Abstract
We report the direct observation of a bent geometry for a non-stabilized nitrile imine in a metal-coordination crystal. The photoinduced tetrazole ring rupture to release N2 appears to depend on the size of voids around the N3-N4 bond in the crystal lattice. We further observed the selective formation of 1,3-addition product when a reactive nitrile imine was photo-generated in water. Taken together, the bent nitrile imine geometry agrees with the 1,3-dipolar structure, a transient reactive species that mediates the photoinduced 1,3-dipolar cycloaddition in the aqueous medium.
The photoinduced ring-opening of 2,5-diphenyltetrazole with the generation of N2 and a nitrile imine was first reported by Huisgen et al. in 1967.1 As a highly reactive dipole, nitrile imine reacts readily with a variety of dipolarophiles2 to form the 5-membered ring heterocycles.3 Recently, we have employed the photo-generated nitrile imines for functionalization of an alkene-containing protein in living cells.4 Whereas crystal structures of the stabilized nitrile imines have been reported,5 the non-stabilized N-aryl nitrile imines have only been spectroscopically observed as transient intermediates in the low-temperature matrices.6
Four alternative structures have been postulated for the non-stabilized nitrile imines: propargylic, allenic, 1,3-dipolar, and carbenic structure (Scheme 1). So far, theoretic calculations of the nitrile imine structures have generated the conflicting results in the literature. For example, in 1993 a high-level calculation study with the configuration interaction (QCISD) and a large basis-set concluded that the stable nitrile imine structure has a non-planar, allenic geometry and that the propargylic structure does not correspond to a local minimum on the potential energy surface.7 More recent DFT calculations in combination with the natural resonance theory indicated that all four resonance structures are necessary for a full description and that the carbenic form dominates for F-CNN-F and H2N-CNN-NH2.8 In contrast, a spin-coupled valence bond calculation using the geometry from a CASSCF calculation suggested that the stable electronic structure of H-CNN-H is predominantly propargylic.9 To provide direct evidence, herein we report the use of photocrystallography10 to observe for the first time the structure of a non-stabilized nitrile imine generated photochemically in situ in the solid state.
Scheme 1.

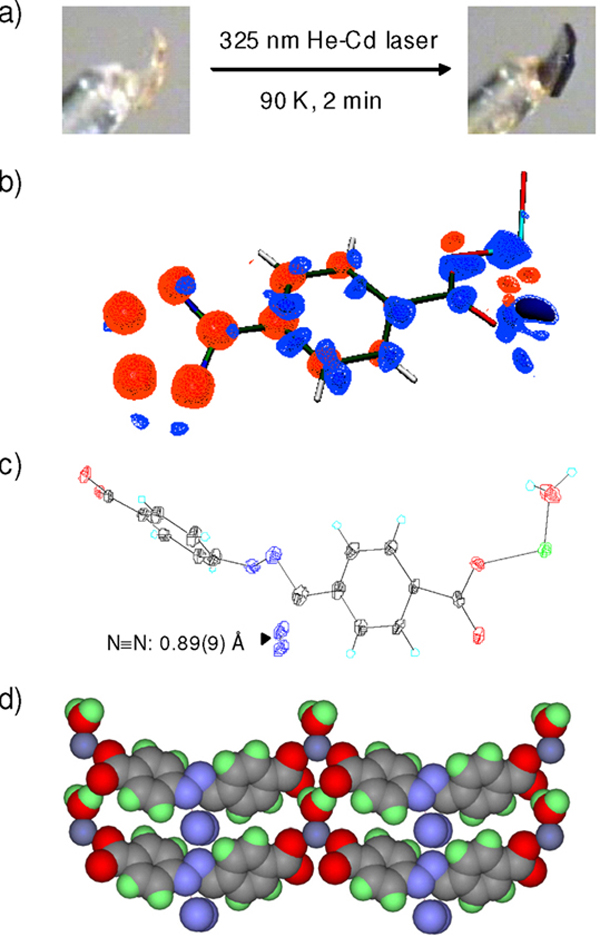
In our initial study, a crystal of 2-(4'-methoxyphenyl)-5-(2”-iso-propoxy-4”-methoxyphenyl)-tetrazole2b was photoirradiated with a 325-nm He-Cd laser (45 mW/cm2) at 280 K for 12 h. While the crystal showed a darkening of its color, no products could be detected in the X-ray photodifference map, defined as the difference in electron density after- minus before-laser exposure. A closer examination revealed that the tetrazole molecules are tightly packed in the crystal lattice with the distance between dissociating N3–N4 atoms and an adjacent methyl group on the neighboring tetrazole equal to 2.65 Ǻ, resulting in a very small void for N2 to escape (Figure S1 in the Supporting Information). To overcome this problem, we envisioned that the void next to N3–N4 can be enlarged by the use of rigid hydrogen-bonded supramolecular frameworks formed by complexing carboxylates with Zn.11 To test this, we prepared a small panel of N-aryl tetrazoles carrying carboxyl groups and/or potential hydrogen-bond donors such as NH2 and OH (1–6) (Figure 1). The crystals of Zn-tetrazole complexes were obtained by allowing ~17 mM tetrazole solutions (dissolved in 2:1 MeOH/H2O and mixed with Zn(NO3)2 and NH4OH) to stand in air at room temperature for 2 weeks (Figure 2; see Table S1 for crystal data and structural refinement). Structural analyses indicated the Zn to be tetra-coordinated with two carboxylates and two waters in all structures except the Zn-tetrazole 4 structure in which two NH3 serve as the ligands (Figure 2d). A close examination of the tetrazole packing revealed that the empty spaces (voids) vary significantly with the distance between N3–N4 and the nearest surrounding atoms being 2.66, 2.90, 3.21, 2.43, 2.70, and 2.35 Å, respectively (Figure 2).
Figure 1.
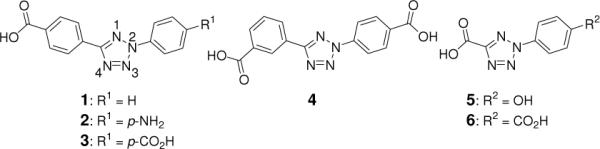
Structures of the tetrazole compounds used in this study.
Figure 2.

Crystal structures of Zn-tetrazole complexes: (a) Zn•12•(H2O)2; (b) Zn•22; (c) Zn•3•(H2O); (d) Zn•4•(NH3)2; (e) Zn•52•(H2O)4•(CH3OH)2; (f) Zn•62•(H2O)4•(NH4)2. Zn is shown in silver. The distances between N3–N4 and the nearest surrounding atoms are marked on the structures.
To test the photoreactivity, all six crystals were exposed to the 325-nm He-Cd laser beam. Whereas the crystals of Zn-2–5 showed slow decay indicated by color darkening (Figure 3a), only the Zn-tetrazole 3 crystal afforded a discrete photodifference map after 2-min photoirradiation at 90 K (Figure 3b). This is consistent with the fact that crystal 3 has the largest void around N3–N4 (3.21 Å in Figure 2c). Subsequent least-square refinement gave a 13% yield of the corresponding nitrile imine product. The dissociated N2 was visible in the photodifference map, with a bond length of 0.89(9) Å (Figure 3c), within experimental error of its value in molecular N2 (1.09 Å). The occupancy of N2 in the crystal lattice was 8%, less than 13% for the nitrile imine, suggesting that part of the N2 has escaped from the crystal lattice. Since apart from the CNN center tetrazole 3 structure is symmetric, the photodifference map showed a two-fold symmetry (Figure 3b). Using a free geometry refinement model,12 we fit the electron density to two symmetry-related nitrile imine geometries (only one is shown in Figure 3c).13 Evidently, in the solid state nitrile imine adopted a bent geometry with an increased twisting of the flanking phenyl rings (dihedral angle = 62.1° for the nitrile imine vs. 38.8° for the tetrazole 3; compare Figure 3c to Figure 2c) to allow the trapping of the escaping N2 in the intra-strand space (Figure 3d). The photoreactivity of 3 is not due to the electronic effect of the carboxylic groups as photoirradiation of the Zinc-free crystal of 3, which has smaller voids around N3–N4 in the crystal (Figure S2), did not yield a recognizable photodifference map.
Figure 3.
Photocrystallography of Zn-tetrazole 3 complex: (a) Color change of the crystal upon laser exposure. (b) Photodifference map based on the Fo,(after)-Fo (before). Blue, 2.0; light blue, 1.0; orange, −1.0; red, −2.0 e/A3. Only one half of the map is shown because of the 2-fold symmetry. (c) ORTEP representation of the geometry-refined nitrile imine structure. (d) Packing of the nitrile imines and molecular N2 in the crystal lattice. The N≡N bonds are perpendicular to the plane of view.
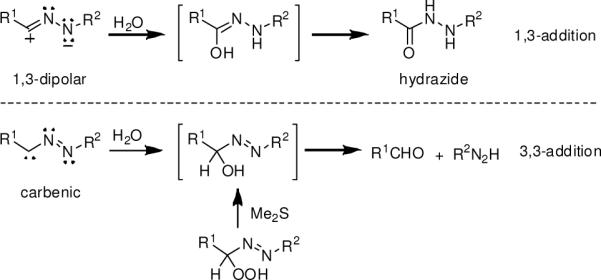
The bent geometry of the nitrile imine can be ascribed to either the 1,3-dipolar or carbenic structure (Scheme 1). These two can be distinguished by a water-quenching experiment; it is expected that the dipolar structure undergoes 1,3-addition to generate a hydrazonic acid intermediate which tautomerizes to afford the stable hydrazide while the carbenic structure undergoes 3,3-addition14 to generate metastable α-hydroxyazobenzene which decomposes slowly to produce benzaldehyde and phenyl-diazene15 (Scheme 2). When tetrazole 3 was irradiated at 302 nm in acetonitrile/water (1:1), the 1,3-addition product was found to be the major product in the product mixture based on 1H-NMR (Figure S3). Moreover, water-quenching of the nitrile imine derived from reactive 2-phenyl-5-p-methoxyphenyl tetrazole4b yielded exclusively the 1,3-addition product with no traces of benzaldehyde (Figure S4), thereby excluding the existence of the carbenic structure. To ensure there is no cross-over between the 1,3- and 3,3-addition pathways, we prepared α-hydroxyazobenzene separately from α-azohydroperoxide and followed its decay in the NMR tube in CD3CN/D2O (1:1). We found the major product to be benzaldehyde with no traces of hydrazide (Figure S5). Hence, we propose that the 1,3-dipolar structure represents the major electronic structure of the photo-generated nitrile imine. The bent geometry in the 1,3-dipolar structure can explain the high reactivity of the photo-generated nitrile imines in the cycloaddition reactions in the aqueous medium4 because of the lower activation barriers, the result of dipole structural pre-organization.16
Scheme 2.
In summary, we report the direct observation of a photo-generated, bent nitrile imine structure in a Zn-coordination crystal. The efficiency of tetrazole ring rupture in the solid state appears to depend on the size of the void around the N3–N4 bond. A water-quenching study suggested that the bent geometry represents the 1,3-dipolar form, a major electronic structure involved in the photoinduced 1,3-dipolar cycloaddition in the aqueous medium.
Supplementary Material
Acknowledgment
We acknowledge the National Science Foundation (CHE0236317 and CHE0843922 to P. C.) and the NIH (GM 85092 to Q. L.) for financial support.
Footnotes
Supporting Information Available: Experimental procedures, structural information with CCDC CIF numbers. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Clovis JS, Eckell A, Huisgen R, Sustmann R. Chem. Ber. 1967;100:60–70. [Google Scholar]
- (2).(a) Wang Y, Vera CIR, Lin Q. Org. Lett. 2007;9:4155–4158. doi: 10.1021/ol7017328. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Hu WJ, Song W, Lim RK, Lin Q. Org. Lett. 2008;10:3725–3728. doi: 10.1021/ol801350r. [DOI] [PubMed] [Google Scholar]
- (3).(a) Padwa A, Nahm S, Sato E. J. Org. Chem. 1978;43:1664–1671. [Google Scholar]; (b) Meier H, Heimgartner H. Helv. Chim. Acta. 1985;68:1283–1300. [Google Scholar]
- (4).(a) Song W, Wang Y, Qu J, Lin Q. J. Am. Chem. Soc. 2008;130:9654–9655. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Song W, Hu WJ, Lin Q. Angew. Chem. Int. Ed. 2009;48:5330–5333. doi: 10.1002/anie.200901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For an excellent review, see: Bertrand G, Wentrup C. Angew. Chem. Int. Ed. 1994;33:527–545.; and references therein.
- (6).(a) Toubro NH, Holm A. J. Am. Chem. Soc. 1980;102:2093–2094. [Google Scholar]; (b) Wentrup C, Fischer S, Maquestiau A, Flammang R. Angew. Chem., Int. Ed. 1985;24:56–57. [Google Scholar]
- (7).Wong MW, Wentrup C. J. Am. Chem. Soc. 1993;115:7743–7746. [Google Scholar]
- (8).Mawhinney RC, Muchall HM, Peslherbe GH. Chem. Commun. 2004:1862–1863. doi: 10.1039/b407302a. [DOI] [PubMed] [Google Scholar]
- (9).Cargnoni F, Molteni G, Cooper DL, Raimondi M, Ponti A. Chem. Commun. 2006:1030–1032. doi: 10.1039/b517030c. [DOI] [PubMed] [Google Scholar]
- (10).(a) Coppens P, Zheng S-L, Gembicky M. Z. Kristallogr. 2008;223:265–271. [Google Scholar]; (b) Coppens P. Synchr. Rad. News. 1997;10:26–30. [Google Scholar]; (c) Kawano M, Hirai K, Tomioka H, Ohashi Y. J. Am. Chem. Soc. 2001;123:6904–6908. doi: 10.1021/ja067306b. [DOI] [PubMed] [Google Scholar]; (d) Kawano M, Hirai K, Tomioka H, Ohashi Y. J. Am. Chem. Soc. 2007;129:2383–2391. doi: 10.1021/ja067306b. [DOI] [PubMed] [Google Scholar]
- (11).Zheng SL, Vande Velde CM, Messerschmidt M, Volkov A, Gembicky M, Coppens P. Chem. Eur. J. 2008;14:706–713. doi: 10.1002/chem.200701037. [DOI] [PubMed] [Google Scholar]
- (12).We did not use the theoretically calculated values of the bond lengths and the bond angles for the structural refinement of nitrile imines because they remain controversial; see discussion in the introduction.
- (13).See Table S2 in the Supporting Information for the bond-lengths and bond angles of the nitrile imine structure.
- (14).Du X, Fan H, Goodman JL, Kesselmayer MA, Krogh-Jespersen K, LaVilla JA, Moss RA, Shen S, Sheridan RS. J. Am. Chem. Soc. 1990;112:1920–1926. [Google Scholar]
- (15).Baumstark AL, Vasquez PC. J. Org. Chem. 1983;48:65–69. [Google Scholar]
- (16).(a) Ess DH, Houk KN. J. Am. Chem. Soc. 2007;129:10646–10647. doi: 10.1021/ja0734086. [DOI] [PubMed] [Google Scholar]; (b) Ess DH, Houk KN. J. Am. Chem. Soc. 2008;130:10187–10198. doi: 10.1021/ja800009z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.