Abstract
Background
Much progress has been made in the treatment of lung cancer in the last ten years (adjuvant chemotherapy, targeted therapy, individualized therapy). Nonetheless, lung cancer is still the leading cause of death due to cancer and thus remains a major medical, scientific, and social problem.
Method
This review is based on national and international recommendations and selected articles from the literature.
Results
Cigarette smoking is the major pathogenic factor for lung cancer. Lung cancer can be divided into two major types that differ in their biological behavior, small cell lung cancer and non-small cell lung cancer. Whenever possible, the diagnosis should be confirmed by biopsy, the extent of disease should be documented in detail (international TNM classification/staging), and the patient’s functional level should be assessed with a view toward treatment planning. Surgery for non-small cell lung cancer with curative intent is possible up to stage IIIA, while stage IIIB is the domain of radiotherapy. Surgery for small cell lung cancer with curative intent is possible for rare cases in early stages (T1N0 and T2N0, i.e., stage IA and IB). As long as small cell lung cancer is restricted to one side of the chest, simultaneous radiation therapy and chemotherapy are indicated. If a malignant pleural effusion or distant metastases are present, both lung cancers are treated palliatively with platinum-based chemotherapy.
Keywords: lung cancer, diagnosis, treatment, targeted therapy, cigarette smoking
Lung cancer is by far the most common malignant tumor originating in the lung. The four major histological types of lung cancer are:
squamous cell carcinoma (30% to 40% of lung cancers)
adenocarcinoma (25% to 30%)
non-small cell lung carcinoma (less than 10%), and
small cell lung carcinoma (15% to 20%).
These four types are subdivided into numerous subtypes (1). A notable subtype is bronchoalveolar carcinoma (synonym: alveolar cell carcinoma), a rare subtype of adenocarcinoma, that lines the alveoli as it grows. Lung cancers can be classified according to a variety of criteria. Histologically a distinction is made between small cell lung carcinoma (15% to 20%) and non-small cell lung carcinoma, because of differences in their biological behavior and the implications of these differences for treatment and prognosis.
Because of the effectiveness of particular therapies, non-small cell lung carcinomas are divided into squamous cell carcinoma and non-squamous cell carcinoma, and they are characterized using techniques of molecular pathology. More than 30% of lung cancers have elements of a variety of histological types (1).
The learning goals for the reader are:
To become familiar with the diagnostic workup for lung cancer
To acquire an overview of the principles of stage-dependent treatment.
This article is based on an evaluation of national and international recommendations and on a selective literature review.
Epidemiology, etiology, and pathogenesis
Lung cancer is the third most frequently diagnosed cancer in Germany in both men and women (2). The annual incidence in Germany is 65 per 100 000 for men and 21 per 100 000 for women. The peak incidence is between the ages of 75 and 80 years (2). At the same time, both in Germany and worldwide, lung cancer is the most frequent cause of death from cancer among men, and in Germany it is the third most frequent cause of death from cancer among women. In men the figures are steady or slightly reducing, but in women the rate is going up. Both incidence and mortality rates reflect cigarette consumption in a given population about 20 years ago (3) (Figure).
Graphic.
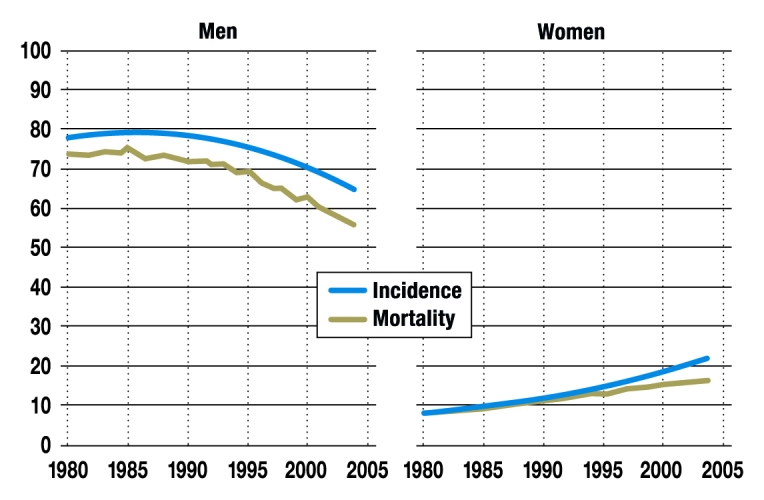
Incidence and mortality
Course over time of age-adjusted incidence (blue) and mortality (green) from lung cancer (left: men; right: women) in Germany from 1980 to 2004 (cases and deaths per 100 000 population) (2). Reproduced by kind permission of the Robert Koch Institute
Basic histological classification.
Because of differences in their biological behavior, the distinction between small cell lung cancer and non-small cell lung cancer is important.
Exogenous noxae have a decisive role in the development of lung cancer—in particular, cigarette smoke inhalation. About 90% of lung cancers may be ascribed to this cause (3). Once smoking has stopped, the risk of developing lung cancer reduces over time. Other relevant etiological factors are occuptional exposure to asbestos, polycyclic hydrocarbons (in soot and tar), chromates, arsenic, and nickel. Radon, a gaseous radioactive decay product of uranium, is naturally present as background radiation that varies in intensity from region to region and is also present in uranium mines. In both cases it can cause local clustering of cases of lung cancer (3). It is estimated that between 9% and 15% of lung carcinomas have causes other than cigarette smoking (3). The carcinogenesis of a lung cancer probably goes back years or even decades. During this period, the affected cells undergo numerous changes at the molecular level, which eventually lead to an invasively growing lung carcinoma.
Smoking as a cause of lung cancer.
Cigarette smoking is by far the most important factor in the development of lung cancer.
Symptoms/clinical presentation
Most patients with lung cancer have symptoms at the time they are diagnosed. However, there are no specific early symtoms. The symptoms of lung cancer (box) may be caused by endobronchial growth, intrathoracic extension, or distant metastases. In addition, systemic signs of cachexia and, occasionally, also symptoms of paraneoplastic syndrome may be encountered.
Box. Symptoms and findings of lung cancer and frequency distribution of important symptoms*1.
Symptoms and findings of endobronchial growth
Cough (8% to 75%), hemoptysis (6% to 35%), pain, wheezing (0% to 2%), poststenotic pneumonia, dyspnea (3% to 60%), stridor (0% to 2%)
Symptoms and findings of intrathoracic extension
Chest pain (20% to 49%), hoarseness, upper airway inflow obstruction, Horner’s triad, pleural effusion, pericardial effusion, dysphagia, raised diaphragm
Systemic signs of cancer
Weight loss (0% to 68%), night sweats, fatigue, fever (0% to 20%)
Symptoms and findings of distant metastases
Bone pain (6% to 25%), headache, neurological or psychiatric abnormalities, paraplegia, hepatomegaly, pathological fractures
Symptoms of paraneoplastic syndromes
Cushing syndrome, syndrome of inappropriate ADH secretion, Lambert-Eaton syndrome, Perre-Marie-Bamberger syndrome, etc.
*1 Frequency data from [11]; ADH, antidiuretic hormone
Diagnosis
The diagnostic workup for lung cancer must include histological confirmation of the diagnosis, evaluation of how far the tumor has spread (staging), and an analysis of the patient’s functional status with a view to treatment possibilities. The scope of the workup must be always governed by the patient’s overall situation and the prognosis that can be achieved. For example, one would not embark on complicated invasive diagnostic procedures for precise N-staging in a patient who already has extensive confirmed distant metastases. In non-small cell lung cancer in particular, important aspects of therapy depend on accurate staging and on accurate evaluation of the patient’s functional status. The sections on evaluating the extent of disease and evaluating functional status therefore relate to non-small cell lung cancer.
Early symptoms.
Lung cancer has no real early symptoms, so it is often only diagnosed when it has reached an advanced stage.
Histological confirmation
For treatment, the most important thing is to distinguish between small cell and non-small cell lung cancer. Distinguishing between various subtypes of non-small cell cung cancer is also becoming increasingly important because of variations in the licensing and effectiveness of particular chemotherapeutics and targeted therapies. For this reason, histological confirmation is carried out in all cases if possible. In situations in which it is impossible to obtain a biopsy specimen by reasonable means, an unambiguous cytology result is adequate. Bronchoscopy allows confirmation of the primary tumor with a sensitivity of 0.88 for central tumors and 0.78 for peripheral tumors (4), and can provide information for T-staging and cytology samples for N-staging. In addition, transthoracic puncture, guided by ultrasound, fluoroscopy, or computed tomography (CT) may be needed. Only exceptionally is endobronchial or esophageal ultrasonography necessary in addition to confirm the diagnosis, but they can be useful for mediastinal lymph node staging. So far as possible without running excessive risks, histological confirmation from a lung specimen is preferable to confirmation on the basis of a metastatic sample.
Evaluating the extent of disease
For small cell and non-small cell lung cancer, the international staging system is used (Tables 1 and 2) (5, 6). In small cell lung cancer a further distinction is made between “limited disease” (i.e., disease limited to one hemithorax) and “extensive disease” (extension beyond one hemithorax) (7).
Table 1. TNM classification of lung cancer in the 6th edition (5, 6) and proposal for the 7th edition (25).
| 6th edition | Proposal for the 7th edition | |||
| T | TX | Primary tumor cannot be assessed, or evidence of malignant cells in sputum or bronchial lavage fluid but no visualization of tumor on imaging or bronchoscopy | TX | Primary tumor cannot be assessed, or evidence of malignant cells in sputum or bronchial lavage fluid but no visualization of tumor on imaging or bronchoscopy |
| T0 | No evidence of primary tumor | T0 | No evidence of primary tumor | |
| Tis | Carcinoma in situ | Tis | Carcinoma in situ | |
| T1 | Tumor ≤ 3 cm greatest diameter, surrounded by lung tissue or visceral pleura, no bronchoscopic evidence of invasion proximal to the lobar bronchus (i.e., main bronchi are free)*1 | T1 | Tumor ≤ 3 cm greatest diameter, surrounded by lung tissue or visceral pleura, no bronchoscopic evidence of infiltration proximal to the lobar bronchus (i.e., main bronchi are free)*1 | |
| T1a | Tumor ≤ 2 cm greatest diameter | |||
| T1b | Tumor > 2 but ≤ 3 cm greatest diameter | |||
| T2 | Tumor > 3 cm or tumor with one of the following features: | T2 | Tumor > 3 but ≤ 7 cm with one of the following features: | |
|
|
|||
| Associated atelectasis or obstructive pneumonia extending as far as the hilus but not involving the whole lung | ||||
| T2a | Tumor > 3 but ≤ 5 cm greatest diameter | |||
| T2b | Tumor > 5 but ≤ 7 cm greatest diameter | |||
| T3 | Tumor of any size with direct invasion of one of the following structures: | T3 | Tumor > 7 cm or any tumor with direct invasion of one of the following structures: | |
|
|
|||
| or tumor in the main bronchus < 2 cm distal to the carina, without involvement of the carina and without associated atelectasis or obstructive pneumonia of the whole lung | or tumor in the main bronchus < 2 cm distal to the carina, without involvement of the carina and without associated atelectasis or obstructive pneumonia of the whole lung or satellite tumor nodule(s) in the same lobe | |||
| T4 | Tumor of any size invading one of the following structures: | T4 | Tumor of any size invading one of the following structures: | |
|
|
|||
| or separate tumor nodule(s) in the same lobe or tumor with malignant pleural* 2 or pericardial effusion | ||||
| N | NX | Regional lymph nodes could not be evaluated | NX | Regional lymph nodes could not be evaluated |
| N0 | No regional lymph node metastases | N0 | No regional lymph node metastases | |
| N1 | Metastasis/metastases in the ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary lymph nodes, including involvement by direct extension of the primary tumor | N1 | Metastasis/metastases in the ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary lymph nodes, including involvement by direct extension of the primary tumor | |
| N2 | Metastasis/metastases in the ipsilateral mediastinal and/or subcarinal lymph nodes | N2 | Metastasis/metastases in the ipsilateral mediastinal and/or subcarinal lymph nodes | |
| N3 | Metastasis/metastases in the contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene or supraclavicular lymph nodes | N3 | Metastasis/metastases in the contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene or supraclavicular lymph nodes | |
| M | MX | Distant metastases could not be evaluated | MX | Distant metastases could not be evaluated |
| M0 | No distant metastases | M0 | No distant metastases | |
| M1 | Distant metastases, including separate tumor nodules in another pulmonary lobe | M1 | Distant metastases | |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural*2 nodes or malignant pleural (or pericardial) effusion | |||
| M1b | Distant metastases | |||
*1 A superficially extending tumor with invasion limited to the bronchial wall is always classified as T1.
*2 In patients with lung cancer, pleural and pericardial effusions are usually due to the tumor. In a few patients, however, if multiple cytological examinations are negative for the presence of tumor cells, the effusion is not hemorrhagic and is not an exudate. If these factors are present, and if the clinical evaluation also indicates that the effusion is not caused by the lung cancer, the effusion should not be taken into account as a staging criterion.
Table 2. Staging of lung cancer according to TNM findings (see Table 1)*1.
| 6th edition | Proposal for the 7th edition | |||||||
| T | N | M | T | N | M | |||
| Occult carcinoma | X | 0 | 0 | X | 0 | 0 | ||
| 0 | is | 0 | 0 | is | 0 | 0 | ||
| I | IA | 1 | 0 | 0 | IA | 1a, b | 0 | 0 |
| IB | 2 | 0 | 0 | IB | 2a | 0 | 0 | |
| II | IIA | 1 | 1 | 0 | IIA | 1a, b | 1 | 0 |
| 2a | 1 | 0 | ||||||
| 2b | 0 | 0 | ||||||
| IIB | 2 | 1 | 0 | IIB | 2b | 1 | 0 | |
| 3 | 0 | 0 | 3 | 0 | 0 | |||
| III | IIIA | 1–3 | 2 | 0 | IIIA | 1,2 | 2 | 0 |
| 3 | 1 | 0 | 3 | 1,2 | 0 | |||
| 4 | 1,0 | 0 | ||||||
| IIIB | 4 | 0–2 | 0 | IIIB | 4 | 2 | 0 | |
| Any | 3 | 0 | Any | 3 | 0 | |||
| IV | Any | Any | 1 | IV | Any | Any | 1a, b | |
T-descriptor: The most significant examination for T-descriptor assessment is contrast-enhanced CT. In a few situations, magnetic resonance imaging (MRI) can deliver more detailed information about invasion of thoracic structures (8). In patients with a Pancoast tumor, MRI is absolutely essential in order to assess invasion of the vascular and neural structures of the brachial plexus and for operative planning.
Diagnosis.
The diagnostic workup of lung cancer includes histological confirmation, staging (diagnosis of extent of disease), and characterization of the patient’s functional status.
N-descriptor: Contrast-enhanced chest CT has a sensitivity between 51% and 64% and a specificity between 74% and 86% in staging mediastinal lymph nodes (8), which makes it inadequate as a sole procedure for evaluating the mediastinum in a patient without distant metastases. The most accurate noninvasive procedure for mediastinal N-staging is positron emission tomography (PET) or PET-CT; however, with a sensitivity of 74% and a specificity of 85% (8), it is by no means perfect. If an enlarged lymph node is present (>1 cm), the sensitivity of PET/PET-CT rises to 100%, with a specificity of 78% (9). If curative therapy is intended, PET-positive mediastinal lymph nodes are biopsied for definitive analysis. Available methods for this, depending on the localization of the target lymph node, are mediastinoscopy (including video-assisted mediastinal lymphadenectomy), endobronchial and esophageal ultrasonography, and transbronchial needle aspiration (TBNA) (10). PET and PET-CT allow exact identification of the target node for biopsy confirmation for lymph node staging.
M-descriptor: In general, distant metastases speak against treatment with curative intent. The most frequent locations of distant metastases are brain, liver, skeleton, lungs, and adrenals.
Suitable imaging procedures for detecting distant metastases are:
Contrast-enhanced cranial CT or MRI
Bone scintigraphy
Ultrasonography
CT or MRI of the liver and adrenals
PET, PET-CT.
The patient history, clinical findings, and lab results can provide important clues to whether there are any distant metastases and point the way to the next diagnostic steps (e1). Especially in patients with N2 or N3 tumors at diagnosis, there must be a raised expectation of asymptomatic distant metastases.
In general, the only reason not to aim at potentially curative therapy is when distant metastases have been confirmed by biopsy or may be regarded as definite on the basis of clinical or radiological findings.
PET and PET-CT in the staging of non-small cell lung cancer: PET/PET-CT imaging is of central importance in tumor staging. Ruling out distant metastases by a negative finding saves further diagnostic procedures, while the detection of structures suggestive of metastasis can guide the next step and move the diagnostic process rapidly forward. In patients in clinical stages IB to IIIB, in whom curative therapy should be attempted, PET/PET-CT scanning (if available) should be carried out for mediastinal N-staging and for M-staging; in stage IA this examination should be considered (8).
Staging.
Staging of the extent of disease follows the internationally accepted staging classification.
Evaluating functional status
The initial evaluation of lung function is governed by the risk carried by the planned treatment and the quality of life that it would achieve. In addition, cardiovascular co-morbidities and any severe impairment of liver or renal function are taken into account. Detailed recommendations exist for the assessment of functional operability (12). A patient with a forced expiratory volume (FEV1) of more than 80% of the norm (FEV1 > 80% of norm) (or >2.0 L) and a diffusion capacity of more than 80% of the norm may be referred without further evaluation for chest surgery up to and including pneumonectomy. If either of these values is below 80% of the norm, further evaluation with spiroergometry is required. If this shows a maximum oxygen consumption above 75% of the norm or if the value is greater than 20 mL/min/kg, the patient is a suitable candidate for surgery up to and including pneumonectomy.
If the maximum oxygen consumption is below 40% of the norm or less than 10 mL/min/kg, functional inoperability must be assumed. If the maximum oxygen consumption is between these limits, postoperative FEV1 values are estimated on the basis of quantitative ventilation/perfusion scintigraphy of the lung. If these values are above 40% of the norm, the operative procedure envisaged can be carried out; if they are below 40% of the norm, the patient must be regarded as inoperable. If one of the values is above and one below 40% of the norm, the decision is guided by the maximum oxygen consumption calculated for the postoperative status, and in this case 35% of the norm or 10 mL/min/kg is taken as the threshold for operability.
Special aspects of the diagnostic workup of small cell lung cancer
In addition to history, clinical exam, and routine laboratory tests, the diagnostic workup of small cell lung cancer should include CT of the chest and abdomen (at least liver and adrenals), bone scintigraphy, and contrast-enhanced cranial CT or cranial MRI. PET is not recommended for regular staging (7). PET/PET-CT has the potential to determine tumor stage more accurately in some patients, and to prevent unnecessary treatment if distant metastases are discovered or, conversely, to allow radiochemotherapy if distant metastases are ruled out. Future guidelines may therefore give PET/PET-CT a larger role in small cell lung cancer.
Basic diagnostic procedures.
Basic diagnostic procedures include CT of the chest and upper abdominal organs, bronchoscopy, and usually bone scintigraphy. Further diagnostic procedures are added as required.
Treatment
Local therapy modalities are surgery and radiotherapy. For systemic therapy, conventional chemotherapy and increasingly also targeted therapies (i.e. interventions that affect tumor-specific structures at the molecular level) are employed. Chemotherapy is polychemotherapy—so long as the patient’s condition permits.
Treatment for lung cancer is often multimodal. Radiotherapy and chemotherapy can be administered simultaneously as radiochemotherapy. Chemotherapy, radiotherapy, and radiochemotherapy may precede surgery (neoadjuvant therapy) or may follow it (adjuvant therapy).
If a lung tumor with mixed histology contains a combination of small cell lung cancer and non-small cell lung cancer, it should be treated as small cell lung cancer.
The German S3 guidelines on lung cancer are in preparation. The guidelines of the American College of Chest Physicians (ACCP) dating from 2007 are based on a systematic analysis of the published data. In these guidelines, the quality of the evidence on which a recommendation is based is graded as high (A), intermediate (B), or low or very low (C). The outline of therapy given below, where it relies on central statements of the ACCP Guidelines, reproduces their grading of the evidence (A–C) (13).
Treatment of non-small cell lung cancer
Stages I and II: In 25% to 30% of cases of non-small cell lung cancer, the diagnosis is made at this early stage (14). For patients without contraindications for surgery, resection is the treatment of choice (A). The oncosurgical procedure includes among other things lobectomy, bilobectomy (removal of two adjacent pulmonary lobes), and pneumonectomy with systematic mediastinal lymphadenectomy. After complete resection, platinum-based adjuvant chemotherapy is recommended for stage II tumors (A), but not generally for stage I tumors except in clinical trials. Adjuvant radiotherapy is not recommended after complete resection. In patients with stage I or II tumors who cannot undergo surgery, radiotherapy with curative intent is indicated (B) (14).
Distant metastases.
Specific abnormalities in the clinical history and examination (see Box) and in laboratory results (blood profile, GOT, AP, and calcium should be determined) indicate the presence of distant metastases.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Where these questions relate to TNM classification and staging, the 6th edition (4, 5) is used.
Question 1
Why is the distinction between small cell lung cancer and non-small cell lung cancer important?
Because it distinguishes lung cancers due to cigarette smoking from those that arise independently of this noxa.
Because it distinguishes lung cancers due to occupational noxae from those that arise independently of such noxae.
Because it allows lung cancers to be distinguished on the basis of their biological properties.
Because it distinguishes lung cancers with early symptoms from those without early symptoms.
Because it distinguishes lung cancers of ectodermal from those of endodermal origin.
Question 2
In the course of the carcinogenesis of lung cancer, changes in cell biology occur. What, according to the current state of knowledge, is required for malignant transformation to take place?
Spontaneous mutation of the EGF receptor, leading to constant activation of receptor tyrosine kinase
Spontaneous mutation of the EGF receptor, leading to constantly reduced responsiveness of receptor tyrosine kinase
Simultaneous malignant transformation of several cells
Several cumulative changes in cell biology
Influence of two separate noxae over a long period
Question 3
What initial test(s) should be carried out in a patient at high risk of lung cancer who is suspected of having lung cancer?
Mediastinoscopy
Transbronchial needle aspiration
Endobronchial ultrasonography
PET or PET-CT
Thoracic CT and bronchoscopy
Question 4
What is the place of PET/PET-CT in the diagnostic workup of lung cancer?
PET/PET-CT is chiefly used for research purposes in lung cancer.
PET/PET-CT is used when available for patients with non-small cell lung cancer for whom curative therapy is being considered.
In most cases of non-small cell lung cancer, PET/PET-CT replaces biopsy confirmation.
In all cases of non-small cell lung cancer, PET/PET-CT replaces biopsy confirmation of suspected distant metastases.
PET/PET-CT is mainly used in small cell lung cancer, less often in non-small cell lung cancer.
Question 5
In a patient with non-small cell lung cancer for whom curative operative therapy is to be attempted if possible, a PET-positive lymph node is discovered. What should be the next step?
Start palliative radiotherapy
Curative resection
Histological/cytological confirmation
CT
PET-CT
Question 6
A patient has a non-small cell lung tumor in the right upper lobe, diameter 2.5 cm. There is no contact with the pleura, no endobronchial tumor growth, and no distant metastasis. The surgical specimen from upper lobe resection with systematic mediastinal lymphadenectomy shows involvement only of intrapulmonary lymph nodes.
In what stage is this tumor according to the 6th edition of the staging and classification system?
Stage IA
Stage IB
Stage IIA
Stage IIB
Stage IIIA
Question 7
In a 65-year-old patient in good general condition and with unrestricted functional status, a non-small cell lung cancer 5 cm in diameter is discovered in the left lower lobe. The tumor is in contact with the visceral pleura; the main bronchi appear free on endoscopy; cytological analysis of a left-side pleural effusion shows malignancy; CT shows markedly enlarged lymph nodes on both sides of the trachea; PET shows no signs of distant metastases.
What is the correct procedure?
Start palliative irradiation
Start palliative chemotherapy
Start radiochemotherapy
Start neoadjuvant chemotherapy followed by curative surgery if the response is good
Perform resection with curative intent followed by adjuvant chemotherapy
Question 8
Which procedure is central to the evaluation of the T descriptor in the diagnosis of disease extent?
Contrast-enhanced CT
PET
Magnetic resonance imaging
Endobronchial ultrasonography
Chest X-ray
Question 9
In which age group is the incidence of lung cancer highest?
45–50 years
55–60 years
65–70 years
75–80 years
85–90 years
Question 10
Which treatment for stage I–III small cell lung cancer, if there is no malignant effusion or distant metastasis, is supported by high-grade evidence, so long as the patient’s general condition allows it?
Platinum-based chemotherapy and radiotherapy
Chest radiotherapy alone
Chemotherapy alone
Chemotherapy with a receptor kinase inhibitor
Surgery followed by adjuvant chemotherapy
Stage IIIA: 15% to 20% of non-small cell lung cancers are diagnosed at this stage (1). This stage corresponds to T3N1M0 status. These tumors are often—except in the presence of contraindications—operated on like stage I and II non-small cell lung cancer. The operation is followed by adjuvant chemotherapy (15). Stage IIIA also includes tumors with N2 status (10% of all non-small cell lung cancers) and those on the borderline between operability and nonoperability (stage IIIB). This is a very heterogenous group, ranging from cases in which N2 lymph nodes were unexpectedly found in the histological analysis of the operative specimen, to N2 lymph nodes unexpectedly found intraoperatively, and single or multiple lymph nodes known about preoperatively, to “bulky disease” in the mediastinum. In cases where an N2 situation is discovered intraoperatively, the operation should be carried out as planned together with systematic lymphadenectomy of the involved nodes (C). When an N2 situation is discovered intra- or postoperatively and the patient’s general condition is good, adjuvant platinum-based chemotherapy should be given (A) and radiotherapy should be considered (C). When the N2 status is confirmed preoperatively and lymph node involvement is not extensive (no bulky disease), treatment is tailored individually on an interdisciplinary basis (C). For these patients, as for those with bulky N2 disease, platinum-based radiochemotherapy is recommended (B), and in patients whose general condition is good both parts of the therapy should be carried out simultaneously.
Functional status.
In addition to determining co-morbidities, the functional part of the diagnostic workup must establish whether adequate lung function would be expected to remain after any planned curative treatment.
Stage IIIB: About 10% to 15% of patients with non-small cell lung cancer have stage IIIB disease at the time of diagnosis (16). This stage is the domain of radiochemotherapy. Patients whose disease is staged as IIIB on the ground of malignant pericardial or pleural effusion (which for the next classification in the future have been proposed to belong to stage IV) can only be treated palliatively, like patients in stage IV. For patients whose disease is staged as IIIB because of satellite disease in the same lobe and who do not have N2 status, operative treatment should be considered (C).
Non-small cell lung cancer Stages I to II.
In these stages treatment is given with curative intent. Stages I and II are the domain of surgical treatment.
In all other cases, in which the IIIB staging is principally on the basis of N3 status (no pericardial or pleural effusion), a platinum-based radiochemotherapy regimen is the method of choice (A), and in patients in good general condition simultaneous therapy should be preferred to sequential therapy. In the case of patients in poor general condition or who have experienced more than 10% weight loss, the decision must be taken whether to carry out radiochemotherapy sequentially (C). In all other cases, if tumor symptoms are present, palliative radiotherapy can be carried out.
Stage IV: In 40% to 50% of patients, those in whom non-small cell lung cancer is diagnosed when it has reached stage IV, only palliative treatment can be offered (17). The success of chemotherapy depends on appropriate selection of the patients. General condition as assessed using the Karnofsky index/ECOG performance status, age, and co-morbidities are decisive factors.
A milestone in the palliative therapy of non-small cell lung cancer occurred when the effectiveness of platinum-based chemotherapy was demonstrated in comparison to supportive therapy (median survival 6.5 vs. 3.6 months) (18). The combination of platinum with a modern combination partner (a taxane, gemcitabine, vinorelbine) leads to survival times of around 10 months. The chemotherapy not only lengthens life, but in most patients also improves symptoms. For patients in good general condition, combination therapy with two substances (usually platinum-based) is recommended (A). At the age of 70 to 80 years, monotherapy is preferred (A) (unless there are no relevant co-morbidities [B]). After the age of 80, treatment decisions are taken on an individual basis (C).
Recent times have seen increasing individualization of the treatment of non-small cell lung cancer. In bevacizumab (a monoclonal antibody against vascular endothelial growth factor, VEGF), for the first time a licensed targeted therapy is available for first-line treatment of non-squamous non-small cell lung cancer in combination with chemotherapy. The chemotherapeutic pemetrexed is licensed for first-line treatment of non-squamous non-small cell lung cancer. Both these substances have increased overall survival in patients with stage IIIB/IV non-squamous non-small cell lung cancer to over 12 months (19, 20).
Non-small cell lung cancer Stage III.
In stage IIIA, management depends on N status Stage IIIB is the domain of radiochemotherapy.
Other factors such as the expression of endothelial growth factor (EGF) receptors or other structures associated with carcinogenesis, and the expression of chemotherapy-resistant factors such as ERCC-1, can also play a role in guiding treatment.
In second and later lines of treatment, the chemotherapeutic docetaxel (e2) and the tyrosine kinase inhibitor erlotinib (e3) are licensed for all non-small cell lung cancers. Pemetrexed is also licensed for second-line therapy for non-squamous cell lung cancer (e4), and the receptor tyrosine kinase inhibitor gefitinib (e5) is licensed for all lines of treatment of non-small cell lung cancer with activating mutations in the EGF receptor. Maintenance or consolidative therapy (e6) is at present not recommended in advanced lung cancer. Recently studies have been published showing that patients in good general condition (ECOG 0 or 1) after four to six cycles of a platinum-based combination treatment have a survival advantage from receiving maintenance/consolidative therapy (with chemotherapy or targeted therapy). Pemetrexed is licensed for non-squamous cell cancers for maintanance therapy (e7). A final evaluation and recommendation on this subject is awaited.
Treatment of small cell lung cancer
Stages I–III: In these stages a combination of platinum-based chemotherapy and radiotherapy is indicated (A). If the patient’s general condition allows and the patient has limited disease, radiochemotherapy should be carried out simultaneously and the irradiation should be accelerated and hyperfractionated (7).
Patients with a malignant pericardial or pleural effusion are treated as for stage IV disease. In stage I (T1N0, T2N0, stages IA and IB), operative treatment followed by adjuvant chemotherapy may be considered (C).
Stage IV: In most patients (60% to 70%) the disease is at this stage when it is diagnosed (7). Palliative chemotherapy takes the form of four to six cycles of platinum-based chemotherapy (B). Etoposide and irinotecan are recommended combination partners, although irinotecan is not licensed for this indication. If complete extrathoracic remission is achieved, consolidative or remission-promoting thoracic radiotherapy may be considered (C).
Non-small cell lung cancer Stage IV.
In 40% to 50% of patients, the diagnosis is made in stage IV. Only palliative therapy can be offered to these patients.
Recurrent or refractory disease: Disease that recurs earlier than 3 months after completion of initial treatment is regarded as refractory. Irrespective of the time of recurrence, a further course of chemotherapy should be offered if the patient’s general condition allows (B) (7). If there is a good response to the first treatment protocol with a relatively long disease-free interval, the first protocol may be repeated. Topotecan is licensed for second-line use, or else an anthracycline-based protocol may be employed (e8, e9).
Prophylactic cranial irradiation: All patients who have achieved complete remission, or have undergone curative resection in stage I, should be offered prophylactic cranial irradiation (PCI) (B, C) (7). There is now reason to believe that a broad group of patients, including those with only partial remission, benefit from PCI (21).
Undesired drug effects during chemotherapy
All chemotherapeutic substances used to treat lung cancer are frequently or very frequently associated with bone marrow depression. This toxicity can require the use of growth factors, transfusion of red or white blood cell concentrate, dose adjustments, and even the interruption of therapy. Because of nausea and vomiting, adequate antiemetic premedication should be provided when any of the chemotherapeutics under discussion are given, especially the platinum-based combinations. In addition, with some substances nephrotoxicity (e.g., cisplatin), neurotoxicity (e.g., of taxanes), or cardiotoxicity (e.g., vinorelbine, taxanes) must be taken into account. Hair loss after chemotherapy is usually reversible. Cisplatin therapy must be accompanied by fluid and electrolyte administration. Treatment with taxanes must be preceded by prophylactic measures against allergic reactions. Treatment with pemetrexed must be accompanied by folic acid and vitamin B12 replacement.
Small cell lung cancer Stages I to III.
In stages I to III a combination of platinum-based chemotherapy and radiotherapy is indicated, if the patient’s general condition allows and if there is no malignant effusion.
Other treatment options
In palliative situations, radiotherapy and in special cases also surgery are used to treat local tumor-related problems. Examples are irraditation to alleviate pain caused by tumor (radiotherapy for pain), radiotherapy or surgical treatment of brain metastases, and palliative resection of infected tumors. Existing or imminent bronchial obstruction can be treated by interventional endoscopy, transcutaneous irradiation, or afterloading intraluminal brachytherapy. Pleurodesis and pericardiodesis are also available for palliative intervention.
Prognosis
The 5-year survival rate in lung cancer is around 15% and is closely dependent on stage. Table 3 gives the prognosis for the various stages of non-small cell lung cancer with appropriate treatment. For small cell lung cancer, the prognosis of which is measured in weeks to months if untreated, the median survival with treatment is 16 to 22 months for limited disease and about 10 months for extensive disease (7).
Table 3. Prognosis of non-small cell lung cancer*1.
| Clinical stage | Pathological stage | |||
| 5-year survival | Median survival | 5-year survival | Median survival | |
| IA | 50% | 60 months | 73% | 119 months |
| IB | 40% | 37 months | 54% | 70 months |
| IIA | 24% | 38 months | 48% | 54 months |
| IIB | 25% | 18 months | 38% | 33 months |
| IIIA | 18% | 14 months | 25% | 23 months |
| IIIB | 8% | 10 months | 19% | 16 months |
| IV | 2% | 6 months | 21% | 18 months |
*1 The cases on which this prognosis classification is based were collected as part of the development of a proposal for a new staging and classification system, but were grouped together into stages according to the current 6th edition of this system for an analysis of prognosis (25). Both clinical stage (evaluated preperatively) and pathological stage (evaluated postoperatively on the basis of the surgical specimen) are shown
Early diagnosis—screening
At the present time, screening for lung cancer is not recommended (22– 24). In studies with regular “low-dose” CT, lung cancers were diagnosed in early stages and thus with a better prognosis. Whether “low-dose” CT can also reduce the disease-related mortality—as is required for a screening program—is currently being investigated in large studies.
If lung cancer is suspected in a patient, especially one at high risk of developing lung cancer, chest CT and bronchoscopy should be carried out.
Small cell lung cancer Stage IV.
In stage IV small cell lung cancer, palliative platinum-based chemotherapy is indicated, if the patient’s general condition allows.
Prevention
All measures that reduce cigarette smoking reduce the incidence of lung cancer (3).
Prognosis.
The 5-year survival in lung cancer is around 15% and is strongly dependent on disease stage.
Screening.
At present, screening examinations for lung cancer are not recommended.
Further information on cme.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 5/2010.
The CME unit „Principles of Pediatric Emergency Care“ (issue 45/2009) can be accessed until 18 December 2009.
For issue 1–2/2010 plan to offer the topic „Drug Resistant Tuberculosis“.
Solutions to the CME questionnaire in issue 41/2009: Suckfüll M: Perspectives on the Pathophysiology and Treatment of Sudden Idiopathic Sensori-neural Hearing Loss:
1b, 2d, 3a, 4b, 5c, 6a, 7e, 8a, 9c, 10b
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA
Footnotes
Conflict of interest statement
Professor Hammerschmidt has received lecture fees and travel expenses from Roche and lecture fees from GlaxoSmithKline. Professor Wirtz declares that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Brambilla E, Lantuejoul S. Pathology and immunhistochemistry of lung cancer. Eur Resp Monogr. 2009;44:15–35. [Google Scholar]
- 2.Batzler WU, Giersiepen K, Hentschel S, et al. Robert Koch Institut, V. GdeKiDe. Contributions to Federal Health Reporting. Berlin: Mercedes Druck; 2008. Cancer in Germany, 2003-2004 Incidence and Trends. [Google Scholar]
- 3.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:29–55. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 4.Rivera MP, Mehta AC. Initial diagnosis of lung cancer: ACCP -evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:131–148. doi: 10.1378/chest.07-1357. [DOI] [PubMed] [Google Scholar]
- 5.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 6.Wittekind C, Meyer HJ, Bootz F. TNM-Klassifikation maligner -Tumoren. Berlin, Heidelberg, New York: Springer; 2002. [Google Scholar]
- 7.Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:324–339. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:178–201. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–889. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 10.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:202–220. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 11.Spiro SG, Gould MK, Colice GL. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:149–160. doi: 10.1378/chest.07-1358. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M, Gatzemeier U, Goerg R, et al. [Recommendations on the diagnosis of bronchial carcinoma. German Pneumology Society] Pneumologie. 2000;54:361–371. doi: 10.1055/s-2000-6949. [DOI] [PubMed] [Google Scholar]
- 13.McCrory DC, Lewis SZ, Heitzer J, Colice G, Alberts WM. Methodology for lung cancer evidence review and guideline development: ACCP evidence-based clinical practice guidelines. Chest. ((2nd Edition)) 2007;132:23–28. doi: 10.1378/chest.07-1346. [DOI] [PubMed] [Google Scholar]
- 14.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:234–242. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 15.Robinson LA, Ruckdeschel JC, Wagner H, Jr., Stevens CW. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:243–265. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 16.Jett JR, Schild SE, Keith RL, Kesler KA. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical prac-tice guidelines. Chest. ((2nd edition)) 2007;132:266–276. doi: 10.1378/chest.07-1380. [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:277–289. doi: 10.1378/chest.07-1381. [DOI] [PubMed] [Google Scholar]
- 18.Socinski MA, Morris DE, Masters GA, Lilenbaum R. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123:226–243. doi: 10.1378/chest.123.1_suppl.226s. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 21.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 22.Bach PB, Silvestri GA, Hanger M, Jett JR. Screening for lung cancer: ACCP evidence-based clinical practice guidelines. Chest. ((2nd edition)) 2007;132:69–77. doi: 10.1378/chest.07-1349. [DOI] [PubMed] [Google Scholar]
- 23.Hammerschmidt S. [Early diagnosis of lung cancer: where do we stand?] MMW Fortschr Med. 2006;148:28–30. 21. [PubMed] [Google Scholar]
- 24.Manser RL, Irving LB, Stone C, Byrnes G, Abramson M, Campbell D. Screening for lung cancer. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD001991.pub2. CD001991. [DOI] [PubMed] [Google Scholar]
- 25.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- e1.Silvestri GA, Littenberg B, Colice GL. The clinical evaluation for detecting metastatic lung cancer. A meta-analysis. Am J Respir Crit Care Med. 1995;152:225–230. doi: 10.1164/ajrccm.152.1.7599828. [DOI] [PubMed] [Google Scholar]
- e2.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- e3.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- e4.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- e5.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatinpaclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. Epub 2009 Aug 2019. [DOI] [PubMed] [Google Scholar]
- e6.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance peme-trexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. Epub 2009 Sep 1418. [DOI] [PubMed] [Google Scholar]
- e7.Stinchcombe TE, West HL. Maintenance therapy in non-small cell lung cancer. Lancet. 2009;374:1398–1400. doi: 10.1016/S0140-6736(09)61598-1. Epub 2009 Sep 1318. [DOI] [PubMed] [Google Scholar]
- e8.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- e9.O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]


