophytes, indicating elevated differentiation of osteoblasts. In addition, blockade of DKK1 decreased osteoclast numbers in the joints; this finding was ascribed to upregulation of OPG levels via Wnt signaling. Further, targeted deletion of one allele of DKK1 in mice was shown to be sufficient to result in increased osteoblast number and bone formation 57. Importantly, these data suggest that boosting osteoblast function through blockade of Wnt antagonists can overcome and repair ongoing bone destruction in arthritis independent of inflammation.
Targeted deletion of sclerostin in mice results in increased bone volume, bone mineral density and bone strength compared with wild type controls 58. Sclerostin-deficient animals had increased osteoblast activity as well as increased serum levels of osteocalcin, an osteoblast marker, but the osteoclast axis was unaffected. A further study using the ovariectomized rat model of postmenopausal osteoporosis showed that administration of an antibody against sclerostin resulted in increased bone formation on multiple bone surfaces after 5 weeks of treatment 59. Further, antibody-mediated blockade of sclerostin in a murine model of chronic colitis was recently shown to halt inflammation-induced bone loss 60. These data support the notion that sclerostin, like DKK1, is an important antagonist of osteoblast formation and that inhibition of this pathway has potential benefit for boosting bone repair.
Clinical data
A monoclonal antibody against RANKL, denosumab, has been developed and tested in the clinic. The development of this antibody has focused on postmenopausal osteoporosis, and has reported positive outcome data 61. A 12 month placebo-controlled phase 2 study of this antibody was also conducted in RA 62. This study showed a dose responsive effect of denosumab on decrease of MRI-determined erosion scores compared with placebo as early as 6 months after treatment, and both tested doses of denosumab decreased X-ray determined Sharp erosion scores after 12 months of treatment. In addition, RANKL blockade resulted in rapid and sustained suppression of the systemic markers of bone turnover C-telopeptide of type I collagen (CTX-I) and N-propeptide of type I collagen (PINP), but only transiently decreased the cartilage marker C-telopeptide of type II collagen (CTX-II) after 3 months of treatment. Consistent with preclinical observations, denosumab treatment had no effect on measures of RA disease activity, such as components of the American College of Rheumatology (ACR) response, Health Assessment Questionnaire (HAQ) scores or the Disease Activity Score 28 (DAS28), as compared with placebo treated patients, suggesting that it does not substantially impact on the inflammatory component of RA.
Future clinical development
As discussed above, there is considerable evidence that inhibiting osteoclast function via blockade of RANKL, and enhancing osteoblast function via blockade of Wnt/BMP antagonists DKK1 and sclerostin, might have benefits for reducing ongoing bone erosion and repairing existing joint damage; however, blockade of these pathways is not likely to have an effect on inflammation-dependent signs and symptoms of RA such as joint swelling and tenderness. These mechanisms of action raise two implications for clinical development of these potential therapies: first, they will likely have to be administered in combination with well established anti-inflammatory therapies such as methotrexate or TNFα antagonists; second, appropriate endpoints such as radiographic and MRI-defined erosion scores rather than signs and symptoms endpoints such as change in American College of Rheumatology and Disease Activity Score 28 scores must be utilized during clinical trials to determine the contribution of these therapies to meaningful clinical outcomes.
The patient population most likely to benefit from such a therapeutic intervention would also have to be carefully considered. For example, there have been reports of RA patients who are in considered to be in a state of disease remission, but yet have progressive radiographic joint destruction 63. Such patients presumably have an inflammation-independent bone destructive disease manifestation, and might benefit more from alteration of the osteoclast–osteoblast balance rather than from other therapies targeting only the inflammatory cascade.
We need to optimize the therapeutic window for these therapies to avoid adverse effects such as pushing bone resorption or bone formation too far in the opposite direction, resulting in inappropriate bone formation characteristic of anabolic bone disorders (e.g. osteoarthritis and ankylosing spondylitis) and neoplastic transformation resulting from excessive activation of the Wnt or BMP pathways, or both 64,65. The risk of development of oncogenesis caused by long term activation of the Wnt pathway through antagonism of DKK1 or sclerostin must also be considered 66.
Outlook
The progress made over the past few years in defining the molecular basis of control of osteoblast and osteoclast differentiation has presented new opportunities to target bone disorders in the clinic. Since bone destruction and concomitant lack of new bone formation is a major clinical manifestation of RA, therapeutic intervention to reverse these pathogenic processes should have considerable clinical benefit for patients with RA. Such therapies will likely need to be administered in combination with well established and cost effective anti-inflammatory therapies such as methotrexate, and might benefit patients who have clear evidence of bone destruction even though their inflammatory disease is well controlled. Since at present joint destruction in RA is irreversible and highly debilitating, this alternative therapeutic intervention, if successful, would be a major addition to existing anti-inflammatory treatment paradigms for this disease.
KEY POINTS
A hallmark of RA pathogenesis is an imbalance of the osteoblast–osteoclast axis that is driven by inflammatory processes
Current treatments for RA are targeted at synovitis but do not adequately target bone repair
The RANKL pathway is a key driver of osteoclastogenesis, whilst the Wnt and BMP pathways drive osteoblastogenesis
DKK1 and sclerostin have been shown to act as endogeneous inhibitors of the Wnt and BMP axes
Targeting RANKL, DKK1 & scelerostin may have clinical benefit in reducing bone destruction and enhancing repair of erosions
ACKNOWLEDGMENTS
No Genentech acknowledgments
Biographies
Yongwon Choi received his Ph.D. from University of Illinois College of Medicine, Chicago, USA, and carried out postdoctoral research with John Kappler and Philippa Marrack (National Jewish Center, Howard Hughes Medical Institue). He is currently a Professor of Pathology and Laboratory Medicine at the University of Pennsylvania, Philadelphia, USA.
Joseph Arron earned a PhD in immunology from The Rockefeller University in 2002 and an MD from Cornell University in 2003. After a postdoctoral fellowship at Stanford University, he started at Genentech in 2006. He directs a laboratory focusing on molecular and clinical heterogeneity and biomarker discovery in allergic and inflammatory diseases.
Michael Townsend gained a PhD in Immunology from the University of Cambridge in 2001 followed by a postdoctoral fellowship at Harvard University. He now directs a laboratory at Genentech that studies disease mechanisms and heterogeneity focused on discovery of biomarkers in autoimmune diseases.
Footnotes
COMPETING INTERESTS
Joseph Arron and Michael Townsend are employees of Genentech.
REVIEW CRITERIA
We searched for articles focusing on bone formation and erosion mechanisms in arthritis in PubMed published by July 2009. The search terms we used alone or in combination were “arthritis”, “rheumatoid arthritis”, “osteoblast”, “osteoclast”, “bone remodelling”, “bone formation” and “bone erosion”. All papers identified were English-language full text papers. We also searched the reference lists of identified articles for further papers.
Contributor Information
Yongwon Choi, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA.
Joseph R. Arron, Immunology, Tissue Growth and Repair Biomarker Group, Genentech Research & Early Development, South San Francisco, CA, USA
Michael J. Townsend, Immunology, Tissue Growth and Repair Biomarker Group, Genentech Research & Early Development, South San Francisco, CA, USA
References
- 1.Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43:2619–2633. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Walsh MC, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 3.Combe B. Progression in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2009;23:59–69. doi: 10.1016/j.berh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Klareskog L, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–710. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SB, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 8.Genovese MC, et al. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis. 2008;67:547–554. doi: 10.1136/ard.2007.074773. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 2000;43:1001–1009. doi: 10.1002/1529-0131(200005)43:5<1001::AID-ANR7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Breuil V, Euller-Ziegler L. Bisphosphonate therapy in rheumatoid arthritis. Joint Bone Spine. 2006;73:349–354. doi: 10.1016/j.jbspin.2005.10.019. doi:S1297-319X(06)00043-1 [pii] 10.1016/j.jbspin.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Romas E. Bone loss in inflammatory arthritis: mechanisms and therapeutic approaches with bisphosphonates. Best Pract Res Clin Rheumatol. 2005;19:1065–1079. doi: 10.1016/j.berh.2005.06.008. doi:S1521-6942(05)00079-3 [pii] 10.1016/j.berh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Lard LR, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med. 2001;111:446–451. doi: 10.1016/s0002-9343(01)00872-5. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann Rheum Dis. 2008;67:1084–1089. doi: 10.1136/ard.2007.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota T, Michigami T, Ozono K. Wnt signaling in bone metabolism. J Bone Miner Metab. 2009;27:265–271. doi: 10.1007/s00774-009-0064-8. doi:10.1007/s00774-009-0064-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. doi:HHV6108EX6P056CA [pii] 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 16.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 17.Suda T, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 18.Glass DA, 2nd, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 20.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 21.Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27:968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- 22.Gravallese EM, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- 23.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47:1635–1640. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 24.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004;63:1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 26.Kotake S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravallese EM, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Kong YY, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam J, et al. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arron JR, et al. A positive regulatory role for Cbl family proteins in tumor necrosis factor-related activation-induced cytokine (trance) and CD40L-mediated Akt activation. J Biol Chem. 2001;276:30011–30017. doi: 10.1074/jbc.M100414200. [DOI] [PubMed] [Google Scholar]
- 33.Kim K, et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005;280:35209–35216. doi: 10.1074/jbc.M505815200. [DOI] [PubMed] [Google Scholar]
- 34.Walsh NC, et al. Osteoblast Function is Compromised at Sites of Focal Bone Erosion in Inflammatory Arthritis. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090320. doi:10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 35.Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. doi:10.1359/jbmr.081235 10.1359/jbmr.081235 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X, et al. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng H, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Miller JR. The Wnts. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3001. REVIEWS3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Si W, et al. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol. 2006;26:2955–2964. doi: 10.1128/MCB.26.8.2955-2964.2006. doi:26/8/2955 [pii] 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 42.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 43.Diarra D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 44.ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am. 2008;90 Suppl 1:31–35. doi: 10.2106/JBJS.G.01183. [DOI] [PubMed] [Google Scholar]
- 45.Lin C, et al. Sclerostin Mediates Bone Response to Mechanical Unloading via Antagonizing Wnt/beta-Catenin Signaling. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090411. doi:10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 46.van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. doi:10.1084/jem.20031454 jem.20031454 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler DG, et al. Sclerostin inhibition of Wnt-3a-induced C3H10T1/2 cell differentiation is indirect and mediated by bone morphogenetic proteins. J Biol Chem. 2005;280:2498–2502. doi: 10.1074/jbc.M400524200. [DOI] [PubMed] [Google Scholar]
- 48.Li X, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 49.Semenov MV, He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281:38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- 50.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 51.Zwerina J, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50:277–290. doi: 10.1002/art.11487. [DOI] [PubMed] [Google Scholar]
- 52.Redlich K, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46:785–792. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- 53.Schett G, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48:2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- 54.Pettit AR, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolon B, Campagnuolo G, Feige U. Duration of bone protection by a single osteoprotegerin injection in rats with adjuvant-induced arthritis. Cell Mol Life Sci. 2002;59:1569–1576. doi: 10.1007/s00018-002-8530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H, et al. Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest. 2009;119:813–825. doi: 10.1172/JCI36809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morvan F, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. doi:10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 58.Li X, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 59.Li X, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 60.Eddleston A, et al. A Short Treatment with an Antibody to Sclerostin can Inhibit Bone Loss in an Ongoing Model of Colitis. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090403. doi:10.1359/jbmr.090403. [DOI] [PubMed] [Google Scholar]
- 61.Miller PD. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7:18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 62.Cohen SB, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 63.Cohen G, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis. 2007;66:358–363. doi: 10.1136/ard.2006.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson JA, Girish G, Jiang Y, Resnick D. Radiographic evaluation of arthritis: inflammatory conditions. Radiology. 2008;248:378–389. doi: 10.1148/radiol.2482062110. doi:248/2/378 [pii] 10.1148/radiol.2482062110. [DOI] [PubMed] [Google Scholar]
- 65.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. doi:10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 66.Kansara M, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]