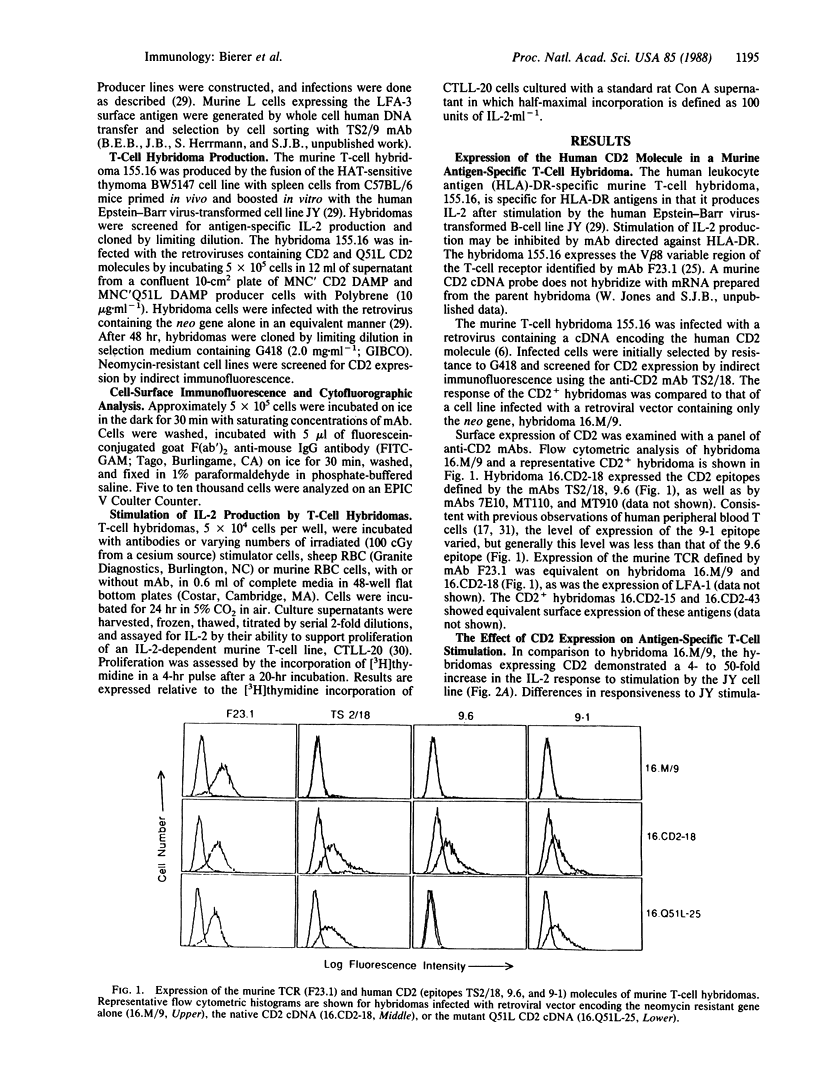
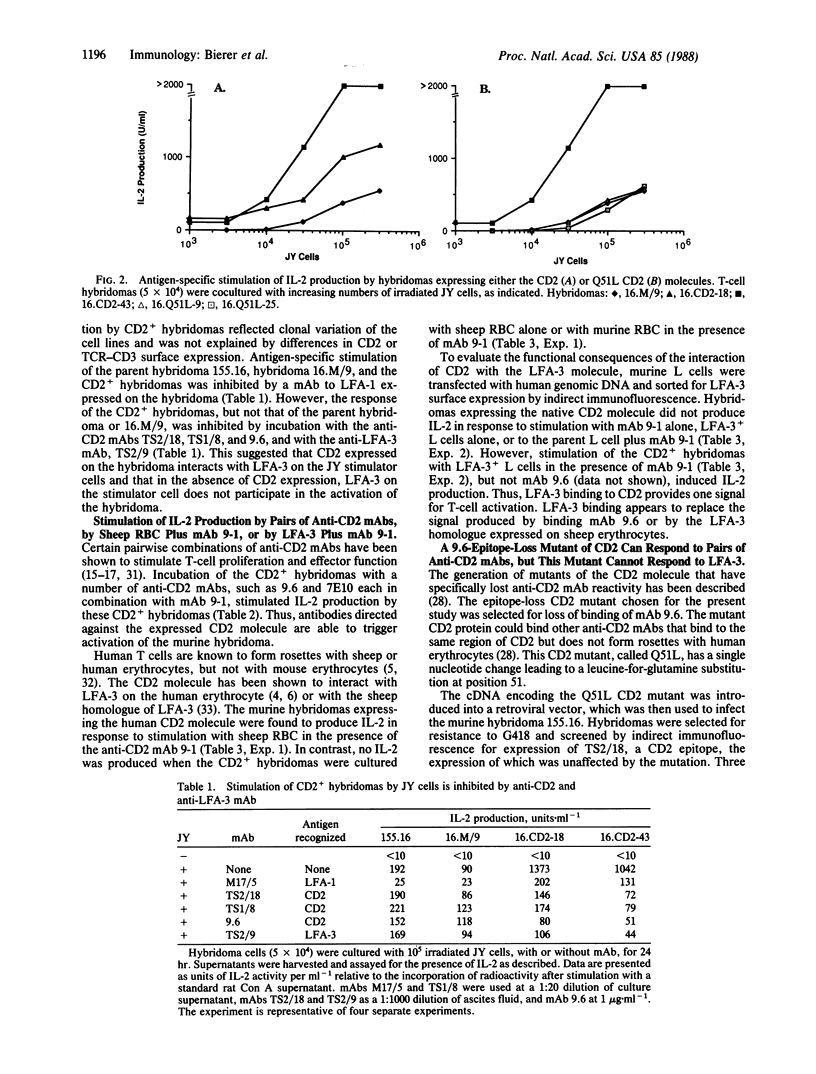
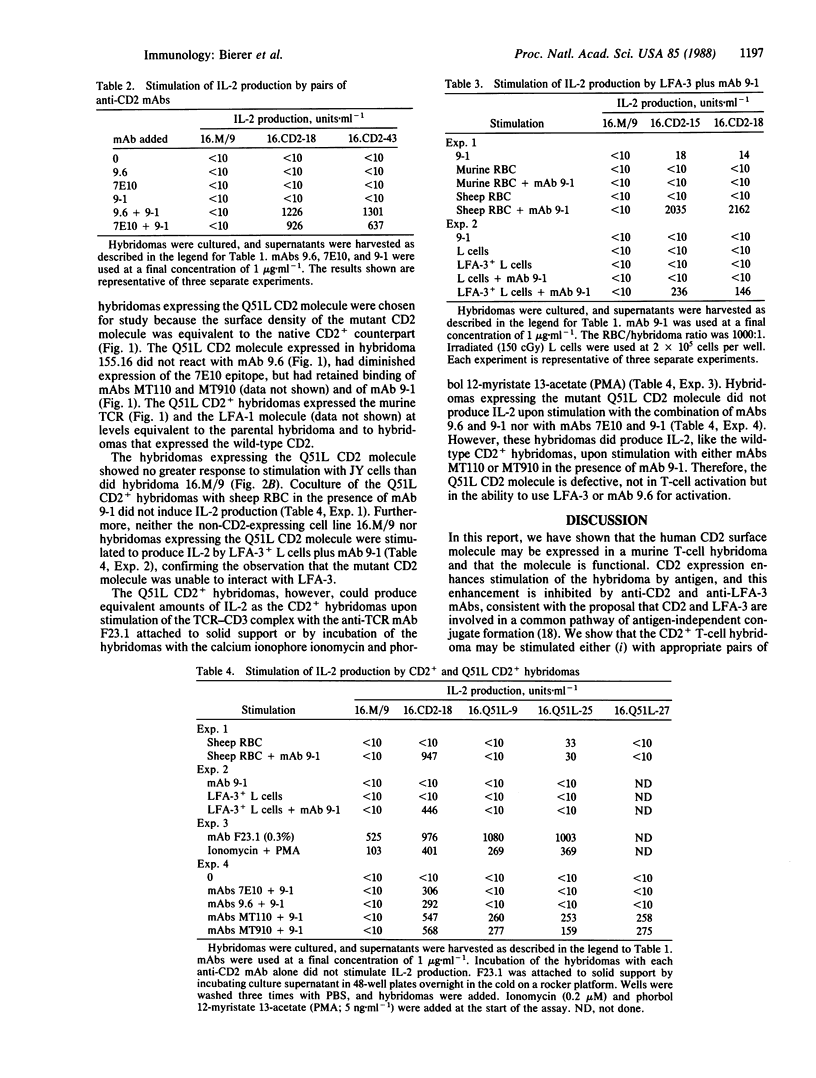
Abstract
To define the role of the CD2-lymphocyte function-associated antigen 3 (LFA-3) interaction in T-cell activation, we have expressed a cDNA encoding the human CD2 molecule in a murine antigen-specific T-cell hybridoma. Expression of the CD2 molecule greatly enhances T-cell responsiveness to antigen; this enhancement is inhibited by anti-CD2 and anti-LFA-3 monoclonal antibodies (mAbs). CD2+ hybridomas produce interleukin 2 in response to combinations of anti-CD2 mAbs 9.6 and 9-1 and, in the presence of mAb 9-1, to sheep erythrocytes or to the LFA-3 antigen. Furthermore, hybridomas expressing a mutant CD2 molecule that has lost mAb 9.6 binding do not exhibit the enhanced response to antigen or the ability to respond to LFA-3 plus mAb 9-1, but these hybridomas retain the ability to respond to combinations of anti-CD2 mAbs. The role of the CD2-LFA-3 interaction in T-cell activation and the potential for other physiologic ligands for CD2 are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Baxley G., Bishop G. B., Cooper A. G., Wortis H. H. Rosetting of human red blood cells to thymocytes and thymus-derived cells. Clin Exp Immunol. 1973 Nov;15(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Bernard A., Gelin C., Raynal B., Pham D., Gosse C., Boumsell L. Phenomenon of human T cells rosetting with sheep erythrocytes analyzed with monoclonal antibodies. "Modulation" of a partially hidden epitope determining the conditions of interaction between T cells and erythrocytes. J Exp Med. 1982 May 1;155(5):1317–1333. doi: 10.1084/jem.155.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Brottier P., Boumsell L., Gelin C., Bernard A. T cell activation via CD2 [T, gp50] molecules: accessory cells are required to trigger T cell activation via CD2-D66 plus CD2-9.6/T11(1) epitopes. J Immunol. 1985 Sep;135(3):1624–1631. [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Holter W., Fischer G. F., Majdic O., Stockinger H., Knapp W. T cell stimulation via the erythrocyte receptor. Synergism between monoclonal antibodies and phorbol myristate acetate without changes of free cytoplasmic Ca++ levels. J Exp Med. 1986 Mar 1;163(3):654–664. doi: 10.1084/jem.163.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Johansen K. S., Johansen T. S., Talmage D. W. T cell rosette formation in primates, pigs, and guinea pigs. The influence of immunosuppresive agents. J Allergy Clin Immunol. 1974 Aug;54(2):86–93. doi: 10.1016/0091-6749(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Nalet V., Fournier C. Human autologous rosette-forming cells. III. Binding of erythrocytes from different species to the T-cell receptors for autologous red blood cells. Cell Immunol. 1985 Nov;96(1):126–136. doi: 10.1016/0008-8749(85)90345-4. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O. Is the E receptor on human T lymphocytes a "negative signal receptor"? J Immunol. 1982 Dec;129(6):2479–2485. [PubMed] [Google Scholar]
- Peterson A., Seed B. Monoclonal antibody and ligand binding sites of the T cell erythrocyte receptor (CD2). 1987 Oct 29-Nov 4Nature. 329(6142):842–846. doi: 10.1038/329842a0. [DOI] [PubMed] [Google Scholar]
- Plunkett M. L., Sanders M. E., Selvaraj P., Dustin M. L., Springer T. A. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3). J Exp Med. 1987 Mar 1;165(3):664–676. doi: 10.1084/jem.165.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Jones W. K., Foran J. A., Greenstein J. L., Seed B., Burakoff S. J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Davignon D., Ho M. K., Kürzinger K., Martz E., Sanchez-Madrid F. LFA-1 and Lyt-2,3, molecules associated with T lymphocyte-mediated killing; and Mac-1, an LFA-1 homologue associated with complement receptor function. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Takai Y., Reed M. L., Burakoff S. J., Herrmann S. H. Direct evidence for a receptor-ligand interaction between the T-cell surface antigen CD2 and lymphocyte-function-associated antigen 3. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6864–6868. doi: 10.1073/pnas.84.19.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wauwe J., Goossens J., Decock W., Kung P., Goldstein G. Suppression of human T-cell mitogenesis and E-rosette formation by the monoclonal antibody OKT11A. Immunology. 1981 Dec;44(4):865–871. [PMC free article] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]



